Abstract
Exercise leads to the production of reactive oxygen species (ROS) via several sources in the skeletal muscle. In particular, the mitochondrial electron transport chain in the muscle cells produces ROS along with an elevation in the oxygen consumption during exercise. Such ROS generated during exercise can cause oxidative modification of proteins and affect their functionality. Many evidences have been suggested that some muscle proteins, i.e., myofiber proteins, metabolic signaling proteins, and sarcoplasmic reticulum proteins can be a targets modified by ROS generated due to exercise. We detected the modification of carnitine palmitoyltransferase I (CPT I) by Nε-(hexanoyl)lysine (HEL), one of the lipid peroxides, in exercised muscles, while the antioxidant astaxanthin reduced this oxidative stress-induced modification. Exercise-induced ROS may diminish CPT I activity caused by HEL modification, leading to a partly limited lipid utilization in the mitochondria. This oxidative protein modification may be useful as a potential biomarker to examine the oxidative stress levels, antioxidant compounds, and their possible benefits in exercise.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Generation of Reactive Oxygen Species in Exercise
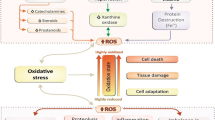
Exercise leads to the production of reactive oxygen species (ROS), mainly via the mitochondrial electron transport chain, xanthine oxidase, and phagocytes (Fig. 15.1). A small percent of the oxygen utilized in the mitochondria is converted to superoxide during the electron transport chain reaction. It is known that oxygen consumption during aerobic exercise is elevated 10 to 20-fold in the whole body and over 100-fold in the skeletal muscle, which causes ROS generation that correlates with the duration and intensity of exercise (Blomstrand et al. 1997; Ferguson et al. 2001). In addition, xanthine oxidase is activated via the ischemia-reperfusion process during exercise, a process known as “blood flow redistribution,” resulting in the production of ROS by the capillary endothelium in contracting muscles (Duarte et al. 1993; Flamm et al. 1990). Oxidative stress generated during exercise also leads to further ROS production due to the invasion of phagocytes into the muscles after exercise via the redox-sensitive inflammatory cascade (Aoi et al. 2004).
Exercise leads to ROS production via mitochondrial electron transport chain, endothelium xanthine oxidase, and phagocytes in muscle tissues. Exercise elevates oxygen consumption over 100-fold in skeletal muscle, leading to production of reactive oxygen species (ROS) via mitochondrial electron transport chain. Xanthine oxidase is activated via the ischemia-reperfusion process during exercise, a process known as “blood flow redistribution,” resulting in the production of ROS by the capillary endothelium in contracting muscles. Following exercise, further ROS production is increased by an invasion of phagocytes into the muscles
Previously, studies have shown elevated levels of ROS in response to exercise by using various methods. Electron-spin-resonance (ESR) studies have demonstrated that the ESR signal is markedly increased in muscle homogenates obtained immediately after exercise (Ashton et al. 1999; Davies et al. 1982). Other studies have shown a decrease in diet-derived antioxidants, and a reduced level of glutathione in tissues and blood (Aikawa et al. 1984; Liu et al. 2000; Rietjens et al. 2007), caused by its usage for ROS elimination. As in the above antioxidant capacity, ROS act as oxidative stressors and oxidize cell components such as proteins, lipids, and DNA in tissues such as skeletal muscle, blood, and internal organs, and oxidation results in various oxidative products. Specifically, peroxidation of polyunsaturated fatty acids such as arachidonic acid, which are a large constituent of the membranes around cells and their organelles, occurs via a radical chain reaction and has been used as a useful marker of exercise-induced oxidative stress. Numerous previous animal and human studies have shown that acute exercise such as running, cycling, and resistance exercise, markedly elevates thiobarbituric acid reactive substances (TBARS), one of the oldest and most frequently used methods for measuring the peroxidation of fatty acids in the skeletal muscle immediately after exercise and on the next day after the exercise (Aoi et al. 2004; Liao et al. 2010; Miyazaki et al. 2001). In addition, this elevation in TBARS is also shown in high-intensity exercise training (Chang et al. 2007; Couillard et al. 2003). On the other hand, dietary supplementation with antioxidants such as vitamin E, vitamin C, carotenoids, and polyphenols can decrease oxidative damage induced by acute and chronic exercises (Aoi et al. 2003, 2004; Bryer and Goldfarb 2006; Goldfarb et al. 2011). These observations clearly demonstrate that exercise induces oxidative stress and causes the oxidation of cellular components. Such oxidative stress is not always negative, and many evidences suggest that moderate oxidative stress is necessary for beneficial adaptations induced by dietary exercise, as mentioned below. The effect of oxidative stress on bodily functions in living body would be due to the extent of this stress, and whether it acts a damaging factor or a protective factor is dependent on the intensity, duration, frequency, and habits of exercise.
