Abstract
Bioerosion, involving the weakening and breakdown of calcareous coral reef structures, is due to the chemical and mechanical activities of numerous and diverse biotic agents. These range in size from minute, primarily intra-skeletal organisms, the microborers (e.g., algae, fungi, bacteria) to larger and often externally-visible macroboring invertebrate (e.g., sponges, polychaete worms, sipunculans, molluscs, crustaceans, echinoids) and fish (e.g., parrotfishes, acanthurids, pufferfishes) species. Constructive coral reef growth and destructive bioerosive processes are often in close balance. Dead corals are generally subject to higher rates of bioerosion than living corals, therefore, bioerosion and reef degradation can result from disturbances that cause coral mortality, such as sedimentation, eutrophication, pollution, temperature extremes, predation, and coral diseases. The effects of intensive coral reef bioerosion, involving El Niño-Southern Oscillation, Acanthaster predation, watershed alterations, and over-fishing, are re-examined after ~20 years (early 1990s–2010). We review the evidence showing that the biologically-mediated dissolution of calcium carbonate structures by endolithic algae and clionaid sponges will be accelerated with ocean acidification. The CaCO3 budget dynamics of Caribbean and eastern tropical Pacific reefs is reviewed and provides sobering case studies on the current state of coral reefs and their future in a high-CO2 world.
The question at once arises, how is it that even the stoutest corals, resting with broad base upon the ground, and doubly secure from their spreading proportions, become so easily a prey to the action of the same sea which they met shortly before with such effectual resistance? The solution of this enigma is to be found in the mode of growth of the corals themselves. Living in communities, death begins first at the base or centre of the group, while the surface or tips still continue to grow, so that it resembles a dying centennial tree, rotten at the heart, but still apparently green and flourishing without, till the first heavy gale of wind snaps the hollow trunk, and betrays its decay. Again, innumerable boring animals establish themselves in the lifeless stem, piercing holes in all directions into its interior, like so many augurs, dissolving its solid connexion with the ground, and even penetrating far into the living portion of these compact communities.
L. Agassiz (1852)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Coral reefs are among the Earth’s most biologically diverse ecosystems, and many of the organisms contributing to the high species diversity of reefs normally weaken them and convert massive reef structures to rubble, sand and silt. The various activities of those reef species that cause coral and coralline algal erosion are collectively termed bioerosion , a name coined by Neumann (1966). A bioeroder is any organism that, through its assorted activities, erodes and weakens the calcareous skeletons of reef-building species. Although an extensive terminology has been adopted only during the past three decades, bioerosion has been recognized as an important process in reef development and maturation for more than a century (e.g., Darwin 1842; Agassiz 1852). Traces of biologically-induced erosion in ancient reef structures indicate that bioerosion has probably had some effect on reef carbonate budgets since Precambrian and Cambrian times (Vogel 1993).
Most bioeroder species are both small in size and secretive in living habits. Although the majority of bioeroders and other cryptic organisms are not visible on coral reefs, it has been suggested that their numbers and combined mass equal or exceed that of the surface biota (Grassle 1973; Ginsburg 1983). Recent research supports this hypothesis (Enochs 2012). Ginsburg has coined the term coelobite to refer to the profusion of organisms inhabiting cavities on reefs. For convenience, bioeroders that are usually present and visible on reef surfaces are termed external bioeroders and those living within calcareous skeletons are termed internal bioeroders (Fig. 4.1a). The feeding scars produced by an external bioeroding pufferfish ( Arothron ) can become permanently incorporated in the skeleton of a massive coral (Fig. 4.2a). A heavily infested coral by internal bioeroders, e.g. lithophagine bivalves, can severely damage and weaken the colony skeleton (Fig. 4.2b).
(a) X-ray photograph of Porites lobata slab cut parallel to the skeletal growth axis. Lunate pufferfish feeding scars, produced externally, are now permanently embedded in the skeleton (6–8 m depth, Clipperton Atoll). (b) Cross section of Porites panamensis extensively bored by lithophagine bivalves (5 m depth, Pearl Islands, Panama)
Several studies have shown that bioeroders are important in sculpting coral reef growth and in producing the sediments (rubble, sand, silt and clay) that characterize coral reef environments. Indeed, carbonate budget studies have demonstrated that constructive and destructive processes are closely balanced on many reefs with net reef accumulation barely ahead of net reef loss (Scoffin et al. 1980; Glynn 1988; Fig. 4.3). Bioerosion proceeds at high rates in certain zones which have high living coral cover and high rates of accretion (Kiene 1988). Sometimes, however, an imbalance develops with erosional processes gaining the upper hand. When environmental conditions decline abruptly, for example during a stressful thermal bleaching event, or over an extended period, such as years of increasing sedimentation or eutrophication, coral recruitment and growth decline or cease, limestone foundations are compromised and reef death ensues.
A generalized scheme illustrating the principal components of coral-reef construction and destruction. In order for reef growth to occur, rates of bioerosion and mechanical erosion must not exceed the rate of net reef accumulation. The relative contribution of inorganic and biological dissolution (= biodissolution) to total reef dissolution is presently unknown. Both components are likely to increase with ocean acidification
The aim of this chapter is to (a) illustrate the diversity of bioeroders on coral reefs, (b) identify the most destructive bioeroder groups, (c) describe the more prevalent modes of limestone destruction, and (d) highlight some case studies of intensified bioerosion on particular reef systems. In this updated review, with reference to the diversity of bioeroding taxa ‘(a)’, protistan foraminiferans are now included as agents of reef carbonate breakdown although it is not yet possible to assess their overall importance. Under case studies ‘(d)’, the effects of continuing, global-scale disturbances that impact coral communities and accelerate bioerosion, namely ENSO warming events and eutrophication, are re-examined in the light of recent findings. For example, the recovery of the Kān’eohe Bay Porites fringing reef, from 1993 to the present (2013), is examined. More recently attention has turned to ocean acidification and its effects on coral carbonate structures. This newly recognized factor, affecting calcification and cementation, can potentially exacerbate bioerosive processes and is also considered below.
In light of the many well documented studies of accelerating coral reef decline during the past decade, it is now all the more critical to understand the conditions that promote bioerosion , a pivotal process affecting the growth potential of coral reefs. For more technical information on this subject, the reader may consult the articles in Carriker et al. (1969) and Barnes (1983), and the reviews by Golubic et al. (1975, 2005), Warme (1975, 1977), Risk and MacGeachy (1978), Trudgill (1983), Macintyre (1984), Hutchings (1986, 2011), Perry and Hepburn (2008), Tribollet (2008), and Tribollet and Golubic (2011). An online bibliographic review of the bioerosion literature is provided by Wilson (2008).
4.2 Bioeroder Diversity
Bioeroders are abundant and diverse members of coral reef communities, belonging to four of the five kingdoms of life on earth, and to most animal phyla. Why have so many taxa become bioeroders? By far, the bioeroders hidden within coral skeletons, the cryptic biota, have the greatest taxonomic diversity . It is probable that intense competition and predation have led to the selection and evolution of cryptic life styles. Many of these secretive species are without toxins, armature, spines and thick shells, traits that are so common to their congeners living on reef surfaces and exposed to predators.
Depending upon their location on calcareous substrata, bioeroders can be classified as epiliths, chasmoliths and endoliths (Golubic et al. 1975). Epilithic species live on exposed surfaces, chasmoliths occupy cracks and holes, and endoliths are present within skeletons. Assignment to these categories is not always clear, however, for some bioeroders may belong to more than one microhabitat or change microhabitats during feeding, reproduction and development .
Bioeroders breakdown calcareous substrata in a variety of ways. The majority of epilithic bioeroders are herbivorous grazers that scrape and erode limestone rock while feeding on associated algae. In terms of eroding capabilities, grazers range from non-denuding and denuding herbivores that remove mainly algae and cause little or no damage to substrata to excavating species that remove relatively large amounts of algae, including calcareous algae, and the underlying limestone substrata (Steneck 1983a). Most endoliths are borers that erode limestone mechanically, chemically or by a combination of these processes. The important role of bioeroders can be appreciated when one realizes that coral reefs are predominantly sedimentary features made up of calcareous particles that are generated in large measure by the activities of bioeroders (Sects. 2.2 and 3.4).
Many species that bioerode calcareous skeletons are minute, requiring microscopical methods for study, and are referred to as microborers or endolithic microorganisms (Golubic et al. 1975; Macintyre 1984). To this group belong three kingdoms, namely bacteria and cyanobacteria (PROKARYOTAE), FUNGI, and eukaryotic microorganisms such as protozoans and algae (PROTOCTISTA). The macroborers are generally more conspicuous on coral reefs, and include numerous invertebrate and vertebrate taxa in the kingdom ANIMALIA. Most endolithic invertebrates are suspension feeder s, gathering their food passively or actively from the water column.
Endolithic microborers, possibly Cyanobacteria , are among the first recognizable bioeroders in the fossil record, having left minute borings in late Precambrian ooids of Upper Riphean/Vendian age, 570–700 Myr (Campbell 1982). While endolithic borers increased steadily during the Paleozoic era, from five to nine classes, they comprised only a small part of hard-ground communities and penetrated structures superficially, i.e., to maximum depths of 2–3 cm (Vermeij 1987). A notable increase in endolithic taxa occurred during the Mesozoic era with the appearance of deep borers, such as pelecypods, gastropods and lithotryid barnacles, capable of penetrating substrates to depths of 15 cm. Excavating bioeroders, comprising mobile epifaunal invertebrates and herbivorous fishes, made their first appearance during the Late Mesozoic and Early Cenozoic (70–60 Myr) and have persisted until today. These animals – chitons, limpets and other gastropods, sea urchins and parrotfishes – are dominantly herbivores whose feeding activities incidentally produce large quantities of sediment . Herbivory and bioerosion by these groups are probably more intense now than at any time in the past (Steneck 1983b). Vermeij (1987) has argued that this Mesozoic increase in the size and extent of excavation among vagile bioeroders can be interpreted as an evolutionary response to escalating predation and competition on open rock surfaces.
4.2.1 Bacteria
Although our knowledge of the bioeroding potential of bacteria and the various taxa involved is very limited, preliminary observations suggest that these organisms may be important under certain conditions. A pilot study in Hawai’i indicated that brownish areas inside the skeletons of massive corals contained from 104 to 105 bacteria per gram dry weight (DiSalvo 1969). Boring sponges also were closely associated with bacteria, which could possibly have assisted the sponges’ penetration into the coral. Different workers have shown that bacteria can etch the surface of limestone crystals and dissolve the organic matrix of coral skeletons, causing internal bioerosion (DiSalvo 1969; Risk and MacGeachy 1978).
Several species of Cyanobacteria , formerly known as blue-green algae, are capable of eroding reef rock from the splash zone to depths of at least 75 m. Species of Hyella, Plectonema, Mastigocoleus, and Entophysalis, for example, have been found on limestone surfaces, inside cavities, and penetrating reef rock (Fig. 4.4a, b). A close relative of Hyella has been found in Precambrian algal reefs that existed 1.7 billion years ago (Vogel 1993). The boring is a dissolution process accomplished by the terminal cells of specialized filaments. Cyanobacteria have been implicated in the erosion of lagoon floor sediments on the Great Barrier Reef, amounting to the dissolution of between 18 and 30 % of the sediment influx rate (Tudhope and Risk 1985) (Table 4.1). (It should be stressed that most of the rates of erosion listed in Tables 4.1 and 4.2 were obtained with different methods and therefore should be compared with due caution. See Kiene [1988] for an assessment of the strengths of the methods and some problems with the intercomparisons.)
Photomicrographs of endolithic microborers in limestone substrates. Cyanobacteria : (a) Plectonema terebrans Bornet and Flahault, scanning electron micrograph (SEM) of plastic casts of filaments in an acid-etched shell; (b) P. terebrans, transmitted light micrograph (TLM) of filaments isolated by dissolution. Fungi: (c) SEM of plastic casts of fine fungal hyphae intertwined with the larger filaments of P. terebrans; (d) SEM of fungal borings covering and possibly feeding (arrows) on the underlying cyanobacterium. Chlorophyta: (e) Ostreobium brabantium Weber Van-Bosse, SEM of plastic cast of large radiating growth form in an acid-etched shell fragment; (f) O. brabantium, TLM of filaments isolated by dissolution. Scale bars: a = 50 μm, b = 40 μm, c = 5 μm, d = 25 μm, e = 200 μm, f = 100 μm (From May et al. 1982)
4.2.2 Fungi
Boring fungi have been found in modern corals in the Caribbean, French Polynesia and on the Great Barrier Reef (Australia). Twelve genera belonging to the Deuteromycota or Fungi Imperfecti have been isolated from a variety of scleractinian corals and a hydrocoral (Kendrick et al. 1982). Fungi are capable of deep penetration into coral skeletons by chemical dissolution. The hyphae produce narrow borings and penetrate the deepest recesses of coral skeletons, probably because of their ability to utilize the organic matrix of coral skeletons (Fig. 4.4c, d). Endolithic fungi growth can cause unique skeletal protuberances in living corals due to the localized deposition of dense skeletal material, perhaps as defensive barrier (Le Campion-Alsumard et al. 1995). Fungi have also been implicated in the etching of calcareous surfaces, the weakening and dissolution of calcareous sediments as well as the calcareous tube linings of various endoliths. Because of the difficulty of distinguishing between fungal and algal borings, estimates of dissolution rates due to boring fungi alone are not yet available.
