Abstract
Compounds with combined anti-glycation and antioxidant properties may offer therapeutic potential for diabetic and oxidative stress related pathologies. We previously demonstrated significant (p < 0.05) anti-glycation properties of culinary herbs and spices by an optimized in vitro glucose-bovine serum albumin assay. In the present investigation we describe the antioxidant potential of these plants and the therapeutic potential of dietary compounds as possible anti-glycation and antioxidant agents. This study was geared to appraise the total antioxidant and free radical scavenging activities of ten common culinary herbs and spices (Allium sativum, Zingiber officinale, Thymus vulgaris, Petroselinum crispum, Murraya koenigii Spreng, Mentha piperita L., Curcuma longa L., Allium cepa L., Allium fistulosum and Coriandrum sativum L.) commercially available on the Mauritian market. The total antioxidant capacity as measured by the phosphomolybdenum method ranged from 0.76 to 2.49 μg AAE/ml. It was observed that the ethanolic extracts exhibited significant (p < 0.05) free radical-scavenging potential as measured by the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) radical cation assay, ferrous ion chelating assay and the Griess assay. The observed anti-glycation and antioxidant activity of the extracts together with their previously reported total phenolic, flavonoid and tannin contents support the therapeutic value of the plants investigated. Dietary agents interfering in the glycation pathway might offer new lead compounds geared towards glucose-derived and oxidative stress related complications. Our findings showed that the herbs and spices studied possess significant antioxidant and anti-glycation properties and hence can be exploited as functional foods.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Antioxidant Activity
- Free Radical Scavenge Activity
- Curcuma Longa
- Albumin Glycation
- Advanced Glycation Endproducts
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
Plants have historically been an important source of lead molecules in drug discovery [1, 2]. Several plants have been used as dietary adjuvant and in treating and/or managing a number of diseases even without any knowledge of their proper functions and constituents [3]. This practice may be attributed to the cost and side effects of synthetic agents. Medicinal foods are used widely even when their biologically active compounds are unknown, because of their safety, effectiveness, and availability [4]. Various plants have been found to possess significant anti-diabetic properties [5]. Use of these plants may delay the development of diabetic complications. These days great attention is being given to management of diabetes with medicinal plants along with dietary restriction [6]. The World Health Organization (WHO) has recommended the evaluation of traditional plant treatments for diabetes as they are cost-effective, non-toxic, with less or no side effects and are considered to be excellent candidates for oral therapy [7]. There is an increasing demand by patients to use natural products with anti-diabetic activity [8]. Herbal therapy has been used to treat various types of disease including diabetes all over the world successfully [9]. The use of nutraceuticals is an attempt to accomplish desirable therapeutic outcomes with reduced side effects, as compared with other synthetic therapeutic agents [6].
Plants with known antioxidant and anti-glycation properties are receiving a lot of attention as they are believed to have less adverse effects [10]. Some plants contain chemicals that can be used directly as drugs or lead compounds for hypoglycemic properties useful in the management of diabetic complications [2]. For example polyphenolic compounds including tannin are known to possess free radical scavenging properties [10]. Curcumin from turmeric (Curcuma longa) reduces blood glucose levels in type 2 diabetic mice [11]. Green tea (Camellia sinensis) polyphenols are reported as useful agents to protect against protein oxidation and glycation-induced pathogenesis of diabetic complications [12]. In recent years, garlic (Allium sativum) has received attention because of its ability to inhibit formation of advanced glycation endproducts (AGEs) [13, 14]. In this report we emphasize the anti-glycation as well as antioxidant properties from plant material. However, many other active agents obtained from plants have not been well characterized. More investigations must be conducted to evaluate the mechanism of action of medicinal plants with anti-diabetic and antioxidant effects. Consequently, it is necessary to perform toxicological investigation of all plants empirically used in order to avoid the risk of the side effects related to phytotherapy [15].
In searching for new therapeutic agents, our research aimed to evaluate anti-glycation and antioxidant potential of ethanolic extracts of ten selected culinary herbs and spices and to measure the impact of polyphenolic constituents on their effectiveness. We have previously reported the total phenolic, flavonoids and condensed tannins of these herbs [16]. Free radical scavenging activities had also been demonstrated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric-reducing antioxidant power (FRAP) assays. In this report, we complement those data with additional antioxidant assays such as the total antioxidant capacity (TAC), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) ABTS assay, nitric oxide (NO) scavenging assay and ferrous ion chelating (FIC) assay.
