Abstract
It has been established long time ago that among many changes in cellular activity the most remarkable event in stressed cells of all know organisms is massive production of highly conserved set of heat shock proteins (Schlesinger et al. 1982a, b). Soon after their discovery heat shock proteins (Hsps) have been implicated in thermotolerance based on the ability to recover heat-induced denatured proteins to their native state (Maloyan et al. 1999).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
It has been established long time ago that among many changes in cellular activity the most remarkable event in stressed cells of all know organisms is massive production of highly conserved set of heat shock proteins (Schlesinger et al. 1982). Soon after their discovery heat shock proteins (Hsps) have been implicated in thermotolerance based on the ability to recover heat-induced denatured proteins to their native state (Maloyan et al. 1999).
It has been demonstrated by many groups that Hsps help cells and organism as a whole to adapt to elevated temperatures and other forms of stress. Thus, preliminary treatment of culture cells or individuals (e.g. flies) with moderate temperature enables them to survive the consequent severe heat shock treatment which would have been otherwise lethal. This phenomenon demonstrated in various organisms was termed “induced thermotolerance” (see Feder and Hofmann 1999 for review). There are abundant data suggesting that accumulation of Hsps after mild heat shock is accounted for the induced thermotolerance phenomenon. Along these lines, it was shown that artificial increase of Hsp70 concentration after transfection of the cells with correspondent constitutive expressed plasmids also led to the resistance against thermal, oxidative and other stresses (Angelidis et al. 1991; Chong et al. 1998; Li et al. 1992; Park et al. 2000). Transgenic organisms with an increased Hsp70 gene copy number are more thermoresistant compared with wild-type (Feder et al. 1996). On the contrary, inhibition of protein synthesis in the cells after HS as well as knockout of certain heat shock genes results in reduced thermoresistance and prevents functional recovery of cellular genes after stress (Johnson and Kucey 1988). Furthermore, mutations in individual Hsp genes or their deletions as well as mutations in the gene encoding correspondent transcription factor (HSF) ablate induced thermotolerance (Craig and Jacobsen 1984; Gong and Golic 2006; Jedlicka et al. 1997). In the following years it was well established that cellular heat shock protection mechanism is dependent on the ability of Hsps to prevent misfolded proteins to form aggregates and effectively regulate degradation and translocation of various proteins to cellular compartments (Feder and Hofmann 1999; Hightower 1991; Hartl and Hayer-Hartl 2002; Pelham 1986).
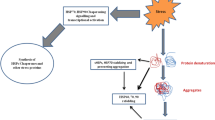
After HS, hypoxia, various chemicals, including heavy metals and many other forms of stressful stimuli cellular proteins undergo denaturation which often results in the exposure of hydrophobic regions of polypeptide chains normally packed inside protein three-dimensional structure to cytosolic hydrophylic environment. Because of such exposure proteins begin “to stick” to each other by their hydrophobic regions and, hence, form insoluble sedimenting aggregates highly toxic for the cells (Szalay et al. 2007). Besides, denatured proteins lose their ability to exercise normal activity in the cells which leads to the irreversible damage of most house-keeping systems and eventual death of the cells. It was demonstrated that Hsps may non-specifically interact with hydrophobic regions of denatured proteins and thus facilitate the restoration of their native tertiary structure and prevent insoluble aggregates formation (Fig. 2.1a). Other representatives of Hsps do not directly interact with misfolded proteins but may work as cofactors (or co-chaperones) with other Hsps. Thus, dissociation of Hsp70 and protein substrate requires ATP hydrolysis which takes place with participation of Hsp40. The product of this reaction (ADP) is subsequently exchanged for ATP with the involvement of co-chaperones (see below).
(а) Fluctuations of free energy in the course of proteins folding and aggregates formation. Molecular chaperones stimulate the formation of normal tertiary structures and prevent aggregation process. (b) The proteostasis network. Molecular chaperones participate in the folding and transport of synthesizing proteins, restoration of misfolfed peptides and disaggregation of denatured proteins. While prevention of aggregates formation definitely represents the major role of chaperones, representatives of AAA + family (e.g. yeast Hsp104) are able to dissolve already formed aggregates and facilitate their degradation by UPS (ubiquitin proteasome system) pathway (Modified from Hartl et al. 2011 with permission)
Besides Hsp70, several other groups of Hsps including small Hsps, Hsp60, Hsp90 and Hsp110 protect cellular proteins from misfolding and aggregation. While prevention of aggregates formation is definitely the major function of various Hsps, certain Hsps are able to dissolve already formed aggregates (Mayer 2010). Furthermore, chaperones together with ubiquitin-proteasome system (UPS) are involved in degradation of many cellular proteins (Bercovich et al. 1997; Hartl et al. 2011). Major pathways of proteins homeostasis maintained in the cells with Hsps involvement are depicted in Fig. 2.1b.
Hsps and especially certain members of Hsp70 family do not only prevent aggregates formation after HS or other stress but under normal conditions may bind newly synthesized proteins in parallel with their translation processing. They assist translocation of these proteins to different cellular compartments and help to maintain the right conformation. Hsp70 and other proteins capable to non-specifically bind with highly diverse polypeptides and help them to maintain native structure and be transported to their destination in the cell are named “chaperones” after French word “chaperone” – companion or duenna (Fig. 2.2). However, as we mentioned above while not all Hsps have this specific chaperone function part of them are named co-chaperones because they are also induced by stress and assist other proteins to bind peptides and translocate them to various cellular compartments.
Below we shall briefly describe the major functions of different heat shock proteins belonging to basic families mentioned above.
All small stress proteins (sHsps) contain α-crystallin domain 80–100 amino acids in length located at the C-terminal domain of the proteins. The homology of this domain within the family may vary from 20 to 60 % depending on the compared shps relationship. Additionally, some of small Hsp family members at the N-terminal end contain a short conserved region with phosphorylation sites implicated in the regulation of small Hsps activity (Kampinga et al. 2009).
The most remarkable feature of all sHsps is the ability to form oligomeric complexes: thus nine β-sheet structures comprising α-crystallin domain form stable intermolecular contacts with other small Hsps. Furthermore, both homo- and heterooligomeric complexes with molecular weights varying from 200 to 400 kD may be formed (de Jong et al. 1998). Such large complexes exist in the cells under normal physiological conditions and serve as a depot where small heat shock proteins are stored. The temperature elevation and other forms of stress result in dissociation of these preexisting complexes and fast release of small Hsps. Increased expression of sHsps during HS response correlates with better survival from cytotoxic stress. Small Hsps in the form of dimers interact with denatured cellular proteins in ATP-independent manner producing large granules. In the course of such interaction hydrophobic regions of denatured proteins become shielded from each other and loss the ability to form insoluble aggregates. At the next step the restoration of native tertiary structure of stress-damaged proteins is accomplished by Hsp70 and Hsp60 family members (Carver et al. 1995; Fernando and Heikkila 2000).
Besides chaperoning function small Hsps display other diverse roles. For example in response to growth factors stimulation they regulate the formation of actin microfilaments and stabilize actin network damaged by stress (Gusev et al. 2002; Khlebodarova 2002). αB-crystallin, a small Hsp family member closely related to Hsp27 is constitutively expressed and is especially abundant in eye where it is involved in lens formation (Horwitz 1976; Ingolia and Craig 1982). Moreover, members of small Hsp family and especially Hsp27 regulate apoptosis exploring several pathways. It was demonstrated, that Hsp27 negatively regulates the activation of procaspase-9 and can block release of cytochrome c from mitocondria in cells exposed to various pro-apoptotic factors (Arrigo et al. 1998; Mehlen et al. 1996; Kamradt et al. 2001). Besides, small Hsps may inhibit apoptosis induced by various other factors such as activators of receptors sFAS/APO-1 (Khlebodarova 2002). In addition, ubiquitously present Hsp27 modulates TNF-induced apoptosis by inhibiting IkB degradation (Kammanadiminti and Chadee 2006).