2 Involvement of Oxidative Stress on Fatigue and Damage in Muscle
The possibility that oxidative stress induced by exercise causes a health disorder is a debatable matter. However, it has been demonstrated that the excess ROS generated by exercise are involved in muscle fatigue and muscle damage. It is well established that ROS have important influences on force production in the skeletal muscle. A study using both spin traps and vitamin E in animals demonstrated that scavenging ROS in muscles during exercise delays the onset of muscular fatigue (Novelli et al. 1990). Moreover, many reports have shown that administration of the antioxidant N-acetylcysteine (NAC), which acts as a reduced thiol donor supporting glutathione re-synthesis, delays muscular fatigue during a variety of submaximal exercise tasks such as electrically stimulated fatigue of the muscle, cycling exercise, and repetitive handgrip exercise (Cobley et al. 2011; Matuszczak et al. 2005; McKenna et al. 2006; Medved et al. 2004; Reid et al. 1994). In animal studies, NAC administration has also been shown to delay fatigue in both in vitro and in situ muscle preparations (Kobzik et al. 1995; Perkins et al. 1997). Studies using excised muscle fiber bundles also revealed that force production during submaximal tetanic contractions is decreased by nitric oxide (NO) donors (Morrison et al. 1998; Richmonds and Kaminski 2001) and increased by nitric oxide synthase (NOS) inhibitors and NO scavengers (Joneschild et al. 1999; Kobzik et al. 1994).
Unaccustomed and strenuous exercise can cause muscle damage that presents clinically as muscular pain and involves protein degradation and ultrastructural changes. As such, muscle damage usually occurs sometime after exercise and not during or immediately after exercise; this is called as “delayed-onset muscle damage.” Previous studies have demonstrated that delayed-onset muscle damage is mainly induced by mechanical stress, especially eccentric muscle contraction (Komulainen et al. 1998; Proske and Morgan 2001), and disturbances in calcium homeostasis (Chen et al. 2007; Gissel and Clausen 2001). In addition, we demonstrated that delayed-onset muscle damage induced by prolonged exercise is partly related to inflammation via phagocyte infiltration caused by ROS generated during exercise (Aoi et al. 2004). In an in vitro study using myotube cells, addition of H2O2 induced the translocation of p65, a component of the redox-sensitive transcription nuclear factor-kappa B (NF-κB), into the nucleus and subsequently increased the expression of cytokine-induced neutrophil chemoattractant-1 (CINC-1) and monocyte chemoattractant protein-1 (MCP-1). Prolonged acute exercise caused an increase in the amount of nuclear p65, a constitutive protein of NF-κB, in rat gastrocnemius muscles at 1 h after exercise, which was similar to the in vitro results, and it caused muscle damage with neutrophil invasion on the next day. Therefore, delayed-onset muscle damage after prolonged exercise is related to inflammation secondary to phagocyte infiltration caused by ROS generated during exercise. In contrast, dietary antioxidants such as vitamin E and carotenoids can partly attenuate delayed-onset muscle damage along with inflammatory changes (Aoi et al. 2003, 2004; Rosa et al. 2009), though several studies reported that supplementation of dietary antioxidants does not have this preventative effect.
