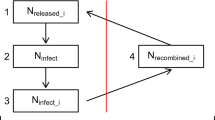
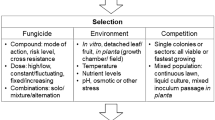
Summary
Knowledge of resistance at the molecular, cellular and organismal level is a basis for understanding resistance at the population level, but does not completely explain resistance at the population level. Other processes or mechanisms operate at the population level increasing or decreasing the significance of resistance. This chapter deals with the various mechanisms operating at the population level influencing host — pathogen interactions. The role of the mechanism of resistance is clarified considering the set of mechanisms determining host — pathogen interactions at the population level.
Populations and their attributes are described in general terms to allow development of an understanding of what is meant by the phenomenon “population”. Subsequently, escape, avoidance, and the paired effects of predisposition and immunisation are described as mechanisms operating before resistance comes into play. Compensation and tolerance are described as mechanisms operating after resistance and relevant to the phenomena of selection and fitness, which are treated subsequently.
Some thoughts about management of resistance in crops are developed by considering all the mechanisms involved in host — pathogen interactions at the population level, and by contrasting wild and crop pathosystems.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Agrios GN (1980) Escape from disease. In: Horsfall JG and Cowling EB (eds) Plant Disease: an Advanced Treatise, Vol 5: How Plants Defend Themselves, pp 17–37. Academic Press, New York / London
Andrivon D (1996) The origin of Phytophthora infestans populations present in Europe in the 1840s: a critical review of historical and scientific evidence. Plant Pathol 45: 1027–1035
Antonovics J and Miller-Alexander H (1989) The concept of fitness in plant-fungal pathogen systems. In: Leonard KJ and Fry WE (eds) Plant Disease Epidemiology, Vol 2: Genetics, Resistance and Management, pp 185–214. McGraw-Hill Publishing Company, New York
Augspurger CK (1983) Seed dispersal of the tropical tree, Platypodium elegans, and the escape of its seedlings from fungal pathogens. J Ecol 71: 759–771
Biere A and Antonovics J (1996) Sex-specific costs of resistance to the fungal pathogen Ustilago violacea (Microbotryum violaceum) in Silene alba. Evolution 50: 1098–1110
Burdon JJ (1987a) Diseases and Plant Population Biology. Cambridge University Press, Cambridge
Burdon JJ (1987b) Phenotypic and genetic patterns of resistance to the pathogen Phakopsora pachyrhizi in populations of Glycine canescens. Oecologia (Berlin) 73 257–267
Burdon JJ and Jarosz AM (1992) Temporal variation in the racial structure of flax rust (Melampsora lini) populations growing on natural stands of wild flax (Linum marginale): local versus metapopulation dynamics. Plant Pathol 41: 165–179
Chin KM and Wolfe MS (1984) The spread of Erysiphe graminis f. sp. hordei in mixtures of barley varieties. Plant Pathol 33: 89–100
Clarke DD (1986) Tolerance of parasites and disease in plants and its significance in host-parasite interactions. Adv Plant Pathol 5: 161–197
Cooper WS (1984) Expected time to extinction and the concept of fundamental fitness. J Theor Biol 107: 603–629
Crute IR and Pink DAC (1996) Genetics and utilization of pathogen resistance in plants. Plant Cell 8: 1747–1755
Damgaard C. and Giese H (1996) Genetic variation in Danish populations of Erysiphe graminis f.sp. hordei: estimation of gene diversity and effective population size using RFLP data. Plant Pathol 45: 691–696
Dean RA and Kuç JA (1987) Immunisation against disease: the plant fights back. In: Pegg GF and Ayres PG (eds) Fungal Infection of Plants, pp 383–410. Cambridge University Press, Cambridge
Dinoor A and Eshed N (1984) The role and importance of pathogens in natural plant communities. Annu Rev Phytopathol 22: 443–466
Drenth A, Tas ICQ and Govers F (1994) DNA fingerprinting uncovers a new sexually reproducing population of Phytophthora infestans in the Netherlands. Eur J Plant Pathol 100: 97–107
de Nooij MP (1988) The role of weevils in the infection process of the fungus Phomopsis subordinaria in Plantago lanceolata. Oikos 52: 51–58
de Nooij MP and van Damme JMM (1988a) Variation in host susceptibility among and within populations of Plantago lanceolata L. infected by the fungus Phomopsis subordinaria (Desm.) Trav. Oecologia (Berlin) 75 535–538
de Nooij MP and van Damme JMM (1988b) Variation in pathogenicity among and within populations of the fungus Phomopsis subordinaria infecting Plantago lanceolata. Evolution 42 1166–1171
Endler JA (1986) Natural Selection in the Wild. Princeton University Press, Princeton.
