Abstract
One of the more fascinating developments in neuroscience is the recognition of endocrine influences on brain regions unrelated to reproductive and basic homeostatic functions. It is now clear that hormones impact both normal function and dysfunction, including the experience of pleasure and the anhedonia accompanying a number of psychiatric disorders, most notably depression. Brain regions contributing to these functions are rich in receptors for the peptides and steroids of the hypothalamic – pituitary – gonadal (HPG) and hypothalamic – pituitary – adrenal (HPA) axes. Indeed, the brain has evolved new functions for ancient hormones. Examples include the brain adaptive uses of steroid precursors and metabolites for non-reproductive functions and the brain co-opting or “hijacking” peptides of the two axes to serve as neuromodulators and neurotransmitters. The result is that HPA and HPG hormones and their interactions have profound influences on opioids and monoamines, especially dopamine and serotonin. These are the same neurotransmitter pathways underlying activation of the brain reward pathway stretching from midbrain to the prefrontal cortex.
Our ultimate goal is to fulfill the promise of the title, an evaluation of neuroendocrine – anhedonia relations. This requires, first, an overview of the endocrine system, and their steroids and peptides. There, we also provide a brief review of the interaction of the HPA and HPG axes in depression. Before embarking on an evaluation of hormones and anhedonia, we will examine normal neuroendocrine influences on pleasure from natural experiences such as food and sex but also from psychoactive drugs. Logic suggests examining data on pleasure before addressing loss of pleasure. The emphasis throughout will be on animal models with a liberal sprinkling of human findings, mostly psychiatric patients.
This journey will take us through endocrine basics (Sect. 10.1), and the influence of hormones on brain systems underlying the experience of pleasure (Sect. 10.2). In Sect. 10.3, the modest literature on the neuroendocrinology of anhedonia in depression will be reviewed. Finally, future research and directions (Sect. 10.4) will provide ideas on filling in the gaps in our understanding of endocrine – anhedonia relations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endocrine system
- Stress
- HPA axis
- HPG axis
- Corticosteroid
- Testosterone
- Estrogen
- Dopamine
- Mesocorticolimbic pathway
- Brain reward system
- Chronic mild stress
1 Endocrine Basics
Hormones have profound effects on structures and functions of the body, including brain and behavior. The metaphor of a river, the Mississippi or Rhine, for the endocrine system is apt because it is the body’s way to send hormones produced locally in glands to nearby and far away destinations. The result is integrating and coordinating disparate functions in multiple tissues.
Endocrine products begin their influences during early fetal life and continue to exert influences through childhood, adolescence, and adulthood. Yet, the importance of the endocrine system in pathological behaviors is often underappreciated, if not ignored altogether, by journal and book editors. Happily, this editor and book are exceptions.
Because endocrinology is not well known by many researchers of psychiatric conditions, we will provide an introduction to a few of the most important endocrine principles. This overview will use stress as related to depression as the exemplar condition.
1.1 Endocrinology of Stress
As the reader of this volume will have recognized by now, anhedonia is part of the symptomology of a number of psychopathologies. Nonetheless, anhedonia is a hallmark symptom of major depressive disorder (MDD). And, there is a rich literature on depression and stress-related endocrinology.
Early life adversity has shown a clear link to the development of depressive-like symptoms in laboratory animals [1] and to MDD in humans [2]. Moreover, exposure to chronic stress at any ontogenetic stage is one of the few generally accepted antecedents of depression in susceptible individuals [3]. Susceptibility is derived presumably from genotype and its epigenetic activation from the environment and individual experiences [4, 5]. The neuroendocrine mechanisms for stress responses are found in two complementary physiological systems. When activated by stressful conditions, the hypothalamus – pituitary – adrenal (HPA) axis and sympathetic adrenal medullary (SAM) system release a sequence of hormones [6].
1.1.1 HPA Axis
There are three primary HPA hormones, corticosteroid (CORT), adrenocorticotropin hormone (ACTH), and corticotropin-releasing hormone (CRH). CORT and ACTH can be detected and easily measured in the peripheral bloodstream whereas CRH is mainly in the brain, making its measurement much more difficult. Still, the workings of the HPA axis are well known [7].
Most familiar to non-endocrinologists is CORT because of its widespread use as a medication. Also known as glucocorticoid, glucocorticosteroid, corticosterone (in rodents) or cortisol (in humans), CORT carries a heavy load in medicine from the topical treatment of skin rashes with cortisone to a variety of more serious conditions. The analogs of cortisone, prednisone for instance, are widely prescribed for emphysema and asthma [8].
CORT is the endpoint hormone of the HPA axis. The term axis describes a cascade of events with activation of endocrine products. Under the influence of the hippocampus and other upstream brain regions, CRH is one of several “releasing hormones,” so named because they induce the pituitary to release its hormones. Synthesized in CRH neurons originating in the paraventricular nucleus (PVN) of the hypothalamus, CRH is deposited from the median eminence into the hypothalamus – pituitary portal system, a small, one-way blood canal leading into the anterior pituitary. There, CRH binds receptors located on corticotropes that release ACTH into general blood circulation. An interesting factoid is that ACTH is “cleaved” from a larger protein proopiomelanocortin (POMC). Cleaving a different section of POMC yields endorphin and the other endogenous opioids that serve as neurotransmitters and neuromodulators. This is further evidence of the close relation between endocrine and central nervous systems.
Once in the bloodstream, ACTH is carried to the distant adrenal glands embedded in fat above the kidneys. ACTH binds adrenocortical receptors located in the outer, cortical layer of the adrenals. These bindings induce CORT to be released into the bloodstream to increase energy available to cells and other metabolic functions in the periphery.
As the bloodstream circulates throughout the body, CORT makes its passage to the pituitary, the hypothalamus and other brain regions. At all points, CORT binds receptors with one result being decrease in the release of ACTH and CRH. This cascade describes the negative feedback processes of an endocrine axis.
But CORT is not done. CORT binds its two receptors, mineralocorticoid (MR) and glucocorticoid (GR), in the brain, particularly in the hippocampus. Presence of MR and GR provide the hippocampus with a mechanism to regulate the HPA axis. Experiments using lesions or electrical stimulation reveal the hippocampus inhibits HPA activity [6]. Interestingly, CORT has a higher affinity for the MR and binds the GR only after the MR are occupied [9]. Thus, chronically high levels of CORT appear to be required, along with CRH activation, to modify hippocampal function [10].
The far reach of HPA hormones includes CRH. CRH neurons are found throughout the limbic system, in the interneurons of the hippocampus and in the locus coeruleus of the midbrain from which norepinephrine cells arise. The neuropeptide is central to the experience of stress and a major player in the pathology induced by chronic HPA activation [11].
1.1.2 The Sympathetic Adrenal Medullary (SAM) System
The two SAM agents are epinephrine (EPI) and norepinephrine (NE). EPI is well known outside of neuroendocrinology by its alternate name, adrenaline. NE in the brain is a neurotransmitter closely related to its monoamine cousins, dopamine and serotonin.
EPI is synthesized in the same adrenal glands that synthesize CORT, but in the middle segment known as the adrenal medulla. Upon confrontation with a stressful stimulus, peripheral EPI is released into general circulation and NE neurons alert the subcortex and cortex. Together, the SAM system activates the sympathetic segment of the autonomic nervous system.
The most notable feature of the SAM system is its speed. While the HPA axis requires 15–30 min to be fully activated, SAM activation is achieved within seconds [12]. Although often ignored in reviews of stress, SAM is responsible for the immediate physiological reactions to stressful stimuli including increased heart rate, sweating, and pupillary dilation. These are the hallmark features we describe when discussing our stress response to a near accident [13, 14].
Nevertheless, HPA hormones are the focus of most studies of stress, and of its relation to depression. Indeed, elevated CORT levels remain the gold standard for confirming that an individual is exhibiting a stress response [15].
1.2 Reproductive Steroids
Along with corticosteroid, there is another set of familiar hormones, the sex steroids. Estrogens, androgens and progestins are reproductive steroids, although they serve many functions besides reproduction [16]. Although they are often grouped according to gender, all three sex steroids are found in both males and females, albeit in different amounts. The plurals for the sex steroids suggest there are more than one estrogen, androgen and progestin, and there are. Nonetheless, the most biologically active are the familiar estradiol (E2), testosterone (TS) and progesterone (PROG). These are the gonadal hormones that represent the end product of the other major endocrine axis, the hypothalamus – pituitary – gonadal (HPG) axis.
Workings of the HPG axis bear close similarity to the HPA axis. The HPG has a hypothalamic hormone (gonadotropin releasing hormone, GnRH), a pituitary hormone (luteinizing hormone, LH), and a distant hormone synthesizing structure (ovaries or testes). The sequential release and negative feedback are also similar to the HPA axis. Indeed, the similarity with TS to CORT in males is notable. That there are two ovarian steroids, along with separate cycles for each, makes the female HPG system more complicated.
The term “steroids” is heard often in everyday discussions, although the reference is used to describe different substances, e.g., synthetic drug treatments (corticosteroids) or illicit use by athletes of performance enhancing drugs (androgens). Steroids are a large group of related biochemical compounds. Figure 10.1 depicts the metabolic cascade for the multiple branches yielding the many familiar, and unfamiliar, steroids.