3 Oxidative Protein Modification in the Muscle Induced by Exercises
Numerous posttranslational modifications have been characterized as resulting either from direct modification of amino acid residues or through the formation of reactive intermediates by the oxidation of other cellular components (Naito and Yoshikawa 2009). The modification of 20 different of amino acids plays an important role in the manifestation of the function of many proteins. The modification can be subdivided into two general forms: reversible modification and irreversible modifications. Some of the lipid peroxidation products exhibit a facile reactivity with proteins, generating a variety of intra- and intermolecular covalent adducts. In addition, the oxidation of cysteine to sulfenic, sulfinic, and sulfonic acids has been shown to occur frequently, and these sulfenic and sulfinic acids can often be enzymatically reduced. Nitration by reactive nitrogen species, chlorination by hypochlorous acid, and bromination by hypobromous acid of the target protein are also the frequently detected modifications. Many evidences suggest that such protein modifications can be associated with the onset of various common diseases, including cancer, inflammation, and metabolic disorders (Bidasee et al. 2004; Hill and Bhatnagar 2012; Oya-Ito et al. 2011; Sultana and Butterfield 2009). Some muscular proteins are also targets modified by exercise-induced ROS or nitrogen oxide species (Aoi et al. 2003; Kato et al. 2000; Magherini et al. 2012; Veskoukis et al. 2008; Vassilakopoulos et al. 2003; Barreiro and Hussain 2010) (Table 15.1).
The influence of oxidative stress on the sarcoplasmic reticulum (SR), a subcellular organelle, which controls the contractile state of the muscle by regulating the calcium concentration in the cytosol, has been studied extensively in the skeletal muscle, and is associated with the oxidative modification of the membrane proteins (Anzueto et al. 1992; Salama et al. 1992; Xia et al. 2003). Muscle contraction is performed by increasing intracellular calcium concentrations, which are released from the SR via the ryanodine receptor (RyR) calcium-release channel following active potentials during the excitation-contraction coupling process. Afterwards, calcium is immediately taken into the SR via SR calcium-dependent ATPase (SERCA), which relaxes the muscle. It has been known that the responsive proteins in the SR are sensitive to redox modulation (Sun et al. 2001; Zhang et al. 1999). The RyR appears to be in close association with the NADP(H) oxidase(s) found in the SR, and locally generated superoxide appears to be the major ROS capable of influencing this channel (Xia et al. 2003). Each subunit of this large tetrameric protein contains a small number of regulatory cysteines. ROS and NO oxidize thiol residues on neighboring cysteines to form disulfide bonds, which induce channel opening. Disulfide formation is reversed by reducing agents, providing a mechanism for direct redox modulation of channel activity. The SERCA, another potential target, contains a small number of critical sulfhydryls near the SERCA active site, which have been shown to slow the reuptake of calcium into the SR (Daiho and Kanazawa 1994; Xu et al. 1997; Gutierrez-Martin et al. 2004). Exposure to elevated NO also inhibits SERCA activity via thiol oxidation and nitration of tyrosine residues (Viner et al. 1997, 2000). Consequently, oxidation of SR proteins tends to increase cytosolic calcium levels, which causes prevention of muscle relaxation. Therefore, the modification of calcium transport proteins in the SR can causes excess or chronic muscle contraction via increasing intracellular calcium levels, leading to muscle fatigue.
Muscle myofilaments are also sensitive to direct redox modification (Fedorova et al. 2009). Myosin heavy chains contain several sulfhydryl residues, which are useful sites for protein labeling; however, thiol modification generally does not dramatically alter myosin function (Crowder and Cooke 1984). On the other hand, Yamada et al. (2007) reported that a force reduction in the soleus muscle of hyperthyroid rat is associated with carbonylation of the myosin heavy chain. In addition, it has been suggested that the myosin heavy chain is easily glycosylated, which changes the structural and functional properties of the protein (Haus et al. 2007). However, the involvement of these modifications on exercise-induced fatigue is unclear. In contrast, myosin light chains, actin, and tropomyosin appear less sensitive to redox modulation (Liu et al. 1990; Williams and Swenson 1982).