11.2 Methodology
11.2.1 Chemicals
Bovine serum albumin (BSA; fraction V, fatty acid free, low endotoxin), d-glucose, sodium azide, phosphate buffered saline (PBS), aminoguanidine, ascorbic acid, trichloroacetic acid, gallic acid, anhydrous sodium carbonate, ethanol, methanol 100 %, iron(III) chloride 6-hydrate, potassium ferricyanide, potassium dihydrogen phosphate and dipotassium hydrogen phosphate, NaNO2, AlCl3, 1M NaOH, vanillin methanolic solution, concentrated hydrochloric acid (HCl), potassium persulfate, ABTS 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid), naphthyl ethylenediamine dihydrochloride, sodium nitroprusside, sodium phosphate, ammonium molybdate, sulphuric acid, and quercetin were purchased from Sigma-Aldrich (St. Louis, USA).
11.2.2 Plant Materials and Preparation of Extracts
Culinary herbs and spices were purchased from a local market. Ten commercially available herbs and spices were tested namely garlic (Allium sativum), ginger (Zingiber officinale), thyme (Thymus vulgaris), parsley (Petroselinum crispum), curry leaves (Murraya koenigii Spreng), peppermint (Mentha piperita L.), turmeric (Curcuma longa L.), onion (Allium cepa L.), green onion scallion (Allium fistulosum) and coriander (Coriandrum sativum L.). The study was limited to products that are widely available to the public and are in routine use for daily food cooking in Mauritius. Fresh culinary herbs and spices were ground into a paste and 5 g of the latter were extracted with ethanol (50 %) at a ratio of 10 ml per gram at room temperature (28 ± 2 °C) for 1 week. The extracts were centrifuged at 1,000 g for 10 min to remove precipitate.
11.2.3 Anti-Glycation Assay
Albumin glycation was determined using fluorometry as described by Matsuura et al. [17]. Briefly, 1 mg/ml of fatty acid-free BSA was incubated with d-glucose (200–400 mM) ± 100 μl of extracts in 0.2 M potassium phosphate buffered saline (PBS, pH 7.4 containing 0.01 % sodium azide) at 37 °C for defined time periods. Aliquots of the reaction mixture were removed at weekly intervals and fluorescent AGEs were assessed by their emission at 440 nm following excitation at 370 nm using a spectrofluorimeter (F-7000 FL) as described previously [16]. The reactions were stopped by adding 10 μl of 100 % (w/v) trichloroacetic acid and after 10 min the mixture was centrifuged at 1,000 g. The precipitate was re-dissolved in alkaline PBS and quantified for the relative amount of fluorescent AGEs. Aminoguanidine (20 mM) was included as positive control.
11.2.4 Determination of Total Antioxidant Capacity by Phosphomolybdenum Method
The total antioxidant activity was evaluated by the method of Prieto [18]. An aliquot of 0.2 ml of extracts was combined with 2 ml of reagent (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and incubated in a water bath at 80 °C for 90 min. After cooling the sample to room temperature, the absorbance of the solution was measured at 695 nm against blank using a Perkin-Elmer spectrophotometer. For reference, the appropriate solution of ascorbic acid was used and the reducing capacity of the extracts was expressed as ascorbic acid equivalents.
11.2.5 ABTS: 2,2′-Azinobis(3-ethylbenzothiazoline- 6-sulphonic acid) Assay
The ABTS assay according to Chen et al. [19] was used to measure the antioxidant activity of the extracts. ABTS was dissolved in distilled water to a concentration of 7.4 mM, and potassium persulfate was added to a concentration of 2.45 mM. The reaction mixture was allowed to stand at room temperature for 16 h in the dark before use. The resulting intensely colored ABTS•+ radical cation was diluted with 0.01 M PBS (phosphate-buffered saline), pH 7.4, to give an absorbance value of ~0.70 at 734 nm. ABTS•+ cation solution (2 ml) was mixed with 100 μl extract. Absorbance was measured at 734 nm after reaction for 6 min. The ABTS scavenging activity was calculated as inhibition % = (1-(test sample absorbance/blank sample absorbance)) × 100. Quercetin was used as positive control.