Recent experiments with D. melanogaster strains clearly demonstrated that enhanced expression of small Hsps significantly increases the resistance of flies to different forms of stress and extends life span. High concentration of Hsp22 was detected in mitochondria which are very sensitive to reactive oxygen species (ROS). The flies with suppressed synthesis of Hsp22 were more sensitive to moderate stress and were characterized by reduced lifetime (Morrow et al. 2006). When studying chaperoning activity measured as the ability to restore thermally denatured luciferase structure characteristic differences were revealed between individual members of small Hsps family. According to this assay, at the presence of Hsp22 luciferase activity was restored by 50 %, in the case of Hsp23 and Hsp26 by 30 % and in the case of Hsp27 by 40 %. Characteristically, Hsp22 localized in mitochondria which exhibit high sensitivity to oxidative stress manifested maximal restoration activity in these tests (Marcillat et al. 1989; Li et al. 2002). However, it should be noted that the observed differences may be partially attributed to different specificity of individual sHsps to substrate (luciferase in this assay) (Morrow et al. 2006).
Small Hsps may be abundant in certain tissues or organs at specific stages of development under normal physiological conditions. Thus in mammals small heat shock proteins are constitutively expressed in heart and muscles where they are probably involved in the preservation of microfilament network. It was also shown that gonads and heads of the newly hatched young flies are enriched with Hsp23 and Hsp26 while concentration of these small proteins is drastically decreased in these organs in aged flies (Morrow and Tanguay 2003). Furthermore, Hsp23 is actively synthesized at certain stages of ontogenesis in the brain of Drosophila in neuronal cells and in glia (Michaud and Tanguay 2003). Characteristically, the most drastic changes in Hsp22 accumulation take place in flies in the course of aging. Concentration of Hsp22 is increased in the heads and in the thoraxes of aged flies 60 and 20 folds respectively (King and Tower 1999; Wheeler et al. 1995). It was also demonstrated that Hsp22 plays an important role in preserving mitochondria from the consequences of oxidative stress occurring in aged flies (Morrow et al. 2004).
Hsp40 family comprises another group of heat shock proteins belonging to the so-called “J-proteins” family named basing on their similarity with bacterial DnaJ protein described in E. coli (Hartl and Hayer-Hartl 2002). Since the major function of Hsp40 is to serve as co-factor of Hsp70, all representatives of this highly diverse group contain J-domain necessary for interaction with Hsp70 family members. It was demonstrated that Hsp40 stimulates ATP-ase activity of Hsp70 by means of interaction between J-domain of Hsp40 with ATP-ase domain of Hsp70 and stabilizes complexes between Hsp70 and protein substrates (Fan 2003). According to the recent model, Hsp40 also recognizes and binds misfolded proteins before their association with the general chaperone Hsp70 (Summers et al. 2009). Different J-proteins function in cytosol, endoplasmic reticulum or mitochondria and serve as cofactors for cytosolic HSP70 and HSC70, or endoplasmic HSPA5/BiP and mitochondrial HSPA9/GRP75 in mammals (Christis et al. 2008).
The Hsp70 family is probably the most thoroughly studied group of stress proteins. According to x-ray analysis the molecule of canonical human HSPА1А consist of highly concerved 450 a. a. domain at the N-terminal end and rather variable 200 a. a. C-terminal domain. The N-terminal domain manifests ATP-binding activity (behave as ATPase in the presence of Hsp40 cofactor) and in terms of tertiary structure resembles actin ATP-binding domain while variable C-terminal domain represents substrate-binding region of Hsp70 (Flaherty et al. 1990). High affinity of Hsp70 members to ATP enables to easily isolate proteins of this family using ATP-Sepharose for laboratory and practical uses (Welch and Feramisco 1985).
Members of Hsp70 family execute various functions in the cell under stressful or normal conditions mainly related to their well-known chaperoning activity.
The molecular basis of Hsp70 chaperoning activity is based on the ability of these proteins to effectively bind misfolded cellular proteins and, hence, to prevent their aggregation (Nollen and Morimoto 2002). By binding the denatured proteins Hsp70 stabilizes them in partially unfolded state (Hartl and Hayer-Hartl 2002). The ability of Hsp70 proteins to recognize and bind to unfolded polypeptides is defined by peculiarities of their structure: C-terminal domen of Hsp70 contains hydrophobic “pocket” reminiscent in structure of peptide-binding region of the major histocompatibility complex (MHC) proteins, and in particular MHCII. It is of note, however, that peptide-binding site in Hsp70 has more open configuration making possible to interact with much longer peptides (Flajnik et al. 1991; Rippmann et al. 1991). Due to its specific structure Hsp70 recognizes polypeptides enriched with hydrophobic amino acids. Under physiological conditions such hydrophobic motifs are hidden inside normally folded protein and, hence, the appearance of such a. a. sequences on the surface of the protein molecule is usually a landmark of misfolded (or newly synthesized) proteins. It was demonstrated that hydrophobic amino acid sequences recognized by Hsp70 are found in polypeptides on the average every 40 a.a. (Frydman 2001). The interaction with Hsp70 leads to stabilization of protein substrates in unfolded state and, hence, prevents their aggregation.
At the next stage the restoration of native conformation may occur either with direct involvement of Hsp70 and its co-chaperone Hsp40 or partially restored proteins may be transported to chaperonins or Hsp90 (for certain proteins) for complete restoration of their native structure (see below).
In fact the detailed interaction of Hsp70 with substrate was initially described in E. coli for DnaK (Liberek et al. 1991) and later in eukaryotes (Summers et al. 2009). As a rule, folding of proteins with participation of Hsp70 or DnaK (in E. coli) requires several repeated cycles of association-dissociation accompanied by ATP hydrolysis. At the first stage Hsp70 interacts with complex of unfolded protein substrate and Hsp40 (DnaJ) in ATP-bound form (see Fig. 2.3). At the second stage DnaJ stimulates ATP hydrolysis and stabilizes the formed Hsp70-substrate complex. The affinity of Hsp70 to the product of reaction – ADP – is significantly higher than to ATP and, thus, dissociation of ADP requires cochaperone nucleotide exchange factor – GrpE in E. coli. After the dissociation of Hsp70-ADP complex the substrate is released and Hsp70 binds a new ATP molecule. Interestingly, in eukaryotic cells the interaction of Hsp70 with protein substrate also requires J-protein for hydrolysis of ATP, while dissociation of ADP is stimulated by cochaperones HspBP1 (Hsp70 binding protein 1) and BAG-1, and, according to the last data, Hsp110 (Summers et al. 2009). Furthermore, binding of Hsp70 with ATP and substrate is promoted by special protein Hap (Hsp70- and Hsc70-associating protein) (Gebauer et al. 1998; Houry 2001). Certain proteins e.g. steroid hormone receptors and many transcriptional factors after interaction with Hsp70 form stable complexes with Hsp90. The subsequent dissociation of these complexes requires interaction with a specific ligand or phosphorylation (see below). In some cases misfolded proteins bound with Hsp70 do not restore its native state but undergo degradation by UPS (Fig. 2.3). Furthermore, members of Hsp70 family are sometimes involved in degradation of misfolded proteins under normal physiological conditions. For instance, constitutively abundant Hsc70 participates in ubiquitin-dependent degradation of many cellular proteins such as actin, α-crystallins, histone 2A and many others (Bercovich et al. 1997). Therefore, constitutive members of Hsp70 family play important roles under physiological conditions and provide normal folding of newly synthesized proteins (Nelson et al. 1992). Practically all house-keeping proteins at some point during their translation process and release from the polysomes interact with Hsp70 family members (John et al. 1992; Ku et al. 1995). Chaperones and in particular certain Hsp70 family members participate in translocation of cellular proteins to endoplasmic reticulum and mitochondria. Thus, in cytosol HSPA1L and HSPA2 recognize and bind to newly synthesized proteins and during the translocation of such proteins through mitochondrial membrane dissociation coupled with ATP hydrolysis occurs. In cytosol HSPA1L and HSPA2 maintain polypeptides in unfolded state thus prevent formation of tertiary structure which inhibits the translocation through the membrane. It was shown that translocation of proteins denatured by urea across the mitochondrial membrane does not require ATP. In the course of translocation through the mitochondrial membrane and cleavage of N-terminal signal sequence, matrix Hsp70 (HSPA9 or GRP75 associated with mitochondrial Hsp40) binds to polypeptide chain. At the next step polypeptide is released with ATP hydrolysis and got caught by mitochondrial Hsp60 which facilitates its final folding and/or oligomerization (Neupert et al. 1990; Voos 2009, 2013).