In delayed-onset muscle damage, protein modification is also observed. We reported the elevation of 4-hydroxy-2-nonenal (4-HNE), a lipid peroxidation product that covalently modifies the proteins on cysteine, histidine, and lysine residues, in the damaged muscle obtained from mice on the next day after acute running (Aoi et al. 2003). Liu et al. (2005) reported a positive correlate between 4-HNE and creatine kinase activity in blood following strenuous exercise in humans. Recently, insulin receptor substrate-1 (IRS-1) was detected as a 4-HNE-targeted protein (Aoi et al. 2012). IRS-1 is upstream in the PI3K/Akt-dependent insulin-signaling pathway in muscle cells and regulates glucose uptake via glucose transporter 4. In the damaged muscle after strenuous exercise, insulin-stimulated glucose uptake is decreased along with a reduction of insulin signal transduction, which suggests that 4-HNE modification of IRS-1 is involved in the transient impairment in insulin sensitivity. Sahlin et al. (2010) showed a marked elevation of 4-HNE modification of mitochondrial protein after acute endurance exercise. In addition, Kato et al. (2000) have reported that Nε-(hexanoyl)lysine (HEL), which is generated from the reaction between the lysine moiety and 13-hydroperoxyoctadecadienoic acid (13-HPODE), is increased in the muscle obtained from rats, which have performed high-intensity exercise training for 3 weeks. In contrast, the administration of eriocitrin, a flavonoid contained in lemon and lime fruits, to exercise-trained rats suppressed the formation of HEL.
4 HEL-Modification of CPT-I and Lipid Metabolism in Exercise
A major source of ROS production during exercise is the mitochondrial electron transport. Thus, mitochondrial proteins could be a major target of posttranslational oxidative modification during exercise. The HEL moiety is a novel adduct formed by the reaction of linoleic acid hydroperoxide and lysine, and is a marker of lipid peroxidation-derived protein modification in the early stages after oxidative stress (Kato et al. 1999; Osawa and Kato 2005), and therefore may be useful in detecting oxidative modification of mitochondrial protein during acute exercise (Fig. 15.2). Thus, we hypothesized that proteins on the mitochondrial membrane are easily modified by lipid peroxide generated on the membrane. In an analysis of mouse gastrocnemius muscle obtained immediately after running, the modification of carnitine palmitoyltransferase I (CPT I) by HEL was detected (Aoi et al. 2008). CPT I is located on the mitochondrial membrane and is a rate-limiting step in fatty acyl-CoA entry into the mitochondria in the muscle (McGarry and Brown 1997). In contrast, astaxanthin, an antioxidant in the mitochondrial membrane, limits the modification of CPT I by HEL after exercise, suggesting that astaxanthin directly traps the oxygen radicals generated by exercise, and/or that astaxanthin promotes the activation of a defense mechanism such as the induction of antioxidative enzymes, and/or that astaxanthin furthers the excretion of HEL from the skeletal muscle by activation of the proteasome. We previously reported that orally administered astaxanthin is absorbed in the intestine and accumulates in the muscle tissues in mice (Aoi et al. 2008). In addition, Manabe et al. (2008) have demonstrated that astaxanthin accumulates in the mitochondrial fraction and inhibits the oxidative modification of mitochondrial proteins in mesangial cells. Thus, the mitochondria are considered to be a major target of astaxanthin.