4.2.3 Algae
Green (Chlorophyta) and red (Rhodophyta) algae have been implicated in the erosion of coral rock under various reef settings. Green and red algae occur on limestone surfaces, in cavities and within coral skeletons (Fig. 4.4e, f). Freshly fractured corals often reveal layers of green banding a few cm beneath the live coral surface. The green color is due to the presence of chlorophyll pigments, which intercept light passing through the coral’s tissues and skeleton. This greenish layer is often referred to as the “ Ostreobium band”, named after a green alga that is commonly present in coral skeletons. However, the green band may also contain a variety of different kinds of algae, e.g., species of Codiolum, Entocladia, Eugomontia, and Phaeophila. The importance of boring algae as bioeroders is controversial; some workers claim that they are among the most destructive agents of reef erosion whereas others maintain that they cause only minimal damage (Sect. 1.1). Nonetheless, mixtures of internal bioeroder taxa – including green and red algae, bacteria, cyanobacteria, and fungi – can produce similar high-end erosion rates , ranging from 330 g CaCO3 m−2 year-1 on a Caribbean coral reef at Bonaire to 470 g CaCO3 m−2 year-1 on the Great Barrier Reef (Table 4.1).
4.2.4 Foraminifera
Some 20 species of bioeroding foraminiferans, belonging to 11 families, have been reported mainly from turbulent, tropical waters (Vénec-Peyré 1996). The majority of these mostly endolithic species occur in coral reef environments and have been found to excavate a variety of substrates, e.g. coralline algae, foraminifers, corals, bryozoans, molluscs, crustacean carapaces, wood and rocks. Only a single species from the Red Sea, Cymbaloporella tabellaeformis (Brady), has been reported to excavate coral skeletons. Most workers hypothesize that foraminifers penetrate hard substrates by chemical dissolution. Only a few quantitative studies on the abundances of bioeroding foraminifers are available. One such survey estimated population densities of between 150,000 and 250,000 individuals/m2 in bioclasts present in sedimentary biotopes on a coral reef at Moorea, French Polynesia. No information is presently available on the rates of bioerosion by foraminiferans. In addition to the erosion caused directly by these protists, it is likely that the minute depressions excavated on substrates may also facilitate the recruitment of other bioeroding taxa. Clearly, much remains to be learned about the destructive capacity of these organisms.
4.2.5 Sponges
The most important genera of siliceous sponges known to bore into calcareous substrata are Cliona, Anthosigmella and Spheciospongia, order Hadromerida, and Siphonodictyon, order Haplosclerida (Wilkinson 1983). Clionaid sponges (Family Clionaidae) are among the most common and destructive endolithic borers on coral reefs worldwide. Zea and Weil (2003) have revealed that a formerly regarded single species of Cliona in the Caribbean consists of at least three distinct excavating sponge species. Upon splitting open infested corals, clionaid sponges are revealed as brown, yellow or orange patches lining the corroded interiors of the coral skeleton (Fig. 4.5a, d). Most boring sponges form 5–15 mm diameter chambers with smaller galleries branching off the main chambers. Their depth of penetration into the coral skeleton is usually no greater than about 2 cm. Some sponges (Siphonodictyon), however, can form chambers up to 100 mm in diameter that penetrate to 12 cm into coral colonies. Subsurface excavation by clionaid sponges removes the skeletal support of coral calyces, thus causing the collapse and death of polyps. In highly infested colonies, some boring sponges emerge from the skeleton, grow over and even kill live coral tissues on reef surfaces. On western Atlantic reefs, Cliona sp. is sometimes very abundant, forming dark brown patches several meters in extent that kill or overgrow dead surfaces and erode all calcifying organisms (Fig. 4.6). In a comparative study in Bonaire, of leeward and seaward reefs with high and low coral cover respectively, Perry et al. (2012) reported significantly higher clionaid bioerosion rates on the former than the latter (Table 4.1).
Boring sponges in limestone substrates. (a) Two oscula of Pione (formerly Cliona) lampa (Laubenfels) visible on the surface of a massive coral (Diploria). (b) Vertical section through peripheral region of Spheciospongia othella Laubenfels revealing abundant spicules. (c) Chambers of Cliona dioryssa (Laubenfels) in porous coral rock. (d) A large tunnel running below the surface of coral rock excavated by S. othella. (e) Upper scalloped and (f) lower convex surfaces of isolated limestone chips discharged through the osculum of P. lampa. (g) Group of chips etched from substratum by P. lampa but still in place. Magnification: a, c, d ×3; b ×140; e, f ×1,500; g ×600 (a–d from Rützler 1974; e, f, g from Rützler and Rieger 1973)
Sponge boring is accomplished by amoebocytes that etch and chip minute calcareous fragments from limestone substrata (Rützler and Rieger 1973; Pomponi 1979). The ends of etching amoebocytes flatten against the calcareous substratum and extend fine pseudopodial (filopodia) sheets into the limestone at the cell’s periphery. The filopodia coalesce centrally, cutting out a hemispherical carbonate chip (Fig. 4.5e–g). This cutting is accomplished by enzymes that simultaneously dissolve calcium carbonate and the organic matter matrix of skeletons. At the end of this process, both the chip and the etching cell are transported away from the site of erosion and are expelled from the sponge. Based on careful microscopic examination, Rützler and Rieger (1973) estimated that only about 2–3 % of coral skeletons are dissolved with the remainder dispersed as silt-sized chips. These oval-shaped, faceted chips are easily recognized in sediments and can contribute up to 30–40 % numerically to the fine silt fraction of sediments on Pacific and Caribbean reefs. However, a recent study that simultaneously measured chemical and mechanical (chip production) bioerosion found that the rate of chemical dissolution was three times greater than the amount of CaCO3 eroded via chip production (Zundelevich et al. 2007).
4.2.6 Polychaete Worms
Polychaete worms that bore into reef rock are enormously abundant in certain environments, prompting some workers to conclude that they are among the most important endolithic borers on coral reefs (Davies and Hutchings 1983). Various species in the following families typically form circular holes 0.5–2 mm in diameter that penetrate up to 10 cm into the interiors of coral skeletons: Cirratulidae, Eunicidae, Sabellidae and Spionidae. Eunicid holes often form a sinuous and anastomosing network (Fig. 4.1). The mechanism of boring has been reported for a few polychaete species. Some eunicids employ their mandibles to excavate. Spionids bore mainly by chemical dissolution with some removal probably due to mechanical abrasion by chaetae (Haigler 1969). Cirratulid and eunicid species are predominantly deposit-feeders whereas sabellids and spionids are mainly filter-feeders. The close physical association of eunicids and spionids with endolithic algae also has suggested the utilization of boring algae as a food source (Risk and MacGeachy 1978).
A quantitative study of boring polychaetes conducted at Lizard Island, Great Barrier Reef provides numerical abundances and bioerosion rates of a pioneer polychaete community. At various times during the study it was not uncommon to find between 27,000 and 80,000 boring polychaetes per m2 in experimental coral blocks set out in three different reef environments (Davies and Hutchings 1983). These worms caused erosional losses of from 0.7 kg m−2 year-1 on the reef front to 1.8 kg m−2 year-1 on a leeward patch reef (Table 4.1).
4.2.7 Crustacea
Barnacles, shrimp, hermit crabs and other kinds of crustaceans can erode reef rock (Warme 1975). Barnacles and shrimp are endolithic borers, producing cylindrical chambers whereas hermit crabs are external bioeroders that abrade live coral surfaces.
Three groups of barnacles contain species that reside in the skeletons of dead corals, namely thoracicans, acrothoracicans and ascothoracicans. Members of the latter two taxa occupy small, mm-sized cavities that keep pace with the host coral’s growth, i.e., they become embedded within the coral skeleton without causing extensive erosion. Species of Lithotrya, members of the thoracican barnacle taxon, erode 2–10 cm long oval-shaped cavities on the undersides of reef rock and beach rock in shallow, agitated waters (Fig. 4.1). The barnacle’s basal plate is attached at the inner-most end of the cavity and the body hangs downward toward the opening with cirri exposed to food-bearing currents. The cavities are formed apparently by mechanical abrasion effected by calcified plates that cover the barnacle’s body. Unlike other invertebrate endoliths, such as polychaete worms and gastropods, adjacent tubes of boring Lithotrya are commonly interconnected, and heavily infested limestones are thoroughly honeycombed and subject to frequent breakage. An average of one boring per cm2 was observed on beach rock in Puerto Rico, and up to 30 % of the substratum had been removed from some of the samples examined (Ahr and Stanton 1973). Overall, however, results from studies in the Caribbean and Indian Ocean indicate that boring barnacles cause relatively little erosion compared with other internal borers (Table 4.1).
Alpheus simus Guerin-Meneville, a pistol shrimp, bores into coral rock on Caribbean reefs and causes considerable erosion on some Costa Rican reefs (Cortés 1985). Male/female pairs excavate 10–15 mm diameter chambers that penetrate as deep as 15 cm into dead coral rock. Microscopical study of the chamber walls suggests that this shrimp bores mainly by chemical means. Seven pairs of shrimp were found in one 1,500 cm2 block, and each pair occupied an average chamber volume of 20 cm3. This is equivalent to the removal of about 950 cm3 of calcium carbonate m−2 . The life span of the shrimp is about 2 years, but since succeeding generations of shrimp probably occupy the same chambers it is not possible to calculate annual erosion rates .
Two species of hermit crabs that feed on live coral produce large amounts of calcareous sediment when they scrape corals to remove soft tissues (Fig. 4.1). The average mass of coral abraded by a small hermit crab [Trizopagurus magnificus (Bouvier)] was about 10 mg ind−1 day−1, and for a large hermit crab (Aniculus elegans Stimpson) about 1 g ind−1 day−1 (Glynn et al. 1972). Relating hermit crab population densities and erosion rates , it was found that Trizopagurus and Aniculus respectively were responsible for the generation of about 1 and 0.1 metric tons of coral sediment ha−1 year−1 on a fringing reef in Panamá (Table 4.2). Since this rate of coral abrasion by hermit crabs has not been reported elsewhere, it is possible that these high levels of erosion are unique to the eastern Pacific.
4.2.8 Sipuncula
Although it is well known that species in several genera of sipunculans (peanut worms) penetrate coral skeletons, there is no general agreement on the overall importance of this group in the bioerosion of coral reefs. Perhaps this is due to their great variation in abundance from reef to reef and across reef zones (Macintyre 1984).
Sipunculan borings are cylindrical and pencil-sized or slightly smaller, ranging from straight to sinuous and from near-surface to several cm deep in coral skeletons, depending on the species (Fig. 4.1). Sipunculans are abundant on some reefs: nearly 800 inds m−2 were present in reef crest substrata, and 1,200 inds m−2 in Porites coral skeletons in Belize (Rice and Macintyre 1982). Even at 30 m depth, 40 inds m−2 were found. While feeding, sipunculans extended their introverts outside of their cavities and appear to ingest debris, sand and algae. The exact manner of boring is not known, but may involve both chemical dissolution and mechanical abrasion (Rice and Macintyre 1972). An estimated sipunculan erosion rate on a Barbados reef indicated only minor carbonate loss (Table 4.1).
4.2.9 Mollusca
Most bioeroding molluscs are external grazers that abrade reef rock while feeding on algae and associated organisms residing on and within limestone substrata. The eroding capacity of surface enmeshed and endolithic algae, important components of the diet of grazing molluscs, also weakens the substratum and thus facilitates erosion during feeding. A group of mussel-like endolithic borers also is prominent on many reefs worldwide.
Molluscan bioeroders are generally most abundant in the intertidal zone with some species extending their ranges into supratidal and subtidal habitats (Fig. 4.7a). Species abundances also change horizontally with chitons often most plentiful in areas protected from strong wave assault and limpets, certain snails, and echinoids more common in wave swept habitats (Fig. 4.7b). Under quiet to rough water conditions, grazing molluscs are largely responsible for producing the notches and nicks on tropical limestone shores. Most early workers surmised that intertidal notches were formed through strictly physico-chemical processes (e.g., the localized lowering of pH and accompanying carbonate dissolution), which resulted in the erosion of the underlying rock. Under extremely rough conditions, many bioeroders either disappear or their activities are greatly reduced. Calcifying taxa, such as coralline algae and vermetid molluscs, increase in abundance with increasing exposure, probably because of ecologic requirements for high energy habitats and a lower abundance of fish consumers in rough water areas (Fig. 4.7c). Vermetid/coralline algal buildups help protect the underlying limestone, thus limiting bioerosion and the development of intertidal notches and nicks in such areas (Focke 1978).
Vertical (a) and horizontal (b) distributions of bioeroding molluscs and other bioeroder taxa on a limestone shore at Palau, Caroline Islands. Theoretical relationship (c) of coastal profile morphology to water turbulence at Curaçao, Netherlands Antilles. An arrow locates a “transition zone” between the “spray” and “surf zones” (a and b after Lowenstam 1974; c after Focke 1978)
Several species of chitons (Class Polyplacophora), e.g., members of Acanthopleura and Chiton, erode chiefly intertidal limestone substrata while grazing on algae. The grazing is achieved with a magnetite (Fe3O4) or other mineral-enriched radula, a tooth-bearing strap of chitinous material, that effectively abrades the substratum (Lowenstam and Weiner 1989). Some erosion also occurs at homing sites, rock depressions that are occupied by chitons when not foraging. As many as 50–100 sausage-shaped, 1–3 mm long fecal pellets are voided daily by individual chitons (Rasmussen and Frankenberg 1990). Erosion rates vary greatly among sites as they are influenced by local differences in rock type and condition, and ecological factors affecting chiton abundances and feeding activities (Table 4.2).