Metabolic cascade for steroids in the brain. Steroid acronyms are: PREG = pregnanolone, PREG-S = pregnanolone sulfate, PROG = progesterone, 5α DH PROG = 5α DH progesterone, ALLO = allopregnanolone (also known as 3α,5α tetrahydroprogesterone or 3α,5α TH PROG), DEOXYCORT = 11-deoxycorticosterone, 5α TH DOC = 5α dihydroxydeoxycorticosterone, 3α,5α TH DOC = allotetrahydrodeoxycorticosterone, CORT = corticosterone, and 18-OH-CORT = 18-OH- corticosterone. Further steroid acronyms are: 17-OH-PREG = 17-OH-pregnanolone, DHEA-S = dehydroxyepiandrosterone sulfate, DHEA = dehydroxyepiandrosterone, 17-OH-PROG = 17-OH-progesterone, and ANDRO = androstenedione. Enzyme acronyms are: P450-1 through 6 = six different forms of P450, SFT = sulfatransferase, SF = sulfatase, 3β HSD 1/2 = 3β hydroxysteroid dehydrogenase form 1 or 2, 5α-red = 5α-reductase, 3α HSD = 3α hydroxysteroid dehydrogenase, and 17β HSD through 6 = different forms of 17β hydroxysteroid dehydrogenase
First feature to note in the figure is cholesterol as the common origin of the steroids. The lipid backbone of the steroids gives them the capacity to cross with ease the blood-brain barrier and enter the brain. Peptides have a more difficult path into the brain from the periphery, if they get there at all. Second feature to note is that E2 is a metabolite of testosterone. This fact has led to countless experiments and journal articles, particularly after the realization that TS may act as an androgen or, after conversion to E2, as an estrogen. Third, not all steroids in the figure are hormones. Among other requirements to earn the label, hormones must have defined receptors. At this time, two estrogen receptors, ER-α and ER-β, and a single androgen receptor, AR have been identified. There are two isoforms for PROG, PR-A and PR-B [17].
Finally, even though steroids in the cascade may not be elevated to hormonal status, a number of the products in the figure have gained acceptance as having important influences on brain function and behavior. Examples include dehydroepiandrosterone (DHEA) and allopregnanolone (ALLO). DHEA is a precursor of TS that is of such importance to neural functions that the brain synthesizes its own DHEA, earning its designation as a “neurosteroid” [18]. Another important neurosteroid is ALLO, a metabolite of progesterone [19], that appears to modify CORT and CRH releases to stress [20].
1.3 HPA, HPG & Depression
There is a rich literature revealing both endocrine axes as prominent factors in mood disorders, including major depressive disorder (MDD). Recognition of the involvement of HPG hormones comes, first, from epidemiological findings of a dramatic sex difference in MDD incidence. Compared to men, women have 2–3 times the likelihood of being diagnosed with clinical depression sometime during their lifetimes [21]. Further epidemiological evidence for the involvement of the hypothalamic – pituitary – ovarian axis is that depressive episodes in women closely follow major HPG lifetime events. Puberty, the ebb and flow of hormones during the menstrual cycle, and periparturition and menopausal stages are all related to MDD.
Of surprise to many people, depression can develop in children. The gender ratio in younger children is even. The female bias ratio begins in the peripubertal stage. Incidence of depression increases progressively and the female bias ratio increases with the surge of sex hormones with oncoming puberty and incidences continue to rise into young adulthood [22]. The rise and fall of circulating sex hormones during the menstrual cycle track depression symptomology. Women of reproductive age report fewer symptoms during the follicular stage than other phases of their cycles [23]. Finally, depressive episodes are notorious during the post-partum period, and menopause can signal relief for previously depressed women [24, 25].
MDD patients overwhelmingly report chronic stress as antecedent to a depressive episode. Clinical and pre-clinical data support their observations [26]. In an animal model of depression, chronic stress in the form of daily restraint reduces spontaneous locomotor activity and induces weight loss [27], both markers of depression in humans. Cumulative stress exposure in life is a risk factor for the development of a number of psychiatric illnesses, including clinical depression and substance use. It is of interest that both of these disorders often are comorbid and are characterized by anhedonia [28].
Early childhood experiences of parental abuse or neglect seem to be a particularly sensitive period, confirmed in animal models. Repeated separation of neonatal rat pups from their mother result in persistent alterations in biology and behavior mimicking those in human depression [29]. The animals also experience elevated CORT during adulthood, another clue for the importance of the HPA axis in depression.
Basal levels of CORT often are elevated during depressive episodes [30], and return to normal baseline levels upon successful anti-depressive drug treatments [31, 32]. Results of a study in MDD patients and healthy controls indicated increased activity of the intracellular cortisol-deactivating enzymes 5α-reductase and 11β-HSD2 in the depressed individuals. These metabolic changes increase CORT bioavailability within tissues [33].
Other evidence supporting a link between the HPA and MDD is the behavioral similarities in symptomology of the endocrine disorder Cushing’s disease and depressive diseases [34]. Elevated CORT is a distinguishing feature of Cushing’s disease, and many patients have a history of depression. Also, exogenous corticosteroids administered as medicines may have the same effect on mood and the hippocampus [35].
CRH also plays a central role. High densities of CRH receptors have been observed in brain regions important in MDD, including the neocortex, the central nucleus of the amygdala, the bed nucleus of the stria terminalis, the hippocampus, the nucleus accumbens and the hypothalamus [36]. Chronic stress can elevate CRH receptor numbers in rats [37]. Moreover, the effects of CRH are amplified in the rodent pituitary by arginine vasopressin (AVP). Levels of AVP also increase after prolonged stress, magnifying further the functional activity of CRH [38]. These results confirm characterization of activation of the HPA axis by stress as “sluggish but long lasting” [6].
These effects have been observed also in humans. CRH and AVP actions in the hypothalamus of patients are sensitized and their adrenals are enlarged. Depressed patients often fail to reveal the normal negative feedback suppression of cortisol to administration of dexamethasone, a synthetic CORT [39, 40]. Failure to show suppression to a sudden increase in CORT points to HPA axis dysregulation in MDD. It is not clear if dysregulation is a cause or an effect. The former is suggested, however, by the observation that chronic elevation of HPA hormones is an antecedent to the development of MDD. Hyper-reactivity of the HPA axis was detected in people at high genetic risk for developing MDD prior to the onset of clinical symptoms [26].
Structural changes in the brain after exposure to stress have been confirmed in animal research. Chronic stress in rodents produces numerous morphological and physiological changes in a variety of limbic brain regions. Dendritic tree branches are reduced and neurotransmitter responses are less predictable with subsequent stress. Daily restraint modulates GR concentrations in the PFC, hypothalamus and, of particular note, in the hippocampus of rats [27, 41].
The hippocampus is a target for both CORT and CRH and is the single most studied brain region for stress – depression interactions [42]. The most dramatic neural consequence to chronic HPA hyperactivity is atrophy of the hippocampus. As much as 20 % of hippocampal volume is lost in long-term depressed people [43]. Volumetric loss is significantly correlated with total lifetime duration of depression. This suggests that repeated stress during recurrent depressive episodes may result in cumulative hippocampal injury as reflected in volume loss [44]. Moreover, atrophy increases with longer durations of depression and persists up to decades after depression has been resolved.
Neuronal loss is the most likely source for atrophy. The mechanism appears to be an indirect influence of CORT on the excitatory neurotransmitter glutamate. Glutamate is notorious for excitotoxicity when overly activated [45]. Chronically high CORT enhances amounts of glutamate released. The results are neuronal death and reduced neurogenesis in the vulnerable hippocampus [43]. There is evidence that exogenous CORT administered as medicines may have the same effects on the hippocampus [35].
A final note is the participation of DHEA in HPA endocrinology. Released by the adrenal medulla, DHEA can serve to reduce the impact of elevated CORT in experimental animals [46, 47]. In the rat brain, DHEA has anti-glucocorticoid effects and is protective against the neurotoxic effects of CORT both in vivo and in vitro [48]. A prediction from those data is of reduced DHEA in patients and, indeed, there is evidence of low serum DHEA levels in both adolescents and adults diagnosed with MDD [49].
Here, we see the seeds of an intimate interaction between stress hormones and reproductive hormones because DHEA is a precursor of both testosterone and estrogen (Fig. 10.1).
1.4 HPA-HPG Interactions & Depression
The HPA axes of males and females are different and their responses to acute and chronic stress are different. The differences are clearer in laboratory animals than in humans [50]. In humans, physiological and neurological measures are more limited. For example, most endocrine measures are from collection of saliva. Only the unbound, “free” CORT can be detected in saliva, thus failing to consider the bound CORT that can be quickly converted to the unbound form [51].
Relying mostly on animal models, the data point to a sexual dimorphic HPG response to stress. The CORT findings reliably identify greater HPA activity in females. Female rats have higher resting levels of CORT, a greater CORT sensitivity to acute stress [52], and a more persistent CORT response to stressful conditions [53]. Although recovery may be delayed in males exposed to physical stressors, such as restraint, return of CORT to baseline following social stress is longer in female rats [54]. Similarly, women have a greater and longer lasting CORT response when submitted to social rejection challenges, which may contribute to their greater vulnerability to depression [55].
However, there are mitigating factors for findings in both rats and humans. One is stage of the estrous cycles of rodents or menstrual cycles of women. Females have higher CORT during the late follicular phase when circulating estradiol is high, decreasing in the other phases when progesterone is high or both hormones are low [56, 57]. Excising the ovaries (ovariectomy or OVX) reduces plasma levels of CORT and restoration with estrogen therapy restores the levels to pre-OVX levels [54]. Males show the opposite pattern as testosterone appears to suppresses the HPA axis. Castrated male rats tend to have a greater stress response compared to intact males or TS-treated castrates [58].
Pregnancy features dramatic increases in E2, PROG and CORT. In women, the hormonal increases reach a peak in the third trimester and, with birth, the levels fall quickly to markedly low levels, setting the stage for post-partum depression [59]. There are conflicting data on whether there are increased or decreased incidences of depression during pregnancy. It is likely that a key factor is whether or not a woman had a history of MDD prior to pregnancy [60].
Effectiveness of modern anti-depressant drugs is well established. That there are sex differences in drug effectiveness is suggested by clinical and pre-clinical studies. Overall, anti-depressants seem to be more effective in young women than men [61]. Moreover, there is a sexually dimorphic response to the different classes of drugs. Depressed women respond better to selective serotonin receptor inhibitors (SSRI) than to the tricyclic anti-depressants while men responded equally well to both [62] or better to the tricyclics than women [63]. Yet, hypoestrogenic women, as with menopause and its accompanying rapid loss of circulating ovarian hormones, show less sensitivity to SSRIs [64].