Relationship among ROS, oxidative protein modification, and nutrient metabolism. ROS are generated via mitochondria and xanthine oxidase, and then oxidizes lipids during exercise, which results in elevation of Nε-(hexanoyl)lysine, an early stage lipid peroxidation marker. In recovery period following exercise, invasion of phagocytes into muscle tissues occurs, which progresses muscle damage via inflammation. In this process, lipid peroxide reaction is further progressed and aldehyde including 4-hydroxy-2-nonenal is produced. Such lipid peroxides modify particular metabolic protein posttranslationally, leading to modulation of nutrient metabolism
Several studies (Campbell et al. 2004; Holloway et al. 2006) have shown that the fatty acid translocase/cluster of differentiation 36 (FAT/CD36) is associated with CPT I on the mitochondrial membrane and increases its function. We found that the interaction between CPT I and FAT/CD36 in the muscle during exercise was facilitated by astaxanthin (Aoi et al. 2008). Thus, modification of CPT I by HEL may alter the colocalization of CPT I with FAT/CD36 by changing the CPT I structure, which could lead to the regulation of lipid metabolism during exercise. Lipolysis in the body is important during exercise to facilitate lipid utilization in the muscle rather than release it from the adipose tissue. The utilization ratio of carbohydrates and lipids for energy generation is almost equal when exercise is of low to moderate intensity. A possible factor influencing the utilized ratio of these energy substrates is the colocalization of CPT I with FAT/CD36. Exercise-induced ROS may partly limit the utilization of fatty acids via diminishing the CPT I activity caused by HEL modification (Fig. 15.3). Indeed, we and another group found that inhibition of this modification by dietary astaxanthin increased fat utilization during exercise as compared with mice on a normal diet and prolonged the running time to exhaustion (Aoi et al. 2008; Ikeuchi et al. 2006). Therefore, HEL-modification of CPT I can partly suppress lipid metabolism during exercise, which would affect the endurance performance and efficiency of adipose tissue reduction with training.
HEL modification of CPT - I on mitochondrial membrane. Amount of FAT/CD36 that coimmunoprecipitated with CPT I (A) and HEL-modified CPT I (B) in skeletal muscle of ICR mice. A single bout of exercise was performed at 30 m/min for 30 min. Lysate protein from the muscle collected immediately after running was immunoprecipitated with CPT I antibody. Immunoprecipitates were separated by SDS-PAGE and membranes probed for FAT/CD36 (A) or HEL (B). Values are the mean ± SE. *, significant difference at the level of P < 0.05 (Data are from Aoi et al. 2008)
5 Prospective
Many evidences have indicated that oxidative stress has both positive and negative effects. A moderate grade of oxidative stress enhances muscle force production, nutrient metabolism, and antioxidant enzymes (Ristow et al. 2009; Gomez-Cabrera et al. 2008; Powers and Jackson 2008), although excess ROS functions as a damaging factor in cellular components. In contrast, dietary vitamin C and E cancel many exercise-induced adaptive benefits, such as improvements in insulin sensitivity, blood pressure, and endurance capacity, which are caused by suppressing the expression of redox-sensitive proteins, including PPAR gamma coactivator-1 alpha, AMP activated kinase, and superoxide dismutase 2. Therefore, the dietary intake of antioxidants for exercise therapy for the treatment and prevention of diseases and for training to improve the athletic performance is a debatable matter. On the other hand, some antioxidants accelerate energy metabolism and insulin sensitivity induced by exercise via the elevation of key modulators (Aoi et al. 2008; Richards et al. 2010; Henriksen 2006; Dolinsky et al. 2012). Therefore, we cannot group all antioxidants together, and we should consider the respective properties of each antioxidant individually but not their absolute antioxidative capacity. This may be responsible for the beyond-antioxidant properties that each compound has specifically. Oxidative protein modification during early-stage oxidative damage, e.g. HEL, may be useful as a potential biomarker to examine the benefit of antioxidant compounds in the sporting scene as well as in regulating oxidative stress levels, although a study focusing on the relationship between protein modification and physiological function in exercise is underdevelopment. Further research is required regarding the functional analysis of modified proteins along with whether this can be a biomarker of athletic performance levels, fatigue, and health benefits obtained from dietary exercise.
