Summary
In this chapter, we (i) place photoprotective thermal dissipation of excess light into the context of the many adjustments plants employ to maximize photosynthesis and growth while minimizing the destructive potential of excess light and (ii) describe the historical development of key measures of thermal energy dissipation and related processes (e.g., common coefficients and other quantification of non-photochemical quenching, or NPQ, of chlorophyll a fluorescence and chlorophyll a fluorescence transients), emphasizing the theoretical and practical advantages and limitations surrounding the use of NPQ as a measure of dissipation of excitation energy from singlet excited state of chlorophyll a as “harmless” heat. Furthermore, we provide guidance on the proper measurement of NPQ and advise readers of the methodological issues that, if not avoided, can render measures of this parameter non-interpretable.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Photosynthetic Electron Transport
- Thermal Dissipation
- Measuring Light
- Ambient Illumination
- Thermal Energy Dissipation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I Introduction
We will first place photoprotective dissipation of excess absorbed sunlight into the broader context of the many adjustments plants make to maximize both light utilization for photosynthesis and growth as well as the safe avoidance of potentially destructive excess light. We will then focus on the measurement of thermal energy dissipation via non-photochemical quenching (NPQ) of chlorophyll fluorescence. For a book on photoprotection in plants, in a broader context, see Demmig-Adams et al. (2006). For a historical perspective of this field, see Papageorgiou and Govindjee, Chap. 1, and for background in photophysics, see Ostroumov et al., Chap. 4.
II Thermal Energy Dissipation in Context: Many Means of Adjustment for Optimal Utilization of Sunlight While Avoiding its Hazards
Plants employ an evolutionarily conserved photosynthetic pathway to reduce atmospheric CO2 using chemical potential energy generated via the absorption of sunlight (for a general description, see Rabinowitch and Govindjee 1969; Blankenship 2002). Components of the photosynthetic pathway are regulated, and acclimate to prevailing conditions, over time scales ranging across many orders of magnitude, so that photosynthetic production of reduced carbon compounds meets demand from the whole plant across the diverse conditions in which plants grow (Barber and Baker 1985; Baker and Long 1986; Baker 1996; Aro and Andersson 2001; Demmig-Adams et al. 2006). The dynamic nature of photosynthesis has been the subject of research for decades.
Oxygenic photosynthesis requires two light reactions and two photosystems: photosystem I (PS I; see Golbeck 2006) and photosystem II (PS II; see Wydrzynski and Satoh 2005). The evolution of the Z-scheme that runs these reactions is described by Govindjee and Björn (2012). Photosynthetic electron transport, and particularly PS II, is sensitive to imbalances between light absorption by chlorophyll (Chl) and the use of light energy in support of photosynthetic carbon fixation and other reductive processes (Demmig-Adams and Adams 2006). Absorption of light in excess of photosynthetic light utilization can potentially cause oxidative modification of PS II and associated proteins via several molecular mechanisms generally involving reactive oxygen species (reviewed in Melis 1999; Takahashi and Murata 2008; Kornyeyev et al. 2010). Such modifications can render PS II ineffective at electron transfer, thus lowering PS II efficiency (i.e., decreasing the proportion of light absorbed by PS II antenna Chl used to support photosynthetic electron transport). Lasting decreases in PS II efficiency that are not associated with rapidly reversible NPQ and the trans-thylakoid pH gradient, while being associated with lasting decreases in the light- and CO2-saturated photosynthetic capacity are commonly referred to as photoinhibition (reviewed in Kyle et al. 1987). While the causal relationships between photoinhibition and low plant productivity remain unelucidated (Adams et al. 2013), it is clear that photoinhibition occurs in response to lasting imbalances between the absorption and utilization of light in photosynthesis (see also Adams et al., Chap. 23).
Plants have evolved numerous means of avoiding lasting imbalances between light absorption and utilization (and the associated excess light stress and persistent decreases in PS II efficiency). These protective mechanisms are manifest at every scale of the organism hierarchy (Fig. 7.1), and can be clustered into mechanisms minimizing absorption of excess light and mechanisms enhancing utilization of excitation energy via photochemical and non-photochemical pathways (i.e., pathways that do, or do not, lead to the generation of reduced compounds, respectively).
When compared with shade-acclimated plants of the same species, many plants acclimated to direct sunlight grow more steeply angled leaves and/or branches, with shorter petioles, and spaced more densely along the branch (i.e., shorter internodes) (Mooney et al. 1977; Givnish 1988; Valladares and Pearcy 1998). This suite of acclimatory responses results in canopies with lower light absorption efficiencies (Valladares and Pearcy 1998). For the leaves of all plants, direct full sunlight super-saturates photosynthesis and is therefore in excess. The structural features of full-sunlight acclimated canopy architecture described above may limit the absorption of excess light by the majority of leaves without compromising photosynthetic productivity (Valladares et al. 2000). In fact, these architectural features may, in some instances, serve to enhance whole canopy photosynthesis in natural sunlight, since outer-canopy, steeply-angled leaves may intercept more sunlight in the early morning and late afternoon, when sunlight can be limiting for photosynthesis (McMillen and McClendon 1979; Givnish 1988; Marias 2010). On the other hand, certain very rapidly growing (and rapidly photosynthesizing) annual species tend to orient leaves parallel to the ground in full sunlight, and – like sunflower – may even track the sun to further maximize light absorption (Ehleringer and Forseth 1980; Forseth and Ehleringer 1983; Greer and Thorpe 2009).