Although various steroids and peptides are likely involved, estrogen is the one hormone most often believed to be responsible for the relation of the HPG axis to stress and to depression [65]. Findings cited earlier on cycling hormones point to high physiological levels of estrogen having the most favorable response to stress and HPA activation. Depressed women show various HPG deficits, for example lower circulating estrogen, than healthy women [66]. Women who underwent bilateral removal of ovaries (oophorectomy) before the onset of menopause had an increased risk of depressive symptoms [67]. Women with a history of MDD but in remission had lower serum E2 levels but higher PROG at mid-cycle of menstrual cycle than controls [68]. Finally, molecular biology studies have suggested the beneficial effects of estrogens on mood are most likely due to estrogen receptor activation. E2 binding of ERs attenuate the glucocorticoid responses to stress, suggesting that estrogens improve mood by suppressing CORT hyperactivity [69].
The general conclusion is that estrogen protects females from the adverse effects of stress. This points to a paradox. That women suffer MDD at higher rates than men stands in stark contrast to the notion of E2 as a protective agent. The answer to the paradox is that we do not know the answer.
A prominent hypothesis centers on the cyclical nature of ovarian steroids [64, 70]. The rise and fall of the hormones is thought to promote conditions for development of mood disorders in susceptible women. Depression itself may contribute to neuroendocrine dysregulation. Depression suppresses E2 levels that are then normalized with antidepressant treatments [24].
Other hormones surely have involvement in MDD. Depressed women have been reported to have higher baseline serum levels of both TS [71] and PROG [72] than healthy women. The suggestion is that the release of TS and PROG may have effects in opposite directions to those of E2.
More recently, the emphasis has switched to non-gonadal endocrine steroids and peptides influencing the two axes and the development of depression. A prime candidate is the progesterone metabolite ALLO, a neurosteroid that is an agonist of the amino acid neurotransmitter gamma aminobutyric acid (GABA) [73]. Cerebrospinal fluid (CSF) levels of ALLO are decreased in people diagnosed with major depression. This decrease is corrected in patients by SSRIs in doses that improve depressive symptoms [74]. DHEA interacts with the other major amino acid neurotransmitter, glutamate, and influences the response to stress. Ultimately, both precursors and metabolites of steroid hormones affect the biological activity of dopamine. We will now see these are the neurotransmitters that will play prominently in hedonia and, likely, anhedonia.
2 The Neurobiology of Pleasure
2.1 Pleasure
Pleasure is recognized as a basic feature of humans and, likely, most other vertebrates. Seeking pleasurable experiences probably has been recognized as a fundamental force in humans since the very beginnings of Homo sapiens and long before someone characterized it as “wine, women and song.” Loss of the capacity to experience pleasure is sure to have a significant impact on one’s psychological wellbeing. Anhedonia accompanying depression and other psychiatric conditions is, indeed, a fundamental loss.
One logical approach to understanding the neuroendocrine underpinnings of anhedonia is to examine hedonia [75, 76]. The concept of pleasure has been a central topic of interest in psychology, and only slightly less so in philosophy and biology, the two precursors of modern psychology.
Evolutionary principles placed hedonism as a key factor in adaptation. Nature (natural selection) regularly ensured that pleasure was highly correlated with the most critical activities required of the animal. Reproduction would generate the most pleasure with food and avoiding pain not far behind. A familiar example is the energy contained in different foods. If it tastes good, it is almost surely to be highly caloric.
The status of hedonism in the form of reward and punishment was elevated to a basic principle in psychology by the early behaviorists such as E.L. Thorndike and B.F. Skinner. Skinner [77] expanded rewards to define it behaviorally as the now-familiar term, reinforcement. A positive reinforcement is anything that led to an animal repeating a response to gain the stimulus, for instance a treat for a dog for obeying a command. A negative reinforcement is anything the animal would respond to remove, a thorn in the dog’s paw for instance. Note that both positive and negative reinforcers are ultimately based on pleasure. Punishment that led to a decrease in responding was not an important concept for Skinner and, even today, is seldom the focus of a research project in psychology.
Pleasure is a subjective experience. Yet, as with all other subjective states, there are brain regions and circuits responsible for the experience. Neuroscience research has pointed to dopamine pathways and the limbic system as likely candidates. Nonetheless, the underlying mechanisms have proven complex and fundamental questions remain unanswered [78].
Our working assumption is that pleasure and anhedonia are opposite side of the same coin [28]. This suggests it is worthwhile to take a cursory look at the workings of the processing of rewards in the brain and, then, the relation of the reward circuitry to sex and stress hormones.
2.2 Brain Reward System (BRS)
2.2.1 Neuroanatomy
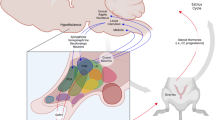
The primary neuroanatomical regions involved in rewards are found in the midbrain and forebrain. The ventral tegmental area (VTA) is located deep in the ancient brain and communicates with the subcortex through a bundle of neuronal axes, known as the medial forebrain bundle (MFB). Serotonin and norepinephrine neurons leave the MFB as it passes near the hypothalamus to make connections with hypothalamic nuclei. Dopamine (DA) neurons in the MFB continue and terminate in the nucleus accumbens (NAcc).
The NAcc is strategically located nearby other subcortical nuclei that subserve limbic activation. Reciprocal connections of the NAcc with the amygdala, hippocampus and, most notably, the prefrontal cortex (PFC) ensure a top-down influence on the NAcc and VTA. Collectively, this route has been dubbed the brain reward system or BRS. Figure 10.2 offers a schematic of the BRS.
The figure also highlights NAcc connections to other brain regions via an array of neurotransmitters [79, 80]. Most of the neurotransmitters thought to be involved in psychopathology are found somewhere along the tract from midbrain VTA to the cortex. Found in the VTA along with DA are serotonin (5HT), acetylcholine, enkephalin, glutamate and GABA. The PFC receives dopaminergic input and sends projections toward the NAcc via glutamate and GABAergic neurons. The amygdala contributes cannabinoid transmitters to both the VTA and PFC [81]. Still, it is DA that holds the spotlight in the BRS.
2.2.2 Focus on Dopamine
The origins of DA neurons are cell bodies located in the substantia nigra and the ventral tegmental area (VTA). The former projects to the striatum of the basal ganglia, thus the term nigrostriatal dopamine pathway. The VTA is the origin of the second DA pathway known as the mesocorticolimbic dopamine system. The VTA originated system often is described in its two segments, mesolimbic or mesocortical. The mesolimbic system sends DA axons into the MFB that terminate in the NAcc. There, the mesocortical pathway makes connections with upstream subcortical structures and continuing onward into the PFC. That processing of rewards depends on an intact mesocorticolimbic DA pathway is well established [75, 82]. Examples are bountiful.
With microdialysis and related technology, neurotransmitter release can be quantified in real time. DA increased in the NAcc and in the medial PFC in rats upon being fed a highly palatable food [83]. DA levels were high in the NAcc prior to and during copulation, followed by increased levels of the DA metabolites, suggesting increased DA turnover [84]. Castrating male rats results in loss of copulatory ability over days that correlates with the loss of DA release to an estrous female. Restoration of copulation with exogenous TS revealed the reemergence of the DA response [85, 86].
There are high concentrations of DA neurons and their receptors in the caudate nucleus of the striatum and in the nearby NAcc, as well as in the central nucleus of the amygdala and several regions of the frontal cortex [87]. Neurophysiological evidence includes increasing firing rates of dopamine neurons in the MFB in the presence of food or a receptive sex partner. Learning plays an important role as there is similar increased neuronal activity in environments in which the animal had previously eaten or copulated [88]. Neuroimaging studies have suggested a similar pattern of activation in humans exposed to pleasurable stimuli [89, 90].
NAcc activation in animal models has been observed to aversive stimuli, which may be a consequence of the rewarding effects of their termination [91]. This “relief” bears notable similarity to Skinner’s negative reinforcement [77].
Additional evidence for the role of the dopamine BRS is found in the drug abuse literature. Cocaine, methamphetamine and many other stimulant drugs target the catecholamines, NE and DA. Other commonly abused drugs, nicotine, marijuana and heroin, also indirectly activate DA neurons in the NAcc [81, 92].
It should be noted before moving on from this section that not everyone is convinced that DA is the pleasure neurotransmitter. The critics cite puzzling and contradictory data. For example, single cell recordings of DA neurons in the VTA indicated DA activation to novel and unexpected rewards and less so to expected ones [93]. Also, depletion of DA in the NAcc failed to interfere with food consumption or effort to obtain food in rats [94]. Finally, because the thalamus receives projections from the NAcc, thalamic neural activity was monitored in rats receiving sucrose rewards. When sucrose access was delayed, thalamic firing rates increased progressively over the delay period. The peak was before the delivery of the reward and firing decreased dramatically during consumption of the sucrose. The same conclusion was suggested in a study of humans [95] using money and social approval as rewards.
The suggestion is that the increase in DA in NAcc and other parts of the BRS is to predict reward, and perhaps not the neurophysiological agent underlying pleasure. In this model, DA is responsible for attention and information processing of salient cues predicting reward that contribute to motivation to obtain the reward [96]. Its role is more questionable in mediating the experience of pleasure [97, 98].
This is not an insignificant semantic debate. Therapies for drug abuse are built on the notion that the mesocorticolimbic DA systems chiefly mediate the intense pleasure of addictive drugs and of anhedonia during drug withdrawal [99].
To better understand the distinction, it is helpful to contrast anticipatory behaviors and consummatory responses [95]. Sexual behavior in male rats can be used as an example. Anticipatory behaviors of the male include increased activity when motivated by cues, e.g., smells of a receptive female or even environmental stimuli from previous learning, indicating a reward awaits the male. Consummation with the acts of copulating and ejaculating is a separate component of the sexual encounter involving separate brain regions [100]. Other researchers have cast this into more familiar language, the difference between “wanting” and “liking” [82, 101].
The critical role played by DA is unquestioned in anticipatory behaviors. The role of DA in consummatory behaviors is less certain. One result is that the search for the ultimate source of pleasure has shifted to the endogenous opiates. Opioid receptors are highly expressed in brain areas of the BRS, including VTA, NAcc, amygdala and PFC. Animal studies demonstrate facilitation of DA release by endogenous opiates binding the opioid receptors. These data have led back to dopamine, and the hypothesis that the mediation of pleasure by endogenous opioids may be secondary to DA release [102].
