Abstract
Gastrointestinal (GI) motility is an essential function of digestive and absorptive processes of the gut, required for propelling intestinal contents, mixing them with digestive juices, and preparing unabsorbed particles for excretion. Pivotal to the discussion of mechanisms for producing defined patterns of motor responses are important and intimately related aspects of intestinal neurophysiology and motor regulation. Many principles presented in this section are intentionally oversimplified to provide a better conceptual framework for understanding both normal physiology and the pathophysiological mechanisms of various intestinal diseases arising from aberrations in intestinal motility and neurophysiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lower Esophageal Sphincter
- Vasoactive Intestinal Peptide
- Enteric Nervous System
- Myenteric Plexus
- Enteric Neuron
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction to Gut Musculature and Neural Innervations
Gastrointestinal (GI) motility is an essential function of digestive and absorptive processes of the gut, required for propelling intestinal contents, mixing them with digestive juices, and preparing unabsorbed particles for excretion. Pivotal to the discussion of mechanisms for producing defined patterns of motor responses are important and intimately related aspects of intestinal neurophysiology and motor regulation. Many principles presented in this section are intentionally oversimplified to provide a better conceptual framework for understanding both normal physiology and the pathophysiological mechanisms of various intestinal diseases arising from aberrations in intestinal motility and neurophysiology.
1.1 Gut Smooth Muscle and Its Organization
With the exception of the upper one third of the esophagus and the external anal sphincter, the muscular layers of the bowel wall are made up of smooth muscle cells. Like striated muscles, smooth muscle contractions of mammalian small intestine are preceded by changes in membrane potential differences. Depolarization of the membrane tends to cause the muscle cell to contract, whereas hyperpolarization has the opposite effect. However, in contrast to skeletal muscles, smooth muscle cells have no striations because they lack well organized arrays of sarcomeres, are smaller, and are organized into discrete muscle bundles surrounded by connective tissue. Each bundle functions as a single unit; all its smooth muscle cells contract simultaneously to produce an efficient motor response. This is possible because of gap junctions , or points of low electrical resistance between cells (Fig. 2.1), through which a depolarizing signal can be instantaneously transmitted to all cells. This also makes unnecessary the neural activation of each individual cell, as each muscle bundle unit could potentially be regulated by a single neuron. In most instances, as will be later evident, each muscle bundle receives several types of neural input that are necessary to coordinate their activities with other motor and intestinal processes.
Most smooth muscles of the GI tract are organized into two layers that make up the muscular sheath of the intestinal wall. Cells of the inner circular muscle layer are circumferentially organized so that each level of the gut muscle fibers simultaneously contracts to produce an annular contraction. The circular muscle layer can be further subdivided into two lamellae, one thick, outer layer and another thin, inner layer. These layers differ electrophysiologically and in the neural innervations they receive: the inner lamella receives a greater proportion of fibers from the submucosal plexus , while the outer is predominantly innervated by the myenteric plexus (see below). Muscles of the outer lamella are also larger, less electron-dense, and have more gap junctions . Thus the two lamellae of the circular muscle layer are believed to have different functional responses; however, understanding of this area remains incomplete.
Longitudinal muscles of the gut are oriented such that their axis runs along the length of the bowel. With the exception of the colon where they are organized into separate thick cords (teniae coli ), the longitudinal muscles form a continuous outer muscle sheet encircling the bowel wall. Contraction and relaxation of the longitudinal muscle layer cause alterations in the length of the bowel, important for complex motor functions such as peristalsis (see Sect. “3.2” below).
1.2 Innervation of Gut Muscle
The gut receives extrinsic sympathetic and parasympathetic innervation, but it also has its own enteric nervous system capable of independent function in many instances. As shown in Fig. 2.2a, preganglionic parasympathetic motor neurons reach the gut either via the vagus nerve or the pelvic nerve, the latter supplying efferent fibers to the distal colon and rectum. Parasympathetic fibers mainly synapse with postganglionic parasympathetic or other enteric neurons located in the intestinal wall plexuses. In contrast, preganglionic efferent fibers emanate from the spinal cord and synapse at paravertebral ganglia, from which postganglionic fibers project to the intestine, following the celiac, superior mesenteric and interior mesenteric arteries in their respective distributions.
Intrinsic enteric neurons are predominantly found within two major gut plexuses (Fig. 2.2b). The myenteric or Auerbach’s plexus, located between the circular and longitudinal muscle layers, is a thin layer array of ganglia, ganglion cells, and inter-ganglionic nerve tracts that serve to interconnect the plexus. Enteric neurons of this plexus largely innervate longitudinal muscles and the outer lamella of the circular muscle layer. A large number of peptides and nonpeptide neurotransmitters are expressed by myenteric plexus neurons. These include vasoactive intestinal peptide (VIP), nitric oxide , peptide histidine isoleucine (PHI), substance P, and neurokinin A (NKA), to name a few. Many of these neurons have projections into adjacent muscle layers, where they are either excitatory or inhibitory, but some are interneurons involved in integrative functions. The submucosal or Meissner’s plexus is the other neuronal array found between the submucosal layers and circular muscle. This plexus has neurons that are functionally distinct from those of the myenteric plexus and, relative to intestinal motor function, appear to be projecting mainly to the inner lamella of the circular muscle layer.
Enteric neurons have been extensively studied and found to be extremely diverse and complex. At least three criteria have been used to classify enteric neurons, i.e. morphology (Dogiel type I, II and III), electrophysiological properties (S and AH neurons), and type of neurotransmitters they express. Because these classifications have been extensively discussed by numerous recent reviews, the following discussion will mostly focus on distinction of enteric neurons by the content of neurotransmitters. This provides some functional connotations meaningful for understanding their role in regulating gut motility. However, basic principles of smooth muscle electrophysiology should be reviewed first.