Exposure to environmental stress (e.g., extreme temperatures, soil nutrient deficiency, or drought) generally lowers photosynthetic CO2 assimilation (see also Adams et al., Chap. 23; Demmig-Adams et al., Chap. 24; Morales et al., Chap. 27). Decreased assimilation can increase excess light absorption since the level of excess light is a function of both irradiance and photosynthetic light utilization. In response to environmental stress, many plants will acclimate by rendering the adaxial (upper) planes of the leaf more optically opaque through various means, thus lowering the effective irradiance experienced by chlorophyllous cells below. Leaves may secrete a thicker layer of reflective cuticular waxes (Barker et al. 1997) or may develop thicker pubescence (Ehleringer and Björkman 1978). Many plants accumulate red anthocyanins in the vacuoles of epidermal or palisade parenchyma cells that absorb light otherwise absorbed preferentially by Chl b (Gould et al. 1995; Hughes et al. 2007; Merzlyak et al. 2008).
Leaf Chl content is also subject to acclimation. In winter, when chilling temperatures curb photosynthesis rates and exacerbate light stress, many broad-leafed evergreen plants decrease leaf Chl content (Adams and Barker 1998; Logan et al. 1998b). Furthermore, soil nutrient deficiency limits growth, which leads to sugar accumulation in the leaves, feedback inhibition of photosynthetic capacity (see Adams et al., Chap. 23), and profound reductions in leaf Chl content (Verhoeven et al. 1997; Logan et al. 1999; Morales et al., Chap. 27).
A minority of plant species is capable of leaf movements apparently enacted to either stave off light stress or to maximize photosynthetic light use (e.g., in sunflower, as mentioned above). Paraheliotropic movements (orienting the leaf lamina parallel to the sun’s rays) minimize excess light absorption during drought or nutrient deficiency (Ludlow and Björkman 1984; Rosa et al. 1991; Kao and Forseth 1991, 1992a, b). Likewise, the movement of Rhododendron leaves from a horizontal to a more vertical orientation in response to low temperature is thought to minimize light absorption on colder winter days (Nilsen 2008; Wang et al. 2008, 2009). In contrast, diaheliotropic leaf movements (orienting the leaf lamina perpendicular to the sun’s rays) have been observed in plants from xeric environments after transient rainfall, which allows higher stomatal conductance in support of photosynthesis (Ehleringer and Forseth 1980).
The intracellular position of chloroplasts is tightly controlled by the actin cytoskeleton (Takagi 2003) and apparently further balances photosynthetic light utilization align the potential for high light stress. During exposure to low light intensities, chloroplasts along the upper and lower planes of the cell (periclinal cell surfaces) to maximize light absorption, whereas during exposure to intense light, they can be found self-shading each other along the lateral (anticlinal) cell surfaces (Haupt and Scheuerlein 1990; Brugnoli and Björkman 1992; Park et al. 1996; Kasahara et al. 2002; Williams et al. 2003).
At the molecular scale, positions and binding associations of some light-harvesting complex protein subunits are also dynamic. For instance, protein phosphorylation cascades trigger migration of light-harvesting complexes between PS II and PS I to balance light inputs across photosynthetic electron transport under limiting light in a process known as state transitions (see, e.g., Williams and Allen 1987; Allen 1992; Krause and Jahns 2004; and citations therein). In addition, protein phosphorylation has recently been invoked in energy transfer from PS II to PS I, followed by net energy dissipation by P700 under excess light (Tikkanen and Aro 2012; Tikkanen et al. 2012).
Utilization of absorbed light energy in photosynthesis clearly benefits plants by (i) supporting their bioenergetic needs and (ii) acting as a sink for excitation energy, thereby reducing potential excess light stress. Herbaceous annuals and biennials employ this approach via rapid growth and high photosynthesis rates when acclimated to full sunlight (Adams and Demmig-Adams 2004; Adams et al. 1995b; Demmig-Adams et al., Chap. 24). However, even electron transport activity not leading to CO2 fixation serves to alleviate excess light stress. Molecular oxygen (O2) can be reduced by iron-sulfur clusters of PS I and/or other photosynthetic electron carriers (Mehler 1951; Asada 1999; Badger et al. 2000). Photoreduction of O2 via the “Mehler reaction” (Figs. 7.1 and 1.1 in Chap. 1 of this volume) generates superoxide, a reactive and potentially cytotoxic molecule, that is further reduced to water by a series of reactions depending directly or indirectly on photosynthetic electron transport for reducing equivalents (Asada 1999). In this series of reactions, water is both the source of reducing equivalents (when oxidized by the Oxygen Evolving Complex) and the product of the final reduction-oxidation reactions, which is why this pathway has been termed the “water-water cycle” (Asada 1999). This cycle is futile insofar as it generates no reduced carbon compounds; since it potentially consumes excess absorbed light energy, it has also been considered photoprotective (Rizhsky et al. 2003). Safe functioning of the water-water cycle depends upon adequate activities for constituent antioxidant enzymes (e.g., superoxide dismutase, ascorbate peroxidase) and enzymes involved in the reduction of electron donors (e.g., glutathione reductase). Plants acclimated to high light environments can possess several-fold higher activities for such enzymes, indicating that the light energy sink represented by the water-water cycle is greater in environments with greater excess light absorption (Grace and Logan 1996; Logan et al. 1998a).
Furthermore, the oxygenation of ribulose bisphosphate, as the initiating step in the process of photorespiration, is also followed by the consumption of electrons from the photosynthetic electron transport chain. Such “wasteful” consumption of energized electrons can therefore contribute to the photoprotection of chloroplasts and leaves (Kozaki and Takeba 1996; Wingler et al. 2000). In fact, utilization of photosynthetic electron transport for the reduction of any protein, metabolite, or compound (e.g., thioredoxin, reduction of nitrate to ammonia, reduction of sulfate to sulfide) has the potential to contribute to the protection of photosynthesis and photosynthetic cells.