11.2.6 Ferrous Ion-Chelating Ability Assay
The ferrous ion-chelating (FIC) assay was carried out according to the method of Viuda-Martos et al. [20], with some modifications. Solutions of 0.2 mM FeCl2·4H2O and 0.5 mM ferrozine were diluted 20 times. Briefly, an aliquot (0.5 ml) of different extracts was mixed with 0.5 ml FeCl2·4H2O. After 5 min of incubation, the reaction was initiated by the addition of ferrozine (0.5 ml). The mixture was shaken vigorously and after a further 10 min incubation period, the absorbance of the solution was measured spectrophotometrically at 562 nm. The percentage inhibition of ferrozine–Fe2+ complex formation was calculated by using the formula: Chelating effect % = [(1 – AS)/AB] × 100, where AS is the absorbance of a tested sample and AB is the absorbance of control sample (the control contains FeCl2 and ferrozine complex formation molecules). Gallic acid was included as a standard.
11.2.7 Nitric Oxide Radical Inhibition Assay
Nitric oxide radical inhibition can be estimated by the use of Griess Illosvoy reaction [20]. In this assay, Griess Illosvoy reagent was modified by using naphthyl ethylenediamine dihydrochloride (0.1 % w/v) instead of 1-napthylamine (5 %). The reaction mixture (3 ml) containing sodium nitroprusside (10 mM, 2 ml), phosphate buffer saline (0.5 ml) and extracts (0.25 ml) or standard solution (quercetin) was incubated at 25 °C for 2.5 h. After incubation, 0.5 ml of the reaction mixture was mixed with 1 ml of sulfanilic acid reagent (0.33 % in 20 % glacial acetic acid) and allowed to stand for 5 min for complete diazotization. Then, 1 ml of naphthyl ethylenediamine dihydrochloride was added, mixed and allowed to stand for 30 min at 25 °C. A pink coloured chromophore was formed in diffused light. The absorbance of these solutions was measured at 540 nm against the corresponding blank solutions.
11.2.8 Statistical Analysis
Results were presented as mean ± S.D. of experiments. Difference between groups was compared using unpaired t-test with one-tailed test. Correlations between variables were quantified by the correlation factor “r”. Correlation and linear regression analysis was performed using Microsoft Excel 2007. In each analysis p < 0.05 was considered statistically significant.
11.3 Results and Discussion
AGE formation and accumulation have been implicated as a major factor in the development of diabetic complications, atherosclerosis, Alzheimer’s disease, and the normal aging process [21]. Linked to the process of AGE formation, lipoxidation reactions, oxidative and carbonyl stress should also be given as much attention where their tight association is now being increasingly reported [22]. Thus, AGE formation and AGE-protein cross-link formation should not be the only target for therapeutic interventions. Highly reactive carbonyl intermediates responsible for the formation of AGEs and advanced lipoxidation end-products (ALEs) should also be targeted. As formation of these end-products involves a complexity of pathways and reactions, inhibitors that react with each step and intermediate products should be evaluated. Another factor to be considered is the fact that glycoxidized proteins generate reactive oxygen species (ROS) and induce oxidative stress through the reaction with receptor for advanced glycation end-products (RAGEs). Also, ROS are generated by other reactions in the cascade of AGE formation such as methylglyoxal (MGO) and Schiff’s base pathways leading to lipoxidation and oxidative damage. Therefore, strategies such as suppression of receptor signaling pathways (e.g. RAGE antagonists), and the use of antioxidants should be targeted. AGEs being complex and having a variety of cross-links, the development of inhibitors and AGE breakers might be difficult. Nonetheless, oxidative and carbonyl stress associated with AGEs can be inhibited by compounds having both metal chelating and carbonyl scavenging properties [23]. Unfortunately, from a large number of naturally occurring and synthetic compounds reported as AGE inhibitors, only the mechanism of action of a few compounds have been studied extensively. Unraveling the mechanism(s) of action of these inhibitors is essential for understanding the roles of AGE in the pathogenesis of a number of age-related chronic diseases and to design more effective therapeutic strategies for these diseases in the future [23].
During the current study, we focused our efforts to evaluate effective anti-glycation as well as antioxidant ability of extracts of culinary herbs and spices with the objective to be incorporated as therapeutic strategy in diabetes control and prevention. Our main approach was to screen a number of natural products. Furthermore, the purpose of this study was also to investigate the antioxidant activity of some local herbs and spices in order to evaluate the scientific basis of their potential therapeutic application as traditional medicine. All the herbs and spices tested in this study showed antioxidant activities. Based on the screening studies, the most promising compounds were selected to conduct further studies. Some of these results are presented below.
Hence, results gathered from our study and a literature search is suggestive of “Formulation” a concept of combining herbs with similar therapeutic activity. There is no doubt that most herbs exhibit their effects owing to a variety of constituents and therefore it becomes important to evaluate the idea of synergy within and between them and to investigate whether a mixture or formulation possesses greater activity than the individual components or whether the overall potency is synergistic in mechanism. However, more detailed pre-clinical and clinical evidences are required to establish these purported potencies.
