Abstract
Dominant tree species from a south-eastern Brazilian savanna showing different leaf phenologies (evergreen, semi-deciduous and deciduous) were characterized regarding photosynthetic potential (A), leaf nitrogen content (% N), specific leaf area (SLA), photosynthetic nitrogen (PN) and photosynthetic nitrogen use efficiency (PNUE). The ecophysiological traits evaluated seasonally (dry and wet season) characterized a gradient of strategies among three species: the evergreen species that dominates lower strata, showed low % N, SLA, Amax and Amass and high PNUE; the semi-deciduous species that dominates intermediate strata, showed medium leaf nitrogen and SLA and high Amax, Amass and PNUE; the deciduous species that dominates the canopy, showed high leaf N, SLA, Amax and Amass and low PNUE. Non deciduous species invested relatively more nitrogen in photosynthesis during the wet season, while the deciduous species maintained higher PN in the dry season. Photosynthetic N and PNUE appear to be the key to a better understanding of the relations among leaf traits, N content and photosynthetic potential in species with different leaf phenologies and subjected to climatic seasonality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerrado Pé-de-gigante
- CO2 response curves
- Deciduous plants
- Leaf nitrogen
- Photosynthetic nitrogen use efficiency
1 Introduction
The Brazilian Savanna (Cerrado) is one of the richest and most threatened tropical savannas in the world and is considered to be a hotspot for biodiversity conservation (Myers et al. 2000) . The Cerrado biome in Brazil covers 2 million km2 representing 23 % of the country’s area (Ratter et al. 1997) . Savanna ecosystems are primarily controlled by the interactions between water and nutrient availability (Medina 1987) with a high influence of seasonality (Nardoto and Bustamante 2003). The basic environmental structure can be modified by changes in fire frequency and land-use (Bustamante et al. 2006). Despite the conversion of the native Cerrado in the recent decades to more intensive land use, few studies have focused on increasing the understanding of the Cerrado ecosystem functioning .
The impact of climatic seasonality on leaf phenology was well documented in the early 1990s. However, the response of trees, particularly trees differing in leaf phenology, to seasonal drought has barely received attention latterly (Eamus et al. 1999). This kind of study is important mainly due to three reasons: as part of climate change research, knowledge of the seasonality of tree behaviour is required to estimate annual carbon (C) fluxes in relation to seasonally dry ecosystems; to manage water resources in such ecosystems; and a complete understanding of any ecosystem can be attained only if the annual cycle is well studied (Eamus et al. 1999).
Phoenix et al. (2006) report substantial increases in nitrogen (N) deposition rates across the tropics over recent decades, as a consequence of fossil-fuel combustion and fertilizer use (Matson et al. 1999). The deposition over Brazilian Atlantic Coast (Galloway et al. 2004), including the Cerrado hotspot has been well documented (Phoenix et al. 2006). Lewis et al. (2004) highlighted several climatic change forces that can act together to modify the forested ecosystems around the world, including increasing CO2 and N deposition, which can promote growth depending on water availability and temperature. But studies on how C and N will possibly interact and on the influence of vegetation on C and N cycles in savanna ecosystem remain scarce (Eamus et al. 1999) . In this context, understanding the assimilation of N and C is pivotal to clarifying these interactions, and photosynthesis is one of the most important physiological processes where these elements interact in plants.
Shipley et al. (2006) showed a unique link between most of the covariation in some leaf traits, across a wide range of environmental conditions and taxonomic groups, such as maximum net photosynthetic rate (Amax), leaf respiration (Rd), leaf N concentration, specific leaf area (SLA), leaf dry matter and leaf life span (LL). Together, this set of leaf traits has been called the ‘worldwide leaf economics spectrum’ (LES) as defined by Wright et al. (2004), because this correlated suite of traits reflects the trade-off between the rapid acquisition of resources and the conservation of captured resources (Marino et al. 2010) . The average values of these traits change predictably along major environmental gradients, but the patterns of covariation are largely (but not completely) unaffected (Marino et al. 2010). Leaf photosynthetic capacity is generally well correlated with leaf N content, but some variations between species occur, and are mainly related to specific leaf area (SLA), i.e. the projected leaf area per unit leaf dry mass (Harrison et al. 2009). In the present study we evaluate the seasonal dynamics of some of the leaf traits involved in the LES and analyze how they interact with N investment into photosynthetic apparatus in dominant tree species belonging to three different phenological groups occurring in a south-eastern Brazilian Savanna.