2 Smooth Muscle Electrophysiology
2.1 Myogenic Control System
2.1.1 Slow Waves and Spike Potentials
Two types of electrical activity are found in gut smooth muscle cells; they are slow waves which contribute to the basic electrical rhythm and spike potentials . Except for the esophagus and proximal stomach , most parts of the bowel have a spontaneous rhythmical fluctuation in resting membrane potential or slow waves, generally between −60 and −65 mV. This fluctuation represents an interval of cyclic depolarization followed by repolarization and is called the basic electrical rhythm (BER). The frequency of BER oscillations varies from one part of the bowel to another (Fig. 2.3), and depolarization is not necessarily accompanied by muscular contractions.
Basic electrical rhythm or BER. The BER is a rhythmical fluctuation in resting membrane potential , characterized by intervals of cyclic depolarization followed by repolarization. BERs arise from specialized pacemaker cells. Depolarization phases are not always accompanied by muscular contraction. Inserts illustrate ion fluxes that are involved in depolarizing phases
The origin of the BER appears to be specialized “pacemaker” cells (interstitial cells of Cajal). These stellate, muscle-like cells interconnect, have projections to muscle cells, and receive neural fibers from nearby myenteric plexuses. In the stomach and small intestine , they are located at the inner circular muscle layer, near the myenteric plexus. In the colon, dominant pacemaker cells appear to be located in the submucosal border of the circular muscle layer. As in the conduction system of the heart, dominant pacemakers, i.e. ones having greater cyclic frequency, capture and entrain subsidiary pacemakers. Thus, in any region of the gut, the electrical characteristics of the dominant regional pacemaker will be not only rapidly propagated circumferentially but also transmitted orad and aborad. However, a descending gradient of pacemaker frequency is known to exist in most regions of the gut, with the possible exception of the colon. Thus, when propagation of a pacemaker BER along the long axis of the gut weakens, more distal subsidiary pacemakers will assume the pacemaker role. Studies have suggested that an aboral ascending gradient may be present in the colon. As will be discussed below, this hierarchical arrangement in BER frequency in various regions of the gut may have important functional significance.
2.1.2 Generation of Slow Waves
Because of the tight circumferential electrical coupling of smooth muscle cells, the BER propagation is extremely rapid and can also be measured in adjacent muscle cells in any given gut segment. The mechanism for generation of the BER is believed to be the consequence of a balance between the depolarizing inward flux of calcium through dihydropyridine-insensitive Ca2+ channels and the repolarizing efflux of K + from the cell through Ca2+-activated K+ channels (Fig. 2.3, insert). The cycle begins with the slow buildup of Ca2+ ions in the cell (a depolarizing process) that progressively inactivates further Ca2+ influx and simultaneously activates Ca2+-activated K+ channels, leading to K+ efflux (a repolarizing process).
Although depolarizations of the BER in stomach and colon can occasionally lead to small motor contractions, most contractions occur after rapid changes in membrane potential differences called spike potentials , which are superimposed on the depolarizing phase of the BER (Fig. 2.4). These “action potentials” are quite different from those seen in mammalian nerve or striated muscle. Their depolarizing phase is caused by the activation of Ca2+-dependent K+ channels or increased activity of nonselective cation or Cl− channels. This is often initiated by stimulation of cells with excitatory ligands. The resulting influx of K+ depolarizes the cells, causing an activation of voltage dependent Ca2+ channels. However, this is only one component contributing to the observed increases in cytosolic Ca2+ necessary for producing muscular contraction. In circular muscle cells, the other component involves inositol 1,4,5-triphosphate (IP 3 )-stimulated Ca 2+ release from intracellular stores, with IP3 generated from ligand-stimulated hydrolysis of membrane phosphatidylinositol . In longitudinal muscle cells, IP3 appears to have little role in activating increases in [Ca2+]i. Little IP3 is generated after ligand stimulation, and addition of IP3 to permeabilized cells has little effect in releasing Ca2+, consistent with the lack of IP3 receptors regulating internal calcium pools. In these cells, excitatory ligands appear to activate Cl− channels, depolarizing the cells and stimulating the opening of voltage-sensitive Ca2+ channels. However, the end result in both circular and longitudinal muscles may be the same. Initial increases in cytosolic calcium augment additional and more sustained increases through the activation of plasma membrane or sarcoplasmic Ca2+ channels.
Stimulated increases in Ca2+ are required for electromechanical coupling. This explains why not all phases of smooth muscle BER are associated with contraction. Even at the peak of membrane depolarization, if the threshold potential for initiating these events is not achieved, contraction will not occur. Spike potentials superimposed on phasic BER depolarization increase the probability that the threshold potential will be reached (Fig. 2.5). The appearance and frequency of spike potentials are greatly affected by hormonal agents and neurotransmitters. The greater the number of spike potentials per BER cycle, the greater is the degree of muscular contraction. Epinephrine , for example, markedly decreases spike potentials and inhibits contraction, whereas acetylcholine stimulates them and promotes contraction. The major function of the BER, therefore, is to dictate when contractions can occur in a certain area in the bowel. Its basic rhythm does not appear to be affected by hormones and neurotransmitters. If there are no spike potentials, contractions will generally not occur. On the other hand, if an agent promotes numerous spike potentials, the frequency of muscular contractions cannot exceed that of the BER.
2.2 Electromechanical Coupling
2.2.1 Contraction of Smooth Muscles
How do stimulated increases in cytosolic Ca2+ cause contraction of smooth muscles? Increased cytosolic Ca2+ binds to the calcium-regulatory protein calmodulin, forming a complex that activates myosin light chain kinase (MLC kinase) (Fig. 2.6). MLC kinase stimulates the phosphorylation of myosin, a critical step in initiating the actin-myosin interaction essential for muscular contraction. Protein kinase C, activated through the generation of diacylglycerol, also appears to have a role, especially in maintaining the duration of contraction.