References
Aikawa KM, Quintanilha AT, de Lumen BO, Brooks GA, Packer L (1984) Exercise endurance training alters vitamin E tissue level and red blood cell hemolysis in rodents. Biosci Rep 4:253–257
Anzueto A, Andrade FH, Maxwell LC, Levine SM, Lawrence RA, Gibbons WJ, Jenkinson SG (1992) Resistive breathing activates the glutathione redox cycle and impairs performance of rat diaphragm. J Appl Physiol 72:529–534
Aoi W, Naito Y, Sakuma K, Kuchide M, Tokuda H, Maoka T, Toyokuni S, Oka S, Yasuhara M, Yoshikawa T (2003) Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid Redox Signal 5:139–144
Aoi W, Naito Y, Takanami Y, Kawai Y, Sakuma K, Ichikawa H, Yoshida N, Yoshikawa T (2004) Oxidative stress and delayed-onset muscle damage after exercise. Free Radic Biol Med 37:480–487
Aoi W, Naito Y, Takanami Y, Ishii T, Kawai Y, Akagiri S, Kato Y, Osawa T, Yoshikawa T (2008) Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem Biophys Res Commun 366:892–897
Aoi W, Naito Y, Tokuda H, Tanimura Y, Oya-Ito T, Yoshikawa T (2012) Exercise-induced muscle damage impairs insulin signaling pathway associated with IRS-1 oxidative modification. Physiol Res 61:81–88
Ashton T, Young IS, Peters JR, Jones E, Jackson SK, Davies B, Rowlands CC (1999) Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J Appl Physiol 87:2032–2036
Barreiro E, Hussain SN (2010) Protein carbonylation in skeletal muscles: impact on function. Antioxid Redox Signal 12:417–429
Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, Besch HR Jr (2004) Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 53:463–473
Blomstrand E, Rådegran G, Saltin B (1997) Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 501:455–460
Bryer SC, Goldfarb AH (2006) Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int J Sport Nutr Exerc Metab 16:270–280
Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A (2004) A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem 279:36235–36241
Chang CK, Huang HY, Tseng HF, Hsuuw YD, Tso TK (2007) Interaction of vitamin E and exercise training on oxidative stress and antioxidant enzyme activities in rat skeletal muscles. J Nutr Biochem 18:39–45
Chen W, Ruell PA, Ghoddusi M, Kee A, Hardeman EC, Hoffman KM, Thompson MW (2007) Ultrastructural changes and sarcoplasmic reticulum Ca2+ regulation in red vastus muscle following eccentric exercise in the rat. Exp Physiol 92:437–447
Cobley JN, McGlory C, Morton JP, Close GL (2011) N-Acetylcysteine’s attenuation of fatigue after repeated bouts of intermittent exercise: practical implications for tournament situations. Int J Sport Nutr Exerc Metab 21:451–461
Couillard A, Maltais F, Saey D, Debigaré R, Michaud A, Koechlin C, LeBlanc P, Préfaut C (2003) Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167:1664–1669
Crowder MS, Cooke R (1984) The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil 5:131–146
Daiho T, Kanazawa T (1994) Reduction of disulfide bonds in sarcoplasmic reticulum Ca2+-ATPase by dithiothreitol causes inhibition of phosphoenzyme isomerization in catalytic cycle. This reduction requires binding of both purine nucleotide and Ca2+ to enzyme. J Biol Chem 269:11060–11064
Davies KJ, Quintanilha AT, Brooks GA, Packer L (1982) Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107:1198–1205
Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JR (2012) Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol 590:2783–2799
Duarte JA, Appell HJ, Carvalho F, Bastos ML, Soares JM (1993) Endothelium-derived oxidative stress may contribute to exercise-induced muscle damage. Int J Sports Med 14:440–443
Fedorova M, Kuleva N, Hoffmann R (2009) Reversible and irreversible modifications of skeletal muscle proteins in a rat model of acute oxidative stress. Biochim Biophys Acta 1792:1185–1193
Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjaer M, Sargeant AJ, Hellsten Y, Bangsbo J (2001) Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J Physiol 536:261–271
Flamm SD, Taki J, Moore R et al (1990) Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 81:1550–1559
Gissel H, Clausen T (2001) Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol Scand 171:327–334