2 Material and Methods
2.1 Study Area and Species
The study area, known as Pé-de-gigante, is located in a Brazilian Savanna (Cerrado) over Red-Yellow Latosol (Pivello et al. 1998) inside the Vassununga State Park, Sao Paulo State, south-eastern Brazil, (21° 36–38’S, 47° 36–39’W; 590–740 m above sea level;1,225 ha), under a Cwag climate (Köppen 1948). A more detailed characterization of the study area can be found in Pivello et al. (1998, 1999). The species were chosen based on a previous phytossociological study (Latansio-Aidar et al. 2010), which identified and characterized the most important species in the area: Anadenanthera falcata (Leguminosae-Mimosoideae), a deciduous species; Xylopia aromatica (Annonaceae), a semi-deciduous species; and Myrcia lingua (Myrtaceae), an evergreen species .
2.2 Gas Exchange Measurements
Net photosynthesis and stomatal conductance were measured simultaneously using a portable photosynthesis analyzer system (Li-6200, LiCor Inc, Lincoln, NE, USA). Light and CO2 response curves were made in leaves under controlled air temperature (25 °C), light and CO2, a flow rate of 500 mmol s−1 and the relative humidity of air entering the leaf chamber maintained between 70 and 80 % . All measurements were performed from 07.00 to 11.00 h local time during late summer and winter in 2006. Photosynthetic rates were measured in ten fully expanded leaves of each species, ten individuals per species. Immediately after the gas-exchange measurements, each leaf was harvested, taken to the laboratory, and its area, dry mass and N concentration determined. From the data obtained, we calculated specific leaf area (SLA), leaf N content per unit mass (% N), net CO2 assimilation per unit leaf area and mass (Aarea and Amass), instantaneous photosynthetic nitrogen (PN) and photosynthetic nitrogen-use efficiency (PNUE) .
2.3 Leaf Analysis
Fresh leaf areas were measured using Leaf Area Measurement software (Version 1.3). Leaves were oven-dried at 65 °C to get the dry mass, then grounded separately in a ball mill to a fine powder and a 1.5–2 mg sub-sample were placed and sealed in a tin capsule and loaded into a ThermoQuest-Finnigan Delta Plus isotope ratio mass spectrometer (Finnigan-MAT; CA, USA) in line with an Elemental Analyzer (Carlo Erba model 1110; Milan, Italy) as described by Stewart et al. (1995). Light curves were fitted with a non-rectangular hyperbola and Rubisco activity was calculated according to Sharkey et al. (2007). The fraction of leaf N allocated to Rubisco or PN was inferred from the relationship between photosynthetic rate per unit leaf N and SLA according to von Caemmerer et al. (1994) using data from CO2curves. Photosynthetic rate per unit N or Photosynthetic nitrogen-use efficiency [PNUE [μmol CO2 mol−1 N s−1] was calculated dividing the CO2 assimilation rate per unit mass by the N content per unit leaf mass (Sharkey et al. 2007) .
2.4 Statistics
The averaged data were analysed using the software packages Origin 5.0 (Microcal Software Corp., Northampton, MA, USA) and Winstat (R. Fitch Software, Cambridge, MA, USA, 2001).