Ca2+-dependent smooth muscle contraction. Increased cytosolic Ca2+ binds to calmodulin, a calcium-regulatory protein important for activating myosin light chain (MLC) kinase. Activating MLC kinase stimulates the phosphorylation of myosin, a critical step in initiating the actin-myosin interaction essential for muscular contraction
2.2.2 Relaxation of Smooth Muscles
Relaxation of smooth muscles involves restoration of basal Ca 2+ levels, decreased MLC kinase activity, and commensurate increase in MLC phosphatase action. Several mechanisms appear to be involved in decreasing cytosolic Ca2+, including the activation of Ca2+-ATPase to pump Ca2+ out of the cells or back into internal Ca2+ stores. In addition, repolarization of the plasma membrane inhibits further entry of Ca2+ via voltage-activated calcium channels. MLC phosphatase causes the dephosphorylatioin of myosin, with cessation of the actin-myosin interaction.
3 Neural and Hormonal Regulation of Gut Motility
3.1 Neurohumoral Regulators
Gut motility is regulated by the balance of effects received from excitatory and inhibitory regulatory agents. These agents can regulate motor functions in several different ways (Fig. 2.7). Some neurotransmitters and hormones have direct effects on smooth muscle, including agents such as acetylcholine , epinephrine , norepinephrine , tachykinins (such as substance P), and opioid peptides such as enkephalins and dynorphins.
3.1.1 Inhibitory Agents
Inhibitory agents generally work by stimulating increases in cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP), secondary messengers that appear to counteract the effects of increased cytolic Ca2+ in several ways. For example, the stimulation of cAMP- and cGMP-dependent protein kinases have been shown to inhibit IP3 formation and its effects on Ca2+ release from internal calcium stores. These kinases may also stimulate mechanisms involved in the re-uptake or lowering of cytosolic calcium, including stimulation of Ca2+-ATPase of plasma membrane or cellular organelles. As a third mechanism, increases in cyclic nucleotides may play a role in stimulatory mechanism, such as Ca2+-dependent K+ channels, which repolarize the cell and inhibit the actions of excitatory stimuli. Inhibitory agents that stimulate increases in smooth muscle cell cAMP include vasoactive intestinal peptide (VIP), glucagon , and peptide histidine isoleucine (PHI) or peptide leucine methionine (PHM), in humans. Nitric oxide is inhibitory because it stimulates soluble guanylate cyclase and increased cellular cGMP levels.
3.1.2 Excitatory Agents
Most excitatory agents of intestinal motility work by stimulating increases in cytosolic Ca 2+ and/or inhibiting the formation of cyclic nucleotides. For instance, acetylcholine ’s excitatory actions are mediated by muscarinic receptor-stimulated increases in cytosolic Ca2+. Other agents such as μ- and δ-opioid receptor agonists also inhibit adenylate cyclase activity, in addition to stimulating increases in cytosolic Ca2+. Serotonin (5-hydroxytryptamine, 5-HT), on the other hand, simultaneously stimulates increases in cytosolic Ca2+ through 5-HT2 receptors and increases in adenylate cyclase activity and cAMP via stimulation of 5-HT4 receptors. These seemingly counterproductive actions probably serve as a form of cellular feedback regulation.
3.1.3 Other Regulatory Agents
Other regulatory agents of intestinal motility act indirectly, either by stimulating or inhibiting the release of other neuropeptides or hormones. As an example of this modulatory role, cholecystokinin (CCK) and bombesin stimulate intestinal motility by enhancing the release of excitatory peptides such as acetylcholine and substance P. By contrast, neuropeptide Y and somatostatin inhibit the release of these agents. As another layer of complexity, agents such as somatostatin can further inhibit motility by inhibiting the release of opioid peptides that normally would inhibit VIP release of nitric oxide production. The net effect, therefore, is decreased inhibitory tone on release of VIP secretion, causing inhibition of motility.
3.2 Types of Gut Motility
Digestive and absorptive functions of the gut are very much dependent on certain characteristic patterns of motility which is present in various parts of the bowel. Each serves important functional roles, including mixing, propulsion, and separation of luminal contents. These actions are possible because of the coordinated interaction of excitatory and inhibitory neurons. As schematically shown in Fig. 2.8, stimulation of intramural baroreceptors by the presence luminal contents simultaneously activates proximal excitatory neurons that stimulate contraction and inhibitory neurons in adjacent segments that induce receptive relaxation. Thus, the regulation of each segment of bowel must be dynamic and integrated with other parts of the gut, or the consequences could be potentially disastrous.
There are three major types of motility pattern in the gut (Fig. 2.9). (1) Segmentation is the non-propulsive annular contraction of the circular muscle layer that is predominantly found in the small and large intestines. Its major function is to mix intestinal chyme through its squeezing action; (2) Tonic contractions characterize certain regions of the gut that serve as sphincters for dividing the gut into functional segments. Important aspects of these regions are mechanisms that can inhibit tonic contraction to allow luminal contents to pass at appropriate times; (3) Peristalsis describes a highly integrated, complex motor pattern marked by sequential annular contractions of gut segments that produce a sweeping, propulsive wave forcefully moving luminal contents distally. Further discussion of these patterns in the context of specific bowel functions is provided below.
4 Chewing and Swallowing
Chewing of food is a voluntary act, though in some de-cerebrate patients chewing responses to food may occur reflexively. Chewing stimulates output from the salivary glands , stomach , pancreas and liver via neural and endocrine reflex pathways. Swallowing of food begins voluntarily but then proceeds reflexively through extrinsic nerves of the pharyngeal muscle.