11.3.1 In vitro Anti-Glycation Activity of Extracts
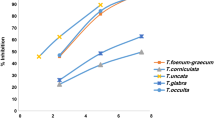
In vitro glycation assays demonstrated that the ten extracts showed marked inhibition of fluorescent AGEs formation as depicted in Fig. 11.1. When glycation was monitored over 12 weeks the percentage inhibition was found to be: garlic (26.1 %), ginger (25.7 %), thyme (42.3 %), parsley (41.2 %), curry leaves (40.9 %), peppermint (39.8 %), turmeric (39.3 %), onion (11.9 %), scallion (27.9 %) and coriander (38.8 %). The correlation between anti-glycation activities of the ten extracts and their total phenolic contents, DPPH and FRAP activities has been presented elsewhere [16].
Anti-glycation activity of the ten food plants. Percentage inhibition of fluorescent AGEs following glycation of BSA with 200 mM glucose in the presence of ten extracts in 0.2 M PBS at 37 °C. Fluorescent AGEs were measured after 12 weeks. Results are presented as means ± S.D. (n = 3) (*Values significantly different (p < 0.05) from control)
11.3.2 Determination of Total Antioxidant Capacity by Phosphomolybdate Method
The antioxidant activities as measured by the TAC assay are depicted in Fig. 11.2 and are as follows: garlic (0.0025 mg AAE/ml), ginger (0.0015 mg AAE/ml), thyme (0. mg AAE/ml), parsley (0.0009 mg AAE/ml), curry leaves (0.0010 mg AAE/ml), peppermint (0.0012 mg AAE/ml), turmeric (0.0010 mg AAE/ml), onion (0.0015 mg AAE/ml), scallion (0.0010 mg AAE/ml) and coriander (0.0008 mg AAE/ml).
11.3.3 ABTS Assay
All the ten extracts displayed antioxidant activity as they were able to scavenge the ABTS•+ radical cation (Fig. 11.3). The ABTS assay involves the oxidation of ABTS to an intensely-coloured nitrogen-centred radical cation, ABTS•+. It is thus useful for testing food extracts because ABTS•+ has an absorption maxima at 734 nm and most food extracts are highly coloured but do not absorb light at 734 nm. It is also viable for both aqueous and lipophilic systems. As the DPPH assay, ABTS measures the total antioxidant activity of extracts. The antioxidant ability of the extracts expressed as the percentage scavenging activity of the radical cation ABTS•+ was as follows: garlic (47 %), ginger (51 %), thyme (95 %), parsley (84 %), curry leaves (98 %), peppermint (97 %), turmeric (95 %), onion (98 %), scallion (69 %) and coriander (38 %).
11.3.4 Ferrous Ion Chelating Ability
Addition of the extracts interferes with the ferrous-ferrozine complex and the red colour of the complex decreased with increasing concentrations of the antioxidant agents. All the extracts captured ferrous ions before ferrozine and thus have ferrous chelating ability at varied extent (Fig. 11.4). Among the extracts tested, the chelating activities were as follows: garlic (37 %), ginger (24 %), thyme (2 %), parsley (18 %), curry leaves (27 %), peppermint (19 %), turmeric (13 %), onion (40 %), scallion (46 %) and coriander (48 %).
Ferrous ion chelating activity. Percentage inhibition of the formation of the ferrous-ferrozine complex of the ten extracts. Results are presented as mean ± S.D. (n = 3) (*Values significantly lower (p < 0.05) compared to positive gallic acid. **Values significantly (p < 0.05) higher than positive control. ***Values not significantly (p > 0.05) different from gallic acid)
11.3.5 Nitric Oxide Radical Scavenging Assay
The extracts effectively reduced the generation of nitric oxide from sodium nitroprusside (Fig. 11.5). The nitric oxide radical scavenging activity expressed as percentage inhibition was as follows: garlic (62 %), ginger (67 %), thyme (84 %), parsley (81 %), curry leaves (79 %), peppermint (73 %), turmeric (71 %), onion (86 %), scallion (85 %) and coriander (83 %).