Hsp70-substrate interaction cycle. 1 Initially, a non-native polypeptide is bound by Hsp40. 2 Hsp40-substrate complex binds with Hsp70 via J-domen of hsp40. 3 J-domain-stimulated ATP hydrolysis in the nucleotide-binding domain induces a conformational shift in the Hsp70 substrate-binding domain, increasing affinity for the non-native polypeptide that is released from the Hsp40. 4 In the case of polyubiquitinated protein substrate Hsp70 can direct them into proteasome for subsequent degradation. 5 and 6 – nucleotide exchange factors (NEFs) such as the BAG1 or Hsp110 replace the ADP with ATP, and the polypeptide releases from Hsp70. 7 If the polypeptide remains in a non-native conformation, the cycle can be repeated until folding is complete. UPS ubiquitin-proteasome pathway, ATP adenosine triphosphate, ADP adenosine diphosphate and Pi phosphate (From Summers et al. 2009 with modification)
Furthermore, BiP in cooperation with GRP94 is involved in the assembly of secreted immunoglobulins (Melnick and Argon 1995). Complexes formed by BiP and GRP94 with antibodies molecules are structurally similar to the complexes formed by Hsp70 and Hsp90 with glucocorticoid receptors and protein kinases in cytosol. Correct folding of MHCI and MHCII molecules also requires chaperones participation. Characteristically, while BiP interacts with both MHCI and MHCII, GRP94 is specific to MHCII and does not interact with MHCI. Furthermore, GRP94 interacts with Toll-like receptors, receptor of insulin-like growth factor and integrins. It is of note that five specific J-proteins (Hsp40 family) function in ER in cooperation with BiP (Christis et al. 2008). In addition, ER-resident molecular chaperones form heterocomplexes with collagen type IV chains and participate in presentation of antigenic peptides by MHCI (Binder et al. 2001; Ferreira et al. 1996). Major stages of proteins folding and transport with participation of Hsp70 and other components of chaperonic machinery are depicted in Fig. 2.4.
General protein folding pathways in mitochondria, cytosol and endoplasmic reticulum of eukaryotic cell. During translocation across the mitochondrial membrane nascent polypeptides interact with cytosolic Hsp70 before and with mitochondrial Hsp70 (mtHsp70) after the transfer through the translocon. After mtHsp70 action, polypeptides may take native conformation immediately, or can interact with GroEL-GroES complex for subsequent folding. Similarly, in cytosol, nascent proteins may fold immediately after interaction with Hsp70, or interact with subsequent Hsp machinery, such as Hsp90 or cytosolic chaperonin CCT, after Hsp70 dissociation. In this case, before transfer to CCT, nascent polypeptide must be marked by prefoldin (PFD). In endoplasmic reticulum, translocated proteins bind with BiP, the ER homolog of Hsp70, and then dissociate with complete folding or interact with ER Hsp90. Therefore, it is evident that chaperonin component of the folding machine is absent in the ER, while substrates of the mitochondrial Hsp90 (Trap1) are unknown. ATP adenosine triphosphate, CCT chaperonins containing t-complex polypeptide 1, ER endoplasmic reticulum (Hartl et al. 2011; Voos 2013)
Proteins of another family of chaperones (HSP110 or HSPH) are rather similar to Hsp70 in general structure. The major difference is larger size of C-terminal peptide-binding domain and linker region between N-terminal ATP-binding domain and C-terminal domains in HSP110 (Kampinga et al. 2009; Lee-Yoon et al. 1995). Recent evidence shows that HSPH members play a role of nucleotide exchange factors for the HSPA family (Dragovic et al. 2006; Raviol et al. 2006). Futhermore, they also show chaperone activity of their own. Unlike Hsp70, Hsp110 lacks the ability to assist in protein folding; however, they exhibit high activity in preventing aggregation of denatured proteins for which is even more efficient than that of Hsp70. For this reason, Hsp110s are called “holdases” but not “foldases”, the term used for Hsp70 family (Xu et al. 2012).
Chaperonins represent another very peculiar family of stress proteins. They include bacterial GroEL and its mitochondrial homologue in eukaryotes (HSPD or Hsp60), as well as eukaryotic cytosolic protein CCT (TRiC). CCT may function without any cochaperones while GroEL requires cofactor (GroES also named Hsp10). Hsp60 of archebacteria is homologous to eukaryotic CCT and also functions without cochaperones (Ditzel et al. 1998). The main feature which differentiates chaperonins from chaperones is their ability to form complex structures resembling a stack consisting of two rings. Each ring consists either of seven like in the case of GroEL or eight like in the case of CCT monomers. Characteristically, GroEL oligomers are formed by identical monomers while CCT complex consist of eight heterosubunits encoded by different genes. Co-chaperone GroES also forms ring-like homooligomeres which are complementary to GroEL complex. In the center of each ring between monomers there is a cavity where folding of protein-substrates takes place (Fig. 2.5). The sequences of each monomer contain several hydrophobic amino acid residues, hidden inside the pore. Protein substrates interact with these residues. Folding of the proteins requires ATP hydrolysis like in the case of typical chaperones. In the case of GroEL the capture of protein substrate inside the pore is accompanied with GroES binding and subsequent ATP hydrolysis. GroES binds the pole of GroEL oligomer and closes the cavity as a lid (Fig. 2.5). Therefore, folding takes place in the environment separated from the cytosol. After this GroES dissociates from the complex and release of protein-substrate takes place. Such mechanism is very efficient and able to completely isolate protein-substrate from cellular environment (Hartl et al. 2011). It is of note, that final folding of many proteins after interaction with members of Hsp70 family requires the involvement of chaperonins (Fig. 2.4).
Proteins folding with participation of GroEL/GroES chaperonins system. (a) Top view of GroEL complex. GroEL represents as an asymmetric stack of two heptameric rings, one of which is situated in the cis-conformation (ADP/ATP and substrate bound), and the other in the trans-conformation. Seven ATP molecules bind anticooperatively between rings. (b) The cycle of Gro-EL/GroES action. Chaperonins bind with protein substrates after its interactions with Hsp70 which can be accompanied by local substrate unfolding. 1 ATP-bound GroEL ring interacts with protein substrate (red squiggle). 2 GroES associates with substrate-bound complex and dissociation of GroES and folded protein takes place from the opposite ring. 3 Folding stage. GroES and protein substrate are bound with the apical GroEL domain, substrate escape is prevented. 4 ATP hydrolysis in cis-complex permits entry of ATP into the trans-complex. 5 and 6 ATP binding is an allosteric signal to the cis-complex for GroES, protein substrate and ADP dissociation
It has been recently demonstrated that over-expression of GroEL and GroES in bacteria helps to maintain mutations which lead to the decrease of protein stability. Apparently chaperonins maintain mutant proteins in active form and prevent aggregation of misfolded proteins. As a result such mutants may survive for some time until compensating mutation occurs which may restore proteins stability. Therefore, chaperonins may have pronounced evolutionary impact promoting the formation of proteins with quite novel activities and characteristics (Tokuriki and Tawfik 2009; Wyganowski et al. 2013).