An illustration of afferent and efferent systems involved in swallowing is shown in Fig. 2.10. The bolus of food forces the soft palate upward, sealing off the nasal pharynx as a possible route of exit for the food. The posterior tongue pushes the bolus backward, using the hyoid bone as a fulcrum and the hard palate as a trough, along which the tongue is able to guide the food. When the bolus reaches the posterior wall of the pharynx, the upper pharyngeal muscle contracts above the bolus and forces it down into the pharyngeal channel away from the nasal pharynx. During this process, the distal pharyngeal muscles relax to make way for the food. Relaxation of the cricopharyngeus muscle and upward movement of the cricoid cartilage open the upper esophageal sphincter for food to enter the esophagus . Concomitantly, the epiglottis moves up and the glottis closes off the trachea to the food. The bolus is pushed to the pharyngeal area by peristaltic action of pharyngeal muscles. Once the bolus passes the upper esophageal sphincter, the pharyngeal muscular structure returns to its resting position and awaits another swallow.
Sequential events involved in chewing and swallowing food. (1) It is initiated voluntarily and swallowing of food thereafter proceeds reflexively. This is achieved by forcing the soft palate upward and the bolus of food seals off the nasal pharynx . (2) The posterior tongue pushes the bolus backward. (3) The upper pharyngeal muscle contracts above the bolus at the posterior wall of the pharynx, forcing it down into the pharyngeal channel away from the nasal pharynx. Distal pharyngeal muscles relax to receive the bolus, and the upper esophageal sphincter opens to allow passage into the esophagus . Concomitantly, the epiglottis moves up and the glottis closes off the trachea to prevent entry of food into the respiratory tract. (4) With passage of the bolus through the upper esophageal sphincter, the pharyngeal muscles return to their resting position and await another swallow
5 Esophagus Motility
The sole purpose of the esophagus , which is about 25 cm in length, is to convey food from the pharynx to the stomach . To achieve this, the esophagus is coordinated at both ends by a structure, called sphincter, the cricopharyngeus or the upper esophageal sphincter (UES) at the top and the lower esophageal sphincter (LES) at the bottom. The UES is easily identified anatomically with the thickening of circular muscle and elevated pressure; while the LES is not evident as an anatomical entity, it exists functionally with elevated pressure.
In humans, the upper one third of the esophagus consists of striated muscle and the lower one third of smooth muscle, while the middle one third is mixed. The sequence of events following a swallow is termed primary peristalsis . When swallowing is initiated, the UES opens, and food is pushed into the upper esophagus by a peristaltic wave that begins in the pharynx , closing the UES after it sweeps over it, and moves distally at about 2–4 cm/s through the body of the esophagus. When the peristaltic wave approaches the lower esophagus, the LES relaxes to let food enter the stomach . Contractions stop at the gastro-esophageal junction and do not proceed into the gastric musculature. Secondary peristalsis waves may also be initiated in the absence of swallowing. These are propulsive muscular contractions that are stimulated by distention of the body of the esophagus. They serve to clear retained food and fluid or remove refluxed gastric contents from the esophagus.
5.1 Neural Regulation of Esophageal Motility
Both intrinsic and extrinsic nerves are necessary for peristalsis to occur. However, their relative roles in initiating and controlling peristalsis remain uncertain. It is likely that cholinergic fibers of the vagus nerve play an important role in modulating the sequence of events. More recently, the role of nonadrenergic, noncholinergic intrinsic neurons in mediating peristalsis has been described, especially neurons that make vasoactive intestinal peptide (VIP) and nitric oxide .
The LES has a very important function in allowing food to enter the stomach and preventing the reflux of gastric contents back into the esophagus . At least two serious diseases namely reflux esophagitis (gastro-esophageal reflux disease or GERD) and achalasia (discussed below) can be attributed to dysfunction of the LES. Sphincter pressure is affected by many endogenous factors in the body, as well as by several food substances (Fig. 2.11). For example, coffee, fats, and ethanol sometimes decrease LES pressure, and many neural and hormonal agents have been shown to affect it, although only a few have been identified as physiologically important. Some appear to directly affect smooth muscle cells, and others modulate interneuron and/or motor activities. Those believed to be physiologically relevant inhibitory regulators include nitric oxide and VIP; they may be secreted from enteric neurons in the LES that receive input from the vagal fibers. Agents such as calcitonin gene-related peptide (CGRP), CCK, somatostatin , and glucagon appear to decrease sphincter tone experimentally, but neural fibers expressing these agents have not been found in the LES. Thus, these agents may work by an endocrine or possibly paracrine pathway and may have relatively minor or predominantly modulatory roles in LES regulation. Although stimulation of the sphincter by prostaglandins can also decrease tone, they are not believed to be important in normal circumstances. Of the agents that increase sphincter pressure, acetylcholine , α-adrenergic agonists, bombesin, and met-enkephalin have been implicated as having important physiological roles. Histamine can both increase and decrease sphincter tone, depending on the receptor subtype stimulated. Gastrin is now believed to have a role in regulating sphincter tone.
5.2 Esophageal Motility Dysfunctions
Given the complexity of the regulatory mechanisms of esophageal motility, it is not surprising that many clinical disorders have been described, some being quite common and other debilitating. Achalasia is a condition characterized by dysphagia (i.e. difficulty in swallowing) that results from the failure of the LES to relax, thereby causing a functional obstruction. Furthermore, there is a loss of peristalsis of the esophageal body. Although the etiology and pathogenesis of the disease are still uncertain, many patients demonstrate a loss of ganglion cells of the myenteric plexus. These findings have led us to believe that loss of critical inhibitory enteric neurons results in a LES that is tonically contracted. Alternatively, there is evidence of neural defects in the vagal dorsal nucleus of the brainstem, which could produce the motor abnormalities observed in achalasia . Tertiary contractions are often seen, particularly with age. These are non-peristaltic, spastic, uncoordinated contractions usually occurring in the distal esophagus . Although they are always abnormal, they usually do not cause symptoms. Reflux esophagitis or GERD is a disease that arises when LES tone is reduced, thereby allowing gastric contents to reflux into the lower esophagus. Since gastric juices are corrosive to the esophageal mucosa , the distal esophagus becomes inflamed and sometimes ulcerated. Patients experience extreme heartburn, and not infrequently severe scarring and stricture at the lower esophagus develop, which can sometimes cause an anatomical obstruction to the passage of food.