Limpets and snails (Class Gastropoda) often occur with chitons on intertidal carbonate substrata. Acmaea, Cellana and Patelloida are common limpet genera, and Cittarium, Littorina, Nerita and Nodilittorina are some common snail genera. Like chitons, limpets and snails utilize a radula to scrape rock surfaces. The radula of patellacean limpets is an especially effective excavating organ with opal (SiO2.nH2O) or goethite (HFeO2)-sheathed radular teeth (Lowenstam and Weiner 1989). The radula of snails contains proteinaceous teeth, but these grazers are still capable of erosion because of the often weakened condition of the rock substratum upon which they feed (Table 4.2). Some gastropods (muricaceans, naticids) and cephalopods (notably Octopus spp.) employ the radula as a drilling tool, producing circular holes in thick shells to help expose the soft tissues of their gastropod and bivalve prey (Ekdale et al. 1984). The contribution of the fine grains thus produced to reef sedimentation rates has not been reported, but drilled mollusc shells are commonly observed in reef sediments.
Species of Lithophaga and Gastrochaena (Class Pelecypoda) bore into dead and live corals, and are most abundant subtidally, with some of these bivalves attacking reef corals to their lower depth limits. Fungiacava spp. penetrate live mushroom corals, but their activities are relatively minor. The siphonal openings of Lithophaga typically have a keyhole-like appearance on coral surfaces and the circular holes penetrate vertically into the skeleton, from 1 to 10 cm deep depending upon the species (Figs. 4.1 and 4.2b). The lithophagines are deposit and suspension feeder s, often most abundant in areas of high productivity . The mantle glands of Lithophaga secrete acid that dissolves and weakens the limestone substratum. The vertical and rotational movements of the shell also assist in boring, resulting in the production of silt/sand-sized sediment . Population densities in productive equatorial eastern Pacific waters range from 500 to 10,000 inds m−2 (Scott et al. 1988), which can lead to rapid reef erosion (Table 4.1).
4.2.10 Echinoidea
Sea urchins (Echinoidea) are the only echinoderms capable of significant bioerosion . Several species in the following genera abrade large amounts of reef rock while feeding and excavating burrows: Diadema, Echinometra, Echinostrephus, and Eucidaris. Sea urchins possess a highly evolved jaw apparatus (Aristotle’s lantern), a flexible and protrusible mastigatory organ consisting of five radially arranged, calcified teeth. The teeth are mineralized, and must be harder than the corroded surfaces they scrape. Sea urchin spines also assist in bioerosion when they are employed in the enlargement of burrows. Sea urchins graze on algae growing on dead coral substrata, but in some areas also attack live coral. On seaward reef platforms where water flow is vigorous, sea urchins usually remain in their burrows and feed predominantly on drift algae. In the Bonaire study by Perry et al. (2012), high echinoid bioerosion occurred on leeward reefs with high coral cover, but none was reported on windward reefs, similar to the habitat differences noted for clionaid sponges (Table 4.2). Sea urchins can cause substantial erosion at low and moderate population densities; at high densities, their destruction of reef substrata rivals clionaid sponge erosion and can lead to rapid framework loss.
4.2.11 Fishes
Numerous fish species erode reef substrata while grazing on algae, and also fragment colonies while feeding on live coral tissues or when extracting invertebrates from coral colonies (Randall 1974). Surgeonfishes (Acanthuridae) and parrotfishes (Scaridae) are the principal grazing groups with some fishes in the latter family capable of scraping and extensive excavation. On western Pacific reefs, excavating parrotfishes primarily bite convex surfaces, thus reducing the topographic complexity of reefs (Bellwood and Choat 1990). Some Atlantic and Pacific parrotfishes occasionally scrape and ingest live coral tissues (Bellwood and Choat 1990; Glynn 1990a). Triggerfishes (Balistidae), filefishes (Monacanthidae) and puffers (Tetraodontidae, Canthigasteridae) are largely carnivorous in feeding habits and are responsible for fragmenting or grazing on live coral colonies (Fig. 4.2a). The jaw muscles and tooth armature are well developed in all of these families. Parrotfishes also have a pharyngeal mill, a gizzard-like organ that further reduces the size of ingested sediment . Fish teeth are composed of dahllite [Ca5(PO4CO3)3(OH)] or francolite (the fluorinated form), both apatite minerals that are harder than CaCO3 (Lowenstam and Weiner 1989).
Parrotfish grazers can produce large amounts of sediment on reefs, especially when their population densities are high. For example, Scarus iserti generated nearly 0.5 kg CaCO3 m− 2 year−1 on a Caribbean reef in Panamá with a high abundance of just under one fish per m2. Entire grazing fish communities, comprised dominantly of parrotfishes , typically erode large amounts of reef substrata. One of the highest erosion rates reported for fishes, 9.1 kg CaCO3 m−2 year−1, occurred in the lagoon of an Australian reef (Table 4.2). It should be recognized, however, that relatively few scarid species in any given fish community are capable of excavating significant amounts of carbonate substrata. For example, Bellwood and Wainwright (2002) noted that only one of 18 scarid species at a Lizard Island site (Great Barrier Reef) effected high rates of erosion. Additionally, at a Red Sea site, three of ten scarids contributed importantly to bioerosion , and at a Caribbean site (Carrie Bow Cay, Belize) only one of six scarids excavated reef substrata. In this regional comparison, the highest rate of bioerosion was effected by the Australian scarid Chlorurus microrhinos, which excavated 6,500 g m−2 year−1 (Table 4.2). Parrotfishes can exhibit interesting spatial differences vis-à-vis grazing activity and consequent erosion (Table 4.2). On the Great Barrier Reef, highest erosion was observed on offshore reefs in oligotrophic waters compared to inshore reefs in eutrophic environments (Tribollet and Golubic 2005). On reefs at Bonaire, parrotfish erosion rates were generally highest on leeward reefs with high coral cover compared to seaward reefs of low coral cover (Perry et al. 2012).
While carnivorous fishes can cause substantial damage locally, their reef-wide effects seem to be relatively minor. For example, a pufferfish ( Arothron ) that erodes about 20 g of coral per day results in a total reef loss of only 30 g CaCO3 m−2 year−1 (Glynn et al. 1972) because of a relatively low population size of 40 individuals per hectare (Table 4.2).
Several other bioeroders known to produce traces or otherwise damage reef rock, e.g. foraminifers, zoanthids, bryozoans and brachiopods (Warme 1975), may contribute to reef degradation under special conditions. To assess the relative importance of the various bioeroders considered in this survey, one may compare their rates of reef destruction with known carbonate production rates. Net carbonate production rates vary greatly among reefs and between reef zones, but 3,000–5,000 g CaCO3 m−2 year−1 have been reported for many of the world’s coral reefs (Kinsey 1983). Among the internal borers, clionaid sponges and lithophagine bivalves can cause a comparable level of bioerosion , and of the external grazers sea urchins are equally destructive. Reef frameworks are generally reduced to silt and fine sand by internal borers and to fine and coarse sand by external grazers. The combined effects of other bioeroders may also contribute importantly to reef erosion in particular areas or zones and at different times.
4.3 Conditions Favoring Bioerosion
Bioerosion increases under a variety of circumstances that can be classified according to (a) conditions causing coral tissue death and (b) conditions that provide a growth advantage to bioeroder compared with calcifying species’ populations. Some of the more important situations that can alter the course of bioerosion are noted here in general terms. Specific examples are considered below in the examination of case studies (Sect. 4.5).
Aside from a few species that invade coral rock directly through living tissues (e.g., some boring sponges, bivalves and barnacles), the great majority of endolithic borers attack dead skeletons (Fig. 4.8). In general, any condition that causes coral tissue death will increase the probability of invasion by borers and grazers. Thus, any natural or anthropogenic disturbances that lead to the loss of live coral tissues will ultimately increase the chances of bioeroder invasion and higher rates of limestone loss. Many disturbances leading to tissue loss are obvious, including storm -generated surge that dislodges and topples corals, sediment scour and burial, tidal exposures, sudden temperature changes, fresh-water dilution, sewage and eutrophication, predation, and disease outbreaks (Endean 1976; Pearson 1981; Grigg and Dollar 1990).
Graphic model showing the probability of excavation of endolithic bioeroders as a function of distance from a coral’s surface (Redrawn from Highsmith 1981a). Curves are illustrated for corals with dead and live surfaces
While violent tropical storms are natural events that are known to seriously affect coral reefs, storm damage certainly must be exacerbated on reefs that have been heavily bioeroded beforehand. Sudden chilling episodes are also natural disturbances that can have devastating effects on tidally exposed or shallow coral assemblages, especially on high latitude reefs. Numerous incidences of coral bleaching (loss of zooxanthellae and/or pigmentation) and mortality were observed world-wide in the 1980s and 1990s, and many of these events occurred during periods of elevated sea temperatures coincident with El Niño-Southern Oscillation activity. Corals that were damaged or killed during these bleaching events have been subject to further damage by bioerosion . Rützler (2002) noted examples of accelerated boring sponge erosion on bleached Caribbean corals stressed by temperature extremes and other suboptimal conditions in recent years. In some parts of the eastern Pacific where coral mortality was high and community recovery slow, extensive damage by both internal and external bioeroders has been observed.
Increases in nutrient loading often cause coral tissue mortality , lowered reproductive success and lower rates of coral settlement and recruitment . Besides such direct negative effects on reef-building corals, nutrient input s can also cause changes in the community structure of epilithic algae . On a La Saline Reef (Reunion Island), increased nutrification has been found to favor the replacement of algal turfs by encrusting calcareous algae and macroalgae (Chazottes et al. 2002). On the one hand, this qualitative change in algal cover can result in reduced bioerosion by external bioeroders and macroborers, but on the other hand it can elevate rates of bioerosion by microendolitic borers. External bioeroders (sea urchins and fishes) may feed less on calcareous algae and macroalgae than on turfs. This reduced grazing in turn allows the proliferation of endolithic borers, whose growth would otherwise be limited under intense grazing pressure. It is cautioned that this sequence of events is not invariant due to other factors that often accompany elevated nutrient conditions (see below).
Predator outbreaks leading to high coral mortality , such as by seastar (Acanthaster) and snail (Drupella) corallivores reported from various areas of the Indo-Pacific, can set the stage for rapid bioerosion . Territorial damselfish that colonize dead reef surfaces can cause complex responses that both increase and decrease bioerosion. Damselfish that invade dead coral patches typically kill nearby corals while enlarging their territories. Studies in Australia have shown that the algal turf communities maintained by damselfish favor the proliferation of internal bioeroders (Risk and Sammarco 1982). However, the territorial defensive behavior of damselfish also limits the bioerosive activities of external grazers such as parrotfishes and sea urchins (Glynn and Wellington 1983; Eakin 1993).
Coral tissue loss due to a variety of diseases can be substantial (Chap. 8; Peters 1984). For example, “black line disease ” or “black band disease”, the result of a cyanobacterial infection (Rützler et al. 1983), may consume one-half of the living tissues of a coral during a single warm season infestation. All live tissues may be sloughed from corals by “white band disease”, “shut-down-reaction” or “stress-related-necrosis”. Though the causative agents of such diseases often remain elusive, their occurrence seems to be influenced by elevated sea water temperature , increased sedimentation and turbidity.
Since the majority of endolith bioeroders are suspension or filter feeders in contrast to calcifying species, which are dominantly autotrophic, generally increases in nutrients, organic matter and plankton biomass tend to favor increases in bioeroder compared with calcifier populations (Fig. 4.9). Because land runoff usually augments siltation and nutrient loading simultaneously (and sometimes pollutant levels), it is often difficult to distinguish between these effects. Unlike La Saline Reef, Pari et al. (1998) found that a polluted reef in Tahiti (at Faaa) is subject to intense grazing by sea urchins . But this South Pacific site is influenced by elevated nutrients, and additionally by terrigenous sediments and chemical pollutants. Moreover, the Pacific reef also exhibits different algal assemblages. Thus, even though both reefs are subject to high nutrient regimes, it is not possible to predict changes in the rates of bioerosion because of potentially numerous confounding influences.
Relationship between the percentage of massive corals infested with boring bivalves and levels of phytoplankton productivity at several geographic locations (Redrawn from Highsmith 1980). Selected areas with values close to the plotted means are indicated. Each mean consists of various sampling areas and colony numbers, respectively, as follows: Tuamotu Islands—6, 212; Gilbert Islands—2, 58; Seychelle Islands—2, 12; Australia—7, 135; Barbados—7, 55; Bahama Islands—2, 64; Panama—4, 70; Singapore—5, 144
There are at least two ways in which bioerosion is self-reinforcing. The first of these is the weakening effect of bioeroders on reef structures and the skeletons of calcifying organisms. For example, as bioerosion increases the volume of internal spaces (porosity) of coral skeletons, less mechanical force is required for breakage, toppling and overturning (Fig. 4.10). Thus, heavily bioeroded reefs are more susceptible to damage by strong surge and projectiles accompanying violent storms . The second kind of positive feedback results from increasing levels of sediment production by bioeroders and its deleterious effects on calcifying populations.
Plot of coral strength to breaking versus amount of bioerosion by Lithophaga (Redrawn from Scott and Risk 1988). The compression and bending tests are two measures of a coral’s strength. N = newton, a unit of force, MN 0.22481 × 106 lbf. Porosity indicates the percent of the skeleton removed
Overfishing can also promote increased bioerosion on reefs. If natural fish predators of some bioeroder populations are eliminated, e.g. triggerfishes that prey on sea urchins , then it is possible for grazing sea urchin populations to increase in size with a devastating effect on reef limestones. Overfishing of parrotfishes causes declines in bioerosion, given their role as important bioeroding agents on reefs. However, the long-term effect of parrotfish exploitation is a depression in the overall carbonate budget because the absence of parrotfishes leads to a decline in coral cover and carbonate production from increases in algal abundance due to reduced grazing (Kennedy et al. 2013). Angelfish predation exerts a strong control on the abundances of clionaid sponges (Hill and Hill 2002), thus overfishing of these species might lead to increases in boring sponge population size.