While GroEL-GroES system functions in bacteria and mitochondria, CCT was found in archea and in eukaryotic cytosol. In eukaryotes this system participates in the folding of major components of cytoskeleton, actin, tubulin and several proteins involved in cell cycle regulation (Brackley and Grantham 2009). Before CCT machinery binding a protein substrate should interact with a special protein named “prefoldin” which marks the substrates for CCT (Vainberg et al. 1998). In contrast to GroEL, CCT activity does not requires co-factors such as GroES. The closing of central cavity in the case of octameric CCT complex occurs through the conformational changes of apical parts of CCT subunits themselves (Mayer 2010).
The Hsp90 family includes abundant constitutive proteins which can be also induced by heat shock and other forms of stress. In contrast to Hsp70 family Hsp90 interacts with substrates proteins in the form of V-like dimers. Typical molecule of Hsp90 consists of three domains: N-terminal domain executes ATP hydrolysis and resembles in general structure DNA-binding sites of topoisomerases and DNA-girases of bacteria; the central domain provides the interactions with protein substrates; and C-terminal domain is required for dimerization (Freeman and Yamamoto 2002; Harris et al. 2004).
It was shown that Hsp90 does not bind newly synthesized proteins and does not participate in the process of denatured proteins refolding. However, under stressful conditions Hsp90 prevents aggregation of denatured proteins and helps to transport them to Hsp70 for subsequent 3D structure restoration (Nollen and Morimoto 2002).
Hsp90 displays a central chaperoning role in the folding, activation and transport of various regulatory proteins which play a key role in signal transduction pathways including normal function of cell cycle. All protein substrates of Hsp90 share the same characteristic feature: they may exist and function in several alternative conformations (Johnson 2012). Association of protein partners with Hsp90 prevents their aggregation and help to maintain the conformation optimal for their interaction with other proteins or low molecular weight ligands. Binding and release of proteins from Hsp90 complexes requires ATP and co-chaperones Hop and р23 (Nollen and Morimoto 2002). Specifically Hsp90 in complex with Hsp70 and several co-chaperones regulates signal protein kinases, NO synthases, telomerase, aminoacyl tRNA synthetases, and multiple transcription factors containing helix-turn-helix motif such as HIF-1, HSF, р53 etc. (Abravaya et al. 1992; Kosano et al. 1998; Sato et al. 2000; Whitesell et al. 1998; Xu and Lindquist 1993). In this respect it is necessary to emphasize an important role playing by Hsp90 in the regulation of major heat shock transcription factor (HSF1) which induces intensive transcription of all heat shock genes after temperature elevation and other stresses (see Chap. 3). The major results regarding interaction between Hsp90 and protein-targets were accumulated in studies of progesterone and glucocorticoid receptors.
It was shown that activation of progesterone receptor begins with its recognition by Hsp70 and Hsp40 with subsequent transfer by Hop protein onto Hsp90 accompanied by ATP hydrolysis. The final result of these events contains dimer of Hsp90, p23 and immunofilins providing optimal conformation of the receptor with the ligand (Nollen and Morimoto 2002). In similar way Hsp90 maintains the optimal conformation of multiple other proteins that participate in signal cascades.
In certain cases Hsp90 may have oncogenic potential and promote malignant transformation of cells. Carcinogenesis may result from the mutations in a few regulatory proteins, such as signal protein kinases, p53 and other regulatory factors that are involved in interaction with Hsp90.
Normally Hsp90 interacts with с-Src protein kinase, which participates in signal transduction which stimulates cell division. Oncogenic variant of SRC contains a few mutations that disturb its normal function. Hsp90 may interact with oncogenic forms of protein kinases and transcription factors involved in cell division and, stabilizing them, promote oncogenesis (Xu and Lindquist 1993). Furthermore, Hsp90 and Hsp70 participate in folding of р53 and by forming stable complexes with mutant variants of p53 prevent its degradation (Nagata et al. 1999). It was shown that p53 may form immunoprecipitates with Hsp70 and Hsp90 after hyperthermia (Nagata et al. 1999; Whitesell et al. 1998). It has been also demonstrated that in certain tumors the level of Hsp90 is increased by two to ten folds in comparison with surrounding normal tissues (Schwartz et al. 2003). Basing on such data Hsp90 is considered to be a perspective target for developing antitumor drugs for the purposes of chemotherapy. The suppression of Hsp90 synthesis leads to inactivation and degradation of signal proteins that promote carcinogenesis and may either restore the normal phenotype of the cell or induce apoptosis. Moreover, Hsp90 inhibition was shown to promote tau protein degradation in a mouse model of tauopathy (Dickey et al. 2007). It was shown that cochaperones CHIP, Hop and Hsp40 are constituents of the Hsp90 chaperone complex that promotes tau degradation (Cook and Petrucelli 2013; Dickey et al. 2007). At the present time there exists a variety of hsp90 inhibitors both natural and synthetic. Several of them are now passing different stages of preclinical or clinical trials and some were approved by FDA. Cisplatin is one of the Hsp90-blocking agents already widely used at the present time for chemotherapy (Rosenhagen et al. 2003). Unfortunately, high cytotoxity common for various Hsp90 inhibitors represents a serious obstacle for their use in clinics (Cook and Petrucelli 2013; Garcia-Carbonero et al. 2013; Patki and Pawar 2013). The other disadvantage of many Hsp90 binders, even such as geldanamycin passing the 3-d stage of clinical trials, is that they often induce the synthesis of Hsp70 probably as compensation for the Hsp90 inhibition and this synthesis causes the enhancement of cell tolerance to a variety of anti-cancer remedies.
It was demonstrated by Suzan Lindquist and other groups studying D. melanogaster and Arabidopsis thaliana that, surprisingly, inhibition of Hsp90 synthesis by rafamycin or geldanamycin leads to the expression of multiple new phenotypes (Rutherford and Lindquist 1998; Queitsch et al. 2002). Moreover, the patterns of expressed phenotypes are strain-specific. The observed phenomenon may be explained by interaction of Hsp90 with mutant regulatory proteins of various signal pathways maintaining them in inactive form. After stress Hsp90 begins to interact with multiple denatured proteins appearing in the cell in high concentration which leads to the release of previous protein-partners including mutant variants that were associated with Hsp90.
This process may result in the increase of possible signal pathways development and their subsequent fixation in the genome (Rutherford and Lindquist 1998; Queitsch et al. 2002; Sangster et al. 2008a, b). Further selection may lead to the appearance of stable phenotypes that will be expressed independently on stress and Hsp90 level in the cell (Rutherford and Lindquist 1998). Therefore, Hsp90 apparently may play important role in the evolution by preserving and accumulating new genetic variants (Cowen and Lindquist 2005).
Later it was shown that Hsp90 prevents phenotypic variation by suppressing transposon-induced mutagenesis through piRNAs. It was demonstrated that deficit in Hsp90 activity reduces piRNA expression, activates TE transposition and causes genotype variation (Specchia et al. 2010).
Furthermore, it was demonstrated that Hsp90 forms a complex with Piwi protein and regulates its phosphorylation. It was proposed that post-translational regulation of Piwi by Hsp90 may allow Piwi to form active complexes with piRNAs and/or epigenetic factors to promote epigenetic and transposon silencing, leading to canalization of certain signaling pathways (Gangaraju et al. 2011).
Hsp104/ClpA/ClpB proteins comprise a separate group characterized by the presence of ААА + domain (ATPases associated with a wide variety of cellular activities). These proteins were designated “unfoldases”, due to their ability to unfold the tertiary structures of other proteins. Bacteria, fungi, protozoa, chromista and plants all harbor homologues of Hsp104, a AAA + ATPase that collaborates with Hsp70 and Hsp40 to promote protein disaggregation and reactivation. Curiously, however, metazoa do not possess an Hsp104 homologue. In mammals, slowly dissolving of protein aggregates may be realized by complexes of Hsp110, Hsp70 and Hsp40 (Shorter 2011).