3 Results
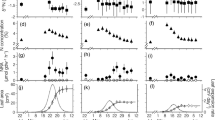
Figure 20.1a shows that the maximum photosynthetic rates based on area (Aarea) do not statistically differ between species neither in the wet, nor in the dry season, however the rates decreased in the dry season to more than half of the wet season values. By contrast, photosynthetic rates based on mass (Amass), shown in Fig. 20.1b, are clearly higher in the wet season for the semi-deciduous species, followed by the deciduous species and evergreen species. In the dry season, values were higher in the deciduous species, followed by the semi-deciduous and evergreen species. All species presented a decrease in photosynthetic rates in the dry season .
a Photosynthetic rate per leaf area (Amax, μmol CO2 m2 s−1). b photosynthetic rate per leaf mass (Amass, μmol CO2 kg−1 s−1). c specific leaf area (SLA, m2 kg−1) and d photosynthetic leaf nitrogen (PN, %) in the wet and dry season of the phenological groups studied in the Brazilian Savanna. Dark bars represent the deciduous species Anadenanthera falcata; grey bars represent the semi-deciduous species Xylopia aromatica; and open bars represent the evergreen species Myrcia lingua. Error bars denote one standard error of the mean
In the semi-deciduous and evergreen species, SLA did not change from the wet to the dry season, and the deciduous species presented a rise in this parameter due to the decrease in leaf mass, typical of deciduous species in senescence period (Fig. 20.1c). Decreases in N invested in photosynthesis (PN) were observed in the evergreen and semi-deciduous species from the wet to the dry season (Fig. 20.1d): X. aromatica PN reduced from 40 to 24 % of total leaf N, and M. lingua PN reduced from 80 to 14 %, following a slight decrease in % N. By contrast, the deciduous species presented an increase in PN amount, rising from 27 % in the wet season to 78 % in the dry season but % N fell substantially between seasons (Table 20.1) .
The PNUE value was lower in the deciduous species and higher in the semi-deciduous species in the wet season. The tendency was reversed in the dry season on the deciduous species that presented higher values just before leaf shedding, when compared with the other species (Table 20.1) .
Based on the phenological and ecophysiological traits, it was possible to characterize a gradient of strategies among species: the evergreen species M. lingua dominating the lower strata, showing low SLA, N content, Amax and Amass; the semi-deciduous species X. aromatica dominating the intermediate strata, and showing intermediate SLA and N content and high Amax and Amass; the deciduous species A. falcata dominating the canopy and showing high SLA, Amax and Amass .
4 Discussion
The results indicate that semi-deciduous and evergreen species invested more nitrogen in photosynthesis in the summer, in agreement with higher photosynthetic assimilation in this season, but they did not change SLA. The increased investment in PN in the winter observed in the deciduous species , even with the lower Amass, suggests that, in spite of the absolute decrease in total leaf N, the remobilization of compounds (including N compounds) preceding senescence, somewhat preserved PN from being withdrawn before leaves started to fall. This result supports the world pattern found by Wright et al. (2004) when describing the LES: deciduous species have high Amass rates, SLA, % N and low LL. Here we have showed that the deciduous species retain some leaf N as PN goes up until leaf abscission .
According to Harrison et al. (2009) PN tends to decrease as SLA decreases because a smaller SLA is associated with greater leaf longevity. Field and Mooney (1986) suggested that there is maybe a trade-off between investing N in photosynthetic proteins such as ribulose1,5-bisphosphate carboxylase/oxygenase (Rubisco) versus compounds required for longevity (Harrison et al. 2009). Our study shows that this relation between PN and SLA is true for the deciduous species but not applicable to the semi-deciduous and evergreen species, which showed similar SLA in both seasons and decreased PN in the dry season, suggesting N reallocation to other functions than photosynthesis, probably to maintain the leaf structure during the dry season, investing in leaf anatomy to protect against water loss. This hypothesis is plausible if we consider the need to cope with water loss imposed by seasonality changes. A study conducted in the same area by Coletta et al. (2009) found that the seasonality of water availability plays a major role in N uptake.
