Abstract
The lampreys (Petromyzontiformes), one of the two surviving groups of agnathan (jawless) vertebrates, currently consist of 41 recognized species. This group has an antitropical distribution, with the 37 species of Northern Hemisphere lampreys assigned to the Petromyzontidae, whereas the four species of Southern Hemisphere lampreys are separated into either the Geotriidae (one species) or Mordaciidae (three species). All lamprey species have a blind and microphagous, burrowing larva (ammocoete), which spends a number of years in the soft sediment of creeks and rivers, after which it undergoes a radical metamorphosis. Eighteen lamprey species then embark on an adult parasitic phase (nine at sea and nine in fresh water) during which they increase markedly in size, whereas the other 23 species do not feed as adults and remain in fresh water. On the basis of morphology, 17 of the 23 non-parasitic species each evolved from a particular parasitic species whose descendants are still represented in the contemporary fauna. The remaining six non-parasitic species, the so-called “southern relict” species, have no obvious potential ancestral parasitic species, implying they have diverged markedly from their parasitic ancestor or that the parasitic ancestor is now extinct. Many of the main taxonomic characteristics reside in features that are associated with parasitic feeding, for example, the type and arrangement of the teeth on the suctorial disc and tongue-like piston. The phylogenetic relationships, derived by maximum parsimony analyses of morphological and anatomical data for the 18 parasitic species, were similar in most respects to those obtained by subjecting molecular data (cytochrome b mitochondrial DNA sequence data) for those species to Bayesian analyses. However, in contrast to the results of morphological analyses, the genera Eudontomyzon and Lampetra were not monophyletic when using molecular analyses. When non-parasitic species were included in the molecular analyses, some of the six relict non-parasitic species formed clades with parasitic species which, from their morphology, had been allocated by taxonomists to different genera. More genes, and particularly nuclear genes, should be used to help resolve the basis for these differences between the morphological and molecular phylogenies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
The lampreys, together with the hagfishes, are the two sole surviving groups of agnathan (jawless) vertebrates (Janvier 1981; Hardisty 2006; see Chap. 1). The possession by these two groups of “round mouths” led to them being termed by Duméril (1806), collectively, as the Cyclostomata, a term retained as a class by Holly (1933) in his important taxonomic treatise on these animals. The implication that lampreys and hagfishes formed a monophyletic group was accepted for many years. However, detailed comparisons of their anatomy, morphology, and physiology, in conjunction with comparisons to the morphology of extinct agnathans, led to an alternative viewpoint (Hardisty 1979, 1982; Janvier 1981). The latter authors came independently to the conclusion that lampreys were more closely related to the gnathostomatous (jawed) vertebrates than to the hagfishes. Since that time, however, the majority of the numerous molecular studies undertaken on the two surviving groups of agnathans have supported the monophyly of lampreys and hagfishes (e.g., Stock and Whitt 1992; Mallatt and Sullivan 1998; Kuraku et al. 1999; Delarbre et al. 2002; Takezaki et al. 2003; Blair and Hedges 2005; Kuraku and Kuratani 2006). The question of whether or not cyclostomes are considered to constitute a monophyletic group was subsequently shown by Near (2009) to be influenced by the characters used and the types of analyses employed. A subsequent study, however, by Heimberg et al. (2010), employing microRNAs and a reanalysis of morphological characters, provided such overwhelming evidence for cyclostome monophyly that it convinced Janvier (2010) that this was indeed the case.
The first fossil lamprey to be described was the beautifully-preserved Mayomyzon pieckoensis from the upper Carboniferous (c. 280 million years ago, mya) deposits of Mazon Creek in Illinois (Bardack and Zangerl 1968, 1971). This fossil clearly possessed many of the morphological and anatomical characters of the adults of extant lampreys, such as an annular cartilage, which maintains the structural integrity of the suctorial disc, a piston cartilage, dorsolateral eyes, and seven gill apertures on either side of the body. Since the landmark discovery of M. pieckoensis, a further three definitive fossil lampreys have been found. The youngest of these is Mesomyzon mengae from the lower Cretaceous of China c. 125 mya (Chang et al. 2006), followed in age by Hardistiella montanensis from lower Carboniferous deposits in Montana c. 320 mya (Janvier and Lund 1983), and then Priscomyzon riniensis from upper Devonian deposits in South Africa c. 360 mya (Gess et al. 2006). The first indisputable fossil hagfish to be discovered was Myxinikela siroka, which was found in the same geological horizon and general locality as the lamprey M. pieckoensis, and thus likewise dates back c. 300 mya (Bardack 1991, 1998). More recently, another hagfish fossil, Myxineidus gononorum, was discovered in upper Carboniferous deposits in France and is therefore also of approximately the same age as the above two fossils (Poplin et al. 2001). Germain et al. (2014) have cast doubt, however, on whether M. gononorum is a hagfish and provide evidence that it could be a lamprey.
Both groups of extant cyclostomes possess a similar body shape (Fig. 2.1) and typically have an antitropical distribution (Hubbs and Potter 1971; Hardisty 1979). Although lampreys are thereby essentially confined to temperate regions of the world, two species (genus Tetrapleurodon) are found in elevated cooler waters in a restricted sub-tropical area (Álvarez del Villar 1966). The living lampreys are represented by three families (Mordaciidae, Geotriidae, and Petromyzontidae) and 41 species (Table 2.1; Potter et al. 2014) and the hagfishes by two subfamilies (Eptatretinae and Myxininae) and approximately 60 species (Fernholm 1998). However, whereas the Mordaciidae and Geotriidae are confined to the Southern Hemisphere and the Petromyzontidae to the Northern Hemisphere, the two subfamilies of hagfishes are represented in both hemispheres.
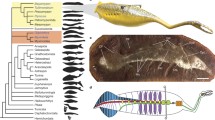
Lateral views of a a larval lamprey (ammocoete), b an adult lamprey, and c a hagfish. This figure was originally published in Hardisty et al. (1989). (Reproduced by permission of The Royal Society of Edinburgh from Transactions of the Royal Society of Edinburgh: Earth Sciences volume 80 (1989), pp. 241–254)
The aim of this chapter is to provide a comprehensive list of the species, genera, subfamilies, and families of extant lampreys, providing details of the types of morphological characters used in taxonomic studies and the distributions of each species. Emphasis is also placed on outlining the schemes that have been proposed for the interrelationships of the various species, based on morphological and molecular criteria, and discussing the implications of any differences between those schemes.
2.2 Life Cycles and “Paired Species”
The ability to describe accurately a species of lamprey and thereby facilitate its allocation to the appropriate genus and family requires both a thorough understanding of the features that characterize the divergent larval and adult stages and recognition that, in some species, the morphology changes markedly during adult life. It should also be recognized that the types of life cycle vary amongst lampreys, with some containing a parasitic adult phase whereas others do not feed after the completion of larval life (see later).
The life cycle of all lamprey species contains a protracted larval phase that is spent in fresh water (Hardisty and Potter 1971a; Potter 1980a; see Chap. 3). The larva, termed an ammocoete, has a worm-like body shape and is blind and toothless (Fig. 2.1a). The ammocoete spends most of its time burrowed in the soft substrata in the slower-flowing regions of streams and rivers, feeding on the detritus and microorganisms (e.g., diatoms) that it extracts from the water overlying its burrow (Moore and Mallatt 1980; Yap and Bowen 2003). After typically between 3 and 7 years, the ammocoete undergoes a radical metamorphosis, which leads to the development of functional eyes, a suctorial disc and protrusible tongue-like piston (both of which are armed with teeth), and enlargement of the dorsal fins (Figs. 2.1b, 2.2, and 2.3; Hardisty and Potter 1971b; Potter 1980a; Youson 1980; see Chap. 4), with metamorphosis typically occurring at body lengths of 80–200 mm (Hardisty and Potter 1971a).
Oral disc of Ichthyomyzon bdellium, showing the different fields and types of teeth and laminae and their nomenclature. Note that alate rows comprise an inner circumoral and an outer marginal, and the intervening intermediate rows of disc teeth: median anterior tooth row (MA), marginal teeth (MG), anterior field (AF), anterior circumoral teeth (AC), supraoral lamina (SO), lateral field (LF), intermediate disc teeth (IT), lateral circumoral teeth (LC), longitudinal lingual lamina (LL), transverse lingual lamina (TL), infraoral lamina (IO), posterior circumoral teeth (PC), and posterior field (PF). (This figure was originally published in Hubbs and Potter (1971) and reproduced with permission of Elsevier)
Following the completion of the larval phase, the life cycle of the lamprey diverges in one of two main directions. One course leads to the development, during metamorphosis, of a sexually immature young adult (Fig. 2.1b) that embarks on a parasitic feeding phase (Renaud et al. 2009a; Renaud and Cochran in press). The young adults of nine of these eighteen parasitic species feed at sea following a downstream migration. When fully grown they cease feeding and return to rivers, but not necessarily their natal systems, where they become sexually mature, spawn and die (Table 2.1; Hardisty and Potter 1971b; Potter et al. 2014; see Chap. 5). Five of these nine anadromous species have given rise to freshwater-resident or landlocked forms, whose immature adults feed in lakes or in the wider regions of large rivers (Table 2.1; Applegate 1950; Nursall and Buchwald 1972; see Docker and Potter in press). The remaining nine parasitic species are confined to fresh water and have essentially the same life cycle as the landlocked forms of anadromous species (Table 2.1; Hubbs and Trautman 1937; Chappuis 1939; Álvarez del Villar 1966; Renaud and Cochran in press). The maximum total length attained by parasitic species varies markedly, ranging from 145 mm in the freshwater Miller Lake lamprey Entosphenus minimus to 310–490 mm in small anadromous species, such as the western and European river lampreys (Lampetra ayresii and La. fluviatilis, respectively) to between 780 and 1,200 mm in the large anadromous pouched lamprey Geotria australis, Pacific lamprey Entosphenus tridentatus, and sea lamprey Petromyzon marinus (Oliva 1953; Vladykov and Follett 1958; Hardisty and Potter 1971b; Potter et al. 1983; Hardisty 1986; Lorion et al. 2000).
The second main direction exhibited by the lamprey life cycle involves a shifting in the timing of sexual maturation relative to metamorphosis, such that it commences during the transition from the ammocoete to the adult rather than after the completion of a parasitic phase as with the species above. The parasitic phase thus becomes eliminated and spawning takes place soon after the completion of metamorphosis (Hardisty 2006; Docker 2009). Consequently, these non-parasitic species breed at a length no greater than that of their longest ammocoetes. As most of these non-parasitic species are morphologically similar to a particular parasitic species in all aspects other than body size, it has been assumed that each evolved from that parasitic species (Potter 1980b; Docker 2009). On this basis, 15 of the 23 non-parasitic species listed in Table 2.1 can be “paired” with a congeneric parasitic species (in some cases, with a single parasitic “stem” species giving rise to more than one non-parasitic “satellite” species; Vladykov and Kott 1979a). An additional two species (Northern California brook lamprey Entosphenus folletti and Pit-Klamath brook lamprey En. lethophagus) also appear to be recent non-parasitic derivatives but, in these cases, it is not clear whether En. tridentatus or En. similis is the ancestor (Potter et al. 2014). The reader is referred to the reviews by Hardisty (2006), Docker (2009), Renaud et al. (2009b), and Docker and Potter (in press) for a comprehensive discussion of the issues surrounding the relationships between non-parasitic and parasitic species. We focus below on the taxonomic status of these species.