Climate change, owing to anthropogenic CO2 emissions since the industrial revolution, is a leading threat to the survival of coral reef ecosystems over the twenty-first Century (Hoegh-Guldberg et al. 2007). Elevated and increasing CO2 concentration in the Earth’s atmosphere is associated with global warming and an increase in extreme weather events and patterns (IPCC 2007). Warm water bleaching events have increased in severity and spatial scale, particularly over the past 30 years (Baker et al. 2008). Bleaching events reduce CaCO3 production by corals, but also can be followed by increases in bioeroder abundances (Glynn 1988). After ENSO -related bleaching and mortality in the eastern tropical Pacific (ETP), echinoid abundances increased greatly, in part due to the resultant increase of algal food sources on dead coral skeletons, and caused significant bioerosion of reef framework structures (Glynn 1988; Eakin 2001). Clionaid sponges have also been reported to increase after bleaching events, perhaps due to the increase in available substrates following coral mortality (Rützler 2002; Schoenberg and Ortiz 2009). This is not limited to warm water bleaching, as clionaid sponge populations also exhibited large increases after cold-water bleaching in the Florida Keys (Manzello, pers. obs.) that caused mass coral mortality (Lirman et al. 2011). An increase in the frequency, severity, and/or duration of warm and cold-water events with climate change negatively impact the CaCO3 budget of coral reefs via reductions in coral calcification and increases in bioerosion.
Anthropogenic CO2 emissions are not only changing the climate of the planet, but they are also altering the chemistry of the world’s oceans. About one-third of all the CO2 released into the atmosphere since the industrial revolution has been taken up by the oceans (Sabine et al. 2004). This process, termed ocean acidification (OA), has caused a decline in oceanic pH of 0.1, and will likely cause a further decline of 0.3–0.4 pH units by the end of the twenty-first century (IPCC 2007). The anthropogenic acidification of the oceans is occurring at a rate that is unprecedented over at least the past 55-300 million years (Hönisch et al. 2012). OA results in a decrease in seawater [CO3 2−] and, consequently, a decrease in the saturation state of carbonate mineral s (Ω = [CO3 2−] [Ca2+]/K′sp, where K′sp is the solubility product for a carbonate mineral). Declines in aragonite saturation state (Ωarag, aragonite is the crystalline form of CaCO3 precipitated by scleractinian corals) lead to reduced rates of coral calcification (Langdon and Atkinson 2005). In this review, we have emphasized that the rate of CaCO3 production only slightly exceeds its rate of loss on healthy coral reefs, therefore any disturbance forcing global-scale declines in calcification, such as OA, is alarming (Kleypas et al. 1999a).
Recent experimental work has shown that high-CO2 conditions lead to accelerated rates of bioerosion by endolithic algae and clionaid sponges. Tribollet et al. (2009) exposed coral blocks to 400 ppm and 750 ppm pCO2 for three months following the recruitment of a natural epilithic and endolithic community over 8 months of field deployment in Hawai’i. The alga, Ostreobium querkettii, was the dominant agent of bioerosion and the depth to which its filaments penetrated the coral rock substrate increased significantly in the high-CO2 treatment, leading to a 48 % increase in CaCO3 dissolution. Reyes-Nivia et al. (2013) observed enhanced biologically-mediated dissolution associated with increases in endolith biomass and respiration during combined exposure to elevated CO2 and temperature . These workers found a significant effect of substrate, as skeletons of the coral Porites cylindrica exhibited a higher increase in endolith bioerosion when compared to the more dense Isopora cuneata, as well as an increase in the relative abundance of O. querkettii within the endolithic community. This is intriguing as previous authors have suggested that internal bioerosion is highest in more dense coral skeletons (Highsmith 1981b; Schönberg 2002), yet the response due to high temperature and high-CO2 may follow a reverse pattern.
Bioerosion by clionaid sponges will also intensify in a high-CO2 world (Wisshak et al. 2012; Fang et al. 2013a, b; Enochs et al. 2015). Biologically-mediated chemical dissolution by the common Caribbean boring sponge Pione lampa (formerly Cliona lampa) is predicted to increase 99 % by the end of the twenty-first century as a result of OA, which is nearly double the expected decline in coral calcification (Enochs et al. 2015). Fang et al. (2013a) examined the combination of high-CO2 and high-temperature and reported increases in both sponge biomass and bioerosion rate by the zooxanthellate Pacific boring sponge Cliona orientalis. However, these workers found that Symbiodinium population abundances within C. orientalis decreased with increasing CO2 and bleached in their experimental treatment, mimicking elevated temperatures and CO2 concentration expected by the end of the century under a business-as-usual emissions scenario. In spite of this, bioerosion rates were still highest in the bleached sponges at the highest exposures of CO2 and temperature, even though biomass was reduced by bleaching and peaked at a lower CO2 scenario. In a complementary study, it was suggested that the stimulation of bioerosion at high-CO2 in C. orientalis may be tempered by high temperatures due to bleaching, reductions in biomass, and an overall negative energy balance, as more carbon is consumed than produced at high temperature (Fang et al. 2013b). This suggests that bioerosion rate could increase up to some thermal threshold and then decline due to bleaching, and potentially cease, if mortality occurs.
Enochs et al. (2015) observed a similar parabolic or asymptotic response in Pione lampa to high-CO2, however this was independent of bleaching , as this species is azooxanthellate, and temperature was held constant at 25 °C. Further work is necessary to better understand the mechanism of CO2 stimulation of biologically mediated chemical dissolution in clionaid sponges to determine if this similarity is a coincidence or represents an optimal pH range for clionaid physiological function.
4.4 Variety of Effects
The chief effect of bioerosion emphasized thus far is the mass of calcium carbonate that is reduced to sediments or is dissolved from reef substrata. The weakening of reef substrata by bioeroders that remove relatively little carbonate, but attack critical supporting structures, can be just as important in promoting reef erosion. Large massive corals may be easily toppled or overturned after their supporting bases have been weakened by endolithic borers such as Cliona, Lithotrya and Lithophaga or by grazers that attack bases and hollow out the interiors of colonies such as Diadema and Eucidaris. Many of the displaced corals on reefs, e.g., those making up emergent, rubble ramparts or deep, forereef talus accumulations, owe their new locations in large measure to bioerosion. Large stands of Acropora corals that collapsed after Acanthaster predation on reefs in Japan, Palau and Australia were presumably destabilized as a result of the weakening of dead skeletons by intensified bioerosion (Moran 1986; Birkeland and Lucas 1990).
Aside from weakening reef substrata, the cavities produced by bioeroders increase habitat complexity and thus the variety and biomass of reef associated organisms (Enochs and Manzello 2012a, b). Numerous reef species live permanently attached to cavity walls, pass particular stages of development in cavities, and reside in cavities by day or night. Reef cavities tend to collect sediments that are produced locally or are transported to reefs from more distant sources. The microenvironmental settings of cavities promote internal cementation and the strengthening of reef substrata. Cycles of internal bioerosion , infilling of cavities and cementation may be repeated so that eventually the reef rock appears quite different from its original condition.
The sediments generated by bioeroders accumulate around reefs and eventually infill and bury frame-building species (Fig. 4.11). This effect leads to the shoaling of reef waters and influences the development of reef zonation . Under moderate regimes of bioerosion , sediment accumulation does not overwhelm reef framework growth, however, excessive bioerosion can lead to premature burial and widespread coral death.
Cross-section views of a fringing reef off the west coast of Barbados showing coral framework growth, bioerosion , and infilling by bioeroded sediments. Panels a–e illustrate seaward (deep) to shoreward (shallow) reef sections. The inset plan view shows the location of the panels (After Scoffin et al. 1980)
When bioerosion is excessive it can reduce the topographic complexity of reefs. The reefs noted above in the western Pacific that were subjected to intense predation by Acanthaster and then bioeroded, lost much of their three dimensional structure with the collapse of the Acropora canopies. The loss of these erect corals would eliminate important microhabitats for fishes. The topographic complexity of eastern Pacific reefs can also be reduced by echinoid bioerosion following El Niño disturbances. Coral reefs in the eastern Pacific, particularly in the Galápagos Islands, have been bioeroded to rubble and fine-grained sediments following high coral mortality and low recruitment , respectively, during and after the 1982–1983 El Niño event (Glynn 1994; Reaka-Kudla et al. 1996). Erect, branching coral frameworks have collapsed and massive corals have detached from the substratum and fragmented. Coral recruitment is now generally severely limited with macrobenthic communities composed dominantly of turf algae, gastropods, sea urchins and sea cucumbers.
Like many kinds of plants that spread from cuttings, it seems that some corals may actually benefit from increased breakage facilitated by bioerosion . A common mode of reproduction in many branching coral species is by asexual fragmentation (Tunnicliffe 1979; Highsmith 1982). It has been argued that propagation by this means, which usually results in local rather than distant dispersal , is advantageous to populations that are well adapted to particular environmental settings. Asexual reproduction occurs most commonly among branching, plate-like and other such colonies of delicate morphology with bioerosion aiding breakage by mechanical and biotic agents. Large clones of corals that dominate certain reef zones have arisen by this means (Highsmith 1982).
4.5 Case Studies
Six documented cases of environmental alterations that have affected or threaten reef-building corals are now examined. The first two examples, disturbances caused by El Niño-Southern Oscillation and predator outbreaks, are ostensibly natural events. Runoff and overfishing effects are then examined, representing two examples caused by humankind. In addition, we discuss how many Caribbean reefs are presently in a net erosional state and how they will further be affected by climate change . Lastly, we show how eastern tropical Pacific reefs represent a real-world climate change model, providing insight into how thermal stress and ocean acidification may affect coral reefs of the future.
4.5.1 El Niño-Southern Oscillation
Elevated seawater temperatures that accompanied the 1982–1983 El Niño-Southern Oscillation (ENSO ) caused high coral mortality on reefs in the equatorial eastern Pacific. Mortality ranged from 50 to 99 %, resulting in the virtual elimination of coral cover on many reefs. Coral recruitment has been low to non-existent on many of the affected reefs, which had shown little signs of recovery after 10 years. A more recent analysis of coral reef recovery in the eastern tropical Pacific, including effects of both the 1982–1983 and 1997–1998 ENSO events, revealed no recovery at most monitored sites for periods of up to 20+ years (Wellington and Glynn 2007; Baker et al. 2008; Glynn et al. 2015).
Sea urchin abundances have increased dramatically on dead reef patches. In Panamá, Diadema population densities have increased from 3 inds m−2 before 1983 to 80 inds m−2 after 1983 (Glynn 1988). Similarly, in the Galápagos Islands Eucidaris population densities increased from 5 to 30 inds m−2 from before to after 1983. Probably contributing to this post-El Niño sea urchin increase were the high mortality of lithophagine molluscs (Scott et al. 1988) and the resulting large numbers of vacant bore holes that became available in massive Porites colonies. Numerous juvenile Eucidaris (≤1 cm test diameter) recruited to these newly available shelter sites. The grazing activities of these sea urchins are very destructive (Table 4.2) and their sudden increases in population size, combined with low coral recruitment , have resulted in severe bioerosion of coral reef frameworks. Post El Niño bioerosion rates for Diadema in Panamá amounted to 10–30 g dry wt CaCO3 m−2 day −1, and for Eucidaris in the Galápagos 50–100 g dry wt CaCO3 m−2 day −1. Carbonate breakdown caused by other external and internal bioeroders was about equal to that caused by sea urchins in Panamá, but only about one-fifth of the erosion caused by sea urchins in the Galápagos Islands. Total bioerosion ranged from 10 to 20 kg CaCO3 m−2 year−1 in Panamá and from 20 to 40 kg CaCO3 m−2 year−1 in the Galápagos Islands. Both of these rates exceed net carbonate production of ~10 kg CaCO3 m−2 year–1, estimated for reefs in these areas before 1983. If bioerosion continues at this pace, without an increase in coral recruitment, it is highly likely that many reef formations in the eastern Pacific will disappear.
Studies in the Galápagos Islands and Panamá to the year 2000, demonstrated virtually total reef frame loss in the central and southern islands (Glynn 1994; Reaka-Kudla et al. 1996; Glynn et al. 2001) and substantial calcium carbonate declines in the latter region (Eakin 2001). Eucidaris population densities have remained high in the central and southern Galápagos Islands through 2012 with continuing bioerosion of any remaining limestone structures. Eakin’s modeling results, incorporating post 1997–1998 data , indicated that the Uva Island reef in Panamá was still in an erosional state in the year 2000, ranging from around –3,000 to –18,000 kg CaCO3 year−1 net. A current assessment of coral recovery on the Uva reef in Panama, up until 2010, has revealed a steady increase in live coral cover of ~35 %, and no increase in populations of echinoid bioeroders (Glynn et al. 2014).
While the coral mortality noted above had been caused primarily by elevated sea temperature extremes during El Niño events, it is necessary to recognize that sudden declines in temperature during La Niña events can also result in widespread and significant coral mortality. A sudden transition from a moderate El Niño warm event to a strong La Niña cold condition in 2007, from 26–28 °C to 16 °C over just 6 days, resulted in island-wide coral bleaching in the Galápagos Islands (Banks et al. 2009). Little recovery was observed after one year, which likely increased the vulnerability of corals to bioerosive processes.
4.5.2 Crown-of-Thorns Seastar (Acanthaster)
This example is instructive because it reveals some of the long-term consequences of coral death and bioerosion at the community level. Between 1981 and 1982, the corallivore Acanthaster planci increased greatly in abundance at Iriomote Island, southern Japan, and by the end of 1982 it had killed virtually all the corals on a large study reef (Sano et al. 1987). This sudden loss of live coral precipitated major changes in the physical and biological character of the coral reef.