A wide range of antioxidants from both natural and synthetic origin have been proposed for use in the treatment of various human diseases [23]. Medicinal plants are used as a source of phytochemicals to cure various illnesses in many parts of the world [24]. Being the backbone of traditional medicine, medicinal plants as a good source of biologically active compounds known as phytochemicals have been found to act as antioxidants by scavenging free radicals and having therapeutic potential for free radical associated disorders [25] as it is well known that free radicals are the major cause of various chronic and degenerative diseases such as coronary heart disease, inflammation, stroke, diabetes mellitus and cancer [26]. ROS have been found to play an important role in the initiation and/or progression of various diseases [27]. Thus, recent studies have investigated the potential of plant products as antioxidants against various diseases induced by free radicals [28]. Additionally, it has been determined that the antioxidant effect of plant products is mainly attributed to phenolic compounds, such as flavonoids, phenolic acids, tannins, and phenolic diterpenes [29]. In this regard, during our study we investigated the glycation inhibitory capacity which was correlated with total phenolic, flavonoids and tannins contents of the extracts [16]. As AGEs are related to reactive species, antioxidants have therapeutic potential by scavenging free radicals but also suppress glycation. Antioxidant compounds such as vitamin C, vitamin E and the carotenoids have been shown to reduce in vitro and in vivo protein glycation [30]. For example, treatment of diabetic rats with vitamin E resulted in a decrease of plasma lipid peroxidation [31]. Therefore, antioxidant activity of the plants used in herbal medicine should be assessed either to elucidate the mechanism of their pharmacological action or to provide information on antioxidant activity of these herbal plants. Dietary intake of antioxidants can help scavenge free radicals and oxidants and protect against diseases as suggested by intervention trial studies [32]. Research on natural antioxidants has become increasingly active in various fields. Accordingly, numerous articles on natural antioxidants, including polyphenols, flavonoids, vitamins, and volatile chemicals, have been published [15]. Phenolics are ubiquitous secondary metabolites in plants and possess a wide range of therapeutic uses such as antioxidant, anti-mutagenic, anti-carcinogenic, free radical scavenging activities and also decrease cardiovascular complications [33]. The scavenging ability of the phenolics is mainly due to the presence of hydroxyl groups [34]. Flavonoids and other phenolic compounds of plant origin have been reported as scavengers of free radicals [35]. Flavonoids are a group of polyphenolic compounds, which exhibit several biological effects such as anti-inflammatory, anti-hepatotoxic, anti-ulcer, anti-allergic, antiviral and anticancer activities [36]. Hence, nowadays search for natural antioxidant sources is gaining much importance.
Medicinal plants have been reported to have not only antioxidant but also anti-inflammatory as well as anti-glycation effects [37]. Natural products are an obvious place to search for potential anti-diabetic regimens. Several plant extracts have been reported to exhibit anti-diabetic activity [38]. Among the various medicinal and culinary plants, some endemic species are of particular interest because they may be used for producing raw materials or preparations containing phytochemicals with significant antioxidant capacities and health benefits [39]. The harmful effects of diabetes have been found to be mediated through increased oxidative stress playing a critical role which leads to increased formation and accumulation of AGEs contributing to diabetic complications [15].
In addition to those endogenously formed, AGEs can also be introduced in the body from exogenous sources [40]. Studies have provided evidence that diet is a significant exogenous source of highly reactive AGEs and as environmental risk factors for diabetic complications [41]. To some extent this can be prevented by dietary AGE restriction and incorporation of edible plants with demonstrated anti-glycation and antioxidant properties suggesting that modulation of food-AGE content could become an important ingredient for the therapeutic management of diabetic patients. Until effective and safe drugs become available, physicians and dietitians can, for instance, advise increased reliance on foods with anti-glycation and antioxidant activity. As temporary normalization of serum glycemia is not sufficient, dietary modification to reduce AGEs and prevent glycation can be exploited as a means for reducing serum AGEs for extended periods of time e.g. months or years [42]. Ethnomedical literature contains a large number of plants that can be used against diseases, in which ROS and free radicals play an important role [43]. There is a plethora of plants that have been found to possess strong antioxidant activity. Recent reports indicate that there is an inverse relationship between the dietary intake of antioxidant rich foods and the incidence of human diseases [44]. Interest in finding naturally occurring antioxidants in foods or medicines to replace synthetic antioxidants has increased considerably, given that synthetic antioxidants have restricted use due to their side effects [6]. The potential toxicity of synthetic antioxidants (e.g. butylated hydroxyanisole, butylated hydroxytoluene, tertiary butylhydroquinone, esters of 3,4,5-trihydroxybenzoic acid, etc.) [45] has aroused an increased interest and scientists have focused on isolation and characterization of natural antioxidants from natural sources such as herbs, spices, seeds, cereals, fruits and vegetables by extraction, fractionation and purification [46].