Harrison et al. (2009) found no trade-off between N associated with cell walls and the N allocated to ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and variation in PNUE could not be explained by variation in cell wall N. But the comparison between evergreen and deciduous Quercus species (Takashima et al. 2004) revealed a clear trade-off between N invested in Rubisco and cell wall proteins . Leaves from evergreen Quercus had greater leaf mass per unit area (LMA, the reciprocal of SLA) and allocated a greater proportion of leaf N to cell wall protein than leaves from deciduous Quercus. Onoda et al. (2004) studying leaves of Polygonum cuspidatum also found a greater proportion of leaf N allocated to cell walls as LMA increased. Ellsworth et al. (2004) calculated that the proportion of N allocated to Rubisco declined as LMA increased in 16 species with LMA ranging from 50 to 300 g m−2. They suggested that this was related to the need for greater investment in structural N. Clearly, there is a need for more data on cell wall N. Still, our results suggest that the semi-deciduous and evergreen species are probably allocating N in the dry season to processes other than photosynthesis. However, we cannot be sure at this time, that investing more N on cell walls, for improvements in leaf structure, is an adaptation to surviving throughout the dry season and low water availability, even with no changes in SLA or total leaf N between the seasons. Further data are needed for a better understanding on the relations among leaf phenology, leaf N content, PNUE and PN in tree species from the Brazilian Savanna .
References
Bustamante, M. M. C., Medina, E., Asner, G. P., Nardoto, G. B., & Garcia-Montiel, D. C. (2006). Nitrogen cycling in tropical and temperate savannas. Biogeochemistry, 79, 209–237.
Coletta, L., Nardoto, G. B., Latansio-Aidar, S. C., Rocha, H. R., Aidar, M. P. M., & Ometto, J. P. H. B. (2009). Isotopic view of vegetation and carbon and nitrogen cycles in a cerrado ecosystem, Southeastern Brazil. Scientia Agricola, 66(4), 477–475.
Eamus, D., Myers, B., Duff, G., & Williams, D. (1999). Seasonal changes in photosynthesis of eight savanna tree species. Tree Physiology, 19, 665–671.
Ellsworth, D.S., P.B. Reich, E.S. Naumburg, G.W. Koch, M.E. Kubiske & S.D. Smith. (2004). Photosynthesis, carboxylation, and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland, and desert. Global Change Biology 10, 2121– 2138.
Field, C., & Mooney, H. A. (1986). The photosynthesis-nitrogen relationship in wild plants. In T. J. Givnish (Ed.), On the economy of form and function (pp. 25–55). Cambridge: Cambridge University Press.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., Asner, G. P., Cleveland, C. C., Green, P. A., Holland, E. A., Karl, D. M., Michaels, A. F., Porter, P. A., Townsend, A. R., & Smarty, C. J. (2004). Nitrogen cycles: Past, present, and future. Biogeochemistry, 70, 153–226.
Harrison, M. T., Edwards, E. J., Farquhar, G. D., Nicotra, A. B., & Evans, J. R. (2009). Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant, Cell & Environment, 32, 259–270.
Köppen, W. (1948) Climatologia. Fondo de cultura económica. México.
Latansio-Aidar, S. R., Oliveira, A. C. P., Rocha, H. R., & Aidar, M. P. M. (2010). Phytossociology of a dense cerrado on the footprint of a carbon flux tower, Pé-de-Gigante, Vassununga State Park, SP. Biota Neotropica, 10(1), 195–207.
Lewis, S. L., Malhi, Y., & Philips, O. L. (2004). Fingerprints the impacts of global change on tropical forests. Philosophical Transactions of the Royal Society B: Biological Sciences, 359(1443), 437–462.
Marino, G., Aqil, M., & Shipley, B. (2010). The leaf economics spectrum and the prediction of photosynthetic light-response curves. Functional Ecology, 24, 263–272.
Matson, P. A., McDowell, W. H., Townsend, A. R., & Vitousek, P. M. (1999). The globalization of N deposition: Ecosystem consequences in tropical environments. Biogeochemistry, 46, 67–83.
Medina, E. (1987). Nutrients: Requirements, conservation and cycles of nutrients in the herbaceous layer. In B. H. Walter (Ed.), Determinants of tropical savannas (pp. 39–66). Paris.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858.
