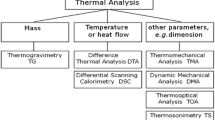
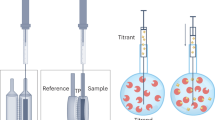
Abstract
Calorimetric techniques constitute a powerful tool to investigate materials. The methods used for the characterization of thermodynamic properties for molten salts include temperature, enthalpy and heat capacity measurements as mixing enthalpy and phase diagram determinations for their mixtures.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
A. Tian, J. Chim. Phys. 20, 132 (1923)
E. Calvet and H. Prat, Microcalorimetrie, Masson, Paris (1955); E. Calvet and H. Prat, Recents progrès en microcalorimetrie, Dunod, Paris (1958)
M. Gaune-Escard and J. P. Bros, Thermochim. Acta 31, 323 (1979)
O. Kubaschewski and E. L. L. Evans, La thermochimie en métallurgie, Gauthier-Villard, Paris (1964)
H. Eslami, Thesis, Universite de Provence, Marseille (1976)
R. Fehrmann, M. Gaune-Escard, and N. J. Bjerrum, Inorg. Chem. 25, 1132 (1986)
M. Gaune-Escard, Thesis, Universite de Provence, Marseille (1972)
O. J. Kleppa, J. Phys. Chem. 64, 1937 (1960)
M. Gaune-Escard, L. Rycerz, A. Bogacz. Enthalpies of mixing in the DyCl3-NaCl, DyCl3-KCl and DyCl3-PrCl3 liquid systems. J. Alloys and Compounds, 204. 185–188, (1994).
R. Takagi, L. Rycerz, M. Gaune-Escard. Mixing enthalpy and structure of the molten NaCl-DyCl3 system. Denki Kagaku, 62, 3, 240, (1994).
M. Gaune-Escard, L. Rycerz, W. Szczepaniak, A. Bogacz. Enthalpies of mixing in the PrCl3-CaCl2 and NdCl3-CaCl2 liquid systems, Thermochimica Acta, 236, 51–58, (1994).
M. Gaune-Escard, L. Rycerz, W. Szczepaniak, A. Bogacz, Calorimetric investigation of the NdCl3-MCl (M = Na, K, Rb, Cs). Thermochimica Acta, 236, 67–80, (1994).
M. Gaune-Escard, L. Rycerz, W. Szczepaniak, A. Bogacz, Calorimetric investigation of the PrCl3-NaCl and PrCl3-KCl liquid mixtures. Thermochimica Acta, 236, 59–66, (1994).
M. Gaune-Escard, A. Bogacz, L. Rycerz, W. Szczepaniak. Formation enthalpies of the MBr-LaBr3 liquid mixtures (M = Li, Na, K, Rb, Cs). Thermochimica Acta, 279, 1–10, (1996).
M. Gaune-Escard, L. Rycerz. Mixing enthalpy of TbCl3-MCl liquid mixtures. M = Li, Na, K, Rb, Cs. High Temp. Material Processes, 2, n°4, 483–496 (1998).
M. Gaune-Escard, L. Rycerz. Calorimetric investigation of the NdI3-MI systems ((M = Li, Na, K, Cs). Molten Salt Forum, 5-6, 217 (1998)