It is also clear that most of the dietary antioxidants have low or minimal toxicity, and that intake can be increased without adverse effects [47]. Many fresh fruits and vegetables have been found to contain natural antioxidants, mainly phenolic compounds such as ferulic acid, catechins, as well as ascorbic acid [48]. Polyphenols are the biggest group of phytochemicals, and many of them have been found in plant-based foods. Polyphenol-rich diets have been linked to many health benefits. Dietary polyphenols have received tremendous attention among nutritionists, food scientists and consumers due to their roles in human health [49]. Some of the reported active compounds of the extracts studied are given in Table 11.1.
Our observation that high values of antioxidant activity and phenolic content were found in ethanol extracts is in line with reported data [16]. In general, there was a good positive correlation between the TPC and antioxidant activity as assessed by FRAP and DPPH-scavenging assays suggesting that polyphenols are important contributors to the antioxidant and free-radical scavenging activities. However, the lack of correlation between TPC and antioxidant activity reported in some studies could be due to the different antioxidant activity parameters determined in these studies, different responses of phenolic compounds in different bioassays used to determine the antioxidant activity and/or the chemical structure of the phenolic compounds present in different plants. Moreover, the total phenolic content estimated by the Folin-Ciocalteu reagent may overestimate TPC because it is known to react with other components present in the plant extracts such as minerals and ascorbic acid [59, 60]. Consequently, the antioxidant/free-radical scavenging activities of the plant extracts cannot be predicted on the basis of their TPC alone, but also requires proper characterization of the individual phenolic components [61].
The results of the current study have shown that most of the studied plants are potentially good sources of free-radical scavenging compounds and support the traditional medicinal application of some of the tested plants. During this study, we systematically screened extracts of medicinal and edible plants used as culinary herbs and spices for their anti-glycation activities through biochemical assay. BSA was incubated with reducing sugar at physiological pH for various durations. The extent of glycation of proteins was measured mainly by the spectrofluorimetric method. As a result, the ten extracts under study were identified as having anti-glycation potential and their activities were compared with aminoguanidine. The total phenolic, flavonoids, tannins contents and antioxidant activity as determined by the DPPH and FRAP assays were correlated with the observed anti-glycation activity. Details of this study have been published elsewhere [16]. The phenolic compounds make a significant contribution to the antioxidant activity in these extracts as evidenced by the positive correlation between phenolic contents and antioxidant activities. Therefore, ingestion of extracts from these plants may help to prevent in vivo oxidative damage associated with diseases and illnesses.
11.4 Conclusions
The present study was aimed at investigating the antioxidant activities of ethanolic extracts of selected culinary herbs and spices. It is reasonable to consider that good glycemic control, in combination with a careful diet in terms of reduced AGE consumption and increased intake of natural products with anti-glycation and antioxidant properties should be among the new goals for optimal management of diabetic patients. Addressing dietary habits from a new perspective, while difficult, could achieve the best long-term effects as novel drug interventions become available for clinical use in the future.
Comparison of nutritional regime and values of AGEs shows that the higher intake of fructose in alternative nutrition of healthy subjects may cause an increase in plasma AGE values. Protective effect of regular consumption of vegetables and fruits dominantly concerns the prevention of free-radical diseases. The risks of alternative nutrition may be reduced by better choice of foodstuffs.
Further studies to evaluate the in vivo potential of these dietary agents in various animal models and the isolation and identification of the antioxidant principles are needed. Nonetheless these studies provide a database as a valuable instrument for available information on natural products with reported therapeutic ability for guiding food choices to reduce AGE and oxidative stress.