Hsp104 functions in the form of ring-like hexamers with six active centers localized inside the pore which is formed in the center of hexamer. Polypeptides substrates in un-folded state are pulled through the pore like a thread through the eye of the needle (Fig. 2.6). Certain representatives of Hsp104 family (ClpA and ClpX in E. coli) function in complex with proteases by means of unfolding of the substrate proteins and translocating them into protease complexes ClpP. Other members of Hsp104 family (ClpB in bacteria and Hsp104 in yeast) dissolve protein aggregates in association with Hsp70 and Hsp40 (Mayer 2010; Zolkiewski et al. 2012). Importantly, yeast Hsp104 plays a significant role in the adaptation to extreme environmental conditions, based on the ability to recover splicing after HS. Thus, S. cerevisiae Hsp104 mutants do not differ in terms of growth kinetics from the wild type cells, while under normal conditions, after acute heat shock or ethanol treatment the mutants die 100–1,000 fold more frequently (Lindquist and Kim 1996; Vogel et al. 1995; Schirmer et al. 1996). AAA + family includes 19S proteasome complex, mitochondrial Hsp78 and MCX1, that work in cooperation with proteases Pim1/LON and ClpP respectively, and many other different eukaryotic proteins (Ciechanover and Stanhill 2014; Voos 2009).
Along with the described above “classical” Hsps, to molecular chaperones can be classified certain enzymes, such as disulfide isomerases (PDIs) involved in the formation of disulfide bonds during protein folding processes, and prolyl-peptidyl isomerases (PPIases), which interconverts the cis and trans isomers of peptide bonds formed by the amino acid proline. These enzymes are found both in prokaryotes and eukaryotes, and are essential for different pathways of the cell physiology (Christis et al. 2008).
It is evident, therefore, that representatives of different groups of Hsps usually function in the cells in cooperation with members of other Hsps families as well as with multiple cofactors. At the present time there are more than 180 components of chaperone system including cofactors that regulate activity of various Hsps groups (Hartl et al. 2011). In other words, it is clear nowadays that enhanced synthesis of a single Hsp group cannot account for thermoresistance of the cell and organism as a whole. Apparently thermotolerance is provided by complex interaction of multiple proteins and each of them plays a specific role in protecting cells from harmful effects of stress.
Apart from their chaperoning functions, Hsps are involved in recovery of normal genome activity after HS and other forms of stress. It was demonstrated exploring different approaches that normal genetic activity fails to recover if protein synthesis after HS is inhibited. It was shown along these lines that following thermal stress Hsp90 is accumulated in the cytoplasm and enters the nuclei in large quantities where it binds histones and performs other protective functions (Prodromou et al. 1997). If the translocation of Hsps into the nuclei after HS is blocked, the chromatin structure is disturbed due to histones aggregation and inactivation of DNA topoisomerases. As a consequence, unwinding of DNA necessary for normal transcription does not occur after stress termination. Hsp70 also plays an important role in recovery of normal genome functioning after stress. Immunofluorescent analysis revealed the transport of Hsp70 from cytoplasm into nuclei, which is especially pronounced after DNA damage by free radicals and adriamycine (Abe et al. 1995; Knowlton et al. 2000). It was demonstrated that after translocation Hsp70 interacts with proteins of nuclear matrix and is predominantly accumulated in the nucleolus (Abe et al. 1995).
Apart from their direct chaperoning functions, majority of the constitutive as well as stress induced heat shock proteins interact with members of the apoptotic cascades since pro-apoptotic stimuli frequently induce Hsps (Li et al. 1999).
At the present time abundant material is accumulated indicating important anti-apoptotic role of certain mammalian HSPs and in particular HSP70 and HSP27. Importantly, such effect depends on the cell line and particular apoptotic-signaling pathway (Ahn et al. 1999; Brar et al. 1999; Lasunskaia et al. 1997; Takano et al. 1998; Wagstaff et al. 1999). Increased expression of HSP27 during stress response correlates with better survival from cytotoxic stress induced by different stimuli. It was shown that HSP27 can efficiently block release of cytochrome c from mitochondria in cells exposed to staurosporine or cytochalasin D (Paul et al. 2002).
On the other hand, HSP70 is able to efficiently block p53-induced apoptosis but is not effective in the case of Fas-mediated apoptosis (Schett et al. 1999). Heat shock-induced HSP70 significantly inhibits the activation of stress kinases of SAPK/JNK family. These kinases are involved in phosphorylation of p53 and antiapoptotic protein bcl-2 and, hence, initiate apoptosis. The latter protein (bcl-2) controls the release of cytochrome c from mitochondria and serves as one of the major inhibitors of apoptosis. Overexpression of HSP70 inhibits JNK activity and prevents translocation of pro-apoptotic protein Bax from cytoplasm to mitochondria where it triggers release of various death factors (Mosser et al. 1997; Stankiewicz et al. 2005). It is of note, that anti-apoptotic effect of HSP70 is realized in this case not by direct inhibiting of JNK substrates phosphorylation but rather by blockade of early stages of its activation by controlling the activity of certain phosphatases that use JNK as a substrate (Gabai et al. 1998; Kumar and Tatu 2003). HSP70 may regulate apoptosis through interaction with BAG-1 protein which activates proapoptotic protein Bax (Mosser et al. 1997). HSP70 may also directly interact with Apaf-1 and, thus, inhibit formation of functional apoptosome complex and activation of initiator caspase-9 (Mosser et al. 2000). Therefore, it was demonstrated that HSP70 function as anti-apoptotic agent at the very early stages of apoptosis which may explain its high protective potential. There are data suggesting that HSP70 may exercise its anti-apoptotic functions at later stages of apoptosis as well. Thus Mosser with coauthors demonstrated that constitutive expression of HSP70 decreases proteolysis of caspase-3 substrates while in vitro HSP70 can not modulate the function of activated caspase-3. Probably HSP70 is able to bind caspases substrates and thus protects them from proteolysis. The ability of HSP70 to protect cells from death due to expression of active caspase-3 corroborates this hypothesis (Jaattela et al. 1998; Komarova et al. 2004a, b; Mosser et al. 1997).
Various death-inducing stimuli such as TNF, may cause apoptosis via activation of transcription factors of NF-kB family. Heat-induced HSP70 interacts with р65 and c-Rel proteins belonging to this family and prevents their nuclear translocation thus hampering the TNF-induced apoptosis in U-937 cells. The protective effect is independent on the presence of NF-kB inhibitor (IkB), because IkB was not detected in complexes of HSP70 with р65 and c-Rel (Komarova et al. 2004a, b).
Furthermore, HSP27 protects fibrocarcinoma cells from apoptosis induced by Fas receptor activation and protein kinase C inhibitor staurosporin and provides resistance to adriamycine to the breast tumor cells. The essential protective anti-apoptotic action of Hsp27 is based on its two important characters: first, HSP27 is able to decrease solubility of multiple transcription factors including p53, thus modulating their activity and transport in the cell. Second, HSP27 is able to efficiently bind cytochrome c released from mitochondria and, thus, prevents its interaction with Apaf-1 and inhibits caspase-9 activity (Arrigo et al. 1998; Kamradt et al. 2001; Mehlen et al. 1996).
The above brief survey clearly shows that various HSPs may modulate apoptosis at different stages and interact with various components of cell death program realization. This may explain high efficiency of anti-apoptotic action of HSPs and strain-specific pattern of their modulating effects. If the intensity of stress does not exceed certain threshold rapid switching on of the battery of heat shock genes and resulted inhibition of apoptosis enables the cell to survive the challenge. However, in the case of a heavy and prolonged stress HSPs are not able to inhibit or block apoptosis and in this instance the cell eventually dies. The cell death is executed due to rapid activation of protein kinases р38 and JNK, that phosphorylate HSF1 at the regulatory domain and, this in turn, blocks its activity and prevents HSPs accumulation after shock (Anckar and Sistonen 2007; Chu et al. 1996; He et al. 1998).