Despite the morphological similarities that link these non-parasitic derivative species with their presumed parasitic ancestor, a number of studies have revealed significant anatomical differences between species in at least some pairs. The differences between the non-parasitic and parasitic members of one or more species pairs include, in the non-parasitic member of the pair, a lower prevalence of pigmentation on the tongue precursor and usually fewer oocytes in the ovaries of the ammocoetes and, following metamorphosis, a less well-developed gut, a relatively smaller eye and suctorial disc, less well-developed teeth and velar tentacles, and fewer trunk myomeres (Hughes and Potter 1969; Hardisty and Potter 1971c; Potter and Osborne 1975; Vladykov and Kott 1976a, 1979a; Potter 1980b; Beamish and Thomas 1983). In exceptional cases, however, a particular trait can go in one direction in some species pairs and in the opposite direction in other species pairs. Thus, while the number of teeth in the anterior field and in the lateral and posterior fields were greater in the non-parasitic European brook lamprey Lampetra planeri than in its parasitic ancestor Lampetra fluviatilis (Hardisty et al. 1970), the number of posterial teeth in the non-parasitic Entosphenus folletti and En. lethophagus were less than in parasitic En. tridentatus and En. similis (Vladykov and Kott 1976b, 1979b).
The above differences between non-parasitic species and corresponding parasitic species indicate that there are genetic differences between such pairs. Yet, as pointed out by Docker (2009) in her extensive review, the use of molecular techniques for analyzing the genetic compositions of a number of species pairs has generally not been able to detect differences between the members of such pairs. An inability to distinguish genetically between an ancestral parasitic and derivative non-parasitic species is widespread, encompassing species in different genera and from different geographical regions (e.g., Docker et al. 1999; Docker 2006; Yamazaki et al. 2006; Espanhol et al. 2007; Blank et al. 2008; April et al. 2011). The techniques used, however, may not have provided sufficient resolution to determine whether the lack of genetic distinction merely reflects a recent divergence of a non-parasitic species from a parasitic species or lack of genetic divergence in the particular markers used (Docker 2009). In their study of the Arctic lamprey Lethenteron camtschaticum—Far Eastern brook lamprey Le. reissneri species pair, Yamazaki et al. (2006) noted that results, based on analyses of nuclear and mitochondrial genomes, were incongruent and suggested that the failure of a mitochondrial-based phylogeny to distinguish between members of a species pair may have been due to incomplete lineage sorting.
In an attempt to resolve this issue, Docker et al. (2012) examined over 10,000 base pairs of the mitochondrial genome in adults of the freshwater parasitic silver lamprey Ichthyomyzon unicuspis and its non-parasitic derivative northern brook lamprey I. fossor in populations across the Laurentian Great Lakes, and concluded that the two taxa were not reciprocally monophyletic. Where I. unicuspis and I. fossor occurred sympatrically in the Lake Huron basin, these authors further found no significant differences in mitochondrial haplotype or microsatellite allele frequencies, suggesting that, at least in this locality, there was gene flow between these species. A recent exciting study by Mateus et al. (2013a), however, has taken analyses of whether there are genetic distinctions between the members of paired species a step further. The results obtained by these authors, using restriction site-associated DNA sequencing, provided incontrovertible evidence of genome-wide divergence between La. fluviatilis and La. planeri. The validity of these conclusions is supported by the fact that the individuals of the two species used for these analyses were obtained from the same spawning site. It is particularly relevant that, in the latter study, most of the genes showing fixed allelic differences between the two species are related to functions implicated in adaptations to a freshwater-resident life style, as with La. planeri, as opposed to a migratory and anadromous mode of life, as with La. fluviatilis. The differences between the outcomes of the above studies may be due to the markers used (i.e., a small number of presumably neutral loci versus a large number of potentially functional loci) or to the species pairs examined (e.g., I. unicuspis and I. fossor are both freshwater residents). However, as these discrepancies reinforce previous suggestions that the taxonomic status of each pair should be determined individually (e.g., Docker 2009; Renaud et al. 2009b), we have adopted a conservative approach in this chapter that taxonomic changes should not be made hastily. We thus consider it appropriate to follow Renaud et al. (2009b) in continuing to regard, as distinct species, each of the non-parasitic species and its presumed parasitic ancestor that are listed in Table 2.1, recognizing that these species are separable on the basis of morphological criteria, particularly body size, and also by life style.
Although mitochondrial DNA sequence data have been unable to differentiate between parasitic and non-parasitic members of many species pairs, such data have provided sufficient resolution to distinguish among brook lamprey populations from different geographic locations, at least in some widespread species such as La. planeri and the western brook lamprey Lampetra richardsoni (e.g., Espanhol et al. 2007; Mateus et al. 2011; Boguski et al. 2012). This poses the question of whether different populations of the same species have originated independently, that is, at different times or different locations (see Docker 2009). The notion that some recognized brook lamprey species may be polyphyletic was suggested by Hubbs (1925) and Hubbs and Trautman (1937). In the absence of distinct morphological differences among such populations, however, we continue to consider these populations (despite molecular synapomorphies) to constitute a single species (see below). In this context, we have decided not to recognize three cryptic “species” which were recently described by Mateus et al. (2013b) from Portugal and belong to the Lampetra planeri complex. At present, we do not recognize these populations as specifically distinct from La. planeri for two reasons: (1) the authors did not compare the putative new species with material of La. planeri from its type locality (i.e., brooks of Thuringia, Germany; Bloch 1784); and (2) none of the putative species is morphologically diagnosable from either of the others at better than 78 %, when using a stepwise discriminant function analysis.
In addition to the 17 recent non-parasitic derivatives discussed above, the contemporary fauna also contains six non-parasitic species for which there is no obvious potential ancestral parasitic species, implying either that these species have diverged markedly from their parasitic ancestor or that the parasitic ancestor is now extinct. These so-called “southern relict” species (non-parasitic lampreys that occur at or near the extreme southern limits of distribution of the Northern Hemisphere lampreys; Hubbs and Potter 1971) are the: Western Transcaucasian brook lamprey Lethenteron ninae; Macedonia brook lamprey Eudontomyzon hellenicus; Epirus brook lamprey Eu. graecus; Kern brook lamprey Lampetra hubbsi; least brook lamprey Lampetra aepyptera; and Po brook lamprey Lampetra zanandreai (see Sect. 2.4.2).
2.3 Taxonomic Characters
A comprehensive list of the morphological characters used in the taxonomy of lampreys has been provided by Holčík (1986a) and Renaud (2011), while a list of the synapomorphies for genera and families are given in Gill et al. (2003). It should be recognized, however, that whatever characters are used, it is far more difficult to distinguish between the ammocoetes than the adults of the various species. Indeed, the ammocoetes of some species belonging to the same genus, and especially of those representing the particular parasitic and non-parasitic species that constitute a species pair, have frequently been unable to be unequivocally separated using morphological criteria (see Sect. 2.2). For example, this is the case with the Mexican lamprey Tetrapleurodon spadiceus and Mexican brook lamprey T. geminis (Álvarez del Villar 1966) and with the short-headed lamprey Mordacia mordax and precocious lamprey M. praecox (Potter 1968; Potter et al. 1968).
The main morphological characters used to describe the ammocoetes of the various species are the number of trunk myomeres, the shape of the caudal fin, and the patterns of pigmentation on various parts of their body surface and tongue precursor (Vladykov 1950; Potter and Osborne 1975; Neira et al. 1988). In contrast, the most important characters for describing the adults of the various species are those involving the dentition on the suctorial disc and piston (Figs. 2.2 and 2.3). Although this disc and dentition are not fully developed until late in metamorphosis (Bird and Potter 1979; see Chap. 4) and the dentition of one species, Lampetra aepyptera, is extremely degenerate (Fig. 2.4), the number of teeth in the various tooth series and the arrangement and shape of those teeth are very useful diagnostic tools for identifying the adults of different species (Hubbs and Trautman 1937; Vladykov and Follett 1967; Potter and Strahan 1968). The number and arrangement of the velar tentacles of adult lampreys (Vladykov and Kott 1976a), structures which guard the entrance to the water tube that leads into the branchial chamber and thus prevent large particles from entering that chamber and potentially clogging the gills (Renaud et al. 2009a), also represent valuable taxonomic tools. As with ammocoetes, the number of trunk myomeres is also often useful for identifying the adults of certain species (Hubbs and Trautman 1937; Zanandrea 1957; Iwata et al. 1985; Renaud and Economidis 2010). Although Bond and Kan (1986) suggested that myomere counts in La. richardsoni and the Pacific brook lamprey La. pacifica followed Jordan’s rule, that is, increasing in number with increasing latitude and thus decreasing temperature, Reid et al. (2011) found no such latitudinal cline in either species. Likewise, Creaser and Hubbs (1922) proposed that the Pacific lamprey comprised a northern subspecies Entosphenus tridentatus tridentatus with 68–74 trunk myomeres and a southern subspecies En. tridentatus ciliatus with 57–67 trunk myomeres, but this proposal was later dismissed as untenable (Hubbs and Potter 1971). Beamish (2010) has shown that the number, size, shape and arrangement of the papillae on the posterior rim of the gill pores of adult lampreys vary among certain species and that the central process, which lies just inside this rim in some species, varies in shape. As this latter suite of characters was capable of distinguishing between even the individuals of closely-related non-parasitic species, it clearly has considerable potential for refining the descriptions of lamprey species.
Oral disc of the least brook lamprey Lampetra aepyptera, showing the highly degenerate dentition of this non-parasitic species. (This figure was originally published in Hubbs and Potter (1971) and reproduced with permission of Elsevier)
In the case of Southern Hemisphere lampreys, a suite of characters can readily be used to distinguish the sole species of Geotria (i.e., G. australis) from those of Mordacia, the only other genus of lamprey in the Southern Hemisphere and with which it co-occurs in the rivers and coastal waters of southeastern Australia (including Tasmania) and Chile (Potter and Strahan 1968; Potter 1986). Thus, as Geotria is monotypic, the differences between G. australis and the three Mordacia species also apply at the generic, and indeed family, levels. In the case of ammocoetes, these characters include differences in body pigmentation, the position of the cloaca relative to the second dorsal fin, and the number of lobes and internal structure of their intestinal diverticula (Neira et al. 1988; Bartels and Potter 1995). The differences between the adults of G. australis and the Mordacia species are even more pronounced, and particularly so in the case of the structure of their teeth and the arrangement of their dentition (Fig. 2.2). Thus, the divergence between the two genera of Southern Hemisphere lampreys, which collectively contain only four species, is far greater than that among Northern Hemisphere lampreys, even though the latter comprise a far greater number of genera (eight) and species (37; Table 2.1). This difference in the extent of divergence is consistent with the separation of the Southern Hemisphere lampreys into two families, Mordaciidae and Geotriidae, and to the Northern Hemisphere lampreys being assigned to a single family, Petromyzontidae (Gill et al. 2003; Potter et al. 2014).
Among Northern Hemisphere lampreys, only the species of Ichthyomyzon possess a single rather than two dorsal fins (Hubbs and Trautman 1937). The ability to readily distinguish the ammocoetes of the six Ichthyomyzon species from those of other genera is particularly useful as Ichthyomyzon has a wide distribution in North America and one or more of its species are often found in the same river system as those of Petromyzon, Lampetra, and Lethenteron (Table 2.1). There are no other characters that are clearly unique to any particular Northern Hemisphere genus.