Regulated removal of excitation energy via its conversion to heat, harmlessly radiated to the environment as thermal energy, is a ubiquitous photoprotective mechanism (reviewed in Demmig-Adams and Adams 2006; Demmig-Adams et al. 2012; Jahns and Holzwarth 2012; Ruban et al. 2012; see also Adams and Demmig-Adams, Chap. 2, Adams et al., Chap. 23 and Demmig-Adams et al., Chap. 24). Thermal energy dissipation, as this process is commonly known, has been observed in diverse algal taxa and every Chl-containing member of the plant kingdom thus far examined.
Thermal energy dissipation appears to be the manifestation of multiple molecular/biophysical mechanisms, many of which involve xanthophylls (i.e., oxygenated carotenoids) with long conjugated carbon backbones. For a discussion of the photochemistry of carotenoids, see Frank et al. (1999). The current view is that xanthophylls facilitate direct de-excitation of singlet-excited Chl a (for detailed discussions on the molecular mechanisms involved, see Polívka and Frank, Chap. 8 and Walla et al., Chap. 9) and/or facilitate a conversion of light-harvesting complexes to a dissipating state (see, e.g., Horton, Chap. 3). Over time-scales of seconds to seasons, plants modulate levels of thermal energy dissipation in response to prevailing conditions (Adams et al. 1995a; for a recent review, see Demmig-Adams et al. 2012), apparently to optimize both photoprotection and the productive photochemical use of absorbed light.
While initial inquiry into the features of thermal energy dissipation was made via other approaches, the advent of pulse-amplitude-modulated analysis of Chl fluorescence emission (described below; Schreiber et al. 1986) has been central to the development of knowledge of photosynthetic dynamics and photoprotection. With this technical advance came the ability to quantify the fate of absorbed photons (including those utilized in photosynthesis and those safely converted to heat via thermal energy dissipation) non-invasively and rapidly in situ (Bilger et al. 1995). As with the early development of most methodologies, the use of Chl fluorescence and the chosen parameters calculated on the basis of Chl fluorescence emission have evolved with the continuing development of improved avenues to exploit the phenomenon and the instruments designed to capture it (for an historical review on fluorescence and instrumentation, see Kalaji et al. 2012). The study of Chl fluorescence emission has grown into a sub-discipline unto itself within photosynthesis research (see Papageorgiou and Govindjee 2004). While important advances have resulted, the literature has unfortunately become unwelcoming to the non-specialist, thus limiting the relevance and utility of a powerful tool to the broader community of plant physiologists, ecologists, horticulturalists, silviculturalists, and plant breeders. In the following, we describe the historical development of parameters designed to capture aspects of thermal energy dissipation, with special attention to NPQ of Chl fluorescence as an estimate of the de-excitation of singlet excited Chl a via thermal energy dissipation. We also describe commonly encountered issues that can interfere with the proper collection of informative and interpretable Chl fluorescence signals. Lastly, we recommend experimental approaches that maximize the usefulness of the study of Chl fluorescence emission.
III Methods of Quantifying Thermal Energy Dissipation
Prior to development of the pulse-amplitude modulation approach, Chl fluorescence emission could only provide interpretable information if the leaves, algae, or other photosynthetic structures under study were pre-darkened before measurement (referred to in the literature as dark acclimation or dark adaptation). Upon abrupt transfer from darkness to illumination, Chl fluorescence emission increases and then quickly decreases to a steady-state level (Walker 1981). The fluorescence transient upon dark-to-light transfer, and the intermediate levels through which fluorescence quickly progresses over the first few seconds, was discovered by Hans Kautsky and later named the “Kautsky effect” (Govindjee 1995; Lichtenthaler 1992). Various features of the transient can be deconvoluted, yielding information about photochemical and non-photochemical processes. A principal disadvantage of this technique, as it was originally conceived, is its inability to capture dynamic processes of photosynthetic structures continuously exposed to ambient illumination.
The resolution of the rapid kinetics of fluorescence induction during exposure to saturating illumination has enabled a further analysis of the function of PS II (Schreiber and Neubauer 1987; Strasser et al. 2004). The resulting OJIP method (so termed for the one-letter names associated with phases of the transient curve) has brought examination of fluorescence transients back into use (see, e.g., Stirbet and Govindjee 2012; Schreiber et al. 2012; and literature cited therein).
Custom-built fluorometers employing tunable amplifiers to detect periodically modulated weak signals on the background of strong continuous signals became available in the mid-sixties of the last century. With the introduction of the pulse-amplitude-modulated (PAM) technique of measuring Chl fluorescence emission, combined with the saturation pulse analysis, commercial fluorescence instruments became more widely available for the study of photosynthesis both in the laboratory and the field (Schreiber et al. 1986; Schreiber 2004). PAM fluorescence analysis employs a weak monochromatic measuring beam that is switched on and off over microsecond time scales. That is, its amplitude (intensity) is modulated in a rapidly repetitive, pulsed manner – hence the name pulse amplitude modulation.
PAM fluorometers report to the user only fluorescence emission exhibiting modulation with the same frequency as the measuring light. Thus, fluorescence resulting from the absorption of ambient light, whose intensity can exceed modulated fluorescence by more than six orders of magnitude, is not reported. This enables the user to make meaningful comparisons of fluorescence emission between tissues exposed to darkness and those exposed to ambient illumination. The measuring light source is non-actinic, i.e., it must be set to such a low intensity that it does not alter the reduction state of PS II reaction centers, and therefore does not introduce an artifact when used to assess the status of PS II.