Basing on the data mentioned above it is possible to assume that involvement of HSPs in apoptosis may play two different roles for the cells and organisms. On one hand, the demonstrated anti-apoptotic effects of HSPs in the case of brain or heart ischemia enable to consider HSPs as one of the most effective protective systems of an organism. On the other hand, the observed inhibition of apoptosis in many types of malignant cells by HSPs allows to consider them as powerful promoters of carcinogenesis. It is well-known that most cancer cells are characterized by high constitutive levels of HSP70 and HSP27 which render them to be highly resistant to many apoptosis-inducing drugs as well as to high temperature (hyperthermia) or hypoxia (Jaattela 1999). We are not going herein to discuss in detail the involvement of different HSPs in carcinogenesis because it is beyond the scope of the book and there are excellent reviews on the subject (Ciocca et al. 2013; Cohen et al. 2010; Murphy 2013; Rappa et al. 2012). However, it is of note that Hsp70 expressing in great quantities in certain cancer or virus-infected cells may be released to culture medium or blood and carry tumor or viral antigens serving as modulator of both innate immunity and adaptive activity and exercise multiple other functions (Calderwood and Ciocca 2008; Didelot et al. 2007; Joly et al. 2010).
It is necessary to mention in this context that for more than two decades after the initial discovery of heat shock proteins including various members of Hsp70, all Hsps were considered to be intracellular proteins and it was generally believed that a protein the size of Hsp70 or more could not pass through the cell membranes without the involvement of a special membrane transporter or a pore. However, several reports appeared indicating apparent release and cell-to-cell transfer of the Hsp70 protein (Hightower and Guidon 1989; Tytell 2005; Tytell et al. 1986, 2010). Later with the discovery that Hsp70 (exogenous Hsp70 or eHsp70) was detectable in the intercellular circulation of humans (Pockley et al. 1998, 2002), a lot of publications appeared demonstrating the presence of Hsp70 in many other extracellular fluids including cerebrospinal fluid (Asea 2007; Tytell et al. 2010). Originally elevated basal levels of eHsp72 were reported in people suffering from a variety of diseases including hypertension, artherosclerosis etc (Reviewed by Johnson and Fleshner 2006; Asea 2007).
Not long after these reports, it was shown by several groups that organisms in the absence of clinical disease may also rapidly respond to acute physical or psychological stressors by pronounced increase in the eHsp70 concentration in the blood (Asea 2007; Johnson and Fleshner 2006; Tytell et al. 2010).
These results enabled to conclude that stress-induced release of eHsp70 into the blood represents quite common feature of the normal stress response exhibited by various organisms including humans that encounter adverse environmental conditions such as high temperature or various xenobiotics. There is no doubt at the present time that eHsp70 has functional significance for both immune system and tolerance to different forms of metabolic stress.
Interestingly, benzene-poisoned workers showed a high incidence of antibodies against Hsp72 which was associated with a decrease in white blood cells and high frequency of lymphocyte DNA damage. These data suggest that antibodies against Hsps can potentially be useful biomonitors to assess if people have experienced abnormal xenobiotic-induced stress within their living or working environment (Wu et al. 1998).
There are several potential release mechanisms for Hsp70 and other Hsps that lack the signal peptide targeting them to secretory vesicles. First of all Hsps may be released from cells whose membranes were damaged by trauma or necrosis induced by various means. Besides such unregulated leakage which may happen in all cells and tissues, regulated release of Hsps and specifically Hsp70 may take place exploring membrane-bounded vesicles called “exosomes” that can be produced in a wide variety of cells (Fevrier and Raposo 2004; Johnson and Fleshner 2006). Though exosomes may account for Hsp70 release by various cells in many cases, recent investigations demonstrated that secretion via conventional secretory vesicles is also an option and can not be excluded (Tytell et al. 2010). Once released, Hsps can interact with the membranes of various cells, be involved in cell-to-cell interactions and enter the cytoplasm of target cells through the membrane by different not yet completely understood mechanisms (Ekimova et al. 2010). It became clear that Hsp72 in human cells apparently has unique releasing signals and immunomodulatory functions when expressed in an extracellular context on the cell surface or in the circulation in blood or other extracellular fluids.
While protective therapeutic role of intracellular Hsp72 was clearly demonstrated by many groups in the treatments of multiple neurodegenerative disorders such as Parkinson’s disease, Huntington’s disease, Alzheimer’s disease and other proteinopathies the ability of eHsp70 to inhibit chronic inflammation and/or exacerbates inflammatory diseases such as Alzheimer’s inflammatory bowel disease or artherosclerosis have only recently been investigated in detail (Bobkova et al. 2013; Ekimova et al. 2010; Tytell et al. 2010; Vinokurov et al. 2012).
As a result of numerous papers describing pronounced anti-inflammatory effects of endogenous intacellular and extracellular heat shock protein 72 exploring various mammalian models of sepsis and neurodegeneration several years ago first attempts were made to apply recombinant Hsp70 as a therapeutic drug and monitor its neuroprotective effects exploring various methods of Hsp70 administration (Bobkova et al. 2014; Ekimova et al. 2010). Although these studies demonstrated that recombinant eHsp70 may be a practical therapeutic agent for treatment of neurodegenerative diseases associated with abnormal protein biogenesis and cognitive disturbances, such as AD, the molecular mechanisms underlying the observed neuroprotection remain unknown.
2.1 Conclusions
Molecular chaperones are highly conserved proteins, providing non-covalent folding, assembling and transport of a wide range of cellular proteins. Expression of many chaperones is stress-induced, and, hence, these proteins protect cells from various forms of metabolic stress, by restoring the conformation of denatured proteins and eliminating of irreversibly damaged proteins. Other constitutively expressed chaperones perform folding and intracellular transport of newly synthesized proteins under normal physiological conditions. In addition, chaperone network is involved in regulation of a large number of cellular signal transduction pathways and cytoskeleton assembling. Different classes of molecular chaperones and their protein co-factors (co-chaperones) work in tight cooperation providing complex house-keeping and anti-stress system, which plays a key role in protein homeostasis in a cell. Recombinant Hsps and in particular Hsp70 represent promising therapeutic agents for treatment of various neurodegenerative diseases associated with abnormal protein biogenesis.