2.4 Current Taxonomic Schemes
The taxonomic scheme employed in this chapter, at the family and generic level, is based predominantly on the results of a detailed cladistic study that employed morphological characters for all parasitic species of lampreys (Gill et al. 2003). This scheme was subsequently adopted by Nelson (2006) in his fourth edition of Fishes of the World, and by Renaud (2011) in his Lampreys of the World. All authorities have recognized, for some time, that the lampreys consisted of three groups, one comprising all Northern Hemisphere species and the other two representing the two Southern Hemisphere genera (e.g., Potter and Strahan 1968; Hubbs and Potter 1971; Bailey 1980; Gill et al. 2003; Renaud 2011). Based on the large number of unique morphological characters that define each of these three groups, we still consider that they are best represented by three families, that is, Petromyzontidae for Northern Hemisphere lampreys and Geotriidae and Mordaciidae for the two Southern Hemisphere genera (Table 2.1; Gill et al. 2003). It should be noted that the common name southern striped lamprey is now used for the Geotriidae following Potter et al. (2014), rather than southern lampreys as in Nelson (2006), in order to avoid confusion with the other family of Southern Hemisphere lampreys, Mordaciidae, the common name for which is southern top-eyed lampreys. The separation of genera in the Petromyzontidae into the subfamilies Petromyzontinae and Lampetrinae follows that of Nelson (2006) in all respects, except that Caspiomyzon is placed in Petromyzontinae rather than Lampetrinae (see Potter et al. 2014 and subsequent text for rationale). The common and scientific names of all parasitic and non-parasitic species and their generic allocations follow those given in Potter et al. (2014), except in the case of Lampetra hubbsi, which was formerly referred to Entosphenus (Vladykov and Kott 1976c; see Docker et al. 1999; Goodman et al. 2009; Boguski et al. 2012). Lampetra hubbsi has now been reconfirmed by the American Fisheries Society (Page et al. 2013a) as the official species name. Other frequently used common names, for example, those adopted by the American Fisheries Society (Page et al. 2013a) or Food and Agriculture Organization (see FishBase; Froese and Pauly 2013), but not used here, are provided in Table 2.1. Renaud (2011) lists additional common names and provides synonyms for each species. A list of the authorities for each lamprey family, genus, and species is given in Appendix 2.1.
Note that, as discussed in relevant parts of the subsequent text, the results of a reanalysis of the molecular data for parasitic species, which was used by Lang et al. (2009) and employed a single gene, sometimes did not match those of the morphological analyses (Fig. 2.5a, b). Although certain implications of the molecular analyses may turn out to be valid, it was decided not to change the and mainly acrocentric generic allocation of any species until more comprehensive genetic analyses have been undertaken. The key differences, as well as similarities, in the implications of cladistic analyses of the morphological and molecular data sets are discussed in the following text.
Phylogenetic relationships among the parasitic species of the three lamprey families, derived from a morphological data using maximum parsimony analyses, and b cytochrome b sequence data using Bayesian analyses. As no molecular data were available for the parasitic Tetrapleurodon spadiceus, the cytochrome b data for Tetrapleurodon geminis, its non-parasitic derivative, were used instead. Bayesian posterior probabilities are given for those nodes where values are greater than 0.95
The taxonomy of the Southern Hemisphere lampreys was in a state of disarray until the late 1960s. There was wide disagreement regarding, not only the number of species present in Australia, New Zealand, and South America, but also the number of genera and even families that they represent (Potter and Strahan 1968). The taxonomic problems posed by Southern Hemisphere species were shown by the latter authors to have arisen largely from taxonomists not having recognized that, during its spawning run, each of these species undergoes far more extreme morphological and other alterations than any of their Northern Hemisphere counterparts. Such pronounced alterations include very marked changes in the structure and arrangement of the teeth and in the body coloration and, depending on the species, the development by males of an exceptionally large gular pouch (Potter and Strahan 1968; Potter and Welsch 1997; see Chap. 6). As a consequence, the species now designated as Geotria australis, for example, was demonstrated by Potter and Strahan (1968) to have previously been considered to constitute a total of 11 species and to represent eight genera! At the family level, there had also been disagreement, for example, as to whether G. australis should be allocated to a family on its own or included with that comprising all Northern Hemisphere species (Potter and Strahan 1968). Eventually, the Southern Hemisphere lampreys were considered to be represented by just four species, Mordacia mordax, M. praecox, Chilean lamprey M. lapicida, and G. australis (Table 2.1). As there are, however, some obvious morphological differences between the ammocoetes of G. australis from Australia, Argentina, and Chile (Neira et al. 1988), it is important that further studies be undertaken to ascertain whether Geotria comprises two or more closely-related species rather than a single species.
The two genera of Southern Hemisphere lampreys were shown by Potter and Strahan (1968) to each possess highly distinctive characteristics and that these differed from those of the group comprising Northern Hemisphere lampreys. Thus, these authors assigned these three groups to the subfamilies Mordaciinae, Geotriinae, and Petromyzoninae, which were later elevated to family level, that is, Mordaciidae, Geotriidae, and Petromyzontidae (Hubbs and Potter 1971), an arrangement that remains widely accepted (Nelson 2006). The morphological differences between the three families are paralleled by differences in their karyotypes. Thus, Mordacia species possess 76 predominantly metacentric or submetacentric chromosomes, whereas G. australis has approximately 180 small and mainly acrocentric chromosomes and the Northern Hemisphere lampreys possess 164–168 largely acrocentric chromosomes (see Potter et al. 2014).
The taxonomy of Northern Hemisphere lampreys was the subject of a number of sound studies during the first half of the last century. Such studies included a remarkably detailed and quantitative analysis by Hubbs and Trautman (1937) of the interrelationships between the various species of the exclusively freshwater genus Ichthyomyzon. These were supplemented, between 1955 and 1982, by the detailed descriptions provided by particularly Vladykov and his co-workers for species belonging to various other genera of holarctic lampreys (see Vladykov and Kott 1979c). The full list of the 37 species of Northern Hemisphere lampreys recognized here is given in Table 2.1. This list includes the 34 Northern Hemisphere species recognized in previous oft-cited reviews (e.g., Renaud 1997), plus the Drin brook lamprey Eudontomyzon stankokaramani, which was subsequently recognized as a valid species (rather than as a synonym of the Ukrainian brook lamprey Eu. mariae) by Holčík and šorić (2004), and two recently-described species, Lethenteron ninae and Eudontomyzon graecus (Naseka et al. 2009; Renaud and Economidis 2010). In his Lampreys of the World, Renaud (2011) included 36 of these species, preferring to leave Eu. stankokaramani as a synonym of Eu. mariae until a more comprehensive study of the variation in the velar tentacle morphology of the wide-ranging Eu. mariae had been undertaken. As discussed above (Sect. 2.2), we consider the three cryptic brook lamprey “species” proposed by Mateus et al. (2013b) as synonyms of La. planeri.
Most species of Northern Hemisphere lampreys have long been recognized as distinct entities on the basis of clear morphological criteria, with the result that only two new species have been described since 1982 (i.e., Naseka et al. 2009; Renaud and Economidis 2010; Appendix 2.1). Furthermore, the monotypic Petromyzon and Caspiomyzon, and also Ichthyomyzon with its six species, have each long been regarded as generically discrete. The taxonomy of Lampetra has had a rather more checkered history (reviewed by Docker et al. 1999). Thus, some workers have considered this genus to contain not only the species that are almost invariably listed for Lampetra, but also those in Lethenteron and Entosphenus, which were regarded by Hubbs and Potter (1971) as subgenera of Lampetra, and also even Tetrapleurodon and Eudontomyzon (Bailey 1980). Following the latter author, the American Fisheries Society Committee on Names of Fishes supported synonymizing Entosphenus and Lethenteron with Lampetra in the fourth and fifth editions of their Common and Scientific Names of Fishes lists (Robins et al. 1980, 1991), and added Tetrapleurodon as another synonym in the sixth edition that was expanded to include the fishes of Mexico (Nelson et al. 2004). The results of cladistic studies using morphological characters supported, however, the separate generic designation of Lethenteron, Entosphenus, Tetrapleurodon, and Eudontomyzon (Gill et al. 2003; Potter et al. 2014), and the seventh edition of the Common and Scientific Names of Fishes recognizes Entosphenus, Lethenteron, and Tetrapleurodon as genera (Page et al. 2013a). Although we follow Docker et al. (1999) and Potter et al. (2014) in also using Lampetra to include aepyptera, we recognize that its dentition, which is the most important of lamprey taxonomic characters (see Sect. 2.3), is highly degenerate and that the arrangement of the few remaining teeth and of other characters do not readily fall under the compass of those of other genera (Fig. 2.4). Indeed, Hubbs and Potter (1971) suggested that this species be allocated to a genus of its own, Okkelbergia, which was originally created as a subgenus of Lampetra by Creaser and Hubbs (1922).
Additionally, a number of putative lamprey species remain undescribed. For example, two non-parasitic species in Japan, which have been referred to as Lethenteron sp. N and Le. sp. S, are morphologically indistinguishable from each other (Yamazaki and Goto 1997) but, on the basis of molecular studies, are clearly distinct (Yamazaki and Goto 1996, 1998; Yamazaki et al. 2003, 2006). Furthermore, Boguski et al. (2012) found four morphologically cryptic, but molecularly-distinct populations of Lampetra spp. in Oregon and California. However, until these putative species have been formally described, taxonomists are not in a position to accept their validity.
2.4.1 Interrelationships Among Parasitic Taxa
A phylogeny of the lampreys was constructed in the early 2000s by subjecting, to maximum parsimony analyses, data for mainly the morphological characteristics of the parasitic species of Southern and Northern hemisphere lampreys (Fig. 2.5a; Gill et al. 2003). The analyses were restricted to the 18 parasitic species, which represent each of the currently recognized genera of lampreys, because only 20 phylogenetically-informative characters were available for analysis, which is far less than the total number of lamprey species (41). Furthermore, apart from body size, the morphological characteristics of the species comprising each pair of parasitic and non-parasitic species are often indistinguishable (see Sect. 2.2). Of the six currently recognized non-parasitic species that are morphologically distinct from extant parasitic species (i.e., the relict species; Sect. 2.2), two had not been described as of 2003 (Eu. graecus and Le. ninae) and one (La. aepyptera) is characterized by extremely degenerate dentition (Sect. 2.3). The outgroups employed for these analyses were three species of fossil from Carboniferous deposits, that is, the lampreys Mayomyzon pieckoensis and Hardistiella montanensis (Sect. 2.1) and the putative lamprey Pipiscius zangerli, and a composite fossil. It was considered inappropriate to use extant hagfishes or gnathostomes as outgroups since these groups share virtually no morphological features that can be used to establish relationships among the living lamprey species.
The above analyses revealed that there was a well-defined clade that contained all Northern Hemisphere parasitic species, which is consistent with the allocation of all Northern Hemisphere lampreys to the single family Petromyzontidae (Fig. 2.5a). Within the clade comprising Northern Hemisphere lampreys, the genera formed two major groups, the first represented by Ichthyomyzon and Petromyzon and the second by the other six genera, that is, Caspiomyzon, Tetrapleurodon, Entosphenus, Lethenteron, Eudontomyzon, and Lampetra (Fig. 2.5a). The analyses failed to resolve, however, the precise relationships between those parasitic species and the two Mordacia species and the monotypic Geotria. It is highly relevant, however, that many of the characteristics of the Northern Hemisphere species differ markedly from those of Mordacia and Geotria, which, in many respects, are also often very different (Potter and Strahan 1968; Hubbs and Potter 1971; Potter and Gill 2003; Renaud et al. 2009a). For this reason we reiterate that it is considered appropriate to continue to regard Geotria and Mordacia as representing separate families, i.e. Geotriidae and Mordaciidae (see Sect. 2.4).