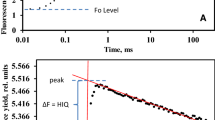
Saturation pulses (that differ from, and are given in addition to, pulse-amplitude modulation of the measuring light) are routinely employed to reveal various aspects of photosynthetic physiology (Bradbury and Baker 1981). Saturation pulses are user-generated exposures of the tissues under study to intense visible light, typically for between 0.7 and 1.0 s (saturation intensities generally exceed 3,000 μmol photons m−2 s−1, or about 1.5 times direct full sunlight). Saturation pulses are intended to be of sufficient intensity to simultaneously reduce all PS II reaction centers (although Loriaux et al. 2013 report that “multiphase flashes” allow better estimation of the fully reduced state under some measurement conditions). Because the intensity of the saturation pulse is many orders of magnitude greater than the intensity of the measuring light, saturation pulses reduce to nearly zero the probability that a photon generated by the measuring light could be utilized in support of photochemistry. During the saturation pulse, there is thus no photochemical use of the measuring light. However, even saturating pulses will not eliminate non-photochemical processes serving as alternative routes for de-excitation of singlet-excited Chl (in leaves exposed to ambient light or in leaves exhibiting sustained non-photochemical quenching in darkness). Indeed, this sensitivity to other processes lies at the heart of the usefulness of the measurement of PAM Chl fluorescence emission. Pulse amplitude modulated fluorescence measured during the saturation pulse is referred to as maximal fluorescence, or Fm (Fig. 7.2). Comparison of Fm with initial minimum fluorescence measured in darkness (after PS II centers are oxidized), Fo, yields the range across which fluorescence can vary (variable fluorescence Fv, equal to Fm − Fo), which can be used to calculate the intrinsic efficiency (Fv/Fm) with which light absorbed by PS II and its antennae can be used in support of photochemistry (for a review of the fundamentals of Chl fluorescence, see Govindjee 2004).
The first approach using PAM fluorometry to measure non-photochemical processes was development of the parameter qN by Schreiber et al. (1986). The q is in reference to quenching, a term commonly used to refer to a decrease in the intensity of fluorescence emission. This coefficient was initially termed qE because its primary mechanism was known to depend on a proton gradient across the photosynthetic (thylakoid) membrane (termed membrane energization). Later, the use of qE was distinguished from qI, a term assigned to slowly-reversible photoinhibitory fluorescence quenching (Krause and Weis 1991). However, it should be noted that qE and qI both show the same relationship with zeaxanthin levels and decreases in Fv/Fm (see Demmig-Adams et al., Chap. 24). Schreiber et al. (1986) developed the equation for qN (qN = 1–F ′v /Fv, where F ′v = F ′m − F ′o , and F ′m is maximal fluorescence collected during a saturation pulse applied during ambient illumination and F ′o is minimal fluorescence collected in darkness immediately following exposure to ambient illumination) in analogy to the equation for photochemical fluorescence quenching, qP. The parameter qN is a dimensionless quantity and varies between values of zero and 1.0. As such, qN exhibits poor sensitivity to changes in non-photochemical processes when they are highly engaged and exhibit a large magnitude (see Fig. 7.3). In addition, qN requires determination of F ′o , which can be difficult to measure in some experimental settings (Baker and Oxborough 2004).
Light response curves of the quantum yield of regulated thermal energy dissipation (ΦNPQ; upper panel), the non-photochemical quenching coefficient (qN; middle panel), and non-photochemical quenching (NPQ; lower panel) calculated from fluorescence data collected from shade-acclimated Oxalis sp. (closed circles) and sun-acclimated Tulipa sp. (open squares). Data were collected from previously dark-acclimated detached leaves exposed to a range of light intensities under an atmosphere of 5 % CO2 in a temperature-controlled chamber.
In a subsequent approach (Bilger and Björkman 1990), non-photochemical fluorescence quenching was calculated in a manner analogous to the Stern-Volmer principle, which relates the extent of fluorescence quenching to the concentration of a quenching molecule, Q (in a solution of the fluorescing molecule and the quencher):
where F and F′ are unquenched and quenched fluorescence, respectively, and k is a proportionality constant. Although the physical conditions in a solution and a highly structured pigment protein complex are not the same, the application of the latter approach to saturation pulse analysis of Chl fluorescence resulted in a highly useful alternative parameter to quantify non-photochemical quenching:
NPQ can be understood from a conceptual standpoint when one considers that photochemical processes cannot be involved in differences between Fm and F ′m , since photochemical utilization of the measuring light cannot occur during exposure to the saturating pulse. Therefore, any differences between Fm and F ′m must be interpreted as due to non-photochemical processes. The magnitude of non-photochemical processes is reflected in the degree to which F ′m is lower than Fm. The parameter NPQ offers advantages when compared with qN; it does not require simultaneous measurement of F ′o (while there still is concurrent quenching of F ′m and F ′o , the latter does not have to be measured to quantify NPQ), it is not constrained arithmetically at its upper ranges to values below 1.0, and, as such, has been shown to correlate linearly with light-induced increases in the rate constant of thermal dissipation KD (Kitajima and Butler 1975).
Shade-acclimated leaves typically do not generate NPQ values exceeding 2.0 (Fig. 7.3), whereas sun-acclimated leaves of evergreens can display values above 4.0 (see Adams and Demmig-Adams, Chap. 2). This shade/sun difference in the capacity for NPQ is consistent with the effects of growth light environment on the conversion state of the VAZ cycle (see Demmig-Adams et al., Chap. 24). Induction of NPQ in a dark-acclimated leaf after transfer to light typically exhibits a biphasic response to irradiance, with a first phase of modest non-photochemical quenching (possibly representing state transitions) saturating at relatively low irradiance and a pronounced second phase under higher light intensities. For state transitions, see Krause and Jahns (2004) and Papageorgiou and Govindjee (2011).