References
Abe T, Konishi T, Hirano T, Kasai H, Shimizu K et al (1995) Possible correlation between DNA damage induced by hydrogen peroxide and translocation of heat shock 70 protein into the nucleus. Biochem Biophys Res Commun 206:548–555
Abravaya K, Myers MP, Murphy SP, Morimoto RI (1992) The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 6:1153–1164
Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS (1999) Suppression of ceramide-mediated apoptosis by HSP70. Mol Cells 9:200–206
Anckar J, Sistonen L (2007) Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594:78–88
Angelidis CE, Lazaridis I, Pagoulatos GN (1991) Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem 199:35–39
Arrigo AP (1998) Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 379:19–26
Asea AAA (2007) Release of heat shock proteins: passive versus active release mechanisms. In: Heat shock proteins: potent mediators of inflammation and immunity. Springer, Dordrecht, pp 3–20
Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A et al (1997) Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem 272:9002–9010
Binder RJ, Blachere NE, Srivastava PK (2001) Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem 276:17163–17171
Bobkova N, Guzhova I, Margulis B, Nesterova I, Medvinskaya N et al (2013) Dynamics of endogenous Hsp70 synthesis in the brain of olfactory bulbectomized mice. Cell Stress Chaperones 18:109–118
Bobkova NV, Garbuz DG, Nesterova I, Medvinskaya N, Samokhin A et al (2014) Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J Alzheimers Dis 38:425–435
Brackley KI, Grantham J (2009) Activities of the chaperonin containing TCP-1 (CCT): implications for cell cycle progression and cytoskeletal organisation. Cell Stress Chaperones 14:23–31
Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS et al (1999) Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptotic as well as against thermal or hypoxic stress. J Mol Cell Cardiol 31:135–146
Calderwood SK, Ciocca DR (2008) Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia 24:31–39
Carver JA, Guerreiro N, Nicholls KA, Truscott RJ (1995) On the interaction of alpha-crystallin with unfolded proteins. Biochim Biophys Acta 1252:251–260
Chong KY, Lai CC, Lille S, Chang C, Su CY (1998) Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J Mol Cell Cardiol 30:599–608
Christis C, Lubsen NH, Braakman I (2008) Protein folding includes oligomerization – examples from the endoplasmic reticulum and cytosol. FEBS J 275:4700–4727
Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK (1996) Sequential phosphorylation by mitogen-activated kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem 271:30847–30857
Ciechanover A, Stanhill A (2014) The complexity of recognition of ubiquitinated substrates by the 26S proteasome. Biochim Biophys Acta 1843:86–96
Ciocca DR, Arrigo AP, Calderwood SK (2013) Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol 87:19–48
Cohen M, Dromard M, Petignat P (2010) Heat shock proteins in ovarian cancer: a potential target for therapy. Gynecol Oncol 119:164–166
Cook C, Petrucelli L (2013) Tau triage decisions mediated by the chaperone network. J Alzheimers Dis 33:145–151
Cowen LE, Lindquist S (2005) Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189
Craig EA, Jacobsen K (1984) Mutations of the heat inducible 70 kilodalton genes of yeast confer temperature sensitive growth. Cell 38:841–849
de Jong WW, Caspers GJ, Leunissen JA (1998) Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int J Biol Macromol 22:151–162
Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM et al (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117:648–658
Didelot C, Lanneau D, Brunet M, Joly AL, De Thonel A et al (2007) Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem 14:2839–2847
Ditzel L, Lowe J, Stock D, Stetter К, Huber H et al (1998) Crystall structure of the thermosome, the Archaeal chaperonin and homolog of CCT. Cell 93:125–138
Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU (2006) Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J 25:2519–2528
Ekimova IV, Nitsinskaya LE, Romanova IV, Pastukhov YF, Margulis BA, Guzhova IV (2010) Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J Neurochem 115:1035–1044
Fan CY (2003) Mechanisms for regulation of hsp70 function by hsp40. Cell Stress Chaperones 8:309–316
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response, evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Feder ME, Cartano NV, Milos L, Krebs RA, Lindquist SL (1996) Effect of engineering hsp70 copy number on Hsp70 expression and tolerance of ecologically relevant heat shock in larvae and pupae of Drosophila melanogaster. J Exp Biol 199:1837–1844
Fernando P, Heikkila JJ (2000) Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones 5:148–159
Ferreira LR, Norris K, Smith T, Hebert C, Sauk JJ (1996) HSP47 and other ER-resident molecular chaperones form heterocomplexes with each other and with collagen type IV chains. Connect Tissue Res 33:256–273
Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16:415–421
Flaherty KM, DeLuca-Flaherty C, McKay DB (1990) Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature 346:623–628
Flajnik MF, Canel C, Kramer J, Kasahara M (1991) Which came first, MHC class I or class II? Immunogenetics 33:295–300
Freeman BC, Yamamoto KR (2002) Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296:2232–2235
Frydman J (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70:603–649
Gabai VL, Meriin AB, Yaglom JA, Volloch V, Sherman MY (1998) Role of HSP70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett 438:1–4
Gangaraju VK, Yin H, Weiner MM, Wang J, Huang XA, Lin H (2011) Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nat Genet 43:153–158
Garcia-Carbonero R, Carnero A, Paz-Ares L (2013) Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol 14:358–369
Gebauer M, Zeiner M, Gehring U (1998) Interference between proteins Hap46 and Hop/p60, which bind to different domains of the molecular chaperone hsp70/hsc70. Mol Cell Biol 18:6238–6244
Gong WJ, Golic KG (2006) Loss of Hsp70 in Drosophila is pleiotropic, with effects on thermotolerance, recovery from heat shock and neurodegeneration. Genetics 172:275–286
Gusev NB, Bogatcheva NV, Marston SB (2002) Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry 67:511–519
Harris SF, Shiau AK, Agard DA (2004) The crystal structure of the carboxy-terminal dimerization domain of htpG, the Escherichia coli Hsp90, reveals a potential substrate binding site. Structure 12(6):1087–1097
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332
He B, Meng Y, Mivechi NF (1998) Glycogen synthase kinase 3β and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol Cell Biol 18:6624–6633
Hightower LE (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66:191–197
Hightower LE, Guidon PT (1989) Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol 138:257–266
Horwitz J (1976) Some properties of the low molecular weight alpha-crystallin from normal human lens: comparison with bovine lens. Exp Eye Res 23:471–481
Houry WA (2001) Chaperone-assisted protein folding in the cell cytoplasm. Curr Protein Pept Sci 2:227–244
Ingolia TD, Craig EA (1982) Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A 79:2360–2364
Jaattela M (1999) Escaping cell death: survival proteins in cancer. Exp Cell Res 248:30–43
Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M (1998) HSP70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 17:6124–6134
Jedlicka P, Mortin MA, Wu C (1997) Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J 16:2452–2462
John NR, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA (1992) The translation machinery and 70kd heat shock protein cooperate in protein synthesis. Cell 71:97–105
Johnson JL (2012) Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta 1823:607–613
Johnson JD, Fleshner M (2006) Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79:425–434
Johnson RN, Kucey BL (1988) Competitive inhibition of hsp70 expression causes thermosensitivity. Science 242:1551–1554
Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C (2010) Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun 2:238–247
Kammanadiminti SJ, Chadee K (2006) Suppression of NF-kappaB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J Biol Chem 281:26112–26120
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM et al (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111
Kamradt MC, Chen F, Cryns VL (2001) The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem 276:16059–16063
Khlebodarova TM (2002) How cells protect themselves against stress? Genetika 38:437–452
King V, Tower J (1999) Aging-specific expression of Drosophila hsp22. Dev Biol 207:107–118
Knowlton AA, Grenier M, Kirchhoff SR, Salfity M (2000) Phosphorylation at tyrosine-524 influences nuclear accumulation of HSP72 with heat stress. Am J Physiol Heart Circ Physiol 278:2143–2149
Komarova EIu, Margulis BA, Guzhova IV (2004a) The role of Hsp70 chaperone in the reaction of human leukemic cells to anticancer drugs. Tsitologiia 46:550–556
Komarova EY, Afanasyeva EA, Bulatova MM, Cheetham ME, Margulis BA, Guzhova IV (2004b) Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones 9:265–275
Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D (1998) The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem 273:32973–32979
Ku Z, Yang J, Menon V, Thomason DB (1995) Decreased polysomal HSP70 may slow polypeptide elongation during skeletal muscle atrophy. Am J Physiol 268:1369–1374
Kumar Y, Tatu U (2003) Stress protein flux during recovery from simulated ischemia: induced heat shock protein 70 confers cytoprotection by suppressing JNK activation and inhibiting apoptotic cell death. Proteomics 3:513–526