References
Patwardhan B, Vaidya ADB, Chorghade M (2004) Ayurveda and natural products drug discovery. Curr Sci 86:789–794
Grover JK, Yadav S, Vats V (2002) Medicinal plants of India with antidiabetic potential. J Ethnopharmacol 81:81–100
Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK (2006) Anti-diabetic agents from medicinal plants. Curr Med Chem 3:1203–1218
Dewanjee S, Das AK, Sahu R, Gangopadhyay M (2009) Anti-diabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol 47:2679–2685
Patel DK, Kumar R, Laloo D, Hemalatha S (2012) Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac J Trop Biomed 2:411–420
Singh U, Singh S, Kochhar A (2012) Therapeutic potential of antidiabetic nutraceuticals. Phytopharmacol 2:144–169
Shokeen P, Anand P, Murali YK, Tandon V (2008) Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol 46:3458–3466
Wadkar KA, Magdum CS, Patil SS, Naikwade NS (2008) Anti-diabetic potential and Indian medicinal plants. J Herb Med Toxicol 2:45–50
Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A (2006) Medicinal plants with potential anti-diabetic activity-A review of ten years of herbal medicine research (1990–2000). Int J Diabetes Metab 14:1–25
Elosta A, Ghous T, Ahmed N (2012) Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 8:92–108
Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nagagawa K, Kitahara M (2005) Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem 53:959–963
Frei B, Higdon JV (2003) Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr 133:3275S–3284S
Morihara N, Nishihama T, Ushijima M, Ide N, Takeda H, Hayama M (2007) Garlic as an anti-fatigue agent. Mol Nutr Food Res 51:1329–1334
Sheikh N, Safari M, Mani Kashani K, Araghchian M, Zeraati F (2004) Study on the effect of garlic on the in vitro albumin glycation reaction. Acta Med Iran 42:113–116
Patel DK, Kumar R, Laloo D, Hemalatha S (2012) Natural medicines from plant source used for therapy of diabetes mellitus: an overview of its pharmacological aspects. Asian Pac J Trop Med 42:239–250
Ramkissoon JS, Mahomoodally MF, Nessar A, Subratty AH (2012) Relationship between total phenolic content, antioxidant potential and antiglycation abilities of common culinary herbs and spices. J Med Food 15:1116–1123
Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, Hasegawa J (2002) Screening system for the Maillard reaction inhibitor from natural product extracts. J Health Sci 48:520–526
Prietto P, Pineda M, Aquilar M (1999) Spectrophotometric quantification of antioxidant capacity through the formation of phosphomolybdenum complex: specification application to the determination of vitamin E. Anal Biochem 269:337–341
Chen YF, Roan HY, Li CK, Huang YC, Wang TS (2011) Relationship between antioxidant and antiglycation ability of saponins, polyphenols, and polysaccharides in Chinese herbal medicines used to treat diabetes. J Med Plants Res 5:2322–2331
Viuda-Martos M, Navajas YR, Zapata ES, Fernández-López J, Pérez-Álvarez JA (2010) Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Frag J 5:13–19
Bandyopadhyay U, Das A, Bannerjee RK (1999) Reactive oxygen species: oxygen damage and pathogenesis. Curr Sci 77:658–666
Rahbar S, Figarola JL (2003) Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys 419:63–79
Cuzzocrea S, Riley DP, Caputi AP, Salvemini D (2001) Antioxidant therapy: a new pharmacological approach in shock, inflammation and ischemia/reperfusion injury. Pharmacol Rev 53:135–159
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Povichit N, Phrutivorapongkul A, Suttajit M, Chaiyasut C, Leelapornpisid P (2010) Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pak J Pharm Sci 23:403–408
Smirin P, Taler D, Abitbol G, Brutman-Barazani T, Kerem Z, Sampson SR, Rosenzweig T (2010) Sarcopoterium spinosum extract as an antidiabetic agent: in vitro and in vivo study. J Ethnopharmacol 129:10–17
Halliwell B (1997) Antioxidants and human diseases: a general introduction. Nutr Rev 55:S44–S52
Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, Chen CH, Hwang SY, Lee MH (2003) Free radical-scavenging activity of Taiwanese native plants. Phytomedicine 10:170–175
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Cameron NE, Cotter MA (1993) Potential therapeutic approaches to the treatment or prevention of diabetic neuropathy: evidence from experimental studies. Diabetes Med 10:593–605
Kinalski M, Sledziewski A, Telejko B, Zarzycki W, Kinalska N (1999) Antioxidant therapy and streptozoticin-induced diabetes in pregnant rats. Acta Diabetol 36:113–117
Frei B (1994) Natural antioxidants in human health and disease. Annu Rev Nutr 16:33–50
Yen GH, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43:27–32
Huang D, Ou B, Proir RL (2005) The chemistry behind the antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Formica JV, Regelson W (1995) Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33:1061–1080