Another approach used to quantify non-photochemical Chl fluorescence quenching considers the fraction of absorbed light energy lost as heat (thermal dissipation or non-radiative decay). This was first addressed by Demmig-Adams et al. (1996) and was further elaborated upon by Kramer et al. (2004) and Hendrickson et al. (2004). This approach assumes that quantum yields (proportions of photons absorbed by PS II-Chl dedicated to a given process) for each process consuming the excited state of Chl must sum to 1.0. The two predominant processes are (i) photochemistry (whose quantum yield for PS II is Φ PS II) and (ii) regulated thermal energy dissipation (Φ NPQ; Fig. 7.3). In addition, the modest non-radiative, presumably thermal, dissipation occurring in all leaves, even under optimal conditions after dark-acclimation, is considered constitutive thermal dissipation, to which any energy lost by fluorescence emission is added (Kramer et al. 2004; Hendrickson et al. 2004). Different equations used by the latter authors vary only slightly, with that of Hendrickson et al. (2004) being the simplest for Φ NPQ:
where Fs represents steady-state fluorescence emission during illumination. This parameter is convenient to use since its calculation does not require determination of F ′o . Fs/Fm represents the constitutive non-radiative thermal energy dissipation, total thermal dissipation is calculated as Fs/F ′m (which equals 1- Φ PS II), and Φ NPQ thus represents the regulated portion of thermal dissipation. Thermal dissipation can, in principle, be directly determined using photoacoustic spectroscopy or micro-calorimetry, but these techniques require extensive equipment and cannot be easily applied to leaves under natural conditions. Moreover, because the photoacoustic changes can arise from multiple sources that have nothing to do with thermal energy dissipation (Malkin and Canaani 1994), some authors have concluded that there is no relationship between zeaxanthin and energy dissipation when utilizing this approach (Havaux et al. 1991; Havaux and Tardy 1997). However, when applied and interpreted properly, good correlations between zeaxanthin and heat emission have been obtained (Buschmann and Kocsányi 1989; Eyletters and Lannoye 1992).
In an unstressed, pre-darkened, sun-grown leaf (i.e., not experiencing a stress-induced, lasting depression in Fv/Fm), Φ PS II (estimated from Fv/Fm) is ~0.83, while regulated thermal energy dissipation Φ NPQ is, by definition, zero. With increasing irradiance, Φ PS II typically declines and both regulated, harmless thermal energy dissipation and non-regulated, non-radiative decay (largely reflecting de-excitation of singlet Chl via triplet conversion and the formation of potentially destructive singlet oxygen) increases. The relationship between Φ NPQ and decay via the triplet route depends on exposure conditions (e.g., irradiance and the presence of other stresses), plant genetics, and growth light environment.
IV Proper Measurement and Interpretation of NPQ
Generating fluorescence signals is relatively easy. Generating interpretable signals, and interpreting those signals properly, is more challenging. The need for methodological guidance grows as the tools for Chl fluorescence analysis have become more widely available (Logan et al. 2007). Below, we describe common pitfalls associated with the measurement of NPQ and offer advice on the design of studies employing this parameter.
Calculation of NPQ assumes unquenched Fm values in dark-adapted leaves. Use of already quenched Fm values in the calculation of NPQ will lead to an underestimate of NPQ. Such underestimation will occur if (i) the period of dark adaptation before measurement of Fm is not long enough, (ii) overly frequent saturating pulses are applied, or (iii) there is pre-existing, lasting quenching of Fm. Fm quenching contributing to nocturnally-sustained depressions in Fv/Fm is commonly observed in plants experiencing environmental stresses such as low temperatures during the winter (see Adams et al. 1995a, 2004, 2006; Öquist and Huner 2003; Demmig-Adams and Adams 2006). Under such circumstances, even pre-dawn measurements of Fm are unlikely to provide unquenched values, in which case NPQ cannot be correctly calculated.
There are additional factors that can compromise calculation of NPQ. Inability to hold the fiberoptic probe at uniform distance and angle from the leaf (or any other sample under study) for the measurement of both Fm and F ′m results in inaccuracies in the calculation of NPQ, and so will failure to collect Fm and F ′m fluorescence data from precisely the same region of the sample. Biological processes occurring between the measurement of Fm and F ′m can also introduce measurement artifacts. Both state transitions (Krause and Jahns 2004) and chloroplast movements (Brugnoli and Björkman 1992) driven by exposure to light can reduce the effective absorption cross-section of PS II antenna Chl, decreasing PS II fluorescence emission during the determination of F ′m .
Use of insufficient, non-saturating light pulses for the measurement of Fm and F ′m renders calculations involving their use meaningless and uninterpretable. Non-saturating pulses can result from the use of inappropriate instrument settings, positioning of the instrument fiberoptic probe too far from the sample, or failure to account for the absorptive effects of leaf surface features (e.g., trichomes, cuticular waxes, etc.) or epidermal pigments such as anthocyanins. This latter consideration is especially important to keep in mind when using fluorometers with pulse-modulated measurement light sources whose wavelength falls outside the red region of the visible spectrum (Cessna et al. 2010).
Many fluorometers report weak, non-zero background signals. The influence of saturating pulses and measuring light intensity on the strength of background signals varies by instrument type (B.A. Logan and K.S. Gould unpublished). The possible effect of background signals on calculations of NPQ will thus vary from instrument to instrument, may be affected by the experimental protocol (e.g., alteration of measurement light intensity between samples), and will be greatest when fluorescence signals are weak.
Chl associated with PS I also emits fluorescence, albeit weaker than the emission from PS II-Chl and with a different wavelength distribution (see, e.g., Itoh and Sugiura 2004). The design of many commercial fluorometers allows for the collection of data across a fluorescence emission wavelength band that captures some PS I fluorescence. Under many experimental circumstances, the effect of this PS I contamination on calculations of NPQ is minimal. However, the artifact represented by PS I fluorescence can be exacerbated if wavelength bands of the measuring light and fluorescence detector are not sufficiently selective for PS II (Franck et al. 2002). PS I contamination can also be problematic in the study of plants with unusual PS II/PS I stoichiometries, such as those exhibiting C4 photosynthesis that have a higher level of PS I centers relative to PS II centers compared to C3 plants (Pfündel 1998) and deep shade-acclimated plants, whose PS II/PS I ratio can be closer to unity than is observed in plants acclimated to higher irradiances (Chow and Anderson 1987; Adams et al. 1990; Adams and Demmig-Adams 2004).