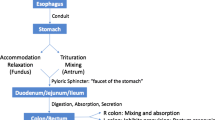
6 Gastric Motility
There are three major important motor functions of the stomach . First, its muscle relaxes to accommodate large volumes during a meal, i.e. receptive relaxation; second, its contraction mixes ingested material with gastric juice, thus facilitating digestion, solubilizing some constituents, and reducing the size of the food particles, i.e. mixing and propulsion; third, its contraction propels gastric content, called gastric chyme, into the duodenum at a regulated rate so as to provide optimal time for intestinal digestion and absorption, i.e . gastric emptying. The first of these functions is possible through a mechanism called receptive relaxation. After the LES opens, the fundus and upper body of the stomach relax to receive the bolus of food. This portion of the stomach has the ability to accommodate a wide range of gastric volumes (0.5–1.5 L) without significant increases in intragastric pressure. Relaxation of the proximal stomach associated with the act of swallowing is mediated by vagal inhibitory fibers because vagotomy (surgical interruption of the vagal nerve to the stomach) reduces distensibility of the proximal stomach. In contrast, distensibility of the proximal stomach is increased by release of GI hormones such as secretin , gastric inhibitory polypeptide (GIP), and CCK.
The distal stomach must mix gastric contents with gastric secretion and slowly empty the gastric chyme into the small intestine . The major motor activity in this part of the stomach is peristalsis , which is initiated and regulated by specialized pacemaker cells in the mid portion of the greater curvature of the stomach (Fig. 2.12). Despite the uniform BER frequency of the gastric motor cells from this area of the stomach to the pylorus, there is a phase lag of distal segments, essential to preventing simultaneous depolarization of all parts of the distal stomach. Muscular contractions occur when spike potentials appear during the depolarizing phase of the BER. The probability of contractions is increased by vagal or gastrin stimulation. With increasing appearance of spike potentials, peristaltic contractions approach and eventually equal the BER frequency of 3–4/min. Conversely, spike potentials are decreased by vagotomy , sympathetic stimulation, and secretin . Because of the phase lag from the midpoint of the greater curvature to the pylorus, peristaltic waves will proceed smoothly in a rostral-to-caudal direction. The motion, referred to as antral systole , can last up to 10 s. Pyloric sphincter function is closely coordinated with antral motor activity, augmenting the grinding process (see below) of gastric contents and slowing the emptying of gastric chyme into the small intestine. During antral systole, the pyloric sphincter partially closes, resulting in retropulsion of antral contents, as the antrum contracts. This process facilitates the breaking up of particulate matter and the mixing of food with gastric secretions (analogous to the agitation cycle of a washing machine). Small amounts of food (usually those that have been digested or are more liquid) pass through the pyloric sphincter and enter the small intestine. These events are summarized in Fig. 2.13.
Pacemaker cells in the regulation of gastric peristalsis . Peristalsis, which is the major motor activity of the distal stomach , is initiated and regulated by specialized pacemaker cells in the mid-portion of the greater curvature. Muscular contractions occur when spike potentials appear during the depolarizing phase of the BER. The probability of spike potentials is increased by vagal or gastrin stimulation
Sequential events during gastric motility. (1) The stomach has distinct anatomical regions. (2) As the bolus of food enters the stomach, the fundus and upper body of the stomach relax to receive it. (3) Through peristalsis , the distal stomach mixes the gastric contents with gastric secretions and slowly empties the gastric chyme into the small intestine . (4) As peristalsis proceeds through the gastric antrum, the pyloric sphincter partially closes, resulting in retropulsion of antral contents as the antrum contracts. This process facilities the breakup of particulate matter and the mixing of food with gastric secretions. Small amounts of liquidized and digested food enter the small intestine through the narrow pyloric channel
6.1 Neural and Hormonal Regulation of Gastric Emptying
The small intestine has a limited capacity and can only accept small amounts of gastric chyme at any one period of time. The pyloric sphincter, therefore, plays a very important role in this respect. However, the small intestine also releases a number of hormonal factors that inhibit gastric emptying. These factors are stimulated by the presence of unsaturated soaps and fatty acids of chain lengths of C 12 or greater. Denervation fails to abolish the effect of fat in the proximal small bowel on gastric motility . Gastric emptying is also inhibited by a number of enterogastric reflexes. For example, the presence in the duodenum and jejunum of hyper- or hypotonic fluids, acid (pH < 3.5), polypeptides, oligosaccharides, and fatty acids result in the inhibition of gastric peristaltic contractions. With the exception of the response to fat, inhibition by these substances in the upper small bowel is abolished by vagotomy , indicating a neurogenic regulatory pathway rather than a hormonal regulatory mechanism. Over-distension of the bowel also causes inhibition of gastric motor activity, as well as the inhibition of the rest of the GI tract. This reflex is called the intestino-intestinal reflex .
In general, receptor activation in the stomach and duodenum sensitive to distension, changes in osmolarity, pH, or lipid content can trigger pathways that regulate gastric emptying. In this regard, the excitatory influences that originate in the stomach are kept in check by inhibitory mechanisms that originate in the duodenum so as to have gastric chyme delivered in metered doses to the duodenum. These inhibitory mechanisms for gastric emptying, i.e. neural and hormonal pathways, prevent the upper small intestine from being overwhelmed by material from the stomach. In addition, various emotional states, such as anger, fear and depression, produce changes in gastric motor activity. Figure 2.14 summarizes the intestinal phase pathways that inhibit gastric emptying.