About two years following the Acanthaster outbreak, most of the erect coral ( Acropora ) canopy had collapsed, a result of bioerosion and water movement. Compared with the live reef, the dead reef exhibited low structural complexity . By 1986 all of the corals were broken apart and the reef formation had been converted into a flat plain of unstructured coral rubble. The degradation of the reef was correlated with marked changes in the fish community. As the topographic complexity of the reef decreased, the numbers of associated fish species and their abundances also declined. Fishes that fed exclusively on live coral tissues disappeared completely from the dead reefs. The declines in fishes with other diets, e.g. planktivores, herbivores and omnivores, were believed due in large measure to the loss of living space and to overall declines in prey on the degraded reef.
More recent studies of large-scale coral predation by Acanthaster, followed by intense bioerosion with reductions in reef fish abundances and diversity , have followed in broad outline the course of events at the Iriomote reef described above. A follow-up study of the degraded Iriomote reef demonstrated rapid recovery under conditions of high coral recruitment and survivorship (Sano 2000). Arborescent Acropora spp. began recruiting in 1989, and by 1995 and following years coral cover had reached about 100 %, closely matching pre-Acanthaster live coral cover values. This buildup in coral cover was accompanied by increases in the species richness and density of adult fish assemblages, to predisturbance levels. It will be instructive to compare this example of rapid recovery, occurring over a period of only eight years, with data from other coral reef areas as they become available.
4.5.3 Runoff (Eutrophication, Sedimentation, Freshwater and Pollutants)
One of the best examples of reef degradation caused by runoff is that reported for the Kāne’ohe Bay, Hawai’i coral reef ecosystem (Banner 1974; Smith et al. 1981; Jokiel et al. 1993). Because the mismanagement of the Kāne’ohe Bay water shed has led to multiple effects, e.g., sewage pollution , agricultural runoff, increased sedimentation and freshwater dilution, it is not always possible to identify individual or combined stressors. However, the occurrence of coral reef mass mortalities during storm floods and a general decline in coral cover during a period of increasing sewage stress implicates these stressors in the degradation of Kāne’ohe Bay coral reefs over two decades (1960–1978).
During the first half of the Twentieth century the coral reefs of Kāne’ohe Bay were in a healthy state, supporting a local artisanal fisheries and offering one of the best underwater vistas of “coral gardens” in the Hawaiian Islands. In 1963, a large sewage outfall was installed in the bay, which had an increasing effect on corals until 1978 when the outfall was moved to the deep ocean outside the bay. The eutrophication caused by increasing sewage loads favored the growth of a bubble alga (Dictyosphaeria cavernosa) and suspension feeding and bioeroding species that combined to degrade the reef communities over a 15 year period (Fig. 4.12). Following the sewage diversion, clear signs of renewed coral growth, reduced bioerosion , and reef community recovery were evident by 1983. Severe storm flooding in 1987 caused extensive coral mortality , but surviving corals quickly resumed rapid growth and the condition of reef communities (as of 1993) had remained favorable.
Another 16 years have passed without a major disturbance event affecting corals, however, notable changes in several reef-associated benthic species have occurred. Thanks to the monitoring efforts of P Jokiel, J Stimson, and N Sukhraj, the recent status of the Coconut Island (= Moku o Loe Island) fringing poritid reef can be briefly reviewed. Since 2006, Dictyosphaera has decreased in abundance, and several species of red algae have become closely associated with Porites compressa, in some instances attached to the peripheral branches of corals (Stimson and Conklin 2008; N Sukhraj pers comm). In addition, a non-boring invasive sponge, Mycale grandis, is sometimes present adhering to the sides of coral branches (Coles et al. 2007). It now appears that interphyletic competition is more prevalent, but rapid coral growth, with branch elongation rates of 2.4–3.5 cm year−1 in reef crest and slope zones (Jokiel 1986), is contributing to vigorous reef progradation. Rapid vertical coral growth at shallow crest depths leads to framework instability, fracturing, and downslope block transport, extending reef foundations seaward. This case history illustrates a degree of resiliency to a disturbance that might have led to reef community collapse in a sewage-stressed environment.
As the human population continues to increase along tropical and subtropical coastal areas, it should be no surprise that reports of associated pollution stress are also on the rise. Indeed, numerous recent studies have documented the deterioration of coral reefs worldwide with eutrophication, related to urbanization (sewage pollution), inappropriate agricultural practices and industrial pollution sources, being the root cause of this decline. It is cautioned that the entry of polluted freshwater into coastal zone communities is not always obvious as sources can include large volumes of groundwater discharge as well as surface effluents. Representative examples of coral reef bioerosion and deterioration under nutrient-rich conditions have been documented in studies in the Indian Ocean (Risk et al. 1993; Chazottes et al. 2002), Indonesia (Tomascik et al. 1997; Holmes et al. 2000), the Great Barrier Reef, Australia (Risk et al. 1995) and at several other Pacific Ocean sites (Hutchings 1994), off Brazil (Leão et al. 1993) and at several localities in the Caribbean Sea (Smith and Ogden 1993).
4.5.4 Overfishing
Several studies in the Caribbean and off the Kenyan coast in the Indian Ocean have presented evidence suggesting that sea urchin abundances are controlled by finfish predators. When fish predators of sea urchins are abundant, urchin abundances tend to be low, but when fishing pressure is high, leading to the disappearance of urchin predators, then urchins can become exceedingly abundant. A study of protected (non-fished) and overfished Kenyan coral reef lagoons indicates that the removal of top, invertebrate-eating, fish carnivores can have cascading effects on coral reef community structure and function (McClanahan and Shafir 1990).
Triggerfish predators of sea urchins were relatively abundant in protected coral reef lagoons, but rare in comparable unprotected environments. The removal of the natural predators of sea urchins by overfishing resulted in several direct effects on the urchin prey and several indirect effects on the condition of the coral reef community. Overfished reefs demonstrated high sea urchin abundances, high urchin survival, and high urchin diversity compared with non-fished reefs. Correlated with the dominance of sea urchins on overfished reefs were declines in (a) live coral cover, (b) calcareous and coralline algal cover, (c) substratum diversity, and (d) topographic complexity . These changes were caused by increased substratum bioerosion , especially by Echinometra mathaei (de Blainville), the competitively dominant sea urchin in unprotected Kenyan reef lagoons. The end result of overfishing is accelerated bioerosion, a reef surface dominated by algal turf, and likely a decline in the reef’s fisheries productivity .
A convincing case of the over-harvesting of sea urchin predators (lobsters, fishes) in the Galápagos Islands, leading to increased external bioerosion , exemplifies the potential for secondary additive effects that could impede coral and reef recovery (Edgar et al. 2010). Low abundances of Eucidaris galapagensis were present in marine protected areas or far from fishing ports, sites supporting high natural abundances of urchin predators. These areas also demonstrated higher levels of coral cover and less disturbance to coral communities recovering from ENSO mortality events.
4.5.5 Caribbean Reef CaCO3 Budgets: Current Status and Future Trends
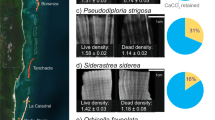
Coral reefs are in decline globally and the state of Caribbean reefs is arguably the most alarming. Caribbean reefs have experienced multiple interacting disturbances that have driven or exacerbated large-scale coral mortality . In no particular order of importance, the loss of acroporids due to white-band disease , the basin-wide ecological extinction of the keystone sea urchin herbivore Diadema antillarum due to an unidentified pathogen , overfishing, coral bleaching , and land-based sources of pollution have all been linked to the collapse of Caribbean coral reefs (Hughes 1994; Aronson and Precht 2001; Jackson et al. 2001; Eakin et al. 2010). Live coral cover has declined by about 80 % since the 1970s, reefs are losing architectural complexity , CaCO3 production has declined to 50 % below historical averages, and more than a third of sites recently surveyed (37 %) were already net erosional (Gardner et al. 2003; Alvarez-Filip et al. 2009; Perry et al. 2013). Many Caribbean reefs are at or close to CaCO3 budget neutral, termed “accretionary stasis,” leading to the concern that the persistence of architecturally complex reef framework structures is in doubt (Perry et al. 2013).
Two recent studies have forecasted future Caribbean reef CaCO3 budgets using different approaches, yet both yielded similarly negative prognoses. Enochs et al. (2015) used the reef budget methodology of Perry et al. (2012) to estimate present day and future CaCO3 budgets for 37 reefs across the Florida Reef Tract when projected atmospheric CO2 levels reach 750 ppm, a conservative estimate for end of the twenty-first century conditions. These workers assessed three differing scenarios: (1) no change in coral cover, no change in coral and coralline algae calcification with OA, increase in bioerosion with OA; (2) no change in coral cover relative to present-day, expected declines in coral and coralline algae calcification due to OA, increase in bioerosion; and (3) 50 % decline in coral cover with expected declines in calcification and increases in bioerosion. The declines in calcification were estimated based on published rates , whereas changes in bioerosion were based on the rate of increase in chemical dissolution shown for the common Caribbean bioeroding sponge P. lampa with high-CO2 (+99 %) in the same study, coupled with the predicted rate of dissolution increase by endolithic algae of 48 % (Tribollet et al. 2009). Under present-day conditions, 89 % of reefs on the Florida Reef Tract were already net erosional. For scenario 1, 92 % of reefs became net erosional, whereas all reefs considered were net erosional under scenarios 2 and 3. This modeling exercise illustrated the potential importance of endolithic algae bioerosion. Even though the rates of dissolution per area coverage and the expected stimulation of bioersoion at high-CO2 are both lower than what is expected for P. lampa, the dominance of bare substrate as a result of very low coral cover leads to a domineering role for endolithic algae in the reef CaCO3 budget in a high-CO2 world. These projections stress the likely importance of local management to safeguard reef ecosystem function in an era of global change. Overfishing of those species that control clionaid populations, such as angelfishes, as well as coastal eutrophication should be controlled and not allowed to exacerbate the expected increase in bioerosion (Rose and Risk 1985; Hill and Hill 2002; Ward-Paige et al. 2005).
Kennedy et al. (2013) combined ecological models with carbonate budgets and assessed the dynamics of simulated Caribbean coral reefs based on the latest climate projections. This study assessed the interacting role of local management of fisheries and land-based sources of pollution . The same trend towards net erosion was apparent under all climate scenarios of increased temperature and CO2, but local management of fisheries, specifically protection of grazing parrtofishes, was found to delay reef loss by a decade. However, positive CaCO3 budgets were only generated when local action was combined with aggressive emission reductions that would limit global warming to less than 2 °C. Changes in coral calcification due to warming and acidification were most important in their CaCO3 budget simulations for healthy, coral-dominated reefs. The controls on bioerosion , such as sea urchin population sizes, sponge boring rates , and nutrification became the dominant drivers of the carbonate budget at low coral cover.
4.5.6 Eastern Tropical Pacific Coral Reefs: A Real-World Climate Change Model
Coral reefs of the eastern tropical Pacific (ETP) provide a real-world example of reef growth, development , structure and function under high-CO2, low-Ωarag conditions that encompass the range of expected changes for the entire tropical surface ocean with a doubling and tripling of atmospheric CO2 (Manzello et al. 2008; Manzello 2009, 2010a). The naturally high-CO2 of the ETP causes reefs in this region to persist near the Ωarag distributional threshold for coral reefs (Kleypas et al. 1999b). Reef structural development is highly limited in the marginal low-Ωarag environment of the ETP and reef structures are ephemeral on geological timescales (Manzello 2009). Calcium carbonate cements, which bind reef frameworks and sediments, do not precipitate above trace levels in the ETP and rates of bioerosion are the highest measured anywhere in the world (Manzello et al. 2008).
Eastern tropical Pacific reef response to ENSO warming varies regionally as a function of CO2, providing possible insight into reef persistence vis-à-vis warming in a high-CO2 world. Galápagos coral reef communities experienced a greater thermal stress (+2 to 3 °C for several months) during the 1982–1983 ENSO when compared to Panamá (+1 to 2 °C for two months) (Podestá and Glynn 2001). As a result, coral bleaching mortality was higher in Galápagos (97–99 %) compared to Panamá (75–85 %; Glynn 1990b). Following this mass mortality, reef framework structures in the southern Galápagos Islands were rapidly bioeroded to rubble and sand and are now non-existent (Glynn 1994; Reaka-Kudla et al. 1996). Conversely, reef framework structures have persisted in Panamá despite net erosion and the additional severe 1997–1998 ENSO event (Eakin 2001). Intriguingly, only one reef has persisted in the northern Galápagos Islands, where pH and Ωarag are regionally elevated (Manzello et al. 2014). The impact of high-CO2, low-Ωarag seawater on carbonate cement precipitation and its apparent inverse relationship to bioerosion rate in the ETP adds a key piece to the puzzle as to why reefs throughout the ETP are poorly developed and ephemeral on geologic time-scales (Manzello et al. 2008).