The cytochrome b gene sequences (1,133 base pairs), derived by Lang et al. (2009) from samples for the parasitic species of lampreys, have been re-subjected to Bayesian analyses (Fig. 2.5b). The outgroups used for these molecular analyses represent the two subfamilies of the other extant agnathan group (i.e., the hagfishes Myxine glutinosa and Eptatretus burgeri), a gnathostome (Chimaera monstrosa) and, as in the study of Lang et al. (2009), the more distantly-related cephalochordate Branchiostoma belcheri. In the following account of the results of molecular analyses, the generic names for each species, which have been traditionally recognized on the basis of morphological criteria, have been retained (Gill et al. 2003; Docker 2009; Renaud et al. 2009a, b). Furthermore, as no molecular data were available for one of the parasitic species, Tetrapleurodon spadiceus, those for its non-parasitic derivative, Tetrapleurodon geminis, were used instead when employing molecular data to analyze the relationships of the parasitic species. It should be noted that a cladogram produced using Maximum Likelihood analysis of the cytochrome b data was essentially the same as that shown in Fig. 2.5b using Bayesian analysis.
Although the number of appropriate morphological characters available for analyses was limited and the molecular analyses were based on data for a single gene, the cladograms produced from both data sets for the parasitic species were similar in several respects (Fig. 2.5a, b). Thus, the molecular analyses also produced very strong support for a clade that comprised all Northern Hemisphere parasitic species and that, within that clade, one group that likewise contained all Ichthyomyzon species and Petromyzon, another with all Entosphenus species, and yet another the species of Lethenteron, Eudontomyzon, and Lampetra (Fig. 2.5b). The molecular analyses placed Geotria australis as the sister to the Northern Hemisphere species, albeit with very low posterior probability or bootstrap support.
The molecular analyses resulted in the “shift” of Caspiomyzon from within a clade that comprises Tetrapleurodon, Entosphenus, Lethenteron, Eudontomyzon, and Lampetra, as in the analyses conducted using morphological data, to the clade that contains Petromyzon and Ichthyomyzon (Fig. 2.5a, b and see above). Furthermore, the relationships of the species within the clade comprising Lethenteron, Eudontomyzon, and Lampetra differ from those traditionally assigned on the basis of morphology, with, for example, La. fluviatilis now being more closely related to Eu. danfordi (Carpathian lamprey) than to La. ayresii, and Eu. morii (Korean lamprey) being more closely related to Le. camtschaticum than to Eu. danfordi. It should be noted, however, that the specimen of Eu. morii used in Lang et al. (2009) was a metamorphosing individual with developing dentition, and thus possibly represents a misidentification since members of Lethenteron from the same broad geographical area are known, in some cases, to possess one or a few exolaterals.
Unlike the trends exhibited by the analyses performed by Gill et al. (2003) using morphological data (Fig. 2.5a), those involving cytochrome b provided overwhelming support for Caspiomyzon wagneri (Caspian lamprey) belonging to the clade that contained Petromyzon marinus and the Ichthyomyzon species and for Tetrapleurodon species being sister to the species of Entosphenus (Fig. 2.5b). The inference that Caspiomyzon is related to Petromyzon is consistent with an earlier proposal that the former species was derived from a Petromyzon-like species that became isolated in the Caspian Sea in probably the pre-Pleistocene (Hubbs and Potter 1971). Moreover, a closer alignment of Tetrapleurodon with Entosphenus is also consistent with an earlier taxonomic scheme in which, on the basis of similarities in their dentitions and geographical distributions, these two genera were placed in the subfamily Entospheninae (Vladykov 1972; Vladykov and Kott 1979c). For the above reasons, it is tentatively proposed that the relationships derived for the above five genera using molecular data, which are consistent with those given in the above much earlier morphological studies, are likely to be valid.
The conflicting results regarding the interrelationships among Lethenteron, Eudontomyzon, and Lampetra are more difficult to reconcile. At the morphological level, the characteristics of the species are consistent within each genus and differ between genera. Indeed, within Lampetra, the morphological characteristics of La. ayresii are so similar to those of La. fluviatilis that they were not regarded as distinct species until comprehensive and careful comparisons were undertaken by Vladykov and Follett (1958), yet several molecular studies (albeit always using mitochondrial DNA sequences; e.g., Docker et al. 1999; Lang et al. 2009) consistently place these two species in separate clades. Lang et al. (2009) were the first to suggest, after using molecular data, that Eu. morii is more closely related to Le. camtschaticum than it is to other Eudontomyzon species. This finding is interesting, particularly since Berg (1931) suggested that Eu. morii may have evolved from Le. camtschaticum but, as noted above, this conclusion was based on a single metamorphosing individual (and single, mitochondrial gene) and requires independent confirmation with other specimens and other (nuclear) genes. Thus, in view of the conflict between the phylogenetic implications of the morphological and molecular analyses regarding the above species/genera, we follow our earlier intention of retaining the original generic allocation of these species until more definitive evidence becomes available. As pointed out by Page et al. (2013b), making changes that are short-lived has the effect of confusing rather than improving the situation.
2.4.2 Relationships of Non-Parasitic Species
The inclusion in the molecular analysis of DNA sequence data for cytochrome b for non-parasitic species had essentially no influence on the interrelationships of the genera of lampreys (Fig. 2.6). Furthermore, this analysis resulted in most non-parasitic species being grouped with the parasitic species which, on the basis of morphology, is their presumed ancestor, as, for example with the three pairings within Ichthyomyzon, as originally proposed by Hubbs and Trautman (1937). Indeed, all 13 of the 17 recently-derived non-parasitic species for which molecular data were available for both parasitic and non-parasitic species (see Table 2.1, Sect. 2.2) grouped with their presumed parasitic ancestor. Additionally, Eu. stankokaramani grouped with Eu. danfordi (Lang et al. 2009). Two non-parasitic species (La. pacifica and En. folletti) and one parasitic species (T. spadiceus) were not included in Lang et al. (2009), but other studies support some of the presumed pairings (e.g., La. pacifica with La. ayresii: Boguski et al. 2012; En. folletti with En. tridentatus and other parasitic species in this genus: Docker and Reid unpublished data).
Phylogenetic relationships of the parasitic and non-parasitic species of the three lamprey families, derived from cytochrome b sequence data using Bayesian analyses: asterisks designate parasitic species. The data were derived from those employed by Lang et al. (2009) together with additional data for Mordacia mordax from New South Wales, Australia (NSW); other abbreviations Victoria (VIC), Western Australia (WA). Bayesian posterior probabilities are given for those nodes where values are greater than 0.95. The clades that included: 1 Lethenteron camtschaticum, Le. kessleri, Le. alaskense, and Le. reissneri; and 2 Le. kessleri, Le. alaskense, and Le. reissneri each had a posterior support of 1
Surprisingly, however, certain non-parasitic and parasitic species, which, from their morphology, had been allocated by taxonomists to different genera, were grouped together by this analysis. For example, analyses using cytochrome b data led to the non-parasitic species classically designated as Eudontomyzon hellenicus being aligned with Caspiomyzon wagneri (Fig. 2.6). Although Eu. hellenicus and C. wagneri both occur in Europe, there is a substantial gap between their present-day distributions (Table 2.1) and their morphological features differ in a number of conspicuous respects (Vladykov et al. 1982; Gill et al. 2003). Note that the Eu. hellenicus from the Ionian Sea basin in the cladogram by Lang et al. (2009) has now been identified as Eu. graecus and that, together with Eu. hellenicus from the Aegean Sea basin, constitute a clade that is the sister group to C. wagneri. However, although Eu. hellenicus and Eu. graecus were shown to form a clade with C. wagneri, they are still genetically very distinct from C. wagneri (i.e., differing by 10.5–10.7 % in their cytochrome b sequences, compared to the above species pairs that differed by 0–3 %; see Docker and Potter in press). Furthermore, the presence of two synapomorphies in the two brook lampreys from Greece, namely, a wide supraoral lamina and a very large median tooth on the transverse lingual lamina (Renaud and Economidis 2010), as well as in the parasitic members of the genus (i.e., Eu. danfordi and Eu. morii), and their absence in C. wagneri (Gill et al. 2003) emphasize the importance of using more than just a single genetic marker in the future to resolve the relationships among the above taxa.
Holčík (1986b) and Bianco (1986) placed Lampetra zanandreai in the genus Lethenteron because its lateral circumorals (endolaterals) are usually bicuspid and because posterior circumorals (posterials) are present in most specimens. This arrangement was followed by Renaud (1997), Potter and Gill (2003), and Renaud (2011). However, Kottelat and Freyhof (2009) argued that, while these two characters may be useful in diagnosing species, they are not useful in defining lineages. We have therefore reverted to the original generic assignment, which is consistent with the molecular-based cladogram that shows Lampetra zanandreai within a Eurasian Lampetra clade (Fig. 2.6).
The Kern brook lamprey was originally assigned by Vladykov and Kott (1976c) to the genus Entosphenus on the basis of its dentition (reviewed in Docker et al. 1999). The molecular analyses of Lang et al. (2009) place this species in a clade together with La. ayresii–La. richardsoni and, as mentioned above (Sect. 2.4), this species is now recognized as Lampetra hubbsi (Page et al. 2013a). As mentioned previously, the very pronounced degeneration of the dentition of La. aepyptera has hindered an unequivocal generic assignment of this species. Molecular analyses suggested that this species, which is confined to eastern North America, resembles more closely La. fluviatilis, which is restricted to Europe, than La. ayresii, which occurs along the western seaboard of North America (Lang et al. 2009; Fig. 2.6). This is consistent with the results of Docker et al. (1999), who used neighbor-joining analysis of cytochrome b and NADH dehydrogenase subunit 3 (ND3) DNA sequences. However, the analyses of Lang et al. (2009) indicate that La. aepyptera is also related to two species of Eudontomyzon, which, like La. fluviatilis, are confined to European waters. It is thus noteworthy that, in the cladogram produced from molecular data, two clades (Fig. 2.6; node with 0.99 posterior probability) tended to comprise species from either the Atlantic Ocean basin (La. aepyptera + Eu. danfordi + Eu. mariae + La. fluviatilis + La. planeri + La. lanceolata + La. zanandreai) or the Pacific Ocean basin (Le. camtschaticum + Le. kessleri + Le. alaskense + Le. reissneri + Eu. morii), with the notable exception of Le. appendix, which has an Atlantic distribution, being grouped with the Pacific clade.
2.5 Distribution
The antitropical distribution of all three families of lampreys within river systems is related to the inability of ammocoetes to tolerate high temperatures. This conclusion is based on the fact that the ultimate incipient lethal temperatures for the three species for which there are such data, that is, Petromyzon marinus from North America, Lampetra planeri from Europe, and Geotria australis from Australia, are only 31.4 °C, 29.4 °C, and 28.3 °C, respectively (Potter and Beamish 1975; Macey and Potter 1978).
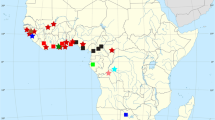
Mordacia is represented by an anadromous species in rivers and coastal marine waters of southeastern mainland Australia and Tasmania (i.e., M. mordax) and by another (M. lapicida) in those of Chile (Table 2.1; Fig. 2.7). The single non-parasitic species in this genus (M. praecox) occurs within creeks and rivers in the same geographical region as its presumed ancestor M. mordax (Potter 1980b). Since preparing this review, we have become aware of isolated pockets of ammocoetes of Mordacia in Queensland over 1,000 km to the north of the previously recorded distribution of this genus. Work is currently in progress to provide details of these populations (Moffat et al. unpublished data). In contrast to Mordacia, Geotria, which is represented solely by the large anadromous parasitic species G. australis, is found in rivers throughout temperate Australasia and southern South America and ranges widely in marine waters (Table 2.1; Fig. 2.7; Potter et al. 1979).