Goldfarb AH, Garten RS, Cho C, Chee PD, Chambers LA (2011) Effects of a fruit/berry/vegetable supplement on muscle function and oxidative stress. Med Sci Sports Exerc 43:501–508
Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87:142–149
Gutierrez-Martin Y, Martin-Romero FJ, Inesta-Vaquera FA, Gutierrez-Merino C, Henao F (2004) Modulation of sarcoplasmic reticulum Ca2+-ATPase by chronic and acute exposure to peroxynitrite. Eur J Biochem 271:2647–2657
Haus JM, Carrithers JA, Trappe SW, Trappe TA (2007) Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103:2068–2076
Henriksen EJ (2006) Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic Biol Med 40:3–12
Hill BG, Bhatnagar A (2012) Protein S-glutathiolation: redox-sensitive regulation of protein function. J Mol Cell Cardiol 52:559–567
Holloway GP, Bezaire V, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL (2006) Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol 571:201–210
Ikeuchi M, Koyama T, Takahashi J, Yazawa K (2006) Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol Pharm Bull 29:2106–2110
Joneschild ES, Chen LE, Seaber AV, Frankel ES, Urbaniak JR (1999) Effect of a NOS inhibitor, L-NMMA, on the contractile function of reperfused skeletal muscle. J J Reconstr Microsurg 15:55–60
Kato Y, Mori Y, Makino Y, Morimitsu Y, Hiroi S, Ishikawa T, Osawa T (1999) Formation of Nε-(hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J Biol Chem 274:20406–20414
Kato Y, Miyake K, Yamamoto Y, Shimomura H, Ochi Y, Mori T, Osawa T (2000) Preparation of a monoclonal antibody to Nε-(hexanonyl)lysine: application to the evaluation of protective effects of flavonoid supplementation against exercise-induced oxidative stress in rat skeletal muscle. Biochem Biophys Res Commun 274:389–393
Kobzik L, Reid MB, Bredt DS, Stamler JS (1994) Nitric oxide in skeletal muscle. Nature 372:546–548
Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS (1995) Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun 211:375–381
Komulainen J, Takala TE, Kuipers H, Hesselink MK (1998) The disruption of myofibre structures in rat skeletal muscle after forced lengthening contractions. Pflugers Arch 436:735–741
Liao P, Zhou J, Ji LL, Zhang Y (2010) Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor-α. Am J Physiol Regul Integr Comp Physiol 298:R599–R607
Liu DF, Wang D, Stracher A (1990) The accessibility of the thiol groups on G- and F-actin of rabbit muscle. Biochem J 266:453–459
Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ, Chyu DW, Brooks GA, Ames BN (2000) Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 89:21–28
Liu JF, Chang WY, Chan KH, Tsai WY, Lin CL, Hsu MC (2005) Blood lipid peroxides and muscle damage increased following intensive resistance training of female weightlifters. Ann N Y Acad Sci 1042:255–261
Magherini F, Abruzzo PM, Puglia M, Bini L, Gamberi T, Esposito F, Veicsteinas A, Marini M, Fiorillo C, Gulisano M, Modesti A (2012) Proteomic analysis and protein carbonylation profile in trained and untrained rat muscles. J Proteomics 75:978–992
Manabe E, Handa O, Naito Y, Mizushima K, Akagiri S, Adachi S, Takagi T, Kokura S, Maoka T, Yoshikawa T (2008) Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J Cell Biochem 103:1925–1937
Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB (2005) Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve 32:633–638
McGarry JD, Brown NF (1997) The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244:1–14
McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X (2006) N-acetylcysteine attenuates the decline in muscle Na+, K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol 576:279–288
Medved I, Brown MJ, Bjorksten AR, McKenna MJ (2004) Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J Appl Physiol 96:211–217
Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H (2001) Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 84:1–6
Morrison RJ, Miller CC 3rd, Reid MB (1998) Nitric oxide effects on force-velocity characteristics of the rat diaphragm. Comp Biochem Physiol A Mol Integr Physiol 119:203–209
Naito Y, Yoshikawa T (2009) Oxidative stress-induced posttranslational modification of proteins as a target of functional food. Forum Nutr 61:39–54
Novelli GP, Bracciotti G, Falsini S (1990) Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic Biol Med 8:9–13