The observation that the highest bioerosion rates ever documented on coral reefs are coincident with very low-Ωarag and bioerosion rates across the ETP are inversely related to Ωarag and CaCO3 cement abundance (Manzello et al. 2008) has been criticized by Tribollet and Golubic (2011). Three specific criticisms were stated: (1) correlations of bioerosion rate with cement abundance and Ωarag were considered “erroneous” because they were not supported experimentally, (2) the presentation of differing types of bioerosion (total, internal, external) were said to be incomparable, and (3) the increase in substrate available for colonization after the 1982–1983 ENSO coral bleaching mortality event was argued to be a more “probable” explanation for the high rates of bioerosion reported by Reaka-Kudla et al. (1996); and they also suggested the rates of bioerosion during the time of study were likely different. Only ETP bioerosion rates were compared and plotted alongside the measured cement abundances in our study because (a) these are where the Ωarag and cement data were collected, and (b) these rates are in fact comparable. Bioerosion research in the ETP has a considerable history and was initiated more than 30 years ago by Glynn et al. (1979), who showed the characteristic very rapid rate of bioerosion by the echinoid Eucidaris galapagensis in the Galápagos Islands. The pioneering research by Glynn and colleagues was subsequently advanced by Scott et al. (1988) in Costa Rica, Eakin (1993, 2001) in Panamá and later by Reaka-Kudla et al. (1996) in the Galápagos Islands. The methodology employed by Glynn and Eakin was the same (Glynn et al. 1979; Glynn 1988; Eakin 1996). The work by Reaka-Kudla et al. (1996) did use different methods, but generated similar rates to those initially published by Glynn (1988). Rates of bioerosion were comparably high prior to the 1982–1983 ENSO (23.5 kg m−2 year−1) in the southern Galápagos Islands (Glynn 1988) to the results reported nearly two decades later by Reaka-Kudla et al. (1996). The assertion that the rates reported by Reaka-Kudla et al. (1996) were most likely just an artifact of an increase in available substrate for colonization is uninformed. Three different studies have shown that bioerosion in the Galápagos is uncharacteristically high and this was known and published before the 1982–1983 ENSO bleaching/mortality disturbance (Glynn et al. 1979; Glynn 1988; Reaka-Kudla et al. 1996).
A comparison of published rates of bioerosion was explored by Manzello et al. (2008) because of the suggestion by pioneering coral reef geologist, Ian Macintyre, that submarine cementation is an important control on the construction and binding of reef framework structures (Macintyre 1997). With this in mind, and the finding that only trace amounts of cement precipitate in ETP reef structures, maximum published rates of bioerosion were compiled to determine if the rates of ETP bioerosion were unique. The ETP bioerosion rates mirrored coral reef cement abundances. The non-ETP maximum rates reported in the literature illustrate that ETP reefs are subject to unprecedented levels of rapid bioerosion, reconfirming that which was known for more than three decades (e.g., Glynn et al. 1979). Differing agents of bioerosion were indeed reported, but the chief objective was to show maximum mean recorded rates from the literature, regardless of bioerosion agent, to confirm the far greater magnitude of rates in the ETP. Bioerosion rates from outside the ETP were listed for qualitative comparison and were not used in the study.
This study was further misrepresented by Tribollet and Golubic (2011) when they claimed that the conclusion was drawn that bioerosion was negatively correlated with Ωarag and cement abundance, when no such statistical test was conducted or reported. In fact, Manzello et al. (2008) solely stated “cement abundance was positively related to Ωarag, but inversely related to bioerosion rate in the ETP.” This statement only reported an apparent trend. The lack of cement in the ETP is later hypothesized to be a factor in the high bioerosion rates of this region, referencing the other published rates to show just how high rates in the ETP are relative to other locations. The publication concludes, “In summary, this study suggests a link between Ωarag, inorganic reef cementation, and coral reef development in the ETP…The ETP examples suggest that coral reefs of the future could be more susceptible to erosion.” Tribollet and Golubic (2011) misinterpreted the hypotheses, unnecessarily attacking claims that were not stated.
Recent research on Galápagos reefs and calcification dynamics provide new insights into reef structure and function in a high-CO2 world. As previously mentioned, only one coral reef persists today within the entire Galápagos archipelago and this reef is located at the remote, northern-most Darwin Island (Glynn et al. 2009, 2015), where pH and Ωarag are regionally elevated (Manzello et al. 2014). Conversely, coral reefs in the southern islands disappeared where pH < 8.0 and Ωarag ≤ 3.0, and have not recovered. We found that high nutrients in the upwelled waters of the southern Galápagos Islands may enhance coral calcification under high-CO2, but ultimately increase reef ecosystem sensitivity to ocean acidification . The warming and acidification that caused the functional collapse of Galápagos reefs is expected to occur world-wide by mid-century for most reefs based on current CO2 trajectories (Frieler et al. 2013; van Hooidonk et al. 2014).
4.6 Conclusions
The fossil record demonstrates that bioerosion and reef growth have always been inseparable. Moderate levels of bioerosion may benefit coral reefs in at least four ways, by (1) creating sedimentary substrata that provide lebensraum for hosts of associated reef species, (2) providing cavities and contributing toward topographic complexity that serve to increase the biodiversity , biomass and productivity of reef communities, (3) structuring reef morphology and growth, and (4) promoting the regeneration and rejuvenation of senescent reef-building organisms.
Except for obvious reef destruction by large populations of sea urchins , bioerosion per se as a possible threat to coral reefs is seldom considered explicitly. This is probably because of the large amount of ‘cryptic’ bioerosion caused by endoliths and the often delayed effects of bioerosion on coral reef communities. For example, descriptions of reef damage caused by violent storms are numerous in the literature, but the contributory effects of bioerosion are seldom mentioned. The prior weakening of reef structures by bioerosion or the accumulation of sediments causing scour and burial during a storm are effects that may have been initiated years before an acute disturbance event resulting in reef devastation.
The existence of coral reefs beyond this century is in jeopardy. The concern has progressed from the dramatic losses of live coral (Gardner et al. 2003), to the likelihood that the underlying framework of coral reefs will erode away (Hoegh-Guldberg et al. 2007; Manzello et al. 2008; Perry et al. 2013). Coral cover has declined across broad geographic scales (Gardner et al. 2003; De’ath et al. 2012), leading to declines in CaCO3 production. Furthermore, declines in coral growth and calcification have been documented in the Indian, Pacific, and North Atlantic Oceans over the past 30 years (Edmunds 2007; Cooper et al. 2008; Bak et al. 2009; De’ath et al. 2009; Tanzil et al. 2009; Manzello 2010b). The production of CaCO3 on coral reefs is depressed at a seemingly global scale. This suggests that the future impacts of OA on the negative side of the coral reef carbonate budget , namely via stimulation of biologically-mediated dissolution, may be more detrimental than the decline of coral calcification. Indeed, Kennedy et al. (2013) explicitly demonstrated that changes to coral calcification are only reflected in the CaCO3 budget when coral cover is high. When coral cover is low, bioerosion of the reef framework becomes the dominant process.
What are some of the measures that can be taken to limit bioerosion ? The most obvious is to reduce coral mortality because numerous bioeroders increase their activities and abundances on dead reef substrata. Direct damage to calcifying organisms can be reduced significantly by several practices already adopted within protected coral reef parks. For example, the use of mooring buoys, navigational markers, the prohibition of destructive fishing techniques, and the banning of coral collecting or touching live corals have all alleviated damage to coral reefs in many areas. The possibility of indirect effects, such as overfishing causing increases in bioerosion, should also be considered in coral reef management plans.
Another method of limiting coral mortality after severe physical damage, e.g., by a ship grounding, involves restoration techniques to stabilize damaged corals and reef substrata (Hudson and Diaz 1988). Hard and soft corals may be transplanted and cemented to stable reef substrata, fractured frameworks may be secured, and the rebuilding of reef topography accomplished by replacing and cementing dislodged corals and sections of framework.
Numerous effects that can accelerate bioerosion are often far-removed from coral reefs and therefore sometimes difficult to link with reef decline. Deforestation, land-clearing and mining activities lead to increased sedimentation, fresh-water dilution and nutrient loading around reefs that may be situated hundreds of kilometers from the affected sites. These sorts of activities may alter reef environments such that certain types of bioeroders could increase in number and possibly accelerate destructive processes. The potential damage of such anthropogenic stresses to coral reefs also may be augmented by natural disturbances such as violent storms , extreme temperature changes, diseases and predator outbreaks. For example, most corals may tolerate low salinities for a few hours or days, but salinity stress in combination with a pathogen could precipitate high coral mortality . Many kinds of runoff include combinations of several pollutants, e.g. sewage, detergents, heavy metals, fertilizers, pesticides, and oil, that may act synergistically to reduce live coral cover.
It is now generally understood that bioerosion will be accelerated in a high-CO2 world via stimulation of biologically-mediated chemical dissolution in endolithic algae and clionaid sponges. It has been suggested that a similar amplification of erosive ability may occur in all bioeroding organisms that utilize chemical dissolution to excavate reef substrates (Enochs et al. 2015). This includes lithophagine bivalves, cirripedes, and various polychaete worms. Many of these bioeroders have greater tolerances to environmental perturbation than corals. In fact, clionaid sponges appear to gain a competitive advantage during thermal stress and coral bleaching events (Rützler 2002; Schönberg and Ortiz 2009). There is much to be learned about coral reef bioerosion, but one thing seems clear – research on bioerosion will most certainly increase over this century because the importance of this fundamental process in coral reef dynamics will likely become increasingly difficult to ignore. Local-scale management of fisheries and watershed pollution would not only benefit corals, but should help limit the proliferation of bioeroders in a high-CO2 world.
In summary, the dynamic balance between reef growth and bioerosion depends on the vitality of numerous calcifying species. If humankind’s activities can be limited to non-intrusive pursuits such as observing and filming reef organisms, and if reef water quality and natural circulation patterns can be safeguarded, then one of the world’s most exquisite ecosystems can be enjoyed by posterity.
References
Agassiz L (1852) Coral reefs. In: Annual report of the Superintendent of the Coast Survey, showing the progress of that work during the year ending November, 1851, pp 153–154, 32d Congr, 1st Sess Senate Ex Doc No 3, R Armstrong, Printer
Ahr WM, Stanton RJ Jr (1973) The sedimentologic and paleoecologic significance of Lithotrya, a rock-boring barnacle. J Sediment Petrol 43:20–23
Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc R Soc Lond B Biol Sci 276:3019–3025
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Bak RPM (1990) Patterns of echinoid bioerosion in two Pacific coral reef lagoons. Mar Ecol Prog Ser 66:267–272
Bak RPM, Nieuwland G, Meesters EH (2009) Coral growth rates revisited after 31 years: what is causing lower extension rates in Acropora palmata? Bull Mar Sci 84:287–294
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Banks S, Vera M, Chiriboga Á (2009) Characterizing the last remaining reefs: establishing reference points to assess long term change in Galápagos zooxanthellate coral communities. Galapagos Res 66:43–64
Banner AH (1974) Kaneohe Bay, Hawaii: urban pollution and a coral reef ecosystem. Proc 2nd Int Coral Reef Symp, Brisbane 2:685–702
Bardach JE (1959) The summer standing crop of fish on a shallow Bermuda reef. Limnol Oceanogr 4:77–85
Bardach JE (1961) Transport of calcareous fragments by reef fishes. Science 133:98–99
Barnes DJ (ed) (1983) Perspectives on coral reefs. Brian Clouston, Manuka, 277 p
Bellwood DR (1995) Direct estimate of bioerosion by two parrotfish species, Chlorurus gibbus and C. sordidus, on the Great Barrier Reef, Australia. Mar Biol 121:419–429
Bellwood DR (1996) Production and reworking of sediment by parrotfishes (family Scaridae) on the Great Barrier Reef, Australia. Mar Biol 125:795–800
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ Biol Fish 28:189–214
