Abstract
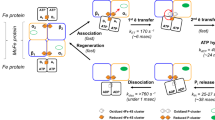
The conveners in their introductions used the Rees model of the nitrogenase protein complex and its metal clusters to formulate the questions that should be addressed in the discussion. These included the site of N2 binding and the nature of metal-bound intermediates, the Russian view favouring a diazene or hydrazine derivative stabilised by electron donation from a metal cluster. It was stressed that although it is generally accepted that substrate reduction occurs on the FeMo-cofactor, there is currently no data to show whether Fe or Mo or both metals are involved in N2 reduction. A key question was the role of homocitrate and the influence of protein environment, in particular hydrogen bonding and electrostatic interactions with the FeMo-cofactor. The mechanism of ATP hydrolysis and energy transduction via the Fe-protein/MoFe-protein interface should be discussed with reference to other systems such as actino-myosin, kinesin and G-proteins. Data to be presented by Dr. Lowe in his lecture (see also his article in this book) shows that phosphate is released from the nitrogenase complex at a rate that is very similar to that measured for these analogous energy transducing, two protein, systems (k for phosphate release in the range 15 to 22 s-1). It is currently not clear how the energy of ATP hydrolysis is used although an attractive hypothesis is that protein conformation changes originating in the Fe-protein transmitted to the MoFe-protein via the interface, modulate the protonation state and redox potential of the FeMo-cofactor.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1995 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Likhtenstein, G.I., Thorneley, R.N.F. (1995). Nitrogenase Catalysis. In: Tikhonovich, I.A., Provorov, N.A., Romanov, V.I., Newton, W.E. (eds) Nitrogen Fixation: Fundamentals and Applications. Current Plant Science and Biotechnology in Agriculture, vol 27. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-0379-4_81
Download citation
DOI: https://doi.org/10.1007/978-94-011-0379-4_81
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-4170-6
Online ISBN: 978-94-011-0379-4
eBook Packages: Springer Book Archive