Lasunskaia EB, Fridlianskaia II, Guzhova IV, Bozhkov VM, Margulis BA (1997) Accumulation of major stress protein 70kDa protects myeloid and lymphoid cells from death by apoptosis. Apoptosis 2:156–163
Lee-Yoon D, Easton D, Murawski M, Burd R, Subjeck JR (1995) Identification of a major subfamily of large hsp70-like proteins through the cloning of the mammalian 110-kDa heat shock protein. J Biol Chem 270:15725–15733
Li GC, Li L, Liu RY, Rehman M, Lee WM (1992) Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A 89:2036–2040
Li S, Chien S, Branemark PI (1999) Heat shock-induced necrosis and apoptosis in osteoblasts. J Orthop Res 17:891–899
Li F, Mao HP, Ruchalski KL, Wang YH, Choy W et al (2002) Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 283:917–926
Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M (1991) Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A 88:2874–2878
Lindquist S, Kim G (1996) Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc Natl Acad Sci U S A 93:5301–5306
Maloyan A, Palmon A, Horowitz M (1999) Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol 276:R1506–R1515
Marcillat O, Zhang Y, Davies KJ (1989) Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. Biochem J 259:181–189
Mayer MP (2010) Gymnastics of molecular chaperones. Mol Cell 39:321–331
Mehlen P, Schulze-Osthoff K, Arrigo AP (1996) Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem 271:16510–16514
Melnick J, Argon Y (1995) Molecular chaperones and the biosynthesis of antigen receptors. Immunol Today 16:243–250
Michaud S, Tanguay RM (2003) Expression of the Hsp23 chaperone during Drosophila embryogenesis: association to distinct neural and glial lineages. BMC Dev Biol 3:9
Morrow G, Tanguay RM (2003) Heat shock proteins and aging in Drosophila melanogaster. Semin Cell Dev Biol 14:291–299
Morrow G, Samson M, Michaud S, Tanguay RM (2004) Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J 18:598–599
Morrow G, Heikkila JJ, Tanguay RM (2006) Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones 11:51–60
Mosser DD, Caron AW, Bourged L, Denis-Larose C, Massie B (1997) Role of the human heat shock protein HSP70 in protection against stress-induced apoptosis. Mol Cell Biol 17:5317–5327
Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY et al (2000) The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol 20:7146–7159
Murphy ME (2013) The HSP70 family and cancer. Carcinogenesis 34:1181–1188
Nagata Y, Anan T, Yoshida T, Mizukami T, Taya Y et al (1999) The stabilization mechanism of mutant-type p53 by impaired ubiquitination: the loss of wild-type p53 function and the HSP90 association. Oncogene 18:6037–6049
Nelson RJ, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA (1992) The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell 71:97–105
Neupert W, Hartl FU, Craig EA, Pfanner N (1990) How do polypeptides cross the mitochondrial membranes? Cell 63:447–450
Nollen EA, Morimoto RI (2002) Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci 115:2809–2816
Park KC, Kim DS, Choi HO, Kim KH, Chung JH et al (2000) Overexpression of HSP70 prevents ultraviolet B-induced apoptosis of a human melanoma cell line. Arch Dermatol Res 292:482–487
Patki JM, Pawar SS (2013) HSP90: chaperone-me-not. Pathol Oncol Res 19:631–640
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP (2002) Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22:816–834
Pelham HR (1986) Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46:959–961
Pockley AG, Shepherd J, Corton JM (1998) Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest 27:367–377
Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J (2002) Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 20:1815–1820
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75
Queitsch C, Sangster TA, Lindquist S (2002) Hsp90 as a capacitor of phenotypic variation. Nature 417:618–624
Rappa F, Farina F, Zummo G, David S, Campanella C et al (2012) HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res 32:5139–5150
Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B (2006) Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J 25:2510–2518
Rippmann F, Taylor WR, Rothbard JB, Green NM (1991) A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J 10:1053–1059
Rosenhagen MC, Sōti C, Schmidt U, Wochnik GM, Hartl FU et al (2003) The heat shock protein 90-targeting drug cisplatin selectively inhibits steroid receptor activation. Mol Endocrinol 17:1991–2001
Rutherford SL, Lindquist S (1998) Hsp90 as a capacitor for morphological evolution. Nature 396:336–342
Sangster TA, Salathia N, Lee HN, Watanabe E, Schellenberg K et al (2008a) HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc Natl Acad Sci U S A 105:2969–2974
Sangster TA, Salathia N, Undurraga S, Milo R, Schellenberg K et al (2008b) HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci U S A 105:2963–2968
Sato S, Fujita N, Tsuruo T (2000) Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 97:10832–10837
Schett G, Steiner CW, Gröger M, Winkler S, Graninger W et al (1999) Activation of Fas inhibits heat-induced activation of HSF1 and up-regulation of HSP70. FASEB J 13:833–842
Schirmer EC, Glover JR, Singer MA, Lindquist S (1996) HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci 21:289–296
Schlesinger MJ, Ashburner M, Tissieres A (1982) Heat shock from bacteria to man. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schwartz J, Pinilla-Ibarz J, Yuan RR, Scheinberg DA (2003) Novel targeted and immunotherapeutic strategies in chronic myeloid leukemia. Semin Hematol 40:87–96
Shorter J (2011) The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One 6:e26319
Specchia V, Piacentini L, Tritto P, Fanti L, D’Alessandro R et al (2010) Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature 463:662–665
Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD (2005) Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 280:38729–38739
Summers DW, Douglas PM, Ramos CH, Cyr DM (2009) Polypeptide transfer from Hsp40 to Hsp70 molecular chaperones. Trends Biochem Sci 34:230–233
Szalay MS, Kovács IA, Korcsmáros T, Böde C, Csermely P (2007) Stress-induced rearrangements of cellular networks: consequences for protection and drug design. FEBS Lett 581:3675–3680
Takano M, Arai T, Mokuno Y, Nishimura H, Nimura Y, Yoshikai Y (1998) Dibutyryl cyclic adenosine monophosphate protects mice against tumor necrosis factor-alpha-induced hepatocyte apoptosis accompanied by increased heat shock protein 70 expression. Cell Stress Chaperones 3:109–117
Tokuriki N, Tawfik DS (2009) Chaperonin overexpression promotes genetic variation and enzyme evolution. Nature 459:668–673
Tytell M (2005) Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int J Hyperthermia 21:445–455
Tytell M, Greenberg SG, Lasek RJ (1986) Heat shock protein is transferred from glia to axon. Brain Res 363:161–164
Tytell M, Robinson MB, Milligan C (2010) Release of heat shock proteins and their effects when in extracellular space in the nervous system. In: Asea AAA, Calderwood SK (eds) Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. Springer, Dordrecht, pp 257–272
Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J et al (1998) Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell 93:863–873
Vinokurov M, Ostrov V, Yurinskaya M, Garbuz D, Murashev A et al (2012) Recombinant human Hsp70 protects against lipoteichoic acid-induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones 17:89–101
Vogel JL, Parsell DA, Lindquist S (1995) Heat-shock proteins Hsp104 and Hsp70 reactivate mRNA splicing after heat inactivation. Curr Biol 5:306–317
Voos W (2009) Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol 160:718–725
Voos W (2013) Chaperone-protease networks in mitochondrial protein homeostasis. Biochim Biophys Acta 1833:388–399
Wagstaff MJ, Collaço-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS (1999) Protection of neuronal cells from apoptosis by HSP27 delivered with a herpes simplex virus-based vector. J Biol Chem 274:5061–5069
Welch WJ, Feramisco JR (1985) Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol 5:1229–1237
Wheeler JC, Bieschke ET, Tower J (1995) Muscle-specific expression of Drosophila Hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci U S A 92:10408–10412
Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH (1998) The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an HSP90-binding agent. Mol Cell Biol 18:1517–1524
Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM (1998) Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones 3:161–167
Wyganowski KT, Kaltenbach M, Tokuriki N (2013) GroEL/ES buffering and compensatory mutations promote protein evolution by stabilizing folding intermediates. J Mol Biol 425:3403–3414
Xu Y, Lindquist S (1993) Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci U S A 90:7074–7078
Xu X, Sarbeng EB, Vorvis C, Kumar DP, Zhou L, Liu Q (2012) Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J Biol Chem 287:5661–5672
Zolkiewski M, Zhang T, Nagy M (2012) Aggregate reactivation mediated by the Hsp100 chaperones. Arch Biochem Biophys 520:1–6
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Evgen’ev, M.B., Garbuz, D.G., Zatsepina, O.G. (2014). Molecular Functions of Heat Shock Proteins. In: Heat Shock Proteins and Whole Body Adaptation to Extreme Environments. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9235-6_2
Download citation
DOI: https://doi.org/10.1007/978-94-017-9235-6_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9234-9
Online ISBN: 978-94-017-9235-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)