Abstract
Age is a very well known prognostic factor in brain tumors. Adults with a medulloblastoma (MB) have a poorer outcome compared to children with a MB. A feature specific to MB’s relationship to age when determining survival is the appearance of differences in survival between age groups after a particular follow-up time. Up to 4 years post-diagnosis, the prognosis remains the same, but between 4 and 10 years of follow-up, adults become significantly more likely to die than children.
The relationship between age and survival may be a confounded relationship due to the genetic basis of the tumor, responses and compliance to treatment protocols, and the anatomical location of the tumor. Each of these factors may be the actual “drivers” of these differences, rather than age itself. When measuring the differences across age groups in MBs, there are two important statistical concepts that are key: relative survival and the proportional hazards assumption. Both of these are discussed within this chapter. This chapter focuses on the factors that may be the drivers of the survival differences, such as clinical factors, genetics, treatment response and/or compliance, and progression patterns.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
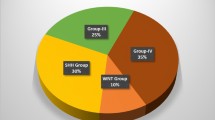
Medulloblastomas (MB) originate from primitive embryonal cells, typically with neurectodermal components, which is why this tumor is classified as a primitive neurectodermal tumor (PNET). The incidence of this tumor is just under 2 per million, and children are 10 times more likely to be affected than adults (Smoll and Drummond 2012). Today, MBs are considered to be distinct from PNETs (Pomeroy et al. 2002), and MBs are considered to be made up of four different subgroups: WNT tumors showing wingless pathway activation (excellent prognosis) make up only 11 % of MBs; SHH showing hedgehog pathway activation (worse prognosis, affects infants and adolescents/adults (affects children less), similar to a “bathtub” distribution) form approximately 28 % of all MBs; group 3 tumors (worst prognosis, rarely found in adults) compose 27 % of all MBs; and group 4 tumors are the most common MB subtype (34 % of MB). Therefore, I suggest that age may be surrogate variable associated with outcomes, because its association with survival is really a relationship confounded by several “driver” variables described here.
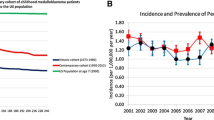
In 2012 I demonstrated how the differences in survival across age-categories is time-dependant, using a large population-based dataset. This means that the differences in hazard rates (and similarly, survival rates) across the age groups only emerge after 4 years of follow-up, also known as a “fork” type interaction because of it’s appearance on Kaplan–Meier curves or similar hazard rate plots. To exemplify this, children and adults had 75 % and 75 % 2-year survival rates, but 57 % and 46 % 10-year survival rates respectively. Thus demonstrating how differences in survival are follow-up time-dependant.
It is suggested that MBs probably develop silently during embryologic phases of development, and mutations after repeated multiplication will accumulate throughout life until a certain combination of mutations (especially those associated with a specific MB subtype) lead to tumor growth (Jones et al. 2012). This is based on the findings that adult MBs have higher frequencies of passenger mutations (Parsons et al. 2010) and confirmed by Jones et al. (2012) finding that the rate of mutations is positively correlated with age.
The question regarding survival differences between adults and children has been a difficult one to answer, as the two patient groups are remarkably different across the entire range of variables. The most obvious example of these important differences is that children and adults get treated in different institutions because of the different requirements of each age group. Since we cannot randomize patients into adult and childhood age groups, the differences across these age groups are best measured using data from observational and/or registry studies. Like all observational/registry studies, there are an infinite number of important variables that cannot be controlled for, and there are probably multiple variables which may confound the relationship. What is important to note, is that age is a variable that is probably confounded by other “driver” variables, which are the variables that are imbalanced across age groups, but are independant factors affecting survival rates.
There are probably several important interactions (effects of an independent variable or predictor which vary across the range of values of another variable) between variables that are known to predict outcomes. Unfortunately, to detect these one must have large sample sizes, or very large effect sizes. For example, a particular type of chemotherapy protocol may show efficacy in children with an MB, but may not show the same efficacy in adults with an MB, revealing an interaction between chemotherapy and age groups (or more precisely, some as yet unknown biological driver variable closely related to age). Interactions are probably (it could be argued certainly) present between genetic subtypes of MBs and their response to chemotherapy and or radiotherapy. While the genetic subtypes of MB are dominating the field of MB research, outcomes are also determined by other factors. There are clinical and/or treatment variables that also impact the differences in survival outcomes between adults and children.