Distributions of the Southern Hemisphere genera of lampreys (Mordacia and Geotria) by polar projection. (Modified from Hubbs and Potter 1971)
The Northern Hemisphere genus Ichthyomyzon, which belongs to the subfamily Petromyzontinae, and comprises three parasitic species and their three respective non-parasitic derivatives (Table 2.1), is confined to river systems and lakes in central and eastern North America (Fig. 2.8). Several lines of evidence indicate that this genus either evolved in fresh water or has been confined to fresh water for a very long period (see Bartels et al. 2012). The anadromous and monotypic Petromyzon is found along the eastern and western seaboards of the North Atlantic Ocean and throughout the Mediterranean Sea and is represented by a landlocked form in North America (Fig. 2.9; Hubbs and Potter 1971; Çevik et al. 2010). Like G. australis, the large anadromous form of P. marinus ranges widely in the marine environment (Halliday 1991). Caspiomyzon, the remaining genus of the subfamily Petromyzontinae (see Sect. 2.4), and which contains only the anadromous parasitic C. wagneri, is restricted to the Caspian Sea basin (Fig. 2.8).
Distributions of four of the eight Northern Hemisphere genera of lampreys (Caspiomyzon, Entosphenus, Eudontomyzon, Ichthyomyzon) by polar projection. (Updated from Hubbs and Potter 1971)
Distributions of two of the eight Northern Hemisphere genera of lampreys (Lethenteron, Petromyzon) by polar projection. (Updated from Hubbs and Potter 1971)
The second subfamily, Lampetrinae, contains five genera (Table 2.1). Although Tetrapleurodon is unique among lampreys in that its distribution is entirely restricted to a sub-tropical area, this apparent anomaly is explained by the fact that the single parasitic and derivative non-parasitic species that comprise this genus occur only in high altitude lakes and rivers, in which the waters are relatively cool (Table 2.1; Fig. 2.10; Álvarez del Villar 1966; Cochran et al. 1996). The four parasitic and two non-parasitic species of Entosphenus are all found in drainages along the west coast of North America (Table 2.1; Fig. 2.8). Entosphenus tridentatus, the large and sole anadromous species in this genus, ranges widely throughout the North Pacific Ocean during its parasitic phase (Hubbs and Potter 1971; Fukutomi et al. 2002; Renaud 2008, 2011). While a few freshwater-resident populations of En. tridentatus have been reported along the western coast of North America, there is some uncertainty regarding their taxonomic status (e.g., Moyle et al. 2009; Taylor et al. 2012; see Docker and Potter in press). The single parasitic species of Lethenteron, Le. camtschaticum, which comprises both anadromous and landlocked forms (Heard 1966; Nursall and Buchwald 1972; Kucheryavyi et al. 2007a, 2007b), is found to the northern tip of Alaska at about 72 °N (McPhail and Lindsey 1970), which is further north than any other lamprey species. This species has a wide distribution in the Arctic Ocean, extending from the White Sea in Russia to the Beaufort Sea in Canada and southwards to Japan in the western North Pacific Ocean (Table 2.1; Fig. 2.9). Although this range encompasses those of three of its non-parasitic derivatives (i.e., Le. alaskense, Le. reissneri, and Le. kessleri), the fourth, Le. appendix, occupies drainages in middle and eastern North America and is thus separated from its presumed ancestor by nearly 2,500 km (Table 2.1; Fig. 2.9; Renaud et al. 2009b). The remaining non-parasitic species of Lethenteron, Le. ninae, whose affinity is unclear, is found in the drainage of the Black Sea (Table 2.1; Fig. 2.9).
Distributions of two of the eight Northern Hemisphere genera of lampreys (Lampetra, Tetrapleurodon) by polar projection. (Updated from Hubbs and Potter 1971)
As with Ichthyomyzon in North America, Eudontomyzon, which is confined to Eurasia, is an exclusively freshwater genus (Table 2.1; Fig. 2.8). Note that we do not recognize Eudontomyzon sp. nov. “migratory,” listed as extinct by the International Union for the Conservation of Nature (IUCN), because it was never formally described (see Kottelat et al. 2005). In Europe, the parasitic Eu. danfordi occurs in tributaries of the Danube River. One of its non-parasitic derivatives, Eu. mariae, has a wide-ranging distribution that includes drainages from the Baltic Sea in the north to the Aegean Sea in the south, whereas the other, Eu. stankokaramani, is restricted to drainages of the Adriatic Sea (Table 2.1). Two other non-parasitic species, Eu. hellenicus and Eu. graecus, whose parasitic ancestries are unclear (see Sect. 2.4.2), each occur in a single drainage on the east and west side, respectively, of the Pindus Mountain range in Greece (Table 2.1). The parasitic species Eu. morii is confined to a single drainage that traverses China and North Korea (Table 2.1).
Within the genus Lampetra, the anadromous parasitic species La. ayresii and its non-parasitic derivative La. richardsoni, co-occur along an extensive strip of the western seaboard of North America, while its analogs, the anadromous parasitic La. fluviatilis and the non-parasitic La. planeri, co-occur and are widely distributed throughout Europe (Table 2.1; Fig. 2.10). In contrast to the above two non-parasitic species, La. pacifica (a second derivative of La. ayresii) and La. lanceolata (a second derivative of La. fluviatilis) both have very restricted distributions. Vladykov (1973) suggested that La. pacifica was distributed in the Columbia River drainage in Oregon and also in the Sacramento and San Joaquin river systems in California, but Reid et al. (2011) recommend restriction of La. pacifica to the Columbia River basin, at least until further systematic information (e.g., regarding unresolved populations of Lampetra brook lampreys; see Sect. 2.4) is available. Nevertheless, while La. pacifica is found within the distribution of its presumed ancestor, that of La. lanceolata is far removed from that of its presumed ancestral species. Although an anadromous lamprey recently discovered in the Sea of Azov and referable to the genus Lampetra might be La. fluviatilis, Naseka and Diripasko (2008) concluded that they were not conspecific because they differed, in admittedly minor morphological respects, and were widely separated geographically. The remaining non-parasitic species of Lampetra, La. zanandreai and La. hubbsi, whose parasitic ancestry have not been established, are both considered southern relicts (see Sect. 2.2). The former species is found in the drainage of the Adriatic Sea and the latter in the Friand-Kern Canal and Merced River, California (Vladykov and Kott 1984), but Boguski et al. (2012) suggest that La. hubbsi may also occur in the upper Sacramento River (Table 2.1; Fig. 2.10).
Trees derived from molecular data (Fig. 2.6; Docker et al. 1999) suggest that La. aepyptera, which has normally been assigned to Lampetra, is more closely related to European species than to any extant North American species. Furthermore, the region where La. aepyptera is found in eastern North America is widely separated from the west coast of this continent where La. ayresii, the only North American parasitic representative of Lampetra, occurs (Docker et al. 1999; Potter et al. 2014). As emphasized previously, future studies should address the question of the ancestry of La. aepyptera and therefore the basis for the geographical distribution.
It is clear from comparisons of the distributions of the various lamprey species that the largest species, P. marinus, G. australis, and En. tridentatus, have the widest distributions and that these can extend well out into oceanic waters. During their parasitic phase, the smaller anadromous species, such as M. mordax, La. fluviatilis, and La. ayresii, occupy coastal waters and those of freshwater species each tend to occur in a restricted number of river systems.
The data compiled for this review emphasize that the lamprey fauna in the Northern Hemisphere, with 37 species and eight genera, is far more diverse than that in the Southern Hemisphere, which contains only four species and two genera. This reflects the presence of a greater number and diversity of rivers in temperate regions of the Northern Hemisphere than in corresponding regions of the Southern Hemisphere.
2.6 Conclusions and Future Directions
The aforegoing accounts and discussion demonstrate that progress is being made in understanding the phylogenetic relationships among extant lampreys (Petromyzontiformes). There is now widespread recognition, for example, that the extant lampreys comprise three families, that is, Geotriidae, Mordaciidae, and Petromyzontidae. However, the precise relationships among the three families remain unresolved. Although there is not a complete consensus at the lower levels of classification, a clearer picture is emerging. Within the Petromyzontidae, the eight genera have either been separated into: (1) three subfamilies by Vladykov and co-workers (e.g., Vladykov 1972; Vladykov and Kott 1979c), namely, Petromyzontinae (Petromyzon, Caspiomyzon, and Ichthyomyzon), Entospheninae (Entosphenus and Tetrapleurodon), and Lampetrinae (Lampetra, Lethenteron, and Eudontomyzon); or (2) two subfamilies by Nelson (2006), namely, Petromyzontinae (Petromyzon and Ichthyomyzon) and Lampetrinae (Caspiomyzon, Lampetra, Lethenteron, Eudontomyzon, Entosphenus, and Tetrapleurodon). Although the three subfamilies proposed by Vladykov and co-workers may be most appropriate (see Sect. 2.4.1), we have adopted a conservative approach in this chapter, placing Caspiomyzon within Petromyzontinae but proposing that other taxonomic changes not be made prematurely.
At the generic level, morphological and molecular data support most of the existing classifications. While some uncertainties remain regarding the relationships among Lampetra, Lethenteron, and Eudontomyzon, we emphasize that taxonomic changes should not be made until the results of more comprehensive studies become available. In particular, the basis for the differences between the phylogenetic schemes produced using morphological and molecular data for Lethenteron, Eudontomyzon, and Lampetra needs to be clarified. This includes determining: (1) whether the parasitic and non-parasitic species designated as Eudontomyzon, which are represented in three different clades on the basis of the molecular data, are appropriately assigned to that genus according to morphological criteria; and (2) whether Lampetra fluviatilis and La. ayresii belong to the same clade, as suggested by their great morphological similarity or to different clades, as suggested by cytochrome b DNA sequence data. The resolution of these questions will require the use of a wider range of genes and particularly of nuclear genes.
Another remaining uncertainty is the phylogenetic relationship between the parasitic and non-parasitic members of species pairs. We recommend that no new non-parasitic species is erected until there has been a thorough morphological and molecular analysis aimed at elucidating the extent of the relationship between the putative new species and its presumed ancestor and comparisons with appropriate type specimens. This is particularly pertinent because the individuals in different populations of non-parasitic species may be genetically divergent but, at present, are morphologically indistinguishable. Furthermore, the phylogenetic positions of the six non-parasitic southern relict species for which there are no obvious ancestors (e.g., La. aepyptera, Le. ninae) need to be investigated using a wide range of independent genetic loci.