V Concluding Remarks: Avoiding Pitfalls when Measuring Fluorescence
Many contemporary fluorometers are computer-operated and automatically calculate fluorescence parameters, such as NPQ, ΦPS II, etc. We urge users to begin each set of measurements on a biological system by carefully examining raw fluorescence traces by hand, to insure that fluorescence signals and instrument-derived parameters were collected at the appropriate times and under the appropriate environmental conditions. Since automated instruments deliver values for parameters irrespective of the quality of the raw fluorescence data, there is no substitute for the informed judgment of the user to insure quality of data.
As stated above, care must be taken to properly measure and interpret Chl fluorescence emission if meaningful insights are to be gained. Here we summarize our advice on how best to avoid common pitfalls when assessing thermal energy dissipation as NPQ. Pre-existing engagement of thermal energy dissipation in dark-acclimated samples generally leads to decreases in Fv/Fm. Verifying that Fv/Fm values are at/near maximum (just slightly above 0.8 for a sun-acclimated leaf and slightly below 0.8 for a shade-acclimated leaf) thus ensures that dark-adapted Fm values used in the calculation of NPQ are not quenched. Since thermal energy dissipation leads to a decrease in PS II quantum efficiency measured during illumination F ′v /F ′m , collecting parallel measurements of NPQ and F ′v /F ′m under illumination offers stronger supporting evidence. One may resort to measurements of F ′v /F ′m alone, when experimental constraints or already quenched dark-adapted Fm values make it impossible to reliably measure NPQ (Adams et al. 1995a, 2006, 2013; see Adams et al., Chap. 23). Concomitant measurements of carotenoid composition, thylakoid protein levels, and photosynthesis rates (O2 evolution or CO2 uptake) can furthermore strengthen the interpretive utility of NPQ measurements.
Abbreviations
- Chl:
-
Chlorophyll
- F:
-
Fluorescence
- Fo, F ′o :
-
Minimal chlorophyll fluorescence in the dark- and light-adapted state, respectively
- Fm, F ′m :
-
Maximal chlorophyll fluorescence in the dark- and light-adapted state, respectively
- Fs :
-
Steady-state chlorophyll fluorescence emission during illumination
- Fv, F ′v :
-
Variable chlorophyll fluorescence in the dark-adapted (Fm − Fo) and light-adapted (F ′m − F ′o ) state, respectively
- Fv/Fm, F ′v /F ′m :
-
Interpreted to be intrinsic efficiency (or quantum yield) of photosystem II in the dark- and light-adapted state, respectively (equivalent to Φ PS II)
- NPQ:
-
Non-photochemical quenching of chlorophyll fluorescence
- OJIP:
-
One-letter names associated with phases of the chlorophyll fluorescence transient curve upon onset of illumination; O is for the initial minimum level, P for peak, and J and I are inflections between the two
- PAM:
-
Pulse-amplitude modulated (fluorescence or fluorometer)
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- Φ NPQ :
-
Quantum yield of the regulated portion of thermal dissipation of the singlet excited state of Chl a
- Φ PS II :
-
Quantum yield (intrinsic efficiency) of photosystem II photochemistry (equivalent to Fv/Fm)
- qE, qI, qN, qP:
-
Quenching coefficients for energy-dependent (E), photoinhibitory (I), non-photochemical (N), and photochemical (P) quenching of chlorophyll fluorescence, respectively; VAZ cycle – the xanthophyll cycle involving the carotenoids violaxanthin, antheraxanthin, and zeaxanthin
References
Adams WW III, Barker DH (1998) Seasonal changes in xanthophyll cycle-dependent energy dissipation in Yucca glauca Nuttall. Plant Cell Environ 21:501–512
Adams WW III, Demmig-Adams B, Winter K, Schreiber U (1990) The ratio of variable to maximum chlorophyll fluorescence from photosystem II, measured in leaves at ambient temperature and at 77 K, as an indicator of the photon yield of photosynthesis. Planta 180:166–174
Adams WW III, Demmig-Adams B, Verhoeven AS, Barker DH (1995a) ‘Photoinhibition’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol 22:261–276
Adams WW III, Hoehn A, Demmig-Adams B (1995b) Chilling temperatures and the xanthophyll cycle. A comparison of warm-grown and overwintering spinach. Aust J Plant Physiol 22:75–85
Adams WW III, Demmig-Adams B (2004) Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 583–604
Adams WW III, Zarter CR, Ebbert V, Demmig-Adams B (2004) Photoprotective strategies of overwintering evergreens. Bioscience 54:41–49
Adams WW III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2006) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht, pp 49–64
Adams WW III, Muller O, Cohu CM, Demmig-Adams B (2013) May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth Res 117:31–44
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Aro E-M, Andersson B (eds) (2001) Regulation of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 11. Springer, Dordrecht
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Phil Trans Roy Soc Lond Ser B 355:1433–1446
Baker NR (ed) (1996) Photosynthesis and the Environment. Advances in Photosynthesis, Volume 5. Kluwer Academic Publishers, Dordrecht
Baker NR, Long SP (eds) (1986) Photosynthesis in Contrasting Environments. Elsevier, Amsterdam
Baker NR, Oxborough K (2004) Chlorophyll fluorescence as a probe in photosynthetic productivity. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 65–82
Barber J, Baker NR (eds) (1985) Photosynthetic Mechanisms and the Environment. Elsevier, Amsterdam
Barker DH, Seaton GGR, Robinson SA (1997) Internal and external photoprotection in developing leaves of the CAM plant Cotyledon orbiculata. Plant Cell Environ 20:617–624
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Bilger W, Schreiber U, Bock M (1995) Determination of the quantum efficiency of photosystem II and of nonphotochemical quenching of chlorophyll fluorescence in the field. Oecologia 102:425–432