6.2 Gastric Motility Dysfunctions
As described, gastric emptying is regulated at a rate optimal for digestion and absorption of a meal. Abnormalities of gastric emptying thus lead to several clinical problems. Rapid emptying of gastric contents into the small intestine , for instance, can cause “ dumping syndrome ,” characterized by nausea, pallor, sweating, vertigo, and sometimes fainting within minutes after a meal or ingestion of a hypertonic solution. The symptoms are very similar to the vasovagal syndrome, in which massive systemic discharge of vagal fibers causes vasodilation and a fall in arterial blood pressure. The pathophysiological basis of this syndrome may be, in part, related to the exaggerated release of enteric hormones such as GIP, neurotensin , VIP, and enteroglucagon, which have significant vascular effects. On the other hand, “ gastroparesis ” is a condition characterized by impaired or absent ability of the stomach to empty. This condition is occasionally observed in severely diabetic patients who develop autonomic neuropathy . The loss of vagal stimulation to the stomach markedly impairs antral systole , preventing the proper digestion and emptying of gastric contents. These patients often complain of early satiety (feeling of being full), abnormal bloating, and nausea.
7 Motility of the Small Intestine
As the major site of digestion and absorption of nutrients, the small intestine has two major motor functions, namely mixing and propulsion. Multiple short (1–2 cm long) annular constrictions, called segmentations, frequently appear in the small bowel and cause an apparent to-and-fro movement of the intestinal chyme. The frequency of segmental contractions is dependent on the regional BER rate, which decreases from proximal to distal small intestine. Thus, the duodenum can contract with a basic rate up to approximately 11cycles/min, whereas contractions in the ileum can only approach 8 cycles/min. The decreasing gradient of BER frequency promotes the distal movement of intestinal chyme (Fig. 2.15).
Like other parts of the bowel, muscular contractions of the small intestine are stimulated by extrinsic and intrinsic factors. For example, CCK, bombesin, opioid peptides (e.g. met-enkephalin), tachykinins such as substance P, and acetylcholine are stimulatory. Sympathetic discharges, α- adrenergic agonists, CGRP, nitric oxide , VIP, and glucagon are inhibitory. Glucagon, for example, is frequently used by radiologists and endoscopists to inhibit intestinal motility during diagnostic studies. Propulsive motor contractions of the small intestine are far less frequent than segmentations. However, after ingestion of a meal and entry of gastric chyme into the proximal small intestine, peristaltic-like waves occur, mostly confined to the upper small bowel. These waves travel relatively short distances but unequivocally move intestinal contents in an aboral direction. The stimulus for these short peristaltic waves appears to be the distention of the intestinal wall by luminal contents.
7.1 Migrating Motor Complex
During fasting or interdigestive periods, a specific pattern of propulsive motor activity in the stomach and small intestine can be observed. This pattern is characterized by cyclic motor activity migrating from stomach to distal ileum . Each cycle consists of a quiescent period (phase I), followed by a period of irregular electrical and mechanical activity (phase II), and finally by a short phase of regular activity (phase III), where electrical spike potentials peak and mechanical frequencies approximate the BER (Fig. 2.16). This specific peristalis, called the migrating motor complex (MMC), can best be followed by measuring phase III events. Occurring about every 90 min, the MMC migrates from the stomach to terminal ileum at a rate of approximately 5 cm/m. Several hormones may be involved in the initiation of MMCs, including motilin , opioid peptides and somatostatin , albeit to varying degrees and in different regions of the bowel. Although their physiological role is still uncertain, MMCs are believed to have a “housekeeping” function, clearing the stomach and small bowel of luminal contents, thus preparing the bowel for the next meal. MMCs are immediately interrupted when a meal is ingested, an inhibitory effect probably mediated by extrinsic neural signals and, in part, by gut peptides such as gastrin and CCK. On the other hand, the orderly propagation of MMCs down the digestive tract is dependent on the regulation by enteric neurons. The enteric nervous system additionally coordinates MMC-motor activities with other intestinal functions such as gastric, biliary, and pancreatic secretions, as well as intestinal blood flow (Fig. 2.17).
Migrating motor complex of the intestine. Seen during fasting or interdigestive periods, this type of motor activity is characterized by cyclic motor activity migrating from the stomach to distal ileum . A quiescent (phase I) period is followed by a period of irregular electrical and mechanical activity (phase II) and finally by a short phase of regular activity (phase III). MMCs are believed to serve a “housekeeping” function, preparing the bowel for the next meal. MMCs are immediately interrupted when a meal is ingested. MMC indicates migrating motor complex
MMC-coordinated digestive functions. The enteric nervous system coordinates MMC-motor activities with other intestinal functions such as gastric, biliary, and pancreatic secretions and intestinal blood flow (Adapted from Wood and Wingate, Gastrointestinal neurophysiology; American Gastroenterological Association Teaching Slide Collection 20, slide 94 ©, Bethesda, Maryland; Used with permission)
7.2 Small Intestinal Dysmotility
Two other motility patterns of the small intestine merit brief discussion, as they are clinically relevant and often occur as a consequence of other pathophysiological processes. The first is “ vomiting ”, which is frequently associated with nausea and can be evoked by many different stimuli. It is a stereotypic response which is dependent on both central and enteric nervous systems. When the vomiting center in the brain is stimulated, a diffuse autonomic neural discharge occurs that activates the enteric neural network responsible for the vomiting reflex. The reflex involves the closing of the glottis after inspiration, contraction of the abdominal muscles causing increased abdominal pressure, and retropulsion of intestinal and gastric contents by retrograde power contractions (reverse peristalsis ) beginning in the upper small intestine. The second pattern is an adynamic state of the small intestine called “ileus” that is often initiated by noxious stimuli such as abdominal trauma or perforation and occasionally by medications such as opiates. During the state of ileus, bowel sounds are absent and air-fluid levels are observed on plain upright X-ray films of the abdomen. MMCs are absent and the bowel motility is characterized by prolongation of phase I, the quiescent phase of the motor activity.
8 Motility of Large Intestine
The colon of an adult human receives 0.5–2.5 L of chyme per day. This consists of undigested and unabsorbed residues of food, in addition to water and electrolytes. The colon must reduce the volume of this intestinal chyme to about 100–200 g of fecal material before excretion. Hence, the movements of the colon are slow and irregular and serve mainly to segment its content to increase contact with the absorbing surface.