Ennos R (1992) Ecological genetics of parasitism. In: Berry RJ, Crawford TJ and Hewitt GM (eds) Genes in Ecology, pp 255–279. Blackwell Scientific Publications, Oxford
Falconer DS (1989) Introduction to Quantitative Genetics. Longman Scientific & Technical, Harlow
Frantzen J (1994a) Studies on the weed pathosystem Cirsium arvense — Puccinia punctiformis. Thesis Agricultural University Wageningen, Wageningen. ISBN 90-5485-211-9
Frantzen J (1994b) The role of clonal growth in the pathosystem Cirsium arvense — Puccinia punctiformis. Can J Bot 72 832–836
Fry WE, Goodwin SB, Matuszak JM, Spielman LJ and Milgroom MG (1992) Population genetics and intercontinental migrations of Phytophthora infestans. Annu Rev Phytopathol 30: 107–129
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63: 151–162
Harry IB and Clarke DD (1986) Race-specific resistance in groundsel (Senecio vulgaris) to the powdery mildew Erysiphe fischeri. New Phytol 103: 167–175
Harry IB and Clarke DD (1987) The genetics of race-specific resistance in groundsel (Senecio vulgaris L.) to the powdery mildew fungus, Erysiphe fischeri Blumer. New Phytol 107: 715–723
Hatcher PE, Paul ND, Ayres PG and Whittaker JB (1994) Interactions between Rumex spp., herbivores and a rust fungus: Gastrophysa viridula grazing reduces subsequent infection by Uromyces rumicis. Func Ecol 8: 265–272
Jennersten O, Nilsson SG and Wästljung U (1983) Local plant populations as ecological islands: the infection of Viscaria vulgaris by the fungus Ustilago violacea. Oikos 41: 391–395
Johnson R (1992) Reflections of a plant pathologist on breeding for disease resistance, with emphasis on yellow rust and eyespot of wheat. Plant Pathol 41: 239–254
Katsuya K and Green GJ (1967) Reproductive potentials of races 15B and 56 of wheat stem rust. Can J Bot 45: 1077–1091
Kolmer JA (1995) Selection of Puccinia recondita f.sp. tritici virulence phenotypes in three multilines of Thatcher wheat lines near isogenic for leaf rust resistance genes. Can J Bot 73: 1081–1088
Linders EGA, van Damme JMM and Zadoks JC (1996) Transmission and overseasoning of Diaporthe adunca on Plantago lanceolata. Plant Pathol 45: 59–69
McDermott JM and McDonald BA (1993) Gene flow in plant pathosystems. Annu Rev Phytopathol 31: 353–373
Murray DC and Walters DR (1992) Increased photosynthesis and resistance to rust infection in upper, uninfected leaves of rusted broad bean (Vicia faba L.). New Phytol 120: 235–242
Nelson RR (1978) Genetics of horizontal resistance to plant diseases. Annu Rev Phytopathol 16: 359–378
Newton AC and Andrivon D (1995) Assumptions and implications of current gene-for-gene hypotheses. Plant Pathol 44: 607–618
Olivieri I, Couvet D and Gouyon P-H (1990) The genetics of transient populations: research at the metapopulation level. TREE 5: 207–210
Parker MA (1987) Pathogen impact on sexual vs. asexual reproductive success in Arisaema triphyllum. Am J Bot 74: 1758–1763
Parlevliet JE (1989). Identification and evaluation of quantitative resistance. In: Leonard KJ and Fry WE (eds) Plant Disease Epidemiology, Vol 2: Genetics, Resistance and Management, pp 215–248. McGraw-Hill Publishing Company, New York
Paul ND and Ayres PG (1984) Effects of rust and post-infection drought on photosynthesis, growth and water relations in groundsel. Plant Pathol 33: 561–569
Robinson (1976) Plant Pathosystems. Springer Verlag, Berlin
Schutz WM and Brim CA (1971) Inter-genotypic competition in soybeans. III. An evaluation of stability in multiline mixtures. Crop Sci 11: 684–689
Schafer JF (1971) Tolerance to plant disease. Annu Rev Phytopathol 9: 235–252
Schoeneweiss DF (1975) Predisposition, stress, and plant disease. Annu Rev Phytopathol 13: 193–211
Thompson JN (1990) Coevolution and the evolutionary genetics of interactions among plants and insects and pathogens. In: Burdon JJ and Leather SR (eds) Pests, Pathogens and Plant Communities, pp 249–271. Blackwell Scientific Publications, Oxford
Van der Putten WH and Troelstra SR (1990) Harmful soil organisms in coastal foredunes involved in degeneration of Ammophila arenaria and Calammophila baltica. Can J Bot 68: 1560–1568
Wennström A and Ericson L (1992) Environmental heterogeneity and disease transmission within clones of Lactuca siberica. J Ecol 80: 71–77
Wolfe MS (1985) The current status and prospects of multiline cultivars and variety mixtures for disease resistance. Annu Rev Phytopathol 23: 251–273
Wolfe MS, Brandie U, Koller B, Limpert E, McDermott JM, Müller K and Schaffner D (1992) Barley mildew in Europe: population biology and host resistance. Euphytica 63: 125–139
Zadoks JC and Schein RD (1979) Epidemiology and Plant Disease Management. Oxford University Press, New York / Oxford
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2000 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Frantzen, J. (2000). Resistance in Populations. In: Slusarenko, A.J., Fraser, R.S.S., van Loon, L.C. (eds) Mechanisms of Resistance to Plant Diseases. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-3937-3_6
Download citation
DOI: https://doi.org/10.1007/978-94-011-3937-3_6
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-0399-8
Online ISBN: 978-94-011-3937-3
eBook Packages: Springer Book Archive