F. da Silva, L. Rycerz, M. Gaune-Escard, Z. Naturforsch 2001 (under press)
H. Aghai-Khafri, J.P. Bros, M. Gaune-Escard, Chem. Thermodynamics, 8, 331–338, (1976)
W. Lukas, M. Gaune-Escard, and J. P. Bros, J. Chem. Thermodyn. 19, 717 (1987)
J. W. Johnson, W. J. Silva, and D. J. Cubicciotti, J. Chem. Phys. 69, 3916 (1965)
D. Chiotti, G. Gartner, E. Stevens, and Y. Saito, J. Chem. Eng. Data 11, 571 (1966)
Z. Benkhaldoun, Thesis, Universite de Provence, Marseille (1985)
G. Hatem, M. Gaune-Escard, J. P. Bros, and T. Ostvold, Ber. Bunsenses Phys Chem 92, 751 (1988).
Y. Fouque, M. Gaune-Escard, W. Szczepaniak, and A. Bogacz, J. Chim. Phys. 75, 360 (1978)
G. N. Papatheodorou and O. J. Kleppa, Z. Anorg. Allg. Chem. 401, 132 (1973)
J. L. Holm and O. J. Kleppa, Inorg. Chem. 6, 645 (1967)
T. Ostvold and O. J. Kleppa, Inorg. Chem. 8, 78 (1969)
B. K. Andersen and O. J. Kleppa, Acta Chem. Scand. Ser. A 30, 751 (1976)
M. Gaune-Escard and J. P. Bros, Can. Met. Q. 13(2), 335 (1974)
G. Hatem, P. Gaune, J. P. Bros, F. Gehringer, and E. Hayer, Rev. Sci. Instrum. 52, 585 (1981)
G. Hatem and M. Gaune-Escard, J. Chem. Thermodyn. 11, 927 (1979)
J. P. Bros, H. Eslami, and P. Gaune, Ber. Bunsenges. Phys. Chem. 85, 333 (1981)
G. Hatem and M. Gaune Escard, 9th Experimental Thermodynamic Conference, London April 16-18, 1980
E. Hayer, K. L. Komarek, J. P. Bros, and M. Gaune-Escard, Z. Metallkde. 72, 109 (1981)
J. M. Miane, M. Gaune-Escard, and J. P. Bros, High Temp.-High Pressures 9, 465 (1977)
G. Hatem, M. Gaune-Escard, and A. Pelton, J. Phys. Chem. 86, 3039 (1982)
G. Hatem and M. Gaune-Escard, J. Chem. Thermodyn. 16, 897 (1984)
G. Hatem, F. Tabaries, and M. Gaune-Escard, Thermochim. Acta 149, 15, (1989)
K. Mahmoud, Thesis, Universite de Provence, Marseille (1989)
P. Peretz, G. Hatem, M. Gaune-Escard, M. Hoch, Thermochimica Acta 262, 45–54 (1995)
G. Hatem, M. Gaune-Escard, J. Chem. Thermodynamics, 25, 219–228 (1993).
E. Hayer, K. Komarek, J. P. Bros, and M. Gaune-Escard, 4th International Conference on Liquid and Amorphous Metals, Grenoble, July 7-11, 1980
D. El Allam, Thesis, Universite de Provence, Marseille (1989)
M. J. O’Neil, Anal. Chem. 38, 1331 (1966)
A. Bogacz, W. Wisniowski, Y. Fouque, J. P. Bros, and M. Gaune-Escard, J. Cat. Anal. Therm XIV, 339 (1983)
A. Bogacz, J. P. Bros, Y. Fouque, M. Gaune-Escard, and W. Szczepaniak, J. Chem. Soc., Faraday Trans1. 80, 2935 (1984)
Y. Fouque, J. P. Bros, M. Gaune-Escard, M. Wisniowski, and A. Bogacz, Ber. Bunsenges Phys. Chem. 89, 777 (1985)
J. P. Bros, M. Gaune-Escard, W. Szczepaniak, A. Bogacz, and A. W. Hewat, Acta Crystallogr. Sect. B, 43, 113(1987)
M. Gaune-Escard, L. Rycerz, W. Szczepaniak, A. Bogacz, Entropies of phase transitions in the the M3LnCl6 compounds (M = K, Rb, Cs; Ln = La, Ce, Pr, Nd) and K2LaCl5, J. Alloys and compounds, 204, 189–192, (1994).
M. Gaune-Escard, L. Rycerz, W. Szczepaniak, A. Bogacz. Enthalpies of phase transition of the lanthanide chlorides LaCl3, CeCl3, PrCl3, NdCl3, GdCl3, DyCl3, ErCl3 and TmCl3. J. Alloys and Compounds, 204, 193–196, (1994).
L. Rycerz, M. Gaune-Escard. Enthalpy of phase transition and heat capacity of stoichiometric compounds in LaBr3-MBr systems (M = K, Rb, Cs). J. Thermal Analysis and Calorimetry, 56, 355–363 (1999).
L. Rycerz, M. Gaune-Escard. Enthalpies of phase transitions and heat capacity of SmCl3, EuCl3, TbCl3, ErCl3 and TmCl3 Z. Naturforsch 2001 (sous presse)
J. L. Holm, B. J. Holm, B. Rinnan, and F. GrOnvold, J. Chem. Thermodyn. 5, 97 (1973)
M. Gaune-Escard, A. Bogacz, L. Rycerz, W. Szczepaniak, Heat capacity of LaCl3, CeCl3, PrCl3, NdCl3,GdCl3,DyCl3. J. Alloys Compounds, 235, 176–181, (1996).
M. Gaune-Escard, A. Bogacz, L. Rycerz, W. Szczepaniak Heat capacity of LaC13, CeC13, PrC13, NdC13,GdC13,DyC13 J. Alloys Compounds, 235, 176–181, (1996).
K. M. Gaune-Escard, L. Rycerz Heat capacity of the K3LnC16 compounds — Ln = La, Ce, Pr, Nd. Z. Naturforsch.54 a, 229–235, (1999).
I. M. Gaune-Escard, L. Rycerz Heat capacity of the Rb3LnC16 compounds — Ln = La, Ce, Pr, Nd. Z. Naturforsch. 54 a, 397–403, (1999).
H.J. Seifert, J. Sandrock and J. Uebach, Z. anorg. Allg. Chem. 555, 143 (1987)
G. Tamman, Z. Anorg. Chem. 37, 303 (1903); Z. Anorg. Chem. 45, 24 (1905); Z. Anorg. Chem. 47, 289 (1905); see also J. E. Ricci, The Phase Rule and Heterogeneous Equilibrium, Dover, New York (1966)
O. J. Kleppa, J. Phys. Chem. 61, 1120 (1957)
O. J. Kleppa, J. Phys. Chem. 65, 843 (1961)
J. P. Bros, Thesis, Universite de Provence, Marseille (1968)
G. Hatem, Thesis, Universite de Provence, Marseille, (1980)
F. da Silva, M; Gaune-Escard, Z. Naturforsch 2001 (under press)
Y. Koyama, R. Takagi, Y. Iwadate and K. Fukushima, J. Alloys Comp. 260, 75 (1997)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2002 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Gaune-Escard, M. (2002). Calorimetric Methods. In: Gaune-Escard, M. (eds) Molten Salts: From Fundamentals to Applications. NATO Science Series, vol 52. Springer, Dordrecht. https://doi.org/10.1007/978-94-010-0458-9_16
Download citation
DOI: https://doi.org/10.1007/978-94-010-0458-9_16
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-0459-9
Online ISBN: 978-94-010-0458-9
eBook Packages: Springer Book Archive