References
Abbott CC (1860) Descriptions of new species of American fresh-water fishes. Proc Acad Natl Sci Phila 12:325–328
Álvarez del Villar J (1966) Ictiología michoacana, IV. Contribución al conocimiento biológico y sistemático de las lampreas de Jacona, Mich., México. An Esc Nac Cienc Biol Méx 13:107–144
Anikin VP (1905) Opisanie novўkh aziatskikh vidov rўb [Description of new Asian species of fish]. Izv Tomsk Univ 1905:1–18
Applegate VC (1950) Natural history of the sea lamprey, Petromyzon marinus, in Michigan. US Fish Wildl Serv Spec Sci Rep Fish 55:1–237
April J, Mayden RL, Hanner RH, Bernatchez L (2011) Genetic calibration of species diversity among North America’s freshwater fishes. Proc Natl Acad Sci U S A 108:10602–10607
Bailey RM (1980) Comments on the classification and nomenclature of lampreys-an alternative view. Can J Fish Aquat Sci 37:1626–1629
Bardack D (1991) First fossil hagfish (Myxinoidea): a record from the Pennsylvanian of Illinois. Science 254:701–703
Bardack D (1998) Relationships of living and fossil hagfishes. In: Jørgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of hagfishes. Chapman and Hall, London, pp 3–14
Bardack D, Zangerl R (1968) First fossil lamprey: a record from the Pennsylvanian of Illinois. Science 162:1265–1267
Bardack D, Zangerl R (1971) Lampreys in the fossil record. In: Hardisty MW, Potter IC (eds) The biology of lampreys, vol 1. Academic Press, London, pp 67–84
Bartels H, Potter IC (1995) Structural organization and epithelial cell types of the intestinal diverticula (protopancreas) of ammocoetes of southern hemisphere lampreys: functional and phylogenetic implications. Cell Tissue Res 280:313–324
Bartels H, Docker MF, Fazekas U, Potter IC (2012) Functional and evolutionary implications of the cellular composition of the gill epithelium of feeding adults of a freshwater parasitic species of lamprey, Ichthyomyzon unicuspis. Can J Zool 90: 1278–1283
Beamish FWH, Thomas EJ (1983) Potential and actual fecundity of the “paired” lampreys, Ichthyomyzon gagei and I. castaneus. Copeia 1983:367–374
Beamish RJ (1982) Lampetra macrostoma, a new species of freshwater parasitic lamprey from the west coast of Canada. Can J Fish Aquat Sci 39:736–747
Beamish RJ (2010) The use of gill pore papillae in the taxonomy of lampreys. Copeia 2010:618–628
Beamish RJ, Wade J (2008) Critical habitat and the conservation ecology of the freshwater parasitic lamprey, Lampetra macrostoma. Can Field-Nat 122:327–337
Bean TH (1887) Descriptions of five new species of fishes sent by Prof. A Dugès from the province of Guanajuato, Mexico. Proc U S Natl Mus 10:370–375 + 1 pl
Berg LS (1906) Ubersicht der Marsipobranchii des Russischen Reiches. Bull Acad Imp Sci 24:169–183
Berg LS (1931) A review of the lampreys of the northern hemisphere. Ann Mus Zool Acad Sci URSS 32:87–116 + 8 pls
Bianco PG (1986) Lethenteron zanandreai (Vladykov, 1955). In: Holčík J (ed) The freshwater fishes of Europe, vol 1, part 1, Petromyzontiformes. AULA, Wiesbaden, pp 237–246
Bird DJ, Potter IC (1979) Metamorphosis in the paired species of lampreys, Lampetra fluviatilis (L.) and Lampetra planeri (Bloch). 1. A description of the timing and stages. Zool J Linn Soc 65:127–143
Blair JE, Hedges SB (2005) Molecular phylogeny and divergence times of deuterostome animals. Mol Biol Evol 22:2275–2284
Blank M, Jürss K, Bastrop R (2008) A mitochondrial multigene approach contributing to the systematics of the brook and river lampreys and the phylogenetic position of Eudontomyzon mariae. Can J Fish Aquat Sci 65:2780–2790
Bloch ME (1784) M. Marcus Elieser Bloch’s …, ausübenden Arztes zu Berlin, Oeconomische Naturgeschichte der Fische Deutschlands, vol 3. Aus Kosten des Verfassers, und in Commission in der Buchlandlung der Realschule, Berlin, viii + 234 + pls 73–108
Boguski DA, Reid SB, Goodman DH, Docker MF (2012) Genetic diversity, endemism and phylogeny of lampreys within the genus Lampetra sensu stricto (Petromyzontiformes: Petromyzontidae) in western North America. J Fish Biol 81:1891–1914
Bonaparte CL (1832) Saggio d’una distribuzione metodica degli animali vertebrati a sangue freddo. Giorn Arcadico 52:129–189
Bond CE, Kan TT (1973) Lampetra (Entosphenus) minima n. sp., a dwarfed parasitic lamprey from Oregon. Copeia 1973:568–574
Bond CE, Kan TT (1986) Systematics and evolution of the lampreys of Oregon. In: Uyeno T, Arai R, Taniuchi T, Matsuura K (eds) Indo-Pacific fish biology, Proc 2nd Int Conf Indo-Pacific Fish. Ichthyol Soc Jpn, Tokyo, p 919
Bonnaterre JP (1788) Tableau encyclopédique et méthodique des trois règnes de la nature. Ichthyologie, Paris, lvi (there is no p iii) + 215 + pls A and B + 100 pls
Çevik C, Ergüden D, Tekelioğlu N (2010) Confirmation of the presence of the sea lamprey, Petromyzon marinus Linnaeus, 1758 in the Levantine Sea (Petromyzoniformes: Petromyzonidae). Zool Middle East 49:107–108
Chang M-m, Zhang J, Miao D (2006) A lamprey from the Cretaceous Jehol biota of China. Nature 441:972–974
Chappuis PA (1939) Über die Lebensweise von Eudontomyzon danfordi Regan. Arch Hydrobiol 34:645–658 + 1 pl
Cochran PA, Lyons J, Merino-Nambo E (1996) Notes on the biology of the Mexican lampreys Lampetra spadicea and L. geminis (Agnatha: Petromyzontidae). Ichthyol Explor Freshw 7:173–180
Creaser CW, Hubbs CL (1922) A revision of the Holarctic lampreys. Occas Pap Mus Zool Univ Mich 120:1–14 + 1 pl
DeKay JE (1842) Zoology of New-York, or the New-York fauna; comprising detailed descriptions of all the animals hitherto observed within the state of New-York, with brief notices of those occasionally found near its borders, and accompanied by appropriate illustrations, part IV, Fishes. W & A White & J Visscher, Albany, xv + 415 + 79 pls
Delarbre C, Gallut C, Barriel V, Janvier P, Gachelin G (2002) Complete mitochondrial DNA of the hagfish, Eptatretus burgeri: the comparative analysis of mitochondrial DNA sequences strongly supports the cyclostome monophyly. Mol Phylogen Evol 22:184–192
Docker MF (2006) Bill Beamish’s contributions to lamprey research and recent advances in the field. Guelph Ichthyol Rev 7:1–52
Docker MF (2009) A review of the evolution of nonparasitism in lampreys and an update of the paired species concept. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management, and conservation of lampreys in North America. American Fisheries Society Symposium 72, Bethesda, pp 71–114
Docker MF, Potter IC (in press) Life history variation in lampreys: alternate feeding and migratory types. In: Docker MF (ed) Lampreys: biology, conservation and control, vol 2. Springer, Dordrecht
Docker MF, Youson JH, Beamish RJ, Devlin RH (1999) Phylogeny of the lamprey genus Lampetra inferred from mitochondrial cytochrome b and ND3 gene sequences. Can J Fish Aquat Sci 56:2340–2349
Docker MF, Mandrak NE, Heath DD (2012) Contemporary gene flow between “paired” silver (Ichthyomyzon unicuspis) and northern brook (I. fossor) lampreys: implications for conservation. Conserv Genet 13:823–835
Duméril AMC (1806) Zoologie analytique, ou méthode naturelle de classification des animaux; rendue plus facile à l’aide de tableaux synoptiques. Allais, Paris, pp xxxiii + 344
Dybowski BN (1869) Vorläufige Mittheilungen über die Fischfauna des Ononflusses und des Ingoda in Transbaikalien. Verh k-k Zool Bot Ges 19:945–958 + 1 table + pls 14–18
Espanhol R, Almeida PR, Alves MJ (2007) Evolutionary history of lamprey paired species Lampetra fluviatilis (L.) and Lampetra planeri (Bloch) as inferred from mitochondrial DNA variation. Mol Ecol 16:1909–1924
Fernholm B (1998) Hagfish systematics. In: Jørgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of hagfishes. Chapman and Hall, London, pp 33–44
Froese R, Pauly D (eds) (2013) FishBase. http://www.fishbase.org, version 04/2013. Accessed 31 May 2013
Fukutomi N, Nakamura T, Doi T, Takeda K, Oda N (2002) Records of Entosphenus tridentatus from the Naka River system, central Japan; physical characteristics of possible spawning redds and spawning behavior in the aquarium. Jpn J Ichthyol 49:53–58
Germain D, Sanchez S, Janvier P, Tafforeau P (2014) The presumed hagfish Myxineidus gononorum from the Upper Carboniferous of Montceau-les-Mines (Saône-et-Loire, France: new data by means of propagation phase contrast X-ray synchroton microtomography. Ann Paléontol 100:131–135
Gess RW, Coates MI, Rubidge BS (2006) A lamprey from the Devonian period of South Africa. Nature 443:981–984
Gill TN (1862) Notes on some genera of fishes of western North America. Proc Acad Natl Sci U S A 14:329–332
Gill TN (1893) Families and subfamilies of fishes. Mem Natl Acad Sci 6:127–138
Gill HS, Renaud CB, Chapleau F, Mayden RL, Potter IC (2003) Phylogeny of living parasitic lampreys (Petromyzontiformes) based on morphological data. Copeia 2003:687–703
Girard CF (1858) Fishes. General report on the zoology of the several Pacific railroad routes. United States Pacific Railroad Route Explorations and Surveys, vol 10, part 4, War Department, Washington, DC, pp 400 + 21 pls
Goodman DH, Kinziger AP, Reid SB, Docker MF (2009) Morphological diagnosis of Entosphenus and Lampetra ammocoetes (Petromyzontidae) in Washington, Oregon, and California. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management, and conservation of lampreys in North America. American Fisheries Society Symposium 72, Bethesda, pp 223–232
Gray JE (1851) List of the specimens of fish in the collection of the British Museum, part 1. Chondropterygii. British Museum (Natural History), London, pp xi + 160 + 2 pls
Günther A (1870) Catalogue of the fishes in the British Museum, vol 8, catalogue of the Physostomi, containing the families Gymnotidae, Symbranchidae, Muraenidae, Pegasidae, and of the Lophobranchii, Plectognathi, Dipnoi, Ganoidei, Chondropterygii, Cyclostomata, Leptocardii in the British Museum. Taylor and Francis, London, pp xxv + 549
Halliday RG (1991) Marine distribution of the sea lamprey (Petromyzon marinus) in the northwest Atlantic. Can J Fish Aquat Sci 48:832–842
Hardisty MW (1979) Biology of the cyclostomes. Chapman and Hall, London, pp 428
Hardisty MW (1982) Lampreys and hagfishes: analysis of cyclostome relationships. In: Hardisty MW, Potter IC (eds) The biology of lampreys, vol 4B. Academic Press, London, pp 165–260
Hardisty MW (1986) Lampetra fluviatilis (Linnaeus, 1758). In: Holčík J (ed) The freshwater fishes of Europe, vol 1, part 1, Petromyzontiformes. AULA, Wiesbaden, pp 249–278
Hardisty MW (2006) Lampreys. Life without jaws. Forrest Text, Tresaith, pp 272
