Abstract
Solutions containing iron (Fe) were reacted by oxidation or by base addition to form precipitates with various amounts of phosphate (P). The reactions were carried out to simulate the following three scenarios which may occur in soil systems: First, a soil undergoes reduction producing high concentrations of ferrous Fe and P, which are then co-precipitated due to aeration; second, Fe hydroxides are precipitated due to aeration of ferrous Fe after which the precipitate reacts with P; and third, Fe hydroxides formed by base addition to ferric nitrate are reacted with P.
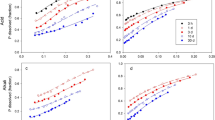
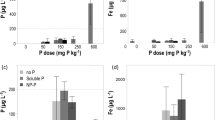
Co-precipitation of Fe and P resulted in a system where pH3PO4 had a value of 7.5 ± 0.2 which was independent of the mole fraction of P in the system. Where P was adsorbed onto iron oxides at low concentrations of P, the solubility of P and the values of pH3PO4 were similar to co-precipitated Fe and P. With increased additions of P, the solubility of P was much greater where it was sorbed than co-precipitated. The increased solubility was attributed to an increase in the activity of solid phase P compounds, in this case described as similar to a regular solid-solution. Fe hydroxides formed by precipitation of ferric nitrate due to base addition adsorbed more P than those formed by aeration of ferrous chloride. Aging by heating at 63°C did not significantly effect the amount of P adsorbed by Fe hydroxide formed from chloride or nitrate.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ainsworth C C, Sumner M E and Hurst V J 1985 Effects of aluminum substitution in goethite on phosphorus adsorption. I. Adsorption and isotropic exchange. Soil Sci. Soc. Am. J. 49, 1142–1149.
Arlidge E Z, Farmer V C, Mitchell B D and Mitchell W A 1963 Infra-red, X-ray and thermal analysis of some aluminum and ferric phosphates. J. Appl. Chem. 13, 17–27.
Benjamin M M and Bloom N S 1981 Effects of strong binding of ionic adsorbate on adsorption of trace metals in amorphous iron oxyhydroxide. In Adsorption from Aqueous Solution. Ed. P H Tewari. Plenum Press, New York
Blanchar R W and Stearman G K 1984 Ion products and solid-phase activity of describe phosphate sorption by soils. Soil Sci. Soc. Am. J. 48, 1253–1258.
Bohn H L and Peech M 1969 Phosphatoiron (III) and phosphatoaluminum complexes in dilute solutions. Soil Sci. Soc. Am. Proc. 33, 873–876.
Corey R B 1981 Adsorption vs precipitation. pp 161–183. In Adsorption of Inorganics at Solid-liquid Interfaces. Eds. M A Anderson and A J Rubin. Ann Arbor Science Publishers, Inc., Ann Arbor, MI.
EPA 1974 Methods for chemical analysis of water and wastes. Method 353.2, Nitrogen, nitrate-nitrite (colorimetric, automated, cadmium reduction). U.S. Environmental Protection Agency, Office of Technology Transfer, Washington, DC.
Feagley S E 1979 Rates of Manganese Oxidation in a Menfro Soil. Ph.D. Thesis. University of Missouri Columbia.
Hansmann D D and Anderson M A 1985 Using elec-trophoresis in modelling sulfate, selenite and phosphate adsorption onto goethite. Environ. Sci. Technol. 19, 544–551.
Hess R E and Blanchar R W 1977 Arsenic determination and arsenic, lead and copper content of Missouri soils. Mo. Ag. Exp. Sta. Bull. 1020, 1–46.
Hingston F J, Atkinson R J, Posner A M and Quirk J P 1968 Specific adsorption of anions on goethite. Trans. Int. Congr. Soil Sci. 9th. I, 669–678.
Langmuir D and Whittemore D O 1971 Variations in the stability of precipitated ferric oxyhydroxides. Adv. Chem. 106, 209–234.
Lindsay W L 1979 Chemical Equilibria in Soils. John Wiley and Sons, New York.
Martin R R and Smart R St C 1987 X-ray photoelectron studies of anion adsorption on goethite. Soil Sci. Soc. Am. J. 51, 54–56.
Martin R R, Smart R St C and Tazaki K 1988 Direct observation of phosphate precipitation in the goethite/ phosphate system. Soil Sci. Soc. Am. J. 52, 1492–1500.
Mortensen J L, Anderson D M and White J L 1965 Infrared spectroscopy. In Methods of Soil Analysis. Part 1. Ed. C A Black. Agron. 9, 743–770.
Munsell Color 1975 Munsell Soil Color Charts. Macbeth Division of Killmorgen Corp., Baltimore, MD.
Nordstrom D K 1977 Thermochemical redox equilibria of ZoBell’s solution. Geochim. Cosmochim. Acta 41, 1835–1841.
Nriagu J O 1972 Solubility equilibrium constant of strengite. Am. J. Sci. 272, 476–484.
Parfitt R L 1978 Anion adsorption by soils and soil materials. Adv. Agron. 30, 1–50.
Sah R N and Mikkelsen D S 1989 Phosphorus behavior in flooded-drained soils. I. Effects on phosphorus sorption. Soil Sci Soc. Am. J. 53, 1718–1722.
Skoog D A and West D M 1974 Analytical Chemistry: An introduction, 2nd ed. Holt, Rinehart and Winston, Inc. New York.
Willet I R and Cunningham R B 1983 Influence of sorbed phosphate on the stability of ferric hydrous oxide under controlled pH and Eh conditions. Aust. J. Soil Res. 21, 301–308.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1991 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Blanchar, R.W., Frazier, M.D. (1991). Soil model of iron phosphate solubility. In: Wright, R.J., Baligar, V.C., Murrmann, R.P. (eds) Plant-Soil Interactions at Low pH. Developments in Plant and Soil Sciences, vol 45. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-3438-5_11
Download citation
DOI: https://doi.org/10.1007/978-94-011-3438-5_11
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-5520-8
Online ISBN: 978-94-011-3438-5
eBook Packages: Springer Book Archive