The colon lacks a continuous layer of longitudinal muscle. Instead, the muscles are organized into three flat bands called the teniae coli . Contractions of teniae coli cause the colon to open and close in a manner similar to an accordion. Segmental contractions of the circular muscle layer divide the colon into segments called haustrations and represent the main motor activity in the colon. Like other parts of the bowels, the frequency of segmentations is dependent on regional BER rates. However, in contrast to the situation in the small intestine , there are increasing gradients of BER frequency from rostral to caudal (Fig. 2.18). This provides an effective means for the more distal part of the colon to mix the luminal contents, allowing the absorptive surface area to maximally extract water and electrolytes and solidify fecal contents.
Gradient of basic electrical rhythm (BER) in human colon. There appears to be an increasing gradient of BER frequency from rostral to caudal in the human colon. This provides an effective means for the mixing of luminal contents in the distal part, allowing the absorptive surface area to extract water and electrolytes efficiently and solidify fecal contents
Three to four times a day, a mass movement occurs wherein the segmental contractions of the left colon disappear and a simultaneous contraction of the right colon propels its content distally. This tends to occur after meals and has been referred to as the gastric colic reflex . True peristaltic waves are extremely rare in the colon. Most often adjacent segments of colon appear to contract independent of each other. These segmental contractions are generally increased in amplitude and stimulated by eating or by the presence of bulk in the colon.
Extrinsic cholinergic nerves can not only directly stimulate mechanical activity of the colon but they also modulate motility by their actions on interneurons and postganglionic enteric motor neurons . In contrast, sympathetic neurons inhibit colonic motility. However, the enteric nervous system plays a major role in regulating colonic motor, transport, and blood flow functions. Like other parts of the bowel, neurotransmitters of the enteric nervous system are inhibitory or excitatory.
9 Rectal Function and Defecation
Motility of the rectum and the anal sphincters are quite distinct from colonic motility. The rectum has two primary functions: (1) to serve as a storage site for feces and (2) to expel feces during defecation. Thus, the rectum must have the ability to accommodate a certain volume of stool. When this capacity is exceeded, intramural stretch receptors are activated, making the individual aware of the urgency of the forthcoming event and relax the internal anal sphincter. The individual then increases intra-abdominal pressure by forcing air against the closed glottis (Valsalva maneuver ) and relaxes the external anal sphincter, which is made of skeletal muscle (Fig. 2.19). The individual can inhibit defecation by voluntarily increasing the tone of external anal sphincter, which transiently increases internal sphincter tone. However, as rectal pressure builds, the urge to defecate increases and the above processes are again initiated.
Defecation involves a series of coordinated motor activities. The figure shows regional pressure measurements (Modified from Davenport [4])
Summary
-
The GI motility is well organized for optimal digestion and absorption functions.
-
It is regulated by neural, hormonal and myogenic control systems.
-
The rhythm of the GI tract is generated by myogenic system of the smooth muscle layer that undergoes spontaneous depolarization and repolarization cycles, known as slow-waves which contribute to the basic electrical rhythm (BER).
-
The frequency of BER is determined by the slow waves which is essentially constant.
-
The force of contractions is determined by the number of spikes fired within each wave, which depends in turn on the neural and hormonal input.
Clinical Correlations
Case Study 1
A 1-week old infant boy presents with a history of vomiting, abdominal distention, and constipation . He has not been nursing well and appears irritable. Physical examination reveals a distended, tympanitic (drum-like) abdomen, and hyperactive bowel sounds, consistent with intestinal obstruction. A plain x-ray film of the abdomen shows a large ovoid mass mottled by small, irregular gas shadows in the right side of the colon, consistent with fecal retention and an area of obstruction is suspected. A barium enema shows narrowing in the distal segment of rectum. An open biopsy of the wall of the narrowed rectum is performed under general anesthesia.
Questions
-
1.
Based on the clinical observation, what is the diagnosis of the patient?
Answer: This child has Hirschsprung’s disease (Congenital megacolon ).
-
2.
Explain briefly the pathogenesis of this disease.
Answer: Congenital megacolon is characterized by a functional obstruction of the colon resulting from aganglionosis (an absence of ganglion cells) of the bowel wall. The rectum and sigmoid colon are the most common sites of involvement. Full thickness biopsy (the diagnostic procedure of choice) shows the hallmark of the disease, which is the absence of ganglion cells in the myenteric and submucosal plexuses. The absence of enteric neurons, especially those secreting nitric oxide and VIP, results in a loss of inhibitory tone necessary to counteract excitatory motor neurons that contract muscle fibers in the anal sphincter and other distal segments of the colon. Consequently, colonic contents cannot pass through the involved segment and the patient experiences symptoms of intestinal obstruction.
-
3.
How do you treat the patient?
Answer: Treatment of the disease involves surgical removal of the involved segment.
Case Study 2
A 20-year-old student has a history of type 1 diabetes mellitus (T1DM: juvenile onset and insulin deficient) since age 15 who presents with severe constipation , abdominal distention, and severe heartburn, especially in the supine position. Occasionally, she experiences dysphagia (difficulty in swallowing), particularly when solid foods are ingested. In the past several months, she has also had abdominal discomfort and fullness immediately after eating small amounts of food. On physical examination, she has diabetic retinopathy, orthostatic hypotension (drop in blood pressure after standing), and moderately severe peripheral neuropathy. Upper GI and small bowel barium X-ray studies reveal a dilated stomach , retained food material, and retention of over half of the barium after 30 min. The small intestine is noted to have some nonspecific dilatations. The colon is filled with feces.
From this case presentation, it is a type of T1DM-induced gut dysmotility. Based on your knowledge of diabetes and on the findings of the physical examination, how would you explain the patient’s symptoms and radiologic findings at different levels of GI tract dysfunction?