Cao G, Sofic E, Prior RL (1997) Antioxidant and pro-oxidant behaviour of flavonoids: structure activity relationships. Free Radic Biol Med 22:749–760
Chua MT, Tung YT, Chang ST (2008) Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour Technol 99:1918–1925
Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte PM, Wang Y, Wu D, Chu W, Qin G, Lam KS (2009) Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology 150:625–633
Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Troganis A, Boskou D (2002) Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J Agric Food Chem 50:5294–5299
Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H (1997) Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Med Sci 94:6474–6479
Ling X, Nagai R, Sakashita N, Takeya M, Horiuchi S, Takahashi K (2001) Immunohistochemical distribution and quantitative biochemical detection of advanced glycation endproducts in fetal to adult rats and in rats with streptozotocin-induced diabetes. Lab Invest 81:845–861
Peppa M, Uribarri J, Vlassara H (2003) Glucose, advanced glycation end products, and diabetes complications: what is new and what works. Clin Diabetes 21:186–187
Huxley RR, Neil H (2003) The relationship between dietary flavonol intake and coronary heart disease mortality: a metaanalysis of prospective cohort studies. Eur J Clin Nutr 57:904–908
Kumar DS, Muthu AK, Smith AA, Manavalan R (2010) In vitro antioxidant activity of various extracts of whole plant of Mucuna pruriens (Linn). Int J PharmTech Res 2:2063–2070
Johnson IT, Fenwick GR (2000) Dietary anticarcinogens and antimutagens: chemical and biological aspects. Royal Society of Chemistry, London, pp 92–95
Umamaheswari M, Chatterjee TK (2008) In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med 5:61–73
Batool F, Sabir SM, Rocha JBT, Shah AH, Saify ZF, Ahmed SD (2010) Evaluation of antioxidant and free radical scavenging activities of fruit extract from Zanthoxylum alatum: a commonly used spice from Pakistan. Pak J Bot 42:4299–4311
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolourisation assay. Free Radic Biol Med 26:1231–1237
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutr 2:1231–1246
Ahmad MS, Ahmed N (2006) Antiglycation properties of aged garlic extract: Possible role in prevention of diabetic complications. J Nutr 80:796–799
Moon JK, Shibamoto T (2009) Antioxidant assays for plant and food components. J Agric Food Chem 57:1655–1666
Suhaj M (2006) Spice antioxidants isolation and their antiradical activity: a review. J Food Compos Anal 19:531–537
Haidari F, Keshavarz A, Shahi MM, Mahboob SA, Rashidi MR (2011) Effects of parsley (Petroselinum crispum) and its flavonol constituents, kaempferol and quercetin, on serum uric acid levels, biomarkers of oxidative stress and liver xanthine oxidoreductase a activity in oxonate-induced hyperuricemic rat. Iran J Pharm Res 10:811–819
Das AK, Rajkumar V, Dwivedi DK (2001) Antioxidant effect of curry leaf (Murraya koenigii) powder on quality of ground and cooked goat meat. Int Food Res J 18:563–569
Wu CH, Huang SM, Lin JA, Yen GC (2011) Inhibition of advanced glycation end-product formation by foodstuffs. Food Funct 2:224–234
Rice-Evans C, Miller N, Bolwell P, Bremley P, Pridham J (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Rad Res 22:375–383
Naidu JR, Ismaill RB, Yeng C, Sasidha S, Kumar P (2012) Chemical composition and antioxidant activity of the crude methanolic extracts of Mentha spicata. J Phytol 4:13–18
Leelarungrayub N, Chanarat N, Rattanapanonel V (2004) Potential activity of Thai shallot (Allium ascalonicum L.) extract on the prevention of hemolysis and glutathione depletion in human erythrocyte from oxidative stress. CMU J 3:225–234
Matthaus B (2002) Antioxidant activity of extracts obtained from residues of different oil seeds. J Agric Food Chem 50:3444–3452
Deepa N, Kaur C, Singh B, Kapoor HC (2006) Antioxidant capacity in some red sweet pepper cultivars. J Food Compos Anal 19:572–578
Molan AL, Faraj AM, Mahdy AS (2012) Antioxidant activity and phenolic content of some medicinal plants traditionally used in northern Iraq. Phytopharmacol 2:224–233
Acknowledgements
We are grateful to the University of Mauritius and the Tertiary Education Commission, Mauritius for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Ramkissoon, J.S., Mahomoodally, M.F., Ahmed, N., Subratty, A.H. (2014). Therapeutic Potential of Common Culinary Herbs and Spices of Mauritius. In: Gupta Bhowon, M., Jhaumeer-Laulloo, S., Li Kam Wah, H., Ramasami, P. (eds) Chemistry: The Key to our Sustainable Future. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7389-9_11
Download citation
DOI: https://doi.org/10.1007/978-94-007-7389-9_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7388-2
Online ISBN: 978-94-007-7389-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)