The most likely opioid candidate (see Fig. 10.2) is enkephalin as it interacts with DA in the NAcc, thus modifying the upstream BRS activity in other limbic areas and into the cortex. The conclusion is that DA and enkephalin are interconnected in the motivation-pleasure cascade [92]. Moreover, these data demonstrate the complexities of brain reward circuitry, and that we do not know yet all the pieces that create the experience of pleasure [78].
Although it may not be the sole contributor, DA is clearly involved in seeking a pleasurable stimulus and, likely, in the experiencing of pleasure. Next, we will examine the interaction of HPA and HPG hormones with the BRS and dopamine.
2.3 Hormones and the Brain Reward System
2.3.1 HPA & the DA BRS
A principle common to both acute and chronic stress is that a hyperactive HPA axis can change DA release and metabolism and, thus, function of the mesocorticolimbic pathway.
Two areas of research have led to that conclusion. Most direct are the studies of changes in DA integrity along the BRS pathways to acute and chronic stress. Second, studies of humans and animal models suggest a close relation between stress hormones and DA-related drugs that have high abuse potential.
A well-functioning HPA axis complements well-functioning DA pathways. The normal synthesis, release and metabolism of DA in the medial forebrain bundle [103] and the NAcc [104] are dependent upon CORT. CORT regulates tyrosine hydroxylase, the rate-limiting enzyme in DA synthesis [105]. Also, CORT is essential for maintaining normal DA metabolism and function of the PFC [106]. Changing CORT levels with stress or administration of psychostimulant drugs can modify those processes.
The bulk of the findings demonstrate that acute stress enhances dopamine BRS activity. Exposure to a brief stressor increases DA activity throughout the BRS. Acute stress activates DA neurons in the VTA [107]. DA activation in the NAcc, measured by increases in DA metabolites, was increased in mice exposed to a single 2 h restraint [108] or a social defeat [109]. Acute tail pressure stress to rats increased DA dialyses in the PFC [110].
The influence of chronic stress on the dopamine BRS is more complicated. Whereas DA activity is increased by acute stress, effects of longer periods of stress on DA are different in mesolimbic and mesocortical segments. With chronic stress the VTA – NAcc segment habituates while the NAcc – cortex segment continues to respond with higher DA activity [111]. One implication is that a sensitized PFC with chronic stress is involved in reward dysfunction and its normalization with anti-depressant drugs [112].
The literature on stress and drug use and abuse has a special connection to the dopamine BRS. An often-stated behavioral model for drug use and abuse recalls the positive – negative reinforcement distinction made by Skinner and others [113]. The model suggests drug use is driven by the good feelings induced by most psychoactive drugs. With continued use and the onset of “addiction,” self-administration of the drug is driven by relief from the unpleasant withdrawal symptoms.
The BRS fits comfortably within the model because all drugs of abuse ultimately increase release of DA in the NAcc. HPA hormones do not have as natural of a fit in this model, yet stress has an important place in drug abuse. Studies in lab animals indicate a variety of stressors accelerate the acquisition of drug self-administration. Moreover, once established, self-administration of cocaine increases to acute or chronic stress. One possible result is the triggering of relapse of drug-seeking even after a period of abstinence [114].
Indications of CORT involvement are the findings that exogenous CORT increased cravings in cocaine dependent people and in an animal model of cravings [115]. Indeed, CORT antagonists decreased cravings for the drug [116].
Research also has focused on genetics and early experiences [117]. The rewarding effects in adulthood to amphetamine, for instance, are increased by neonatal stress in rats [118]. Behavioral reactivity to a stressor may be related to self-administration of abused drugs [119]. Individual rats or humans who are highly reactive to novelty are more prone to drug self-administration. High reactive rats reveal an elevated and prolonged CORT response to acute stress. They also have a lower density of dopamine D2 receptors in the NAcc, but a higher elevation of mesolimbic DA release than those found in low reactive rats [120, 121]. Notably, the high reactive animals also had greater concentrations of dopamine in the NAcc to self-administered cocaine [122].
These data suggested the hypothesis that HPA hormones can enhance the incentive value of cocaine. That is to say stress enhances the reward value of drugs of abuse [123]. However, there is no agreement on the mechanism(s) underlying the hypothesized increased “liking” of a drug with current or previous stressors.
2.3.2 HPG & the Dopamine BRS
Pre-clinical evidence indicates that HPG hormones also are involved in dopamine pathways [124]. Rodent studies have documented sex differences in the depletion, turnover, and extracellular accumulation of dopamine in the striatal pathway following methamphetamine administration [125]. Sex hormones influence the mesocorticolimbic pathways, as well. Concentrations of DA and its metabolite, dihydroxyphenylacetic acid (DOPAC), in the NAcc decreased after castration. Both DA and DOPAC were restored with exogenous TS or, interestingly, with exogenous E2 [126].
The few relevant studies of humans suggest a similar conclusion. Supraphysiological levels of androgens produced by self-administration of anabolic steroids by athletes elicit electrophysiological responses that are similar to the responses to amphetamine. A primary mechanism of action for Cocaine and amphetamine is increases in synaptic DA in the mesocorticolimbic pathway [127]. There is some question about the receptor responsible for these very high dosages of TS. Because the aromatase enzyme that metabolizes TS to E2 is highly concentrated in limbic structures, hippocampus and cortex [128], it may be the hedonic effects are actually from after TS is converted to E2 and the latter binds ERs [129].
Interactions between E2 and DA can be observed at all levels of the dopamine BRS pathways [130]. Reports of decreased DA and 5HT in the VTA of OVX rats indicate activity in both neurotransmitters can be restored by exogenous E2 [131].
Much of the focus in this research area has been on subcortical structures. Other brain regions along the mesolimbic DA segment also reveal sensitivity to estrogenic input. Acute or chronic exposure of OVX rats to E2 enhanced the release of DA in the NAcc to amphetamine or cocaine [129, 132]. Levels of 5-HT and DA in the amygdala were significantly reduced by OVX in rats [133]. OVX rats administered E2 increased DA turnover in both striatum and NAcc. Notably, the increase coincided with peak circulating E2 concentrations [134].
The NAcc is closely linked to the striatum with reciprocal projections between the two structures. DA receptor density in the striatum increased significantly in juvenile rats at puberty. It is interesting that the male juveniles showed a much higher increase in DA receptors than their female counterparts [135]. In adulthood, no such sex differences are observed in DA receptor activity in the striatum, measured by density of DA reuptake sites, is significantly higher in gonadally intact female rats than in OVX, intact and castrated male rats. DA reuptake density sites also fluctuated during the female estrous cycle with a peak occurring in the morning of proestrus when estradiol is elevated [136].
The relation of E2 to the mesocortical segment of the dopamine BRS is more complicated. One example is that phases of the estrous cycle in which there are high levels of E2 can lead to DA and PFC dysregulation [137]. Another study reported that basal DA concentrations in the PFC varied during the estrous cycle, with DA being lowest in proestrus when endogenous E2 levels are highest [138]. On the other hand, administration of an ER-β agonist in OVX rats was reported to increase levels of DA turnover by 100 % in the PFC [139].
DA turnover was elevated in the medial PFC with E2 treatments. These data are notable in that the subjects were castrated male rats [140]. Moreover, the opposite effect, inhibition of DA turnover in the medial PFC, was observed in another group of castrates who were administered dihydrotestosterone (DHT), an androgen that cannot be aromatized into E2.
In summary, E2 has potent influences on DA [141]. Indeed, estrogen has been observed repeatedly to have a larger role in brain functions than the other HPG hormones and, perhaps, any other hormone. A potential reason is found in studies of molecular evolution. The estrogen receptor is reported to be the original member of the steroid receptor family. Moreover, it is probably not a coincidence that E2 is the final product to be synthesized in the metabolic pathway of steroids [142]. One result of its ancient status would be that the brain had a longer evolutionary time to co-opt E2 and its receptors for a diversity of influences on different tissues.
3 Hormones and Anhedonia
Clinical observation, patient comments and various psychological test batteries are used to assess anhedonia in MDD [143]. Animal models of anhedonia are based on reducing MDD symptomology into component parts and then designing a paradigm to induce and assess the depressive-like symptom [144]. Prominent among the animal models of depression is the forced swim test (FST) that is designed to mimic learned helplessness in MDD. The FST, however, does not reliably produce anhedonia [145].
A few animal models marginally related to depression have been proposed to assess anhedonia. Intracranial self-stimulation (ICSS) is based on the propensity of rats to bar press to deliver electrical stimulation to locations in the medial forebrain bundle or related areas. ICSS has been used to assess reward sensitivity with concomitant drug administration and then upon drug withdrawal. Notably, drug withdrawal has been proposed as a model of changes in the dopamine BRS that may underlie anhedonia. The logic is based on the high rates of co-morbidity between drug abuse and depression, suggesting a shared neurobiology. Both conditions can modify dopamine BRS circuitry underlying anhedonia [28].
A typical experiment in ICSS literature is to establish bar pressing for cocaine, followed by disabling the bar or changing the response requirements for self-administration and observing changes in the animal’s behaviors. Findings include reductions in bar pressing for ICSS, increases in the current required to maintain ICSS or shorter time to the “break point” at which the animal stops bar pressing [146, 147]. All three are believed to be indicators of an anhedonia based on a lowered of sensitivity to rewards [118].
Exposure to social defeat in aggressive encounters is a type of chronic stress that can lead to reductions in subsequent social interactions [148]. This outcome is said to model the loss of interest in social interactions accompanying depression, an outcome that could be considered a form of anhedonia. However, the other markers of anhedonia seem less dependent on the complex of factors that can modify the experience of defeat and subsequent social interactions [149, 150]. Because avoidance of social contact could result from a fear induced catatonic-like state, social defeat may better simulate the social withdrawal common in schizophrenia [151].