Hardisty MW, Potter IC (1971a) The behaviour, ecology and growth of larval lampreys. In: Hardisty MW, Potter IC (eds) The biology of lampreys, vol 1. Academic Press, London, pp 85–125
Hardisty MW, Potter IC (1971b) The general biology of adult lampreys. In: Hardisty MW, Potter IC (eds) The biology of lampreys, vol 1. Academic Press, London, pp 127–206
Hardisty MW, Potter IC (1971c) Paired species. In: Hardisty MW, Potter IC (eds) The biology of lampreys, vol 1. Academic Press, London. pp 249–277
Hardisty MW, Potter IC, Sturge R (1970) A comparison of the metamorphosing and macrophthalmia stages of the lampreys Lampetra fluviatilis and L. planeri. J Zool 162:383–400
Hardisty MW, Potter IC, Hilliard RW (1989) Physiological adaptations of the living agnathans. Trans R Soc Edinb Earth Sci 80:241–254
Heard WR (1966) Observations on lampreys in the Naknek River system of southwest Alaska. Copeia 1966:332–339
Heimberg AM, Cowper-Sal·lari R, Sémon M, Donoghue PCJ, Peterson KJ (2010) MicroRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci U S A 107:19379–19383
Holčík J (1986a) Determination criteria. In: Holčík J (ed) The freshwater fishes of Europe, vol 1, part 1, Petromyzontiformes. AULA, Wiesbaden, pp 24–32
Holčík J (1986b) Lethenteron Creaser and Hubbs, 1922. In: Holčík J (ed) The freshwater fishes of Europe, vol 1, part 1, Petromyzontiformes. AULA, Wiesbaden, pp 196–197
Holčík J, šorić V (2004) Redescription of Eudontomyzon stankokaramani (Petromyzontes, Petromyzontidae)-a little known lamprey from the Drin River drainage, Adriatic Sea basin. Folia Zool 53:399–410
Holly M (1933) Cyclostomata. Das Tierreich 59:1–62
Hubbs CL (1925) The life-cycle and growth of lampreys. Pap Mich Acad Sci Arts Lett 4:587–603
Hubbs CL (1971) Lampetra (Entosphenus) lethophaga, new species, the nonparasitic derivative of the Pacific lamprey. Trans S Diego Soc Natl Hist 16:125–164
Hubbs CL, Potter IC (1971) Distribution, phylogeny and taxonomy. In: Hardisty MW, Potter IC (eds) The biology of lampreys, vol 1. Academic Press, London, pp 1–65
Hubbs CL, Trautman MB (1937) A revision of the lamprey genus Ichthyomyzon. Misc Publ Mus Zool Univ Mich 35:7–109 + 2 pls
Hughes RL, Potter IC (1969) Studies on gametogenesis and fecundity in the lampreys Mordacia praecox and M. mordax (Petromyzonidae). Austr J Zool 17:447–464
Iwata A, Goto A, Hamada K (1985) A review of the Siberian lamprey, Lethenteron kessleri, in Hokkaido, Japan. Bull Fac Fish Hokkaido Univ 36:182–190
Janvier P (1981) The phylogeny of the Craniata, with particular reference to the significance of fossil “agnathans”. J Vert Paleontol 1:121–159
Janvier P (2010) MicroRNAs revive old views about jawless vertebrate divergence and evolution. Proc Natl Acad Sci U S A 107:19137–19138
Janvier P, Lund R (1983) Hardistiella montanensis n. gen. et sp. (Petromyzontida) from the lower Carboniferous of Montana, with remarks on the affinities of the lampreys. J Vert Paleontol 2:407–413
Jordan DS (1885) A catalogue of the fishes known to inhabit the waters of North America, north of the tropic of Cancer, with notes on the species discovered in 1883 and 1884. Rep U S Comm Fish 13:789–973
Jordan DS (1923) A classification of fishes including families and genera as far as known. Stanford Univ Publ Univ Ser Biol Sci 3:77-243 + i–x
Karaman MS (1974) Eudontomyzon vladykovi stankokaramani n. ssp., a new subspecies of lamprey from tributaries of the Ohrid-Drim-Skadar System in west Balkan Peninsula. Folia Balc 3:1–10 + 6 tables + 2 figs
Kessler K (1870) Volzhskaya minoga (Petromyzon Wagneri n. sp.) [Volga lamprey (Petromyzon Wagneri n. sp.)]. Trud St-Peterbg Obshchestva Estetvoznaniya 1:207–214
Kottelat M, Freyhof J (2009) Notes on the taxonomy and nomenclature of some European freshwater fishes. Ichthyol Explor Freshw 20:75–90
Kottelat M, Bogutskaya NG, Freyhof J (2005) On the migratory Black Sea lamprey and the nomenclature of the ludoga, Peipsi and ripus whitefishes (Agnatha: Petromyzontidae; Teleostei: Coregonidae). Zoosyst Rossica 14:181–186
Kucheryavyi AV, Savvaitova KA, Pavlov DS et al (2007a) Variations of life history strategy of the Arctic lamprey Lethenteron camtschaticum from the Utkholok River (western Kamchatka). J Ichthyol 47:37–52
Kucheryavyi AV, Savvaitova KA, Gruzdeva MA, Pavlov DS (2007b) Sexual dimorphism and some special traits of spawning behavior of the Arctic lamprey Lethenteron camtschaticum. J Ichthyol 47:481–485
Kuraku S, Kuratani S (2006) Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool Sci 23:1053–1064
Kuraku S, Hoshiyama D, Katoh K, Suga H, Miyata T (1999) Monophyly of lampreys and hagfishes supported by nuclear DNA-coded genes. J Mol Evol 49:729–735
Kux Z, Steiner HM (1972) Lampetra lanceolata, eine neue Neunaugenart aus dem Einzugsgebiet des Schwarzen Meeres in der nordöstlichen Türkei. čas Morav Mus Acta Mus Morav 56-57:375–384 + 10 figs
Lang NJ, Roe KJ, Renaud CB et al (2009) Novel relationships among lampreys (Petromyzontiformes) revealed by a taxonomically comprehensive molecular dataset. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management, and conservation of lampreys in North America. American Fisheries Society Symposium 72, Bethesda, pp 41–55
Linnaeus C (1758) Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, vol 1. Laurentii Salvii, Stockholm
Lorion CM, Markle DF, Reid SB, Docker MF (2000) Redescription of the presumed-extinct Miller Lake lamprey, Lampetra minima. Copeia 2000:1019–1028
Macey DJ, Potter IC (1978) Lethal temperatures of ammocoetes of the Southern Hemisphere lamprey, Geotria australis Gray. Environ Biol Fish 3:241–243
Mallatt J, Sullivan J (1998) 28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes. Mol Biol Evol 15:1706–1718
Mateus CS, Almeida PR, Quintella BR, Alves MJ (2011) MtDNA markers reveal the existence of allopatric evolutionary lineages in the threatened lampreys Lampetra fluviatilis (L.) and Lampetra planeri (Bloch) in the Iberian glacial refugium. Conserv Genet 12:1061–1074
Mateus CS, Stange M, Berner D et al (2013a) Strong genome-wide divergence between sympatric European river and brook lampreys. Curr Biol 23:R649–R650
Mateus CS, Alves MJ, Quintella BR, Almeida PR (2013b) Three new cryptic species of the lamprey genus Lampetra Bonnaterre, 1788 (Petromyzontiformes: Petromyzontidae) from the Iberian Peninsula. Contrib Zool 82:37–53
McPhail JD, Lindsey CC (1970) Freshwater fishes of northwestern Canada and Alaska. Fish Res Board Can Bull 173:1–381
Moore JW, Mallatt JM (1980) Feeding of larval lamprey. Can J Fish Aquat Sci 37:1658–1664
Moyle, PB, Brown LR, Chase SD, Quiñones RM (2009) Status and conservation of lampreys in California. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management, and conservation of lampreys in North America. American Fisheries Society Symposium 72, Bethesda, pp 279–292
Naseka AM, Diripasko OA (2008) A recent record of an anadromous lamprey (Agnatha: Petromyzontidae) from the Sea of Azov. Ichthyol Explor Freshw 19:283–287
Naseka AM, Tuniyev SB, Renaud CB (2009) Lethenteron ninae, a new nonparasitic lamprey species from the north-eastern Black Sea basin (Petromyzontiformes: Petromyzontidae). Zootaxa 2198:16–26
Near TJ (2009) Conflict and resolution between phylogenies inferred from molecular and phenotypic data sets for hagfish, lampreys, and gnathostomes. J Exp Zool (Mol Dev Evol) 312B:749–761
Neira FJ, Bradley JS, Potter IC, Hilliard RW (1988) Morphological variation among widely dispersed larval populations of anadromous southern hemisphere lampreys (Geotriidae and Mordaciidae). Zool J Linn Soc 92:383–408
Nelson JS (2006) Fishes of the world, 4th edn. Wiley, Hoboken, pp 601
Nelson JS, Crossman EJ, Espinosa-Pérez H et al (2004) Common and scientific names of fishes from the United States, Canada, and Mexico, 6th edn. American Fisheries Society Special Publication 29, Bethesda
Nursall JR, Buchwald D (1972) Life history and distribution of the Arctic lamprey (Lethenteron japonicum (Martens)) of Great Slave Lake, N.W.T. Fish Res Board Can Tech Rep 304:1–28
Oliva O (1953) Příspěvek k přehledu našich mihulí (Petromyzones Berg 1940). Věstn Král česk Spol Nauk 9:1-19 + 2 pls
Page LM, Espinosa-Pérez H, Findley LT et al (2013a) Common and scientific names of fishes from the United States, Canada, and Mexico, 7th edn. American Fisheries Society Special Publication 34, Bethesda
Page LM, Espinosa-Pérez H, Findley LT et al (2013b) New seventh edition of common and scientific names of fishes changes include capitalization of common names. Fisheries 38:188–189
Poplin C, Sotty D, Janvier P (2001) A hagfish (Craniata, Hyperotreti) from the Late Carboniferous Konservat-Lagerstätte of Montceau-les-Mines (Allier, France). C R Acad Sci Earth Planet Sci 332:345–350
Potter IC (1968) Mordacia praecox, n. sp., a nonparasitic lamprey (Petromyzonidae), from New South Wales, Australia. Proc Linn Soc N S W 92:254–261 + pl XIV
Potter IC (1980a) Ecology of larval and metamorphosing lampreys. Can J Fish Aquat Sci 37:1641–1657
Potter IC (1980b) The Petromyzoniformes with particular reference to paired species. Can J Fish Aquat Sci 37:1595–1615
Potter IC (1986) The distinctive characters of southern hemisphere lampreys (Geotriidae and Mordaciidae). In: Uyeno T, Arai R, Taniuchi T, Matsuura K (eds) Indo-Pacific fish biology. Proc 2nd Int Conf Indo-Pacific Fish. Ichthyological Society of Japan, Tokyo, pp 9–19
Potter IC, Beamish FWH (1975) Lethal temperatures in ammocoetes of four species of lampreys. Acta Zool 56:85–91
Potter IC, Gill HS (2003) Adaptive radiation of lampreys. J Great Lakes Res 29(Suppl 1):95–112
Potter IC, Osborne TS (1975) The systematics of British larval lampreys. J Zool 176:311–329
Potter IC, Strahan R (1968) The taxonomy of the lamprey Geotria and Mordacia and their distribution in Australia. Proc Linn Soc Lond 179:229–240 + 1 pl
Potter IC, Welsch U (1997) The structure of the gular pouch of mature males of the lamprey Geotria australis. Acta Zool 78:97–106
Potter IC, Lanzing WJR, Strahan R (1968) Morphometric and meristic studies on populations of Australian lampreys of the genus Mordacia. Zool J Linn Soc 47:533–546
Potter IC, Prince PA, Croxall JP (1979) Data on the adult marine and migratory phases in the life cycle of the southern hemisphere lamprey, Geotria australis Gray. Environ Biol Fish 4:65–69
Potter IC, Hilliard RW, Bird DJ, Macey DJ (1983) Quantitative data on morphology and organ weights during the protracted spawning-run period of the Southern Hemisphere lamprey Geotria australis. J Zool 200:1–20