Blankenship RE (2014) Molecular mechanisms of photosynthesis. Wiley Blackwell Publishers, Oxford
Bradbury M, Baker NR (1981) Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve: changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta 635:542–551
Brugnoli E, Björkman O (1992) Chloroplast movements in leaves: influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to ΔpH and zeaxanthin formation. Photosynth Res 32:23–35
Buschmann C, Kocsányi L (1989) Correlation between the induction kinetics of heat dissipation and that of chlorophyll fluorescence and its quenching mechanisms. Photosynth Res 21:129–136
Cessna S, Adams WW III, Demmig-Adams B (2010) Exploring photosynthesis and plant stress using inexpensive chlorophyll fluorometers. J Nat Resour Life Sci Educ 39:22–30
Chow WS, Anderson JM (1987) Photosynthetic responses of Pisum sativum to an increase in irradiance during growth II. Thylakoid membrane components. Aust J Plant Physiol 14:9–19
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Demmig-Adams B, Adams WW III, Mattoo A (eds) (2006) Photoprotection, Photoinhibition, Gene Regulation and Environment. Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht
Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Ehleringer J, Björkman O (1978) Pubescence and leaf spectral characteristics in a desert shrub, Encelia farinosa. Oecologia 36:151–162
Ehleringer J, Forseth I (1980) Solar tracking by plants. Science 210:1094–1098
Eyletters M, Lannoye R (1992) Photoacoustically monitored energy dissipation and xanthophyll cycle compared in two durum wheat varieties exposed to a high-light treatment. In: Bicanic D (ed) Photoacoustic and Photothermal Phenomena III. Springer Series in Optical Sciences, Volume 69. Springer, Heidelberg, pp 62–64
Forseth IN, Ehleringer JR (1983) Ecophysiology of 2 solar tracking desert winter annuals 4. Effects of leaf orientation on calculated daily carbon gain and water-use efficiency. Oecologia 58:10–18
Franck F, Juneau P, Popovic R (2002) Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. Biochim Biophys Acta 1556:239–246
Frank HA, Young AJ, Britton G, Cogdell RJ (eds) (1999) The Photochemistry of Carotenoids. Advances in Photosynthesis and Respiration, Volume 8. Springer, Dordrecht
Givnish TJ (1988) Adaptations to sun and shade: a whole plant perspective. Aust J Plant Physiol 15:63–92
Golbeck JH (ed) (2006) Photosystem I: The Light-driven Plastocyanin: Ferredoxin Oxidoreductase. Advances in Photosynthesis and Respiration, Volume 24. Springer, Dordrecht
Gould KS, Kuhn DN, Lee DW, Oberbauer SF (1995) Why leaves are sometimes red. Nature 378:241–242
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Govindjee (2004) Chlorophyll fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 1–42
Govindjee, Björn LO (2012) Dissecting oxygenic photosynthesis: the evolution of the “Z”-scheme for thylakoid reactions. In: Itoh S, Mohanty P, Guruprasad KN (eds) Photosynthesis: Overviews on Recent Progress and Future Perspective. I.K. Publishers, New Delhi, pp 1–27
Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112:1631–1640
Greer DH, Thorpe MR (2009) Leaf photosynthetic and solar-tracking responses of mallow, Malva parviflora, to photon flux density. Plant Physiol Biochem 47:946–953
Haupt W, Scheuerlein R (1990) Chloroplast movement. Plant Cell Environ 13:595–614
Havaux M, Tardy F (1997) Photoacoustically monitored thermal energy dissipation and xanthophyll cycle carotenoids in higher plant leaves. J Photochem Photobiol B 40:68–75
Havaux M, Gruszecki WI, Dupont I, Leblanc RM (1991) Increased heat emission and its relationship to the xanthophyll cycle in pea leaves exposed to strong light stress. J Photochem Photobiol B 8:361–370
Hendrickson L, Furbank RT, Chow WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82:73–81
Hughes NM, Morley CB, Smith WK (2007) Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytol 175:675–685
Itoh S, Sugiura K (2004) Fluorescence of photosystem I. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 231–250
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Kalaji HM, Goltsev V, Bosa K, Allakhverdiev S, Strasser RJ, Govindjee (2012) Experimental in vivo measurements of light emission in plants: a perspective dedicated to David Walker. Photosynth Res 114:69–96
Kao WY, Forseth IN (1991) The effects of nitrogen, light and water availability on tropic leaf movements in soybean (Glycine max). Plant Cell Environ 14:187–293
Kao WY, Forseth IN (1992a) Responses of gas exchange and phototropic leaf orientation in soybean to soil water availability, leaf water potential, air temperature, and photosynthetic photon flux. Environ Exp Bot 32:152–161
Kao WY, Forseth IN (1992b) Diurnal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant Cell Environ 15:703–710
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kitajima M, Butler WL (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376:105–115
Kornyeyev D, Logan BA, Holaday AS (2010) Excitation pressure as a measure of the sensitivity of photosystem II to photoinactivation. Funct Plant Biol 37:943–951
Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79(2):209–218
Krause GH, Jahns P (2004) Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: characterization and function. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 463–495
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kyle DJ, Osmond CB, Arntzen CJ (eds) (1987) Photoinhibition. Elsever, Amsterdam
Lichtenthaler HK (1992) The Kautsky effect: 60 years of chlorophyll fluorescence induction kinetics. Photosynthetica 27:45–55
Logan BA, Demmig-Adams B, Adams WW III, Grace SC (1998a) Antioxidation and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo and Vinca major acclimated to four growth irradiances in the field. J Exp Bot 49:1869–1879
Logan BA, Grace SC, Adams WW III, Demmig-Adams B (1998b) Seasonal differences in xanthophyll cycle characteristics and antioxidants in Mahonia repens growing in different light environments. Oecologia 116:9–17