A surgical paradigm also has been used to model anhedonia and other symptoms of depression. Olfactory bulbs are removed and the animal is tested in several behavioral paradigms. Results indicate bulbectomized rats show greater startle to a loud noise, elevated serum levels of CORT and reductions in sexual behaviors and a suppressed preference for sucrose [145]. On the other hand, anosmic animals are hyperactive in both familiar and unfamiliar locations, which is uncharacteristic of MDD patients.
A final paradigm, chronic mild stress (CMS), also uses the natural attraction to sucrose as its primary measure. Indeed, CMS is the dominant animal paradigm for the study of anhedonia [152, 153]. The CMS paradigm has the virtues of reliably inducing anhedonia in most laboratories and of face validity. Daily exposure to nuisance events mimics the stressful events of everyday human life. That the animals show a progressive loss of attraction to a formerly pleasurable stimulus over time is a form of anhedonia with which many of us older scientists can identify.
Unpredictable stressors are applied daily over weeks, each typically for 12–24 h duration, to an individually housed rodent. Mild stressor can be a manipulation of home cage, e.g., wet bedding or cage tilted 45°, or of the animal room environment, e.g., a strobe light or a low decibel noise. Quantifying anhedonia is accomplished by measuring once every 5 or 7 days quantities of sweet water in which regular tap water freely available. Some researchers use a relative measure, i.e., sweet vs. tap water percentages, and others use total amount of sweet water consumed. Regardless, the typical findings are the original preference for the sweet solution decreases over several weeks of exposure to the mild stressors.
Clearly, the extensive CMS literature provides a fine segue to this third section. Here, we review the CMS findings related to the main topics presented in Sects. 10.1 and 10.2. First, we examine the CMS literature related to DA and the BRS, and then the CMS findings related to the HPA and HPG axes. Included in the latter are unpublished data from our laboratory on androgenic influences on anhedonia with a CMS paradigm.
3.1 CMS & Dopamine
Given the central role proposed for DA in the neurobiology of rewards, there is a surprisingly small literature measuring DA parameters in the CMS paradigm. One reason may be that the paradigms using severe stressors are not conducive to measurements of consummatory behaviors. Also, as presented in Sect. 10.2, acute stressors such as restraint induce stark increases in HPA activity and DA hyperactivity while chronic stress can suppress DA activity.
Nonetheless, much of the relevant DA research with the chronic mild stress procedure has been directed at brain regions either directly in the mesocorticolimbic pathway or regions closely associated with the pathway [152]. At its simplest, the working hypothesis is that induction of anhedonia with CMS predicts reduced DA activity in this brain reward system.
Although there are notable exceptions, the data support the prediction of reduced DA in the brains of CMS animals [154]. CMS was found to be associated with a reduction in DA and its metabolites or reduced DA turnover in the PFC [155]. There also is evidence of CMS interfering with dopaminergic activity in the NAcc [83], although others report no differences in the NAcc of CMS and no stress controls [152].
A couple of CMS experiments have included assessment of the midbrain and reported decreased DA receptor expression in the VTA [112]. Of particular interest is that the cleanest evidence of DA activity decreasing with CMS is in the hippocampus [152].
Finally, CMS appears to have long-term effects on DA neurotransmission. After CMS has been terminated, an additional acute stressor increased DA release in both PFC and NAcc [83]. The suggestion is of CMS sensitizing the dopamine BRS to a subsequent, more intense stressor.
In summary, a prediction from the hypothesis that the experience of pleasure and anhedonia are opposite sides of the same coin is that the loss of sucrose preference would be associated with DA dysfunction in BRS pathways. Modest empirical support for an hypodopaminergic state comes from examination of dopamine BRS structures. The evidence is clearer for the PFC and hippocampus than for the NAcc. This is consistent with the conclusion in Sect. 10.2 that the mesocortical segment is more sensitive to chronic stress than is the mesolimbic segment of the BRS.
Of course, there remains the data on hyper-activity in DA responses to the more severe stressors standing in opposition to the DA hypo-activation with CMS. One conclusion is validation that the CMS paradigm is markedly different from the paradigms employed in the traditional animal studies with intense stressors. That anhedonia is commonly observed in both CMS rats and MDD patients recommends the paradigm for research on depression.
3.2 CMS & HPA Hormones
The relation of chronic mild stressors to the HPA system is less predictable than the endocrine response to restraint and other more severe stressors. Evidence of a relation of CMS – HPA activity comes from measuring CRH receptors in structures of the DA mesocorticolimbic pathway. An increase in receptor concentrations is predicted by dysregulation of the HPA, and there are reports of upregulation of CRH receptors with CMS in frontal cortex, hippocampus and, especially, hypothalamus [37, 156]. The mesolimbic segment has been less well studied and results are equivocal.
Elevated CORT levels have been reported for most, but not all, studies measuring sucrose preference and endocrine variables. For example, Grippo and colleagues [157, 158] reported on several experiments in which rats were subjected to CMS that induced anhedonia. In male rats exposed, CORT levels increased in male rats relative to controls [157]. In a subsequent study, there was a statistically non-significant trend toward elevated CORT [158]. Still, the overall pattern was a 30 % increase in CORT with CMS which is a much lesser rise compared to more severe chronic stressors [159]. The CMS findings of modest increases in CORT have been replicated in other laboratories [160].
It is to be noted that in the above experiments and most others in the literature [161], blood samples for hormone assays were collected at necropsy, i.e., after the animals had been in the CMS for weeks. More problematic for this discussion is interpretation of the studies that measured CORT, but only after exposure to an intense stressor that followed the weeks of CMS [83, 162, 163].
The absence of a dramatic elevation in CORT after weeks of stress is not surprising. The endocrine system shows partial adaptation with repeated exposure even to severe stressors [159]. A modest elevation of CORT above threshold values after weeks of CMS is consistent with mild stress and adaptation to chronic exposure.
On the other hand, consistent with chronically higher titers of CORT during the earlier weeks of CMS are reports of downregulation of CORT receptors at the end of CMS. The reductions in GR and MR expression have been found primarily in the hippocampus [152]. No one, to our knowledge, has measured CORT or CRH receptors in the PFC, nucleus accumbens or other areas in the mesocorticolimbic pathway after CMS.
Of greater interest is the relation of anhedonia to CORT levels. That is, does HPA activation closely correlate with the loss of sucrose preference, suggesting HPA may contribute to anhedonia. Indirect evidence is that the sweet preference often recovers spontaneously over several weeks without the stressors [164]. Also, administration of anti-depressants that moderate hippocampal and HPA activity speeds the recovery [165, 166] Anti-depressants administered at the start of CMS exposure may prevent the development of anhedonia [167].
An experiment using a social defeat paradigm rather than CMS measured sucrose preference along with CORT levels [150]. Results were that the sucrose preference decreased while CORT increased in defeated rats soon after the experiences. However, 2 weeks later the elevated CORT returned to baseline values along with the recovery of the sucrose preference. The suggestion is of an inverse relation of sucrose preference and CORT in socially defeated, stressed animals.
The relation of anhedonia and CORT also has been examined in humans. In an interesting study, different psychological scales were used to identify MDD patients exhibiting different degrees of severity of symptoms accompanying depression [30]. Results were clearest for individuals with predominantly anhedonia symptoms, i.e., higher anhedonia was associated with higher CORT levels measured upon awakening in the morning. Although CORT was not measured, an acute but moderately intense stressor that normally activates the HPA axis was applied to healthy young women. The women revealed a reduced sensitivity to a reward that was especially evident in individuals with existing anhedonic tendencies [168].
Collectively, the data, mainly from animal models, support the hypothesis that HPA hormones are involved in the development and reversal of anhedonia. However, that conclusion is limited by the paucity of studies of receptors in the mesocorticolimbic pathway and the absence of CORT monitored during the weeks of CMS.
3.3 CMS & HPG Hormones
3.3.1 Sex Differences
Several studies of gender differences in MDD patients have included assessments of anhedonia. Psychological scales of multiple symptoms of depression were used. Contrary to the female bias for other symptoms of depression, the findings were either of no sex differences [169] or an unexpected higher incidence of anhedonia in depressed men [170].
Research with animal models has helped clarify the picture, but only somewhat. Comparing male and female animals has been the focus on studies to evaluate sex differences in behavioral and endocrine outcomes with chronic mild stress. Some also have included sucrose preferences as a measure of anhedonia.
The answer to the question of whether CMS is more stressful to one sex over the other in lab animals seems to depend upon which of the various measures of the stress response is used. Loss of body weight is one measure of stress, and the overall results suggest greater relative loss of body weight with CMS in male rats than in females [161, 171]. Males also were affected more by CMS than females when subsequently observed in the FST. Using an ICSS paradigm to assess anhedonia, Bielajaw and colleagues [171] found no differences between males and females in their rates or thresholds of bar press responses.
Recalling the gold standard for determining HPA activation, assays of circulating CORT reveals a female bias. Female rats are reliably. Female rats are reliably found to have elevated CORT levels in the CMS paradigm [161]. Surprisingly, males may not show a CORT response at all to CMS exposure [160].
Results are more nuanced for relative sucrose consumption in the experiments using both males and females. In an early study [172], both males and females showed a decrease in sucrose preference with CMS exposure. However, there were no sex differences in consumption by the rats. Similar findings of no sex differences in preference with CMS were reported for intact or for gonadectomized male and female rats [173, 174].
Evidence for greater anhedonic response in females has also been reported. Male and female rats from two strains were subjected to a CMS procedure. Overall, females tended to show a gradual reduction of sucrose consumption; males did not [160]. Other findings have indicated sucrose reductions as being greater in males than females in the CMS paradigm [175]. Of interest are other findings that sucrose suppression over weeks of CMS was observed in both genders, but the reductions occurred much earlier in male rats than in the females [157]. For example, Dalla and colleagues [161] measured several neurochemical and behavioral parameters in male and female rats in the CMS paradigm. Although the experimental design was incomplete, sucrose consumption was observed as early as the 1st week of CMS in the males while the females required exposure for several weeks longer to reveal sucrose suppression.