Bellwood DR, Wainwright PC (2002) The history and biogeography of fishes on coral reefs. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, San Diego, pp 5–32
Birkeland C, Lucas JS (1990) Acanthaster planci: major management problem of coral reefs. CRC Press, Boca Raton, 257 p
Bruggemann JH, van Kessel AM, van Rooij JM, Breeman AM (1996) Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: implication of fish size, feeding mode and habitat use. Mar Ecol Prog Ser 134:59–71
Campbell SE (1982) Precambrian endoliths discovered. Nature 299:429–431
Carriker MR, Smith EH, Wilce RT (eds) (1969) Penetration of calcium carbonate substrates by lower plants and invertebrates. Am Zool 9:629–1020
Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M, Cuet P (2002) The effects of eutrophication-related alterations to coral reef communities on agents and rates of bioerosion (Reunion Island, Indian Ocean). Coral Reefs 21:375–390
Choat JH, Bellwood DR (1985) Interactions amongst herbivorous fishes on a coral reef: influence of spatial variation. Mar Biol 89:221–234
Choat JH, Robertson DR (1975) Protogynous hermaphroditism in fishes of the family Scaridae. In: Reinboth R (ed) Intersexuality in the animal kingdom. Springer, Berlin, pp 264–283
Cloud PE Jr (1959) Geology of Saipan, Mariana Islands, Part 4. Submarine topography and shoal-water ecology. Geol Surv Prof Pap 280-K:361–445
Coles SL, Marchetti J, Bolick H, Montgomery A (2007) Assessment of invasiveness of the orange keyhole sponge Mycale armata in Kaneohe Bay, O’ahu, Hawai’i. Final Report, Year 2 for Hawaii Coral Reef Initiative Research Program. Contribution No. 2007-002 to the Hawaii Biological Survey, 30 p
Cooper TF, De’ath G, Fabricius KE, Lough JM (2008) Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Global Change Biol 14:529–538
Cortés J (1985) Preliminary observations of Alpheus simus Guerin-Meneville, 1856 (Crustacea: Alphaeidae): a little-known Caribbean bioeroder. Proc 5th Int Coral Reef Congr Tahiti 5:351–353
Darwin C (1842) The structure and distribution of coral reefs. Smith Elder & Co., London, 214 p
Davies PJ, Hutchings PA (1983) Initial colonization, erosion and accretion on coral substrate. Experimental results, Lizard Island, Great Barrier Reef. Coral Reefs 2:27–35
De’ath G, Lough JM, Fabricius KE (2009) Declining coral calcification on the Great Barrier Reef. Science 323:116–119
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci U S A 109:17995–17999
DiSalvo LH (1969) Isolation of bacteria from the corallum of Porites lobata (Vaughan) and its possible significance. Am Zool 9:735–740
Donn TF, Boardman MR (1988) Bioerosion of rocky carbonate coastlines on Andros Island, Bahamas. J Coastal Res 4:381–394
Eakin CM (1993) Post-El Niño Panamanian reefs: less accretion, more erosion and damselfish protection. Proc 7th Int Coral Reef Symp Guam 1:387–396
Eakin CM (1996) Where have all the carbonates gone? A model comparison of calcium carbonate budgets before and after the 1982–83 El Niño at Uva Island in the eastern Pacific. Coral Reefs 15:109–119
Eakin CM (2001) A tale of two ENSO events: carbonate budgets and the influence of two warming disturbances and intervening variability, Uva Island, Panamá. Bull Mar Sci 69:171–186
Eakin CM, Morgan JA, Heron SF, Smith TB, Liu G, Alvarez-Filip L, Baca B, Bartels E, Bastidas C, Bouchon C, Brant M, Bruckner AW, Bunkley-Williams L, Cameron A, Causey BC, Chiappone M, Christensen TRL, Crabbe MJC, Day O, de la Guardia E, Diaz-Pulido G, DiResta D, Gil-Agudelo DL, Gilliam D, Ginsburg R, Gore S, Guzman HM, Hendee JC, Hernandez-Delgado E, Husain E, Jeffrey CFG, Jones RJ, Jordan-Dahlgren E, Kaufman DM, Kline DI, Kramer P, Lang J, Lirman D, Mallela J, Manfrino C, Marechal J, Marks K, Mihaly J, Miller J, Muller EM, Muller M, Orozco-Toro CA, Oxenfor HA, Ponce-Taylor D, Quinn N, Ritchie KB, Rodriguez S, Rodriguez-Ramirez A, Romano S, Samhouri JF, Sanchez JA, Schmahl GP, Shank B, Skirving WJ, Steiner SCC, Villamizar E, Walsh S, Walter C, Weil E, Williams EH, Woody K, Yusuf Y (2010) Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One 5:e13969
Ebbs NK (1966) The coral-inhabiting polychaetes of the northern Florida reef tract. Bull Mar Sci 16:485–555
Edgar GJ, Banks SA, Brandt M, Bustamante RH, Chiriboga Á, Earle SA, Garske LE, Glynn PW, Grove JS, Henderson S, Hickman CP, Miller KA, Rivera F, Wellington GM (2010) El Niño, grazers and fisheries interact to greatly elevate extinction risk for Galapagos marine species. Global Change Biol 16:2876–2890
Edmunds PJ (2007) Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals. Mar Ecol Prog Ser 341:1–13
Ekdale AA, Bromley RG, Pemberton SG (eds) (1984) Ichnology: the use of trace fossils in sedimentology and stratigraphy. SEPM short course notes, Ch 10, 15:108–128. doi: 10.2110/scn.84.15
Endean R (1976) Destruction and recovery of coral reef communities. In: Jones OA, Endean R (eds) Biology and geology of coral reefs. III. Biology 2. Academic, New York, pp 215–254
Enochs IC (2012) Motile cryptofauna associated with live and dead coral substrates: implications for coral mortality and framework erosion. Mar Biol 159:709–722
Enochs IC, Manzello DP (2012a) Species richness of motile cryptofauna across a gradient of framework erosion. Coral Reefs 31:653–661
Enochs IC, Manzello DP (2012b) Responses of cryptofaunal species richness and trophic potential to coral reef habitat degradation. Diversity 4:94–104
Enochs IC, Manzello DP, Carlton R, Graham D, Ruzicka R, Colella M (2015) Ocean acidification enhances the bioerosion of a common coral reef sponge: implications for persistence of the Florida Reef Tract. Bull Mar Sci 91(2):271–290
Fang JKH, Mello-Athayde MA, Schönberg CHL, Kline DI, Hoegh-Guldberg O, Dove S (2013a) Sponge biomass and bioerosion rates increase under ocean warming and acidification. Global Change Biol. doi:10.1111/gcb.12334
Fang JKH, Schönberg CHL, Mello-Athayde MA, Hoegh-Guldberg O, Dove S (2013b) Effects of ocean warming and acidification on the energy budget of an excavating sponge. Global Change Biol. doi:10.1111/gcb.12369
Focke JW (1978) Limestone cliff morphology on Curaçao (Netherlands Antilles) with special attention to the origin of notches and vermetid/coralline algal surf benches (“cornices”, “trottoirs”). Z Geomorphol 22:329–349
Frieler K, Meinhausen M, Golly A, Mengel M, Lebek K, Donner SD, Hoegh-Guldberg O (2013) Limiting global warming to 2°C is unlikely to save most coral reefs. Nat Clim Chang 3:165–170
Frydl P, Stearn GW (1978) Rate of bioerosion by parrotfish in Barbados reef environments. J Sediment Petrol 48:1149–1157
Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Ginsburg RN (1983) Geological and biological roles of cavities in coral reefs. In: Barnes DJ (ed) Perspectives on coral reefs. Brian Clouston, Manuka, pp 148–153
Glynn PW (1970) On the ecology of the Caribbean chitons Acanthopleura granulata Gmelin and Chiton tuberculatus Linné: density, mortality, feeding, reproduction, and growth. Smithson Contrib Zool 66:1–21
Glynn PW (1988) El Niño warming, coral mortality and reef framework destruction by echinoid bioerosion in the eastern Pacific. Galaxea 7:129–160
Glynn PW (1990a) Feeding ecology of selected coral-reef macroconsumers: patterns and effects on coral community structure. In: Dubinsky Z (ed) Ecosystems of the world, vol 25, Coral reefs. Elsevier Science, New York, pp 365–400
Glynn PW (1990b) Coral mortality and disturbance to coral reefs in the eastern tropical Pacific. In: Glynn PW (ed) Global ecological consequences of the 1982–83 El Niño-Southern Oscillation. Elsevier, Amsterdam, pp 55–126
Glynn PW (1994) State of coral reefs in the Galápagos Islands: natural vs anthropogenic impacts. Mar Pollut Bull 29:131–140
Glynn PW, Wellington GM (1983) Corals and coral reefs of the Galápagos Islands (with an annotated list of the scleractinian corals of the Galápagos by JW Wells). University of California Press, Berkeley, 330 p
Glynn PW, Stewart RH, McCosker JE (1972) Pacific coral reefs of Panamá: structure, distribution and predators. Geol Rundsch 61:483–519
Glynn PW, Wellington GM, Birkeland C (1979) Coral growth in the Galápagos: limitation by sea urchins. Science 203:47–49
Glynn PW, Maté JL, Baker AC, Calderón MO (2001) Coral bleaching and mortality in Panamá and Ecuador during the 1997–1998 El Niño-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–101
Glynn PW, Riegl B, Correa AMS, Baums IB (2009) Rapid recovery of a coral reef at Darwin Island, Galápagos Islands. Galapagos Res 66:6–13
Glynn PW, Enochs IC, Afflerbach JA, Brandtneris VW, Serafy JE (2014) Eastern Pacific reef fish responses to coral recovery following El Niño disturbances. Mar Ecol Prog Ser 495:233–247
Glynn PW, Riegl B, Purkis S, Kerr JM, Smith TB (2015) Coral reef recovery in the Galápagos Islands: the northernmost islands (Darwin and Wenman). Coral Reefs 34:421–436
Golubic S, Perkins RD, Lukas KJ (1975) Boring microorganisms and microborings in carbonate substrates. In: Frey RW (ed) The study of trace fossils. Springer, New York, pp 229–259
Golubic S, Radtke G, Le Campion-Alsumard T (2005) Endolithic fungi in marine ecosystems. Trends Microbiol 13:229–235
Grassle JF (1973) Variety in coral reef communities. In: Jones OA, Endean R (eds) Biology and geology of coral reefs. II, Biology 1. Academic, New York, pp 247–270
Grigg RW, Dollar SJ (1990) Natural and anthropogenic disturbance on coral reefs. In: Dubinsky Z (ed) Ecosystems of the world, vol 25, Coral reefs. Elsevier Science, New York, pp 439–452
Haigler SA (1969) Boring mechanism of Polydora websteri inhabiting Crassostrea virginica. Am Zool 9:821–828
Highsmith RC (1980) Geographic patterns of coral bioerosion: a productivity hypothesis. J Exp Mar Biol Ecol 46:177–196
Highsmith RC (1981a) Lime-boring algae in hermatypic coral skeletons. J Exp Mar Biol Ecol 55:267–281
Highsmith RC (1981b) Coral bioerosion: damage relative to skeletal density. Am Nat 117:193–198
Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226
Hill MS, Hill AL (2002) Morphological plasticity in the tropical sponge Anthosigmella varians: responses to predators and wave energy. Biol Bull 202:86–95
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Holmes KE, Edinger EN, Limmon GV, Risk MJ (2000) Bioerosion of live massive corals and branching coral rubble on Indonesian coral reefs. Mar Pollut Bull 40:606–617
Hönisch B, Ridgwell A, Schmidt DN, Thomas E, Gibbs SJ, Sluijs A, Zeebe R, Kump L, Martindale RC, Greene SE, Kiessling W, Ries J, Zachos JC, Royer DL, Barker S, Marchitto TM, Moyer R, Pelejero C, Ziveri P, Foster GI, Williams B (2012) The geological record of ocean acidification. Science 335:1058–1063
Hudson JH, Diaz R (1988) Damage survey and restoration of M/V WELLWOOD grounding site, Molasses Reef, Key Largo National Marine Sanctuary, Florida. Proc 6th Int Coral Reef Symp Townsville 2:231–236
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hutchings PA (1986) Biological destruction of coral reefs. Coral Reefs 4:239–252
Hutchings PA (1994) The Pacific reefs: a paradise lost? Mar Pollut Bull 29:1–140
Hutchings PA (2011) Bioerosion. In: Hopley D (ed) Encyclopedia of modern coral reefs: structure, form and process. Springer, Dordrecht, pp 139–156
IPCC (2007) Intergovernmental panel on climate change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis: contribution of working group I to the fourth assessment report of the IPCC. Cambridge University Press, New York
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Jokiel PL (1986) Growth of the reef coral Porites compressa on the Coconut Island reef, Kaneohe Bay. In: Jokiel PL, Richmond RH, Rogers RA (eds) Coral reef population biology, Tech Rept No 37, Hawaii Inst Mar Biol, Oahu, pp 101–110
Jokiel PL, Hunter CL, Taguchi S, Watarai L (1993) Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu, Hawaii. Coral Reefs 12:177–184
Kendrick B, Risk MJ, Michaelides J, Bergman K (1982) Amphibious microborers: bioeroding fungi isolated from live corals. Bull Mar Sci 32:862–867
Kennedy EV, Perry CT, Halloran PR, Iglesias-Prieto R, Schönberg CHL, Wisshak M, Form AU, Carricart-Ganivet JP, Fine M, Eakin CM, Mumby PJ (2013) Avoiding coral reef functional collapse requires local and global action. Curr Biol 23:912–918
Kiene WE (1988) A model of bioerosion on the Great Barrier Reef. Proc 6th Int Coral Reef Symp Townsville 3:449–454
Kinsey DW (1983) Standards of performance in coral reef primary production and carbonate turnover. In: Barnes DJ (ed) Perspectives on coral reefs. Brian Clouston, Manuka, pp 209–220
Kleypas JA, Buddemeier RW, Archer D, Gattuso J-P, Langdon C, Opdyke BN (1999a) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Kleypas JA, McManus JW, Meñez LAB (1999b) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159
Langdon C, Atkinson MJ (2005) Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res 110:1–16
Le Campion-Alsumard T, Golubic S, Priess K (1995) Fungi in corals: symbiosis or disease? Interaction between polyps and fungi causes pearl-like biomineralization. Mar Ecol Prog Ser 117:137–147
Leão ZMAN, Telles MD, Sforza R, Bulhões HA, Kikuchi RKP (1993) Impact of tourism development on the coral reefs of the Abrolhos area, Brazil. In: Ginsburg RN (compiler) Global aspects of coral reefs: health, hazards and history. Rosenstiel School of Marine and Atmospheric Science, University of Miami, Coral Gables, FL, pp 254–260
Lirman D, Schopmeyer S, Manzello D, Gramer LJ, Precht WF, Muller-Karger F, Banks K, Barnes B, Bartels E, Bourque A, Byrne J, Donahue S, Duquesnel J, Fisher L, Gilliam D, Hendee J, Johnson M, Maxwell K, McDevitt E, Monty J, Rueda D, Ruzicka R, Thanner S (2011) Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida Reef Tract and reversed previous survivorship patterns. PLoS One 6:e23047
Lowenstam HA (1974) Impact of life on chemical and physical processes. In: Goldberg ED (ed) The sea, vol 5, Marine Chemistry. Wiley, New York, pp 715–796