Using relative survival (RS) to remove the effect of expected mortality rates seen in the general population, it appears that adults and children have the same survival outcomes up to 4 years post-diagnosis. After 4 years, adults become significantly more likely to die than children (Smoll 2012). Although recent randomized studies are showing impressive results, with some finding 5-year survival rates in children of up to 95 % (Packer et al. 2006; Rutkowski et al. 2005). Most importantly, this chapter will focus on discussing the drivers of survival differences between children and adults.
Genetic Drivers of Survival Differences Between Children and Adults
The most promising factor in MB research currently is the four genomic subtypes of MB, first discussed by Kool et al. in 2008. Kool et al.’s recent study demonstrated impressive survival rates in patients with metastases positive and metastases negative wingless (WNT) tumors (greater than 95 % 5-year survival for both age categories). Across all age categories, the WNT subtypes were virtually always found in the Classical histology category, which is typically known to have a worse prognosis than the Desmoplastic histology subtype. Nonetheless, this genetic subtype has similarly excellent outcomes in both adults and children (Kool et al. 2012).
The sonic hedgehog (SHH) driven subtype which is more likely to be found in infants and adults similar to a “bathtub”-type age distribution, may be the driver of the differences in survival between adults and children seen after 4 years. This was remarkably different at 10 years post-diagnosis, with survival rates for SHH tumors at 34 % in adults and 51 % in children. Therefore, the differences in survival seen after 5-years post-diagnosis may be because adults are more likely to be affected by the SHH-driven subtype, and the differences in survival in this subtype begins to appear after 5-years post-diagnosis, and are clear by 10 years (Table 10.1).
Treatment Drivers of Survival Differences Between Children and Adults
Investigators must remember that patients are exposed to treatment regimens that are often different for adults when compared to children. Children and adults differ in their ability to withstand different treatment protocols. Greenberg et al. 2001 and Packer et al. 1994 found that 44 % of children were able to complete a multi-agent CDP protocol (to the 8th cycle), while no adult patients in Greenberg et al.’s study were able to complete the CDP protocol (Greenberg et al. 2001; Packer et al. 1994). While the inability to complete therapy may have something to do with the differences in survival, perhaps a more complex mechanism is at work. Adults are typically offered the same post-operative adjuvant therapy protocols as children, but because they are more likely to have SHH-driven tumors, the effect of chemotherapy is less, because SHH-driven tumors may not have the same response as WNT, group 3, and group 4 tumor subtypes. Thus, while we suspect that WNT tumors respond well to typical adjuvant therapies because of their impressive survival rates, the interaction between the latest radiochemotherapy protocols and MB subtypes is unclear when survival or progression-free survival is the measured outcome.
Anatomical Location Drivers of Survival Differences Between Children and Adults
It is well known that adults get more MBs in the cerebellar hemispheres, while children are more likely to have tumors located in the cerebellar vermis. While it is often thought that it is easier to obtain gross-total resection in hemispherically located tumors, there is little evidence that location is associated with survival (Greenberg et al. 2001). Lastly, the effect of anatomical location on survival is probably a relationship confounded by gross-total resection rates or genetic factors.
To briefly recap: confounding is considered to be the situation in which the study exposure groups (in this case, age-categories) differ in their hazard rates or in relative survival-excess hazard rates for reasons other than the effects of the exposure group variable (Greenland et al. 1999). To relate this to the ideas presented here, if anatomical location was known to have an impact on excess hazard rates, when gross-total resection rates are taken into account (controlled for), the effect of anatomical location on excess hazard rates may disappear. In other words, the anatomical location has a relationship with survival, only because it affects the surgeons ability to achieve a gross-total resection at surgery.