These latter data suggest an explanation for the inconsistent findings on sex differences of sucrose preferences. Males may experience stress in the CMS paradigm earlier than females and, thus, are more habituated to the stressors by the end of stress exposure. In that scenario, males would reveal CORT elevation and anhedonia earlier than females. The males could have habituated and returned to baseline levels of sucrose consumption by the end of the 6 week of CMS.
3.3.2 Testosterone and Anhedonia
As reviewed in the previous section, there is a notable influence of HPG hormones, especially ovarian hormones, on dopaminergic activity in the BRS. Those data would suggest considerable research interest in manipulating hormone levels of animals in the CMS paradigm. That has not been the case.
There is a small literature on androgenic effects on anhedonia. A sensitive marker of testicular function is sexual behavior, and exposure to CMS increased latency both to intromission and ejaculation in male rats after 4 week of CMS [176]. That sucrose preference also progressively decreased in those males suggested development of anhedonia coinciding with the reduced HPG function. In a series of experiments [177], gonadally intact middle-aged male rats were given TS supplements to match the circulating TS levels of young males. Animals were subjected to CMS either before or after TS supplementation began. Findings were that TS blocked the onset of anhedonia. However, if the loss of sucrose preference was established before TS supplementation, TS could not reverse the anhedonia.
Evidence supporting that conclusion came from a study of castrated males in another anhedonia paradigm [178]. Although CMS was not used, castrated rats were administered either TS or vehicle only and sucrose preference was measured with daily FST exposures. The vehicle control animals developed anhedonia while the males restored with TS maintained their normal preferences for sucrose. Because hormone metabolism was also manipulated, the authors concluded the estrogen receptor was responsible, that is, TS maintained sucrose preferences in male rats only after being metabolized to estrogen [178]. Finally, there is a report that CMS was associated with elevations of circulating TS in male rats relative to untreated controls. However, this was serum obtained after only a brief, 10 day exposure to CMS [179].
Dehydroepiandrosterone (DHEA) appears to be a good candidate for androgens blocking the development of anhedonia. In a report of middle-aged depressed patients administered DHEA for 6 week, depressive symptoms, and prominently anhedonia, improved in 60 % of the DHEA group compared to a 20 % improvement in placebo controls [180].
Results of an unpublished experiment by the authors on recovery from anhedonia in male rats. Groups of rats were either exposed or not exposed to chronic mild stress for 3 weeks. Beginning in the 4th week, groups from CMS exposed and non-CMS exposure were SC administered daily either saline vehicle or dehydroepiandrosterone (DHEA) at a dosage of 800 μg/kg body weight
An unpublished experiment from our lab attempted to examine the role of DHEA in depression in an animal model. We used a CMS paradigm similar to our earlier, published study in which our goal was assessing the capacity of a kappa opioid agonist to promote a faster recovery of anhedonia after CMS had ended [181]. Gonadally intact rats were exposed to CMS over 3 weeks and, as expected, revealed progressive reductions in percent preference of sucrose water over tap water. Beginning in the 4th week, with CMS halted, half the animals received daily SC injections of DHEA (800 μg/kg body weight). Results are depicted in Fig. 10.3. DHEA induced a more complete recovery of sucrose preference than the control animals without DHEA treatments.
3.3.3 Estrogen and Anhedonia
A few experiments in the animal literature included direct manipulation of E2 in females. In one experiment, young adult and middle-aged rats were ovariectomized and exposed over 7 weeks to CMS. Beginning in the second week and continuing until the end of CMS exposure, the females were administered E2 alone or E2 plus a SSRI antidepressant. Both ages of OVX rats receiving E2 + SSRI increased their relative sucrose preferences. E2 alone failed to significantly influence sucrose consumption in either age group [182]. Of note, the middle-aged females showed earlier recovery from CMS-induced anhedonia than the young adult females. However, the conclusion that E2 alone is unable to influence anhedonia can be questioned. The low dosage of hormone used (2.5 μg) was unlikely to restore the animals to normal circulating E2 levels and are certainly unable to simulate proestrus [183].
In another experiment, E2 (1 mg or 2 mg) was administered as a single bolus to OVX middle-aged females after the first of 3 weeks of CMS exposure. No significant differences in sucrose preferences were observed between hormone-treated and untreated controls [184]. It appears, however, that the hormone may have been effective in inhibiting development of anhedonia during the early weeks with the lower of the two dosages. However, the E2 dosages, 1–2 mg, would have produced dramatically supraphysiological levels of hormone [183]. Thus the two direct tests of the hypothesis that E2 could prevent or relieve anhedonia could be questioned on their choices of restoration dosages of hormone.
Less direct tests of the hypothesis have yielded data suggesting a more profound estrogenic influence on CMS females. Although intact animals were not included for comparison, female rats ovariectomized a month before introduction of CMS showed an unusually rapid development of anhedonia. By the second week of CMS, the OVX animals were drinking less sucrose water than non-CMS OVX rats [173].
In a recent ICSS experiment [185], OVX rats had higher sensitivity thresholds, indicative of anhedonia, than gonadally intact females. With E2 restoration therapy, the stimulation threshold was restored to the levels of the intact animals. Studies of the reward value of drugs of abuse also have pointed to E2 being capable of inhibiting anhedonic behaviors [186]. For example, Galankin and colleagues [187] used stimulation thresholds in a ICSS paradigm and found that E2 to OVX female rats enhanced the hedonic value of cocaine.
OVX decreased reward value of cocaine in females while castrating male rats had no effect [131]. OVX also decreased DA and 5HT in the VTA. The authors interpreted the findings as gonadal hormones influencing reward differently in males and females with the primary mechanism being E2 altering monoamine neurotransmitter systems.
Finally, circulating progesterone was elevated in female rats after a shortened 10 day exposure to CMS that produced anhedonia. Their E2 levels were no different than untreated control females [179]. In a review of ovarian hormonal influences on drug-seeking behavior, the authors [186] concluded that both PROG and its metabolite ALLO reduce drug seeking, a form of anhedonia. Indeed, PROG often is found to oppose the effects of estrogen. For example, PROG counteracts the enhanced effects of estrogen on cocaine self-administration and psychomotor activation [131, 188].
Our own conclusion is a hypothesis of inhibition of anhedonia from estrogenic binding of the ER in brain regions of the DA mesocorticolimbic pathways. This influence on the dopamine BRS can be direct of E2 can be directly from circulating estrogens from the periphery or indirectly from metabolic conversion of DHEA to TS and then to E2 in the brain.
4 Conclusions and Future Directions
The doggedly persistent reader who makes it to this section is acutely aware of the glaring gaps in our knowledge of neuroendocrine influences on anhedonia. Here we cite a few questions awaiting answers from future research efforts. Researchers working with either humans or other animal species can find rich fodder for projects from the list.
-
What are the common endocrine elements with other psychiatric conditions that often include anhedonia?
Schizophrenia and MDD appear to be distinct diseases but share anhedonia symptomology [143]. It is entirely possible that anhedonia arises from distinct processes “with only an apparent resemblance of (anhedonia) expression in the two groups of patients” [189]. We know there are sex differences in incidence, timing, and/or severity in both diseases. Examination of circulating HPG hormones and degree of anhedonia in patient groups would be a first start.
Usefulness of animal models of psychiatric conditions is also suggested. One approach would be to manipulate fetal and perinatal HPA and HPG hormones. Subsequently, the animals would be evaluated as an adult with one of the several measures of anhedonia.
Another approach would be to systematically administer E2 and TS to gonadectomized animals before, during and after exposure to the CMS paradigm. The same approach could be used in an animal model of schizophrenia [190]. Finally, DHEA is the most plentiful circulating sex steroid in humans that begins a steady, predictable decline in the 30’s with rates of decline faster in men than women [191]. Studies are sorely needed on the influence of DHEA on the mesocorticolimbic system.
-
What are the systematic changes in anhedonia symptomology with therapy?
We know that medications used to treat psychiatric disorders such as MDD and schizophrenia are effective, more or less, in relieving symptoms the diseases. However, there is surprisingly little study of the time courses of relief from the various symptoms. For example, anhedonia symptoms may resolve faster, or slower, than the other symptoms of MDD under the different SSRI drugs [192]. Although there is some work already done with SSRIs in the CMS paradigm [112, 182], we recommend more systematic comparisons of established and new psychiatric medications with lab animals.
-
Is anhedonia at base a disorder of the dopamine brain reward system?
An initial goal for both animal and human researchers would be to better understand the neural basis of anhedonia. Modern neuroimaging techniques can be used to search for blunted responses to reward signals in the dopamine BRS in clinically depressed people [75]. Gender differences should always be an independent variable in these studies, along with awareness of current oral contraceptive use and stage of the menstrual cycle of women participants.
Animal models offer a wider range of options to examine dopamine BRS function using behavioral indicators of hedonia and anhedonia. Of particular interest would be the interaction of the HPA and HPG axis in such experiments. There are often surprising influences of one axis on the other [193]. Researchers should take care, however, to ensure physiological dosages of replacement hormones and to include gonadally intact animals as comparison groups.
-
Do understudied groups differing in endocrine status show differences in development and recovery from anhedonia?
Not only are there endocrine differences depending on stage of the menstrual cycle in women, there are many other natural lifetime phases in which humans and lab animals have markedly different endocrine states. Juvenile rats and adolescent humans provide opportunities for researchers to examine the relation of anhedonia with the onset and suddenly high levels of HPG hormones. Pregnancy is characterized by remarkably high levels of steroidal hormones with the highest levels observed during the third trimester in women and 18–21 days of the rodent gestation period [194]. Their dramatic drop with parturition and ensuing mood changes in women are legendary [195]. Yet, we found no studies in the literature of anhedonia in teenagers or depressed pregnant women or in their animal models in the CMS paradigm.
-
Are there simple experiments without necessity of sophisticated technology that will help us to better understand the neuroendocrinology of anhedonia?