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press, New York, 324 p
Macintyre IG (1984) Preburial and shallow-subsurface alteration of modern scleractinian corals. In: Oliver WA Jr, Sando WJ, Cairns SD, Coates AG, Macintyre IG, Bayer FM, Sorauf JE (eds) Recent advances in the paleobiology and geology of the Cnidaria. Palaeontographica Americana, Ithaca, New York, 54, pp 229–244
Macintyre IG (1997) Reevaluating the role of crustose coralline algae in the construction of coral reefs. Proc 8th Intl Coral Reef Symp Panama 1:725–730
Manzello DP (2009) Reef development and resilience to acute (El Niño warming) and chronic (high-CO2) disturbances in the eastern tropical Pacific: a real-world climate change model. Proc 11th Intl Coral Reef Symp Ft Lauderdale 1:1299–1304
Manzello DP (2010a) Ocean acidification hotspots: spatiotemporal dynamics of the seawater CO2 system of eastern Pacific coral reefs. Limnol Oceanogr 55:239–248
Manzello DP (2010b) Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs 29:749–758
Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C (2008) Poorly cemented coral reefs of the eastern tropical Pacific: possible insights into reef development in a high-CO2 world. Proc Natl Acad Sci U S A 105:10450–10455
Manzello DP, Enochs IC, Bruckner A, Renaud P, Kolodziej G, Budd DA, Carlton R, Glynn PW (2014) Galápagos coral reef persistence after ENSO warming across an acidification gradient. Geophys Res Lett 41:9001–9008
May JA, Macintyre IG, Perkins RD (1982) Distribution of microborers within planted substrates along a barrier reef transect, Carrie Bow Cay, Belize. In: Rützler K, Macintyre IG (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize I: structure and communities, Smith Contrib Mar Sci, Washington DC,12, 539 p
McClanahan TR, Shafir SH (1990) Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia 83:362–370
McLean RF (1967) Measurement of beach rock erosion by some tropical marine gastropods. Bull Mar Sci 17:551–561
Moran PJ (1986) The Acanthaster phenomenon. Oceanogr Mar Biol Ann Rev 24:379–480
Neumann AC (1966) Observations on coastal erosion in Bermuda and measurements of the boring rates of the sponge Cliona lampa. Limnol Oceanogr 11:92–108
Ogden JC (1977) Carbonate-sediment production by parrotfish and sea urchins on Caribbean reefs. In: Frost SH, Weiss MP, Saunders JB (eds) Reefs and related carbonates—ecology and sedimentology. Studies in geology 4. American Association of Petroleum Geologists, Tulsa, pp 281–288
Pari N, Peyrot-Clausade M, Le Campion-Alsumard T, Hutchings P, Chazottes V, Golubic S, Le Campion J, Fontaine MF (1998) Bioerosion of experimental substrates on high islands and on atoll lagoons (French Polynesia) after two years of exposure. Mar Ecol Prog Ser 166:119–130
Pearson RG (1981) Recovery and recolonization of coral reefs. Mar Ecol Prog Ser 4:105–122
Perry CT, Hepburn LJ (2008) Syn-depositional alteration of coral reef framework through bioerosion, encrustation and cementation: taphonomic signatures of reef accretion and reef depositional events. Earth Sci Rev 86:106–144
Perry CT, Edinger EN, Kench PS, Murphy GN, Smithers SG, Steneck RS, Mumby PJ (2012) Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31:853–868
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nature Commun 4:1–7
Peters EC (1984) A survey of cellular reactions to environmental stress and disease in Caribbean scleractinian corals. Helgoländer Meeresun 37:113–137
Podestá GP, Glynn PW (2001) The 1997–98 El Niño event in Panamá and Galápagos: an update of thermal stress indices relative to coral bleaching. Bull Mar Sci 69:43–60
Pomponi SA (1979) Ultrastructure and cytochemistry of the etching area of boring sponges. In: Lévi C, Boury-Esnault N (eds) Biologie des Spongiaires, Colloques Internationaux du Centre National de la Recherche Scientifique, Paris, 291, pp 317–323
Randall JE (1974) The effects of fishes on coral reefs. Proc 2nd Int Coral Reef Symp Brisbane 1:159–166
Rasmussen KA, Frankenberg EW (1990) Intertidal bioerosion by the chiton Acanthopleura granulata; San Salvador, Bahamas. Bull Mar Sci 47:680–695
Reaka-Kudla ML, Feingold JS, Glynn PW (1996) Experimental studies of rapid bioerosion of coral reefs in the Galápagos Islands. Coral Reefs 15:101–107
Reyes-Nivia C, Diaz-Pulido G, Kline D, Hoegh-Guldberg O, Dove S (2013) Ocean acidification and warming scenarios increase microbioerosion of coral skeletons. Global Chang Biol 19:1919–1929
Rice ME, Macintyre IG (1972) A preliminary study of sipunculan burrows in rock thin sections. Carib J Sci 12:41–44
Rice ME, Macintyre IG (1982) Distribution of Sipuncula in the coral reef community, Carrie Bow Cay, Belize. In: Rützler K, Macintyre IG (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize I: structure and communities. Smithson Contrib Mar Sci, Washington, DC, pp 311–320
Risk MJ, MacGeachy JK (1978) Aspects of bioerosion of modern Caribbean reefs. Rev Biol Trop 26(suppl 1):85–105
Risk MJ, Sammarco PW (1982) Bioerosion of corals and the influence of damselfish territoriality: a preliminary study. Oecologia 52:376–380
Risk MJ, Dunn JJ, Allison WR, Horrill C (1993) Reef monitoring in Maldives and Zanzibar: low-tech and high-tech science. In: Ginsburg RN (compiler) Global aspects of coral reefs: health, hazards and history. Rosenstiel School of Marine and Atmospheric Science, University of Miami, Coral Gables, pp 66–72
Risk MJ, Sammarco PW, Edinger EN (1995) Bioerosion in Acropora across the continental shelf of the Great Barrier Reef. Coral Reefs 14:79–86
Rose CS, Risk MJ (1985) Increase in Cliona delitrix infestation of Montastraea cavernosa heads on an organically polluted portion of the Grand Cayman fringing reef. PSZNI Mar Ecol 6:345–363
Russo AR (1980) Bioerosion by two rock boring echinoids (Echinometra mathaei and Echinometra aciculatus) on Enewetak Atoll, Marshall Islands. J Mar Res 38:99–110
Rützler K (1974) The burrowing sponges of Bermuda. Smithson Contrib Zool 165:1–32
Rützler K (1975) The role of burrowing sponges in bioerosion. Oecologia 19:203–216
Rützler K (2002) Impact of crustose clionid sponges on Caribbean reef corals. Acta Geol Hisp 37:61–72
Rützler K, Rieger G (1973) Sponge burrowing: fine structure of Cliona lampa penetrating calcareous substrata. Mar Biol 21:144–162
Rützler K, Santavy DL, Antonius A (1983) The black band disease of Atlantic reef corals. III. Distribution, ecology, and development. PSZNI Mar Ecol 4:329–358
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Tilbrook B, Millero FJ, Peng T-H, Kozyr A, Ono T, Rios AF (2004) The oceanic sink for anthropogenic CO2. Science 305:367–371
Sano M (2000) Stability of reef fish assemblages: responses to coral recovery after catastrophic predation by Acanthaster planci. Mar Ecol Prog Ser 198:121–130
Sano M, Shimizu M, Nose Y (1987) Long-term effects of destruction of hermatypic coral by Acanthaster planci infestation on reef fish communities of Iriomote Island, Japan. Mar Ecol Prog Ser 37:191–199
Schönberg CHL (2002) Substrate effects on the bioeroding demosponge Cliona orientalis. 1. Bioerosion rates. PSZNI Mar Ecol 23:313–326
Schönberg CHL, Ortiz JC (2009) Is sponge bioerosion increasing? Proc 11th Int Coral Reef Symp, Ft. Lauderdale, USA, pp 520–523
Scoffin TP, Stearn CW, Boucher D, Frydl P, Hawkins CM, Hunter IG, MacGeachy JK (1980) Calcium carbonate budget of a fringing reef on the west coast of Barbados. Part II—Erosion, sediments and internal structure. Bull Mar Sci 30:475–508
Scott PJB, Risk MJ (1988) The effect of Lithophaga (Bivalvia: Mytilidae) boreholes on the strength of the coral Porites lobata. Coral Reefs 7:145–151
Scott PJB, Risk MJ, Carriquiry JD (1988) El Niño, bioerosion and the survival of east Pacific reefs. Proc 6th Int Coral Reef Symp Townsville 2:517–520
Smith SR, Ogden JC (eds) (1993) Status of recent history of coral reefs at the CARICOMP network of Caribbean marine laboratories. In: Ginsburg RN (compiler) Global aspects of coral reefs: health, hazards and history. Rosenstiel School of Marine and Atmospheric Science, University of Miami, Coral Gables, FL, pp 73–79
Smith SV, Kimmerer WJ, Laws EA, Brock RE, Walsh TW (1981) Kaneohe Bay sewage diversion experiment: perspectives on ecosystem response to nutritional perturbation. Pac Sci 35:279–402
Steneck RS (1983a) Quantifying herbivory on coral reefs: just scratching the surface and still biting off more than we can chew. In: Reaka ML (ed) The ecology of deep and shallow coral reefs. NOAA Symp Ser Undersea Res, Walpole, Maine 1:103–111
Steneck RS (1983b) Escalating herbivory and resulting adaptive trends in calcareous algal crusts. Paleobiology 9:44–61
Stimson J, Conklin E (2008) Potential reversal of a phase shift: the rapid decrease in the cover of the invasive green macroalga Dictyosphaeria cavernosa Forsskål on coral reefs in Kāne’ohe Bay, Oahu, Hawai’i. Coral Reefs 27:717–726
Tanzil JTI, Brown BE, Tudhope AW, Dunne RP (2009) Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand between 1984 and 2005. Coral Reefs 28:519–528
Tomascik T, Mah AJ, Nontji A, Moosa MK (1997) The ecology of the Indonesian seas. Vol. 8, part II, chapters 13–23, Oxford University Press, Oxford, pp 1231–1239
Tribollet A (2008) The boring microflora in modern coral reef ecosystems: a review of its roles. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 67–94
Tribollet A, Golubic S (2005) Cross-shelf differences in the pattern and pace of bioerosion of experimental carbonate substrates exposed for 3 years on the northern Great Barrier Reef, Australia. Coral Reefs 24:422–434
Tribollet A, Golubic S (2011) Reef bioerosion: agents and processes. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 435–449
Tribollet A, Godinot C, Atkinson M, Langdon C (2009) Effects of elevated pCO2 on dissolution of coral carbonates by microbial euendoliths. Global Biogeochem Cycles 23:1–7
Trudgill ST (1976) The marine erosion of limestones on Aldabra Atoll, Indian Ocean. Z Geomorph NF Suppl Bd 26:164–200
Trudgill ST (1983) Measurements of rates of erosion of reefs and reef limestones. In: Barnes DJ (ed) Perspectives on coral reefs. Brian Clouster, Manuka, pp 256–262
Tudhope AW, Risk MJ (1985) Rate of dissolution of carbonate sediments by microboring organisms, Davies Reef, Australia. J Sediment Petrol 55:440–447
Tunnicliffe V (1979) The role of boring sponges in coral fracture. In: Levi C and Boury-Esnault N (eds) Biologie des spongiaires. Centre National de la Recherche Scientifique, Paris, No. 291, pp 309–315
van Hooidonk R, Maynard J, Manzello DP, Planes S (2014) Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Global Chang Biol. doi:10.1111/gcb.12394
Vénec-Peyré M-T (1996) Bioeroding foraminifera: a review. Mar Micropaleontol 28:19–30
Vermeij GJ (1987) Evolution and escalation, an ecological history of life. Princeton University Press, Princeton, 531 p
Vogel K (1993) Bioeroders in fossil reefs. Facies 28:109–114
Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC (2005) Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Mar Pollut Bull 51:570–579
Warme JE (1975) Borings as trace fossils, and the processes of marine bioerosion. In: Frey RW (ed) The study of trace fossils. Springer, New York, pp 181–227
Warme JE (1977) Carbonate borers—their role in reef ecology and preservation. In: Frost SH, Weiss MP, Saunders JB (eds) Reefs and related carbonates—ecology and sedimentology. AAPG Stud Geol 4, American Association of Petroleum Geologists, Tulsa, pp 261–279
Wellington GM, Glynn PW (2007) Responses of coral reefs to El Niño-Southern Oscillation sea-warming events. In: Aronson RB (ed) Geological approaches to coral reef ecology. Springer, New York, pp 342–385
Wilkinson CR (1983) Role of sponges in coral reef structural processes. In: Barnes DJ (ed) Perspectives on coral reefs. Brian Clouston, Manuka, pp 263–274
Wilson MA (2008) An online bibliography of bioerosion references. In: Wisshack M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 473–478
Wisshak M, Schönberg CHL, Form A, Freiwald A (2012) Ocean acidification accelerates reef bioerosion. PLoS One 7:e45124
Zea S, Weil E (2003) Taxonomy of the Caribbean excavating sponge species complex Cliona caribbaea-C. aprica-C. langae (Porifera, Hadromerida, Clionaidae). Carib J Sci 39:348–370
Zundelevich A, Lazar B, Ilan M (2007) Chemical versus mechanical bioerosion of coral reefs by boring sponges-lessons from Pione cf. vastifica. J Exp Biol 210:91–96
Acknowledgments
Thanks are due Charles G Messing, Klaus Rützler, Paul L Jokiel and other referenced workers for the illustrations in this chapter. Michael J Risk and Charles Birkeland are acknowledged for helping with various literature leads, and Ann Campbell for her diligence in providing numerous published sources. Updating of the condition of the Coconut Island fringing reef was made possible by C Birkeland, PL Jokiel, John Stimson, and Nadiera Sukhraj. Joshua Levy kindly assisted in up-dating Fig. 4.12 and Michael P. C. Fuller with page proofing. Research reported by PW Glynn was supported by the Smithsonian Tropical Research Institute, National Science Foundation (Biological Oceanography Program), and the National Geographic Society. DP Manzello has received support from the National Oceanic and Atmospheric Administration via the Coral Reef Conservation and Ocean Acidification Programs.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Glynn, P.W., Manzello, D.P. (2015). Bioerosion and Coral Reef Growth: A Dynamic Balance. In: Birkeland, C. (eds) Coral Reefs in the Anthropocene. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7249-5_4
Download citation
DOI: https://doi.org/10.1007/978-94-017-7249-5_4
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7248-8
Online ISBN: 978-94-017-7249-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)