Potter IC, Gill HS, Renaud CB (2014) Petromyzontidae: lampreys. In: Burr BM, Warren ML Jr (eds) North American freshwater fishes: natural history, ecology, behavior, and conservation. Johns Hopkins University Press, Baltimore, pp 106–139
Regan CT (1911) A synopsis of the marsipobranchs of the order Hyperoartii. Ann Mag Natl Hist 7:193–204
Reid SB, Boguski DA, Goodman DH, Docker MF (2011) Validity of Lampetra pacifica (Petromyzontiformes: Petromyzontidae), a brook lamprey described from the lower Columbia River basin. Zootaxa 3091:42–50
Reighard J, Cummins H (1916) Description of a new species of lamprey of the genus Ichthyomyzon. Occas Pap Mus Zool Univ Mich 31:1–12 + 2 pls
Renaud CB (1997) Conservation status of Northern Hemisphere lampreys (Petromyzontidae). J Appl Ichthyol 13:143–148
Renaud CB (2008) Petromyzontidae, Entosphenus tridentatus: southern distribution record, Isla Clarión, Revillagigedo Archipelago, Mexico. Check List 4:82–85
Renaud CB (2011) Lampreys of the world. An annotated and illustrated catalogue of lamprey species known to date. FAO Species Cat Fish Purposes 5, FAO, Rome
Renaud CB, Cochran PA (in press) Post-metamorphic feeding in lampreys. In: Docker MF (ed) Lampreys: biology, conservation and control, vol 2. Springer, Dordrecht
Renaud CB, Economidis PS (2010) Eudontomyzon graecus, a new nonparasitic lamprey species from Greece (Petromyzontiformes: Petromyzontidae). Zootaxa 2477:37–48
Renaud CB, Gill HS, Potter IC (2009a) Relationships between the diets and characteristics of the dentition, buccal glands and velar tentacles of the adults of the parasitic species of lamprey. J Zool 278:231–242
Renaud CB, Docker MF, Mandrak NE (2009b) Taxonomy, distribution, and conservation of lampreys in Canada. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management, and conservation of lampreys in North America. American Fisheries Society Symposium 72, Bethesda, pp 293–309
Richardson J (1836) Fauna Boreali-Americana; or the zoology of the northern parts of British America: containing descriptions of the objects of natural history collected on the late northern land expeditions under the command of Captain Sir John Franklin, R.N., part 3, the fish. J Murray, London, pp xv + 327 + pls 74–97
Richardson J (1846) Ichthyology of the voyage of H.M.S. Erebus & Terror. In: Richardson J, Gray JE (eds) The zoology of the voyage of the H.M.S. Erebus & Terror, under the command of Captain Sir James Clark Ross, R.N., F.R.S., during the years 1839 to 1843, vol 2. EW Janson, London, pp 53-74 + pls 31–44
Robins CR, Bailey RM, Bond CE et al (1980) A list of common and scientific names of fishes from the United States and Canada, 4th edn. American Fisheries Society Special Publication 12, Bethesda
Robins CR, Bailey RM, Bond CE et al (1991) Common and scientific names of fishes from the United States and Canada, 5th edn. American Fisheries Society Special Publication 20, Bethesda
Stock DW, Whitt GS (1992) Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group. Science 257:787–789
Takezaki N, Figueroa F, Zaleska-Rutczynska Z, Klein J (2003) Molecular phylogeny of early vertebrates: monophyly of the agnathans as revealed by sequences of 35 genes. Mol Biol Evol 20:287–292
Taylor EB, Harris LN, Spice EK, Docker MF (2012) Microsatellite DNA analysis of parapatric lamprey (Entosphenus spp.) populations: implications for evolution, taxonomy, and conservation of a Canadian endemic. Can J Zool 90:291–303
Tilesius von Tilenau WG (1811) Piscium camtschaticorum descriptions et icones. Mém Acad Imp Sci St-Pétersbg 3:225–285 + pls 8–13
Vladykov VD (1950) Larvae of eastern American lampreys (Petromyzonidae). 1. Species with two dorsal fins. Nat Can 77:73–95
Vladykov VD (1955) Lampetra zanandreai, a new species of lamprey from northern Italy. Copeia 1955:215–223 + 4 pls
Vladykov VD (1972) Sous-division en trois sous-familles des lamproies de l’hémisphère-nord de la famille Petromyzonidae. ACFAS 39:148
Vladykov VD (1973) Lampetra pacifica, a new nonparasitic species of lamprey (Petromyzontidae) from Oregon and California. J Fish Res Board Can 30:205–213 + 9 figs
Vladykov VD, Follett WI (1958) Redescription of Lampetra ayresii (Günther) of western North America, a species of lamprey (Petromyzontidae) distinct from Lampetra fluviatilis (Linnaeus) of Europe. J Fish Res Board Can 15:47–77
Vladykov VD, Follett WI (1965) Lampetra richardsoni, a new nonparasitic species of lamprey (Petromyzonidae) from western North America. J Fish Res Board Can 22:139–158 + 9 figs
Vladykov VD, Follett WI (1967) The teeth of lampreys (Petromyzonidae): their terminology and use in a key to the Holarctic genera. J Fish Res Board Can 24:1067–1075
Vladykov VD, Kott EE [sic] (1976a) The taxonomic significance of velar tentacles in Holarctic lampreys (Petromyzonidae). Rev Trav Inst Pêches Marit 40:787–789
Vladykov VD, Kott E (1976b) A second nonparasitic species of Entosphenus Gill, 1862 (Petromyzonidae) from Klamath River system, California. Can J Zool 54:974–989
Vladykov VD, Kott E (1976c) A new nonparasitic species of lamprey of the genus Entosphenus Gill, 1862, (Petromyzonidae) from south central California. Bull South Calif Acad Sci 75:60–67
Vladykov VD, Kott E (1978) A new nonparasitic species of the holarctic lamprey genus Lethenteron Creaser and Hubbs, 1922, (Petromyzonidae) from northwestern North America with notes on other species of the same genus. Biol Pap Univ Alaska 19:1–74
Vladykov VD, Kott E (1979a) Satellite species among the holarctic lampreys (Petromyzonidae). Can J Zool 57:860–867
Vladykov VD, Kott E (1979b) A new parasitic species of the holarctic lamprey genus Entosphenus Gill, 1862 (Petromyzonidae) from Klamath River, in California and Oregon. Can J Zool 57:808–823
Vladykov VD, Kott E (1979c) List of northern hemisphere lampreys (Petromyzonidae) and their distribution. Dep Fish Ocean Misc Spec Publ 42:1–30
Vladykov VD, Kott E (1984) A second record for California and additional morphological information on Entosphenus hubbsi Vladykov and Kott 1976 (Petromyzontidae). Calif Fish Game 70:121–127
Vladykov VD, Renaud CB, Kott E, Economidis PS (1982) A new nonparasitic species of Holarctic lamprey, genus Eudontomyzon Regan 1911 (Petromyzontidae), from Greece. Can J Zool 60:2897–2915
Yamazaki Y, Goto A (1996) Genetic differentiation of Lethenteron reissneri populations, with reference to the existence of discrete taxonomic entities. Ichthyol Res 43:283–299
Yamazaki Y, Goto A (1997) Morphometric and meristic characteristics of two groups of Lethenteron reissneri. Ichthyol Res 44:15–25
Yamazaki Y, Goto A (1998) Genetic structure and differentiation of four Lethenteron taxa from the Far East, deduced from allozyme analysis. Environ Biol Fish 52:149–161
Yamazaki Y, Goto A, Nishida M (2003) Mitochondrial DNA sequence divergence between two cryptic species of Lethenteron, with reference to an improved identification technique. J Fish Biol 62:591–609
Yamazaki Y, Yokoyama R, Nishida M, Goto A (2006) Taxonomy and molecular phylogeny of Lethenteron lampreys in eastern Eurasia. J Fish Biol 68(Suppl B):251–269
Yap MR, Bowen SH (2003) Feeding by northern brook lamprey (Ichthyomyzon fossor) on sestonic biofilm fragments: habitat selection results in ingestion of a higher quality diet. J Great Lakes Res 29(Suppl 1):15–25
Youson JH (1980) Morphology and physiology of lamprey metamorphosis. Can J Fish Aquat Sci 37:1687–1710
Zanandrea G (1957) Esame critico e comparativo delle lamprede catturate in Italia. Arch Zool Ital 42:249–307 + 3 pls
Acknowledgments
We gratefully acknowledge Dr Halina Kobryn for generating the maps of the distribution of lampreys, Dr David Bird for kindly supplying Figs. 2.2b–d and Dr James Tweedley for helping to prepare the figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix 2.1 List of Lamprey Families, Genera and Species and Their Authorities
Appendix 2.1 List of Lamprey Families, Genera and Species and Their Authorities
Mordaciidae Gill 1893 |
Mordacia Gray 1851 |
Mordacia mordax (Richardson 1846) |
Mordacia praecox Potter 1968 |
Mordacia lapicida (Gray 1851) |
Geotriidae Jordan 1923 |
Geotria Gray 1851 |
Geotria australis Gray 1851 |
Petromyzontidae Bonaparte 1832 |
Caspiomyzon Berg 1906 |
Caspiomyzon wagneri (Kessler 1870) |
Petromyzon Linnaeus 1758 |
Petromyzon marinus Linnaeus 1758 |
Ichthyomyzon Girard 1858 |
Ichthyomyzon unicuspis Hubbs and Trautman 1937 |
Ichthyomyzon fossor Reighard and Cummins 1916 |
Ichthyomyzon castaneus Girard 1858 |
Ichthyomyzon gagei Hubbs and Trautman 1937 |
Ichthyomyzon bdellium (Jordan 1885) |
Ichthyomyzon greeleyi Hubbs and Trautman 1937 |
Tetrapleurodon Creaser and Hubbs 1922 |
Tetrapleurodon spadiceus (Bean 1887) |
Tetrapleurodon geminis Álvarez del Villar 1966 |
Entosphenus Gill 1862 |
Entosphenus tridentatus (Gairdner in Richardson 1836) |
Entosphenus minimus (Bond and Kan 1973) |
Entosphenus similis Vladykov and Kott 1979c |
Entosphenus macrostomus (Beamish 1982) |
Entosphenus folletti Vladykov and Kott 1976b |
Entosphenus lethophagus (Hubbs 1971) |
Lethenteron Creaser and Hubbs 1922 |
Lethenteron camtschaticum (Tilesius 1811) |
Lethenteron alaskense Vladykov and Kott 1978 |
Lethenteron appendix (DeKay 1842) |
Lethenteron reissneri (Dybowski 1869) |
Lethenteron kessleri (Anikin 1905) |
Lethenteron ninae Naseka et al. 2009 |
Eudontomyzon Regan 1911 |
Eudontomyzon danfordi Regan 1911 |
Eudontomyzon mariae (Berg 1931) |
Eudontomyzon stankokaramani Karaman 1974 |
Eudontomyzon morii (Berg 1931) |
Eudontomyzon hellenicus Vladykov et al. 1982 |
Eudontomyzon graecus Renaud and Economidis 2010 |
Lampetra Bonnaterre 1788 |
Lampetra ayresii (Günther 1870) |
Lampetra pacifica Vladykov 1973 |
Lampetra richardsoni Vladykov and Follett 1965 |
Lampetra hubbsi (Vladykov and Kott 1976c) |
Lampetra aepyptera (Abbott 1860) |
Lampetra fluviatilis (Linnaeus 1758) |
Lampetra planeri (Bloch 1784) |
Lampetra lanceolata Kux and Steiner 1972 |
Lampetra zanandreai Vladykov 1955 |
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Potter, I., Gill, H., Renaud, C., Haoucher, D. (2015). The Taxonomy, Phylogeny, and Distribution of Lampreys. In: Docker, M. (eds) Lampreys: Biology, Conservation and Control. Fish & Fisheries Series, vol 37. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9306-3_2
Download citation
DOI: https://doi.org/10.1007/978-94-017-9306-3_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9305-6
Online ISBN: 978-94-017-9306-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)