Logan BA, Demmig-Adams B, Adams WW III, Rosenstiel TN (1999) Effect of nitrogen limitation on foliar antioxidants in relationship to other metabolic characteristics. Planta 209:213–220
Logan BA, Adams WW, Demmig-Adams B (2007) Avoiding common pitfalls of chlorophyll fluorescence analysis under field conditions. Funct Plant Biol 34:853–859
Loriaux SD, Avenson TJ, Welles JM, McDermott DK, Eckles RD, Riensche B, Genty B (2013) Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant Cell Environ 36:755–770
Ludlow MM, Björkman O (1984) Paraheliotropic leaf movement in Siratro as a protective mechanism against drought-induced damage to primary photosynthetic reactions: damage by excessive light and heat. Planta 161:505–518
Malkin S, Canaani O (1994) The use and characteristics of the photoacoustic method in the study of photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45:493–526
Marias DE “Assessing light capture and photosynthesis in sun and shade Abies blasamea branches using a three-dimensional canopy model.” Undergraduate thesis, Bowdoin College, 2010
McMillen GG, McClendon JH (1979) Leaf angle: an adaptive feature of sun and shade leaves. Bot Gaz 140:437–442
Mehler AH (1951) Studies on the reaction of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Archiv Biochem Biophys 33:65–77
Melis A (1999) Photosystem II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4:130–135
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59:3903–3911
Mooney HA, Ehleringer J, Björkman O (1977) The leaf energy balance of leaves of the evergreen desert shrub Atriplex hymenelytra. Oecologia 29:301–310
Nilsen ET (2008) Thermonastic leaf movements: a synthesis of research with Rhododendron. Bot J Linn Soc 110:205–233
Öquist G, Huner NPA (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54:329–355
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht
Papageorgiou GC, Govindjee (2011) Photosystem II fluorescence: slow changes – scaling from the past. J Photochem Photobiol B Biol 104:258–270
Park YI, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111:867–875
Pfündel E (1998) Estimating the contribution of photosystem I to total leaf chlorophyll fluorescence. Photosynth Res 56:185–195
Rabinowitch EI, Govindjee (1969) Photosynthesis. Wiley, New York, http://www.life.illinois.edu/govindjee/photosynBook.html and at <http://www.life.illinois.edu/govindjee/g/Books.html>
Rizhsky L, Liang HJ, Mittler R (2003) The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem 278:38921–38925
Rosa LM, Dillenburg LR, Forseth IN (1991) Responses of soybean leaf angle, photosynthesis and stomatal conductance to leaf and soil water potential. Ann Bot 67:51–58
Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 279–319
Schreiber U, Neubauer C (1987) The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: II. Partial control by the photosystem II donor side and possible ways of interpretation. Z Naturforsch 42c:1255–1264
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Schreiber U, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res 113:127–144
Stirbet A, Govindjee (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I-P rise. Photosynth Res 113:15–61
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Volume 19. Springer, Dordrecht, pp 321–362
Takagi S (2003) Actin based photo-orientation movement of chloroplasts in plant cells. J Exp Biol 206:1963–1969
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Tikkanen M, Aro E-M (2012) Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim Biophys Acta 1817:232–238
Tikkanen M, Suorsa M, Gollan PJ, Aro E-M (2012) Post-genomic insight into thylakoid membrane lateral heterogeneity and redox balance. FEBS Lett 586:2911–2916
Valladares F, Pearcy RW (1998) The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia 114:1–10
Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81:1925–1936
Verhoeven AS, Demmig-Adams B, Adams WW III (1997) Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and nitrogen stress. Plant Physiol 113:817–824
Walker DA (1981) Secondary fluorescence kinetics of spinach leaves in relation to the onset of photosynthetic carbon assimilation. Planta 153:273–278
Wang X, Arora R, Homer HT, Krebs SL (2008) Structural adaptations in overwintering leaves of thermonastic and nonthermonastic Rhododendron species. J Amer Soc Hort Sci 133:768–776
Wang X, Peng YH, Singer JW, Fessehale A, Krebs SL, Arora R (2009) Seasonal changes in antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: a comparison of photoprotective strategies in overwintering plants. Plant Sci 177:607–617
Williams WE, Gorton HL, Witiak SM (2003) Chloroplast movements in the field. Plant Cell Environ 26:2005–2014
Williams WP, Allen JF (1987) State 1/State 2 changes in higher plants and algae. Photosynth Res 13:19–45
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Phil Trans Roy Soc B 355:1517–1529
Wydrzynski T, Satoh K (eds) (2005) Photosystem II: The Light-driven Water: Plastoquinone Oxidoreductase. Advances in Photosythesis and Respiration, Volume 22. Springer, Dordrecht
Acknowledgments
Work presented in this chapter was supported by the University of Colorado. We thank Dr. Christopher Cohu and Mr. Jared Stewart for assistance with the generation of the figures and George Papageorgiou and Govindjee for constructive comments on this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Logan, B.A., Demmig-Adams, B., Adams, W.W., Bilger, W. (2014). Context, Quantification, and Measurement Guide for Non-Photochemical Quenching of Chlorophyll Fluorescence. In: Demmig-Adams, B., Garab, G., Adams III, W., Govindjee, . (eds) Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration, vol 40. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9032-1_7
Download citation
DOI: https://doi.org/10.1007/978-94-017-9032-1_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9031-4
Online ISBN: 978-94-017-9032-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)