The short answer is a resounding yes. A few examples include obtaining blood samples from rats every week of CMS to assay for circulating CORT that likely change over time and changes differently in males and females. Also the SAM system is a critical part of the stress response. It would be easy to examine epinephrine (adrenaline) in the periphery or to use another marker of SAM activation in patients with anhedonia exposed to an acute stressor or in animals exposed vs. not exposed to CMS. Evaluating pregnancy – anhedonia relations would be straightforward with a sample of women in different trimesters using one of the psychological batteries that probe for anhedonia. Similarly, pregnant rats exposed to CMS could be examined for sucrose preference and recovery, with special attention paid to the last days of their 21 days gestation period.
There surely are many other experiments wanting for empirical study by more clever researchers. We hope the long journey through this chapter will inspire them.
Abbreviations
- 5HT:
-
Serotonin
- ACTH:
-
Adrenocorticotropin hormone
- ALLO:
-
Allopregnanolone
- AVP:
-
Arginine vasopressin
- BNST:
-
Bed nucleus of the stria terminalis
- BRS:
-
Brain reward system
- CMS:
-
Chronic mild stress
- CORT:
-
Corticosteroid
- CRH:
-
Corticotropin-releasing hormone
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopamine
- DHEA:
-
Dehydroepiandrosterone
- DOPAC:
-
3, 4-Dihydroxyphenlacetic acid
- E2:
-
Estradiol
- EPI:
-
Epinephrine
- FST:
-
Forced swim test
- GABA:
-
Gamma-Aminobutyric acid
- GnRH:
-
Gonadotropin releasing hormone
- GR:
-
Glucocorticoid receptor
- HPA:
-
Hypothalamic-pituitary-adrenal
- HPG:
-
Hypothalamic-pituitary-gonadal
- HVA:
-
Homovanillic acid
- ICSS:
-
Intracranial self-stimulation
- LH:
-
Luteinizing hormone
- MDD:
-
Major Depressive Disorder
- MFB:
-
Medial forebrain bundle
- MR:
-
Mineralocorticoid receptor
- NAcc:
-
Nucleus accumbens
- NE:
-
Norepinephrine
- OVX:
-
Ovariectomy
- PFC:
-
Prefrontal cortex
- POMC:
-
Proopiomelanocortin
- PROG:
-
Progesterone
- PVN:
-
Paraventricular nucleus of the hypothalamus
- SAM:
-
Sympathetic adrenal medullary system
- SSRI:
-
Selective serotonin reuptake inhibitors
- TH:
-
Tyrosine hydroxylase
- TS:
-
Testosterone
- VTA:
-
Ventral tegmental area
References
Ruedi-Bettschen D, Zhang W, Russig H, et al. Early deprivation leads to altered behavioural, autonomic and endocrine responses to environmental challenge in adult Fischer rats. Eur J Neurosci. 2006;24:2879–93.
Frodl T, Reinhold E, Koutsouleris N, et al. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44:799–807.
McEwen BS, Eiland L, Hunter RG, et al. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12.
Heim C, Owens MJ, Plotsky PM, et al. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull. 1997;33:185–92.
Pohl J, Olmstead MC, Wynne-Edwards KE, et al. Repeated exposure to stress across the childhood–adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121:462–74.
de Kloet ER. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–300.
Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions an introduction. Ann N Y Acad Sci. 2004;1024:1–8.
Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci. 2009;1179:41–55.
de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–76.
Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313.
Stern CM. Corticotropin-releasing factor in the hippocampus: eustress or distress? J Neurosci. 2011;31:1935–6.
Sgoifo A, DeBoer SF, Haller J, et al. Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: relationship with aggression. Physiol Behav. 1996;60:1403–7.
Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84.
Sapolsky RM. Stress, the aging brain, and the mechanisms of neuronal death. Cambridge, MA: The M.I.T. Press; 1992.
Taylor G, Bardgett M, Csernansky J, et al. Male rat reproductive systems under chronic fluoxetine or trimipramine treatment. Physiol Behav. 1996;59:479–85.
Taylor GT, Weiss J, Zimmermann F. Animal models of sex differences in nonreproductive brain function. In: Tatlisumak T, Fisher M, editors. Handbook of experimental neurology: methods and techniques in animal research. New York: Cambridge University Press; 2006. p. 239–56.
Conneely OM, Mulac-Jericevic B, DeMayo F, et al. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55.
Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32.
Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–70.
Brunton PJ, Russell JA. Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress. 2011;14:6–12.
Kessler RC. Gender differences in major depression: epidemiologlcal findings. In: Frank E, editor. Gender and its effects on psychopathology. Washington, DC: American Psychiatric Press; 2000. p. 61–84.
Taylor GT, Boggiano J, Cabrera O, et al. Steroidal influences on anxiety disorders in childhood and their animal models. Curr Top Steroid Res. 2011;8:47–64.
Gonda X, Telek T, Juhasz G, et al. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1782–8.
Bloch M, Daly RC, Rubinov DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–46.
Meltzer-Brody S. Understanding and treating mood disorders across the reproductive years. Sex Reprod Menopause. 2010;8:12–8.
Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109.
Chiba S, Numakawa T, Ninomiya M, et al. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:112–9.
Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32.
Post RM, Weiss SR, Li H, et al. Neural plasticity and emotional memory. Dev Psychopathol. 1998;10:829–55.
Wardenaar KJ, Vreeburg SA, van Veen T, et al. Dimensions of depression and anxiety and the hypothalamo-pituitary-adrenal axis. Biol Psychiatry. 2011;69:366–73.
Barden N. Implication of the hypothalamic – pituitary – adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–93.
Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501.
Romer B, Lewicka S, Kopf D, et al. Cortisol metabolism in depressed patients and healthy controls. Neuroendocrinology. 2009;90:301–6.
Wolkowitz OM, Burke H, Epel ES, et al. Mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19–40.
Hoschl C, Hajek T. Hippocampal damage mediated by corticosteroids–a neuropsychiatric research challenge. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 2:II81–8.
DeSouza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819.
Duncko R, Kiss A, Skultetyova I, et al. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89.
Muller MB, Keck ME. Genetically engineered mice for studies of stress-related clinical conditions. J Psychiatr Res. 2002;36:53–76.
Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol. 1991;38:537–58.
Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–6.
Krugers HJ, Lucassen PJ, Karst H, et al. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci. 2010;2:24.
Kubera M, Obuchowicz E, Goehler L, et al. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:744–59.
Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–65.
Sheline YI, Sanghav M, Mintun M, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43.
Olney JW. Excitotoxic amino acids and neuropsychiatric disorders. Annu Rev Pharmacol Toxicol. 1990;30:47–71.
Kaminska M, Harris J, Gijsbers K, et al. Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP: implications for depression. Brain Res Bull. 2000;52:229–34.
Naert G, Maurice T, Tapia-Arancibia L, et al. Neuroactive steroids modulate HPA axis activity and cerebral brain derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 2007;32:1062–78.
Maninger N, Wolkowitz OM, Reus VI, et al. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Endocrinol. 2009;30:65–91.
Herbert J. Neurosteroids, brain damage, and mental illness. Exp Gerontol. 1998;33:713–27.
Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–32.
Matsumoto AM, Bremner WJ. Serum testosterone assays–accuracy matters. J Clin Endocrinol Metab. 2004;89:520–4.
Becker JB, Monteggia LM, Perrot-Sinal TS, et al. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–5.
Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–76.
McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–33.
Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–27.
Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–8.
Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–8.
McCormick CM, Linkroum W, Sallinen BJ, et al. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47.
Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:766–76.
Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–33.
Yang S-J, Kim S-Y, Stewart RB, et al. Gender differences in 12-week antidepressant treatment outcomes for a naturalistic secondary care cohort: the CRESCEND study. Psychiatry Res. 2011;189:82–90.
Baca E, Garcia-Garcia M, Porras-Chavarinoc A. Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:57–65.
Kornstein SG, Sloan DME, Thase ME. Gender-specific differences in depression and treatment response. Psychopharmacol Bull. 2002;36 Suppl 3:99–112.
Osterlund MK. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta. 2010;1800:1136–44.
Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor α messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95:333–42.
Young EA, Midgley AR, Carlson NE, et al. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57:1157–62.
Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–9.
Holsen LM, Spaeth SB, Lee JH, et al. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord. 2011;131:379–87.
Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–8.
Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83.
Baischer W, Koinig G, Hartmann B, et al. Hypothalamic–pituitary–gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20:553–9.
Hardoy MC, Serra M, Carta MG, et al. Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol. 2006;26:379–84.
Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol. 2011;2:73. doi:10.3389/fendo.2011.00073.
Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30.
Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem Soc Trans. 2009;37:313–7.
Nestler EJ, Carlezon JWA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9.
Skinner BF. The behavior of organisms. New York: Appleton; 1938.
Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25:235–51.
Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–41.
Russell VA. Dopamine hypofunction possibly results from a defect in glutamate-stimulated release of dopamine in the nucleus accumbens shell of a rat model for attention deficit hyperactivity disorder–the spontaneously hypertensive rat. Neurosci Biobehav Rev. 2003;27:671–82.
Parolaro D, Realini N, Vigano D, et al. The endocannabinoid system and psychiatric disorders. Exp Neurol. 2010;224:3–14.
Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11.
DiChiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–33.
Pfaus JG, Damsma G, Wenkstern D, et al. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30.
Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm Behav. 2003;44:419–28.
Putnam SK, Sato S, Riolo JV, et al. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Horm Behav. 2005;47:513–22.
Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:435–51.
Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115.
Andersen ML, Sawyer EK, Howell LL. Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol. 2012;20:2–15.
Aron A, Fisher HE, Mashek D, et al. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–37.
Levita L, Hare TA, Voss HU, et al. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–87.
Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neurosci Biobehav Rev. 2004;27:777–86.
Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:303–10.
Salamone J, Cousins M, Snyder B. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–59.
Rademacher L, Krach S, Kohls G, et al. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–85.
Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–26.
Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–41.
Becker JB. Sexual differentiation of motivation: a novel mechanism? Horm Behav. 2009;55:646–54.
Volkow ND, Wang G-J, Fischman MW, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–30.
Everitt BJ. Sexual motivation: a neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses in male rats. Neurosci Biobehav Rev. 1990;14:217–32.
Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209.
Colasanti A, Searle GE, Long CJ, et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry. 2012;72:371–7.
Nakahara D, Nakamura M, Oki Y, et al. Lack of glucocorticoids attenuates the self-stimulation-induced increase in the in vivo synthesis rate of dopamine but not serotonin in the rat nucleus accumbens. Eur J Neurosci. 2000;12:1495–500.
Barrot M, Marinelli M, Abrous DN, et al. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–80.
Lindley SE, Tasha G, Bengoechea TG, et al. Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology. 1999;21:399–407.
Mizoguchi K, Ishige A, Takeda S, et al. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–9.
Chocyk A, Dudys D, Przyborowska A, et al. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience. 2011;173:1–18.
Cabib S, Puglisi-Allegra S, Damato F. Effects of postnatal stress on dopamine mesolimbic system responses to aversive experiences in adult life. Brain Res. 1993;604:232–9.
Cabib S, D’Amato FR, Puglisi-Allegra S, et al. Behavioral and mesocorticolimbic dopamine responses to non aggressive social interactions depend on previous social experiences and on the opponent’s sex. Behav Brain Res. 2000;112:13–22.
Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–28.
Cabib S, Puglisi-Allegra S. Different effects of repeated stress on mesocortical and mesolimbic dopamine metabolism. Neuroscience. 1996;73:375–80.
Dziedzicka-Wasylewska M, Willner P, Papp M. Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol. 1997;8:607–18.
Koob GF, Caine SB, Parsons L, et al. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–21.
Boutrel B. A neuropeptide-centric view of psychostimulant addiction. Br J Pharmacol. 2008;154:343–57.
Elman I, Lukas SE, Karlsgodt KH, et al. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–9.
Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–94.
Goodman A. Neurobiology of addition: an integrative review. Biochem Pharmacol. 2008;75:266–322.
Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–98.
Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–12.
Dellu F, Mayo W, Vallee M, et al. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly – a life-span study of rats. Psychoneuroendocrinology. 1996;21:441–53.
Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8.
Hooks MS, Jones GH, Smith AD, et al. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–8.
Piazza P, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74.
Dluzen DE, Salvaterra TJ. Sex differences in methamphetamine-evoked striatal dopamine output are abolished following gonadectomy: comparisons with potassium-evoked output and responses in prepubertal mice. Neuroendocrinology. 2005;82:78–86.
Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–8.
Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116–27.
Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86:354–67.
Yague JG, Wang AC, Janssen WG, et al. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–27.
Shieh KR, Yang SC. Effects of estradiol on the stimulation of dopamine turnover in mesolimbic and nigrostriatal systems by cocaine- and amphetamine-regulated transcript peptide in female rats. Neuroscience. 2008;154:1589–97.
McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307.
Russo SJ, Festa ED, Fabian SJ, et al. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–33.
Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine: implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:173–87.
Izumo N, Ishibashi Y, Ohba M, et al. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav Brain Res. 2012;227:1–6.
DiPaolo T, Rouillard C, Bedard P. 17B-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203.
Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–41.
Morris ME, Lee HJ, Predko LM. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol Rev. 2003;55:220–40.
Shansky RM, Glavis-Bloom C, Lerman D, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–8.
Dazzi L, Seu E, Cherchi G, et al. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2007;32:892–901.
Jacome LF, Gautreaux C, Inagaki T, et al. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–98.
Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-ß-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13:282–96.
Sanchez MG, Bourque M, Morissette M, et al. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e43–71.
Maggi M, Ciana P, Belcredito S, et al. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313.
Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22.
van der Staay FJ. Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Res Rev. 2006;52:131–59.
Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57.
Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–82.
Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–14.
Von Frijtag JC, Reijmers LG, Van der Harst JE, et al. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117:137–46.
Miczek KA, Nikulina EM, Takahashi A, et al. Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet. 2011;41:787–802.
Razzoli M, Carboni L, Arban R. Alterations of behavioral and endocrinological reactivity induced by 3 brief social defeats in rats: relevance to human psychopathology. Psychoneuroendocrinology. 2009;34:1405–16.
Taylor GT, Smith SE, Kirchhoff BA. Differential effects of antipsychotics on lateral bias and social attention in rats. Psychopharmacology. 2013;225:453–60.
Hill MN, Hellemans KG, Verma P, et al. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–117.
Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29.
Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43.
Dang H, Chen Y, Liu X, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1417–24.
Pan Y, Wang FM, Qiang LQ, et al. Icariin attenuates chronic mild stress-induced dysregulation of the LHPA stress circuit in rats. Psychoneuroendocrinology. 2010;35:272–83.
Grippo AJ, Francis J, Beltz TG, et al. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706.
Grippo AJ, Sullivan NR, Damjanoska KJ, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl). 2005;179:769–80.
Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev. 2009;33:1089–98.
Konkle ATM, Baker SL, Kentner AC, et al. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–38.
Dalla C, Antoniou K, Drossopoulou G, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–14.
Bachis A, Cruz MI, Nosheny RL, et al. Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neurosci Lett. 2008;442:104–8.
Garcia LS, Comim CM, Valvassori SS, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:450455.
Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013;354(1):155–69. doi:10.1007/s00441-013-1664-0.
Elizalde N, Garcia-Garcia AL, Totterdell S, et al. Sustained stress-induced changes in mice as a model for chronic depression. Psychopharmacology. 2010;210:393–406.
Muscat R, Papp M, Willner P. Reversal of stress – induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology. 1993;109:433–8.
Grippo AJ, Beltz TG, Weiss RM, et al. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–16.
Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–54.
Troisi A, Alcini S, Coviello M, et al. Adult attachment style and social anhedonia in healthy volunteers. Personal Individ Differ. 2010;48:640–3.
Bennett DS, Ambrosini PJ, Kudes D, et al. Gender differences in adolescent depression: do symptoms differ for boys and girls? J Affect Disord. 2005;89:35–44.
Bielajew C, Konkle AT, Kentner AC, et al. Strain and gender specific effects in the forced swim test: effects of previous stress exposure. Stress. 2003;6:269–80.
D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–7.
Benelli A, Filaferro M, Bertolini A, et al. Influence of Sadenosyl-L-methionine on chronic mild stress-induced anhedonia in castrated rats. Br J Pharmacol. 1999;127:645–54.
Pitychoutis PM, Dalla C, Sideris AC, et al. 5-HT1A, 5-HT2A, and 5-HT2C receptor mRNA modulation by antidepressant treatment in the chronic mild stress model of depression: sex differences exposed. Neuroscience. 2012;210:152–67.
Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, et al. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98:215–22.
Gronli J, Murison R, Fiske E, et al. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–7.
Herrera-Perez JJ, Martinez-Mota L, Chavira R, et al. Testosterone prevents but not reverses anhedonia in middle-aged males and lacks an effect on stress vulnerability in young adults. Horm Behav. 2012;61:623–30.
Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry. 2012;71:642–51.
Hellemans KG, Verma P, Yoon E, et al. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75.
Bloch M, Schmidt PJ, Danaceau MA, et al. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry. 1999;45:1533–41.
Harden M, Smith SE, Niehoff JA, et al. Anti-depressive effects of the κ-opioid receptor agonist salvinorin A in a rat model of anhedonia. Behav Pharmacol. 2012;23:710–5.
Recamier-Carballo S, Estrada-Camarena E, Reyes R, et al. Synergistic effect of estradiol and fluoxetine in young adult and middle-aged female rats in two models of experimental depression. Behav Brain Res. 2012;233:351–8.
Taylor GT, Farr S, Klinga K, et al. Chronic fluoxetine suppresses circulating estrogen and the enhanced spatial learning of estrogen-restored ovariectomized female rats. Psychoneuroendocrinology. 2004;29:1241–9.
Romano-Torres M, Fernandez-Guasti A. Estradiol valerate elicits antidepressant-like effects in middle-aged female rats under chronic mild stress. Behav Pharmacol. 2010;21:104–11.
Schiller CE, O’Hara MW, Rubinow DR, et al. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav. 2013;119:137–44.
Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56.
Galankin T, Shekunova E, Zvartau E. Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm Behav. 2010;58:827–34.
Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm Behav. 2010;58:22–32.
Auriacombe M, Reneric JP, LeMoal M. Animal models of anhedonia. Psychopharmacology. 1997;134:337–8.
Weiss JM, Kilts CD. Animal models of depression and schizophrenia. In: Schatzberg A, Nemeroff C, editors. Textbook of psychopharmacology. 2nd ed. Washington, DC: American Psychiatric Press; 1998. p. 89–131.
Morley JE, Kim MJ, Haren MT. Frailty and hormones. Rev Endocr Metab Disord. 2005;6:101–8.
Lam RW. Onset, time course and trajectories of improvement with antidepressants. Eur Neuropsychopharmacol. 2012;22 Suppl 3:S492–8.
Taylor GT, Weiss J, Rupich R. Male rat behavior, endocrinology and reproductive physiology in a mixed-sex, socially stressful colony. Physiol Behav. 1987;39:429–33.
Altemus M. Hormone-specific psychiatric disorders: do they exist? Arch Womens Ment Health. 2010;13:25–6.
Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126:73–85.
Acknowledgments
Preparation of this manuscript was supported in part by grants from the University of Missouri Research Board and the College Dean’s Faculty Research Awards at UM-St. Louis. The authors thank Joseph Boggiano, now at the Washington University School of Medicine in St. Louis, Missouri, for assistance in the conduct of the unpublished experiment cited in the manuscript. Also, we thank Dr. Juergen Weiss of the University of Heidelberg (Germany) for assistance in preparing the figure (Fig. 10.1) depicting the metabolic cascade in the brain.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Taylor, G.T., Cabrera, O., Hoffman, J. (2014). The Neuroendocrinology of Anhedonia. In: Ritsner, M. (eds) Anhedonia: A Comprehensive Handbook Volume I. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8591-4_10
Download citation
DOI: https://doi.org/10.1007/978-94-017-8591-4_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8590-7
Online ISBN: 978-94-017-8591-4
eBook Packages: MedicineMedicine (R0)