Nardoto, G. B., & Bustamante, M. M. C. (2003). Effects of fire on soil nitrogen dynamics and microbial biomass in savannas of Central Brazil. Pesquisa Agropecuária Brasileira, 38, 955–962.
Onoda, Y., Hikosaka, K., & Hirose, T. (2004). Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Functional Ecology, 18, 419–425.
Phoenix, G. K., Hicks, W., Cinderby, S., Kuylenstierna, J. C., Stock, W. D., Dentener, F. J., Giller, K. E., Austin, A. T., Lefroy, R. D., Gimeno, B. S., Ashmore, M. R., & Ineson, P. (2006). Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Global Change Biology, 12, 34–70.
Pivello, V. R., Bitencourt, M. D., Mantovani, W., de Mesquita, H. N. Jr., Batalha, M. A., & Shida, C. (1998). Proposta de zoneamento ecológico para a Reserva de Cerrado Pé-de-Gigante (Santa Rita do Passa Quatro, S.P.). Brazilian Journal of Ecology, 2(2), 108–118.
Pivello, V. R., Bitencourt, M. D., de Mesquita, H. N. Jr., & Batalha, M. A. (1999). Banco de dados em SIG para ecologia aplicada: Exemplo do Cerrado Pé-de-Gigante. S.P. Caderno de Informações Georreferenciadas, 1(3), 1–2.
Ratter, J. A., Ribeiro, J. F., & Bridgewater, S. (1997). The Brazilian Cerrado vegetation and threats to its biodiversity. Annals of Botany, 80, 223–230.
Sharkey, T. D., Bernacchi, C. J., Farquhar, G. D., & Singsaas, E. L. (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell and Environment, 30, 1035–1040.
Shipley, B., Lechowicz, M. J., Wright, I., & Reich, P. B. (2006). Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology, 87, 535–541.
Stewart, G. R., Schmidt, S., Handley, L., Turnbull, M. H., Erskine, P. D., & Joly, C. A. (1995). 15N natural abundance of vascular rainforest epiphytes: Implications for nitrogen source and acquisition. Plant, Cell & Environment, 18, 85–90.
Takashima, T., Hikosaka, K., & Hirose, T. (2004). Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant, Cell & Environment, 27, 1047–1054.
von Caemmerer, S., Evans, J. R., Hudson, G. S., & Andrews, T. J. (1994). The kinetics of ribulose-1,5-bisphosphate carboxylase/ oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta, 195, 88–97.
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., Cavender-Bares, J., Chapin, T., Comelissens, J. H. C., Diemer, M., Flexas, J., Garnier, E., Groom, P. K., Gulias, J., Hikosaka, K., Lamont, B. B., Lee, W., Lusk, C., Midgley, J. J., Navas, M. L., Niinemets, U., Oleksyn, J., Osada, N., Poorter, H., Poot, P., Prior, L., Pyankov, V. I., Roumet, C., Thomas, S. C., Tjoelker, M. G., Veneklaas, E. J., & Villar, R. (2004). The worldwide leaf economics spectrum. Nature, 428, 821–827.
Acknowledgments
We thank H.J. Ribeiro, the Park director who gave permission to work in Vassununga State Park and provided logistic support, Biota Fapesp Program which provided full financial support of this project and a research masters fellowship for the first author.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Latansio-Aidar, S., Colleta, L., Ometto, J., Aidar, M. (2014). Seasonal Changes in Photosynthetic Nitrogen of Tree Species Differing in Leaf Phenology in a South-eastern Brazilian Savanna. In: Sutton, M., Mason, K., Sheppard, L., Sverdrup, H., Haeuber, R., Hicks, W. (eds) Nitrogen Deposition, Critical Loads and Biodiversity. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7939-6_20
Download citation
DOI: https://doi.org/10.1007/978-94-007-7939-6_20
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7938-9
Online ISBN: 978-94-007-7939-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)