Questions
-
1.
Esophageal dysfunction?
Answer: A major consequence of severe and long-standing diabetes mellitus is the development of peripheral and autonomic neuropathy . The latter has numerous effects on bowel function, a major manifestation being the development of abnormal bowel motility. Because the extent and severity of autonomic dysfunction vary considerably from patient to patient, the clinical presentation of patients are quite protean. Many patients experience esophageal and swallowing symptoms as a consequence of altered or aberrant sphincter functions and/or peristalsis . Tertiary contractions may become more pronounced and frequent. Loss of lower esophageal sphincter tone can result in symptoms of reflux esophagitis (heartburn, chest pain) because of acid-pepsin injury of the esophageal lining. The barium X-ray study shows reflux of the barium column into the esophagus .
-
2.
Gastric dysfunction?
Answer: Autonomic neuropathy can also affect gastric emptying. The stomach becomes flaccid and antral peristalsis is defective or lost. This condition, called diabetic gastroparesis , is not uncommon. Barium remains in the stomach for 10 hours after ingestion, indicative of delayed emptying. Gastroparesis can result in the formation of a bezoar , a conglomerated mass of undigested vegetable and fruit fiber.
-
3.
Small intestinal dysfunction?
Answer: Autonomic neuropathy of the small intestine can cause intestinal stasis , a condition that favors luminal colonization and overgrowth by bacteria. These organisms compete for luminal nutrients, thus causing nutrient malabsorption and diarrhea .
-
4.
Colonic dysfunction?
Answer: Finally, the normal function of the colon and rectum is frequently affected by diabetic autonomic neuropathy . Patients can develop severe constipation or diarrhea . In addition, the loss of anal sphincter tone and the afferent nerves required for sensing the presence of stool in the rectal vault may develop. These patients may lose normal defecation function and experience fecal incontinence . The treatment of intestinal complications associated with diabetes is particularly problematic, as the neuropathy is usually irreversible.
Case Study 3
A 40-year-old woman presents with a progressive history of dysphagia. She has difficulty swallowing both solids and liquids and finds that it is worse when she experiences periods of emotional stress or eats too rapidly. She has found that alcohol is of some benefit in helping the food pass into the stomach . Occasionally, when she is supine or is exercising, she regurgitates food particles from meals eaten hours before. Several weeks ago, she was treated with antibiotics for bronchopneumonia. On physical examination, she appears to be normal. The only significant finding is that she has halitosis (bad breath). On chest x-ray film, an air-fluid level is noted in the posterior mediastinal area. A barium swallow is taken which reveals a dilated lower esophagus . Esophageal manometry of the patient is subsequently ordered.
Questions
-
1.
What would you expect these examinations to show? Explain the pathophysiology of this disease.
Answer: This patient has achalasia , a condition in many ways similar to Hirschsprung’s disease . It is a neuromuscular disorder that affects the LES and is characterized by the failure of the sphincter to relax during the process of swallowing. Normally, the LES is richly innervated by VIP- and nitric oxide -secreting enteric neurons of the myenteric plexus that provide inhibitory tone during the terminal phase of esophageal peristalsis , allowing the food bolus to pass into the stomach . The loss of inhibitory tone and impairment of sphincter relaxation causes a functional obstruction of the esophagus . Alcohol can result in transient symptomatic relief, as it causes proximal dilation of the esophagus and retention of luminal contents, which explain the patient’s halitosis (fermentation of retained esophageal contents) and the air-fluid level observed radiographically. On occasion, especially at night when the patient is supine, the luminal contents of the esophagus are regurgitated and aspirated. This can cause bronchopneumonia, as was the case with this patient.
-
2.
What would you recommend for the treatment of this patient?
Answer: The treatment of this disease is either esophageal dilation or surgical myotomy of the lower esophageal sphincter . Esophageal dilation is performed with an inflatable balloon or luminal dilator, which splits the sphincter. Patients often require repeated dilation over time, as the condition recurs. Recently, botulinum toxin injection is an alternative approach to treating the disease; it is achievable based on the rationale that the toxin is to decrease cholinergic input, thus reducing the LES pressure.
Further Reading
Bharucha AE, Brookes SJH (2012) Neurophysiologic mechanisms of human large intestinal motility. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 5th edn. Academic, New York, pp 977–1022
Bitar KN, Gilmont RR, Raghavan S, Somara S (2012) Cellular physiology of gastrointestinal smooth muscle. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 5th edn. Academic, New York, pp 489–509
Brierley SM, Hughes P, Harrington A, Blackshaw LA (2012) Innervation of the gastrointestinal tract by spinal and vagal afferent nerves. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 5th edn. Academic, New York, pp 703–731
Davenport HW (1982) Physiology of the digestive tract. Year Book Medical Publishers, Chicago, p 93
Goyal RK, Chaudhury A (2008) Physiology of normal esophageal motility. J Clin Gastroenterol 42:610–619
Koeppen BM, Stanton BA (2008) Gastrointestinal physiology. In: Berne & Levy’s physiology, 6th edn. Mosby-Elsevier, Philadelphia, pp 487–553
Mittal RK (2012) Motor function of the pharynx, the esophagus, and its sphincters. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 5th edn. Academic, New York, pp 919–950
Poole DP, Furness JB (2012) Enteric nervous system structure and neurochemistry related to function and neuropathology. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 5th edn. Academic, New York, pp 557–581
Sanders KM, Koh SD, Ordög T, Ward SM (2004) Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol Motil 16:100–105
Tack J, Janssen P (2010) Gastroduodenal motility. Curr Opin Gastroenterol 26:647–655
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Chang, E.B., Leung, P.S. (2014). Gastrointestinal Motility. In: Leung, P. (eds) The Gastrointestinal System. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8771-0_2
Download citation
DOI: https://doi.org/10.1007/978-94-017-8771-0_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8770-3
Online ISBN: 978-94-017-8771-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)