Osawa T, Kato Y (2005) Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann N Y Acad Sci 1043:440–451
Oya-Ito T, Naito Y, Takagi T, Handa O, Matsui H, Yamada M, Shima K, Yoshikawa T (2011) Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochim Biophys Acta 1812:769–781
Perkins WJ, Han YS, Sieck GC (1997) Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol 83:1326–1332
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537:333–345
Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA (1994) N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94:2468–2474
Richards JC, Lonac MC, Johnson TK, Schweder MM, Bell C (2010) Epigallocatechin-3-gallate increases maximal oxygen uptake in adult humans. Med Sci Sports Exerc 42:739–744
Richmonds CR, Kaminski HJ (2001) Nitric oxide synthase expression and effects of nitric oxide modulation on contractility of rat extraocular muscle. FASEB J 15:1764–1770
Rietjens SJ, Beelen M, Koopman R, VAN Loon LJ, Bast A, Haenen GR (2007) A single session of resistance exercise induces oxidative damage in untrained men. Med Sci Sports Exerc 39:2145–2151
Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106:8665–8670
Rosa EF, Ribeiro RF, Pereira FM, Freymüller E, Aboulafia J, Nouailhetas VL (2009) Vitamin C and E supplementation prevents mitochondrial damage of ileum myocytes caused by intense and exhaustive exercise training. J Appl Physiol 107:1532–1538
Sahlin K, Shabalina IG, Mattsson CM, Bakkman L, Fernström M, Rozhdestvenskaya Z, Enqvist JK, Nedergaard J, Ekblom B, Tonkonogi M (2010) Ultraendurance exercise increases the production of reactive oxygen species in isolated mitochondria from human skeletal muscle. J Appl Physiol 108:780–787
Salama G, Abramson JJ, Pike GK (1992) Sulphydryl reagents trigger Ca2+ release from the sarcoplasmic reticulum of skinned rabbit psoas fibres. J Physiol 454:389–420
Sultana R, Butterfield DA (2009) Proteomics identification of carbonylated and HNE-bound brain proteins in Alzheimer’s disease. Methods Mol Biol 566:123–135
Sun J, Xu L, Eu JP, Stamler JS, Meissner G (2001) Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem 276:15625–15630
Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN (2003) Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol 284:L452–L457
Veskoukis AS, Nikolaidis MG, Kyparos A, Kokkinos D, Nepka C, Barbanis S, Kouretas D (2008) Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl Physiol Nutr Metab 33:1140–1154
Viner RI, Krainev AG, Williams TD, Schoneich C, Bigelow DJ (1997) Identification of oxidation-sensitive peptides within the cytoplasmic domain of the sarcoplasmic reticulum Ca2+-ATPase. Biochemistry 36:7706–7716
Viner RI, Williams TD, Schoneich C (2000) Nitric oxide-dependent modification of the sarcoplasmic reticulum Ca-ATPase: localization of cysteine target sites. Free Radic Biol Med 29:489–496
Williams DL Jr, Swenson CA (1982) Disulfide bridges in tropomyosin effect on ATPase activity of actomyosin. Eur J Biochem 127:495–499
Xia R, Webb JA, Gnall LL, Cutler K, Abramson JJ (2003) Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol Cell Physiol 285:C215–C221
Xu KY, Zweier JL, Becker LC (1997) Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPase function by direct attack on the ATP binding site. Circ Res 80:76–81
Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M (2007) Myofibrillar protein oxidation and contractile dysfunction in hyperthyroid rat diaphragm. J Appl Physiol 102:1850–1855
Zhang JZ, Wu Y, Williams BY, Rodney G, Mandel F, Strasburg GM, Hamilton SL (1999) Oxidation of the skeletal muscle Ca2+ release channel alters calmodulin binding. Am J Physiol Cell Physiol 276:C46–C53
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Aoi, W., Naito, Y., Yoshikawa, T. (2014). Potential Role of Oxidative Protein Modification in Energy Metabolism in Exercise. In: Kato, Y. (eds) Lipid Hydroperoxide-Derived Modification of Biomolecules. Subcellular Biochemistry, vol 77. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7920-4_15
Download citation
DOI: https://doi.org/10.1007/978-94-007-7920-4_15
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7919-8
Online ISBN: 978-94-007-7920-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)