Relapse-Free Survival as a Driver of Survival Differences Between Children and Adults
Incidence of late relapse appears to be greater in adults, with relapses in children tending to occur before 3 years post-diagnosis. Khalil (2008) presents a series of 51 pediatric MBs in which all patients that relapsed (10, or 20 %) relapsed before 2 years. Brandes et al. 2007 demonstrated 17 relapses in 36 adult patients (47 %), with a median recurrence time of 3 years post-diagnosis (Brandes et al. 2007). In addition, when one reviews the progression-free curves of various studies including children, plateaus are noted to start at or before 4 years for children (Allen et al. 2009; Evans et al. 1990; Rutkowski et al. 2010; Zeltzer et al. 1999). When compared to adults the progression-free curve of adults continues to decrease and reaches a plateau at 10 years (Padovani et al. 2007). While these progression-free curves are consistent with the finding of survival differences between adults and children (because adults appear to progress later than children), although it may seem logical, but we are as yet unclear if later progressions explain the survival difference seen after 4 years.
Relative Survival and Measuring Differences Between Children and Adults
To measure survival differences between adults and children, investigators must take into account that adults, and especially elderly people in the general population are already more likely to die than children. The mortality rate of the general population is called the expected mortality rate. Subtracting the expected mortality rate from the hazard rate in a population of cancer patients gives us a measure known as the excess hazard rate, and when transformed into the survival scale this gives us the measure known as relative survival (RS). RS is considered to be the gold standard of cause-specific survival estimation because of its robustness and non-reliance on death certificates for correct descriptions of the cause of death.
When one compares the survival rates of children to those of adults, the relationship between the categories changes during follow-up (at least in population-based studies). As mentioned previously, survival rates are virtually identical before 4 years. After 4 years the survival rates begin to differ, with adults faring worse. This concept is known as non-proportional hazards, and is key to understanding changing relationships between two groups. As a brief recap, hazard rates are what underpin survival rates. A hazard rate is the instantaneous event per unit of time. In other words, it is the amount of deaths per smallest unit of time. Some might call this the “speed of death” or “speed of mortality”. Regression models that present hazard ratios generally present a ratio of two hazard rates. For example, if during a particular month 5 children per 100 children died, and this was compared with 10 adults per 100 died, a hazard ratio of 2 would be present.
Proportional hazards models (such as the Cox proportional hazards model) assume that this difference will be present throughout the entirety of follow-up, and therefore only one estimate is presented, like if it was an average over time. This is unanimously, and I believe erroneously considered to be appropriate for almost all brain tumors and all situations. This is evidenced by most analyses of data published on brain tumors relying on Cox’s proportional hazards model to provide regression estimates. The assumption of “proportional hazards” has been found to be violated for MBs and low grade gliomas (Smoll 2012; Smoll et al. 2012). In these studies, the differences in hazard rates changed throughout follow-up. For example, when young adults are compared to the elderly, the excess hazard rates for low-grade gliomas (adjusted for expected mortality) are enormously different (magnitude of 30 times in the first year) for the first 2 years, and as time progresses the excess hazard rates re-approximate for what is termed the “reverse fork-type interaction”. For a more extensive discussion of this problem, see the article by Miguel Hernan (2010).
In addition to non-proportionality, adults and children have much different expected mortality rates when all-cause mortality is considered. Adults are simply more likely to die from all causes. Therefore, to truly extract differences in survival between adults and children, the use of relative survival methodology is required. Relative survival is considered the gold-standard of cause-specific survival, because all deaths in a particular population that are above the rate normally seen in the general population can be considered to be due to the tumor, irrespective of the listed cause of death. This is particularly important when we measure the differences between adults and children because it is well known that adults and children have different expected mortality profiles, and relative survival methods intrinsically control for this factor.
Excess hazard rates of MBs have therefore demonstrated the quality of non-proportionality when adults and children are compared, which means the investigator must beware when using proportional hazards models when modeling MB data. Thus, modelling of age differences requires the use of specialized models such as a discrete-time survival models or Dickman’s piecewise constant hazards model for relative survival data to accurately model such data (Dickman et al. 2004; Singer and Willett 1993).
In conclusion, the differences in survival between children and adults with MB/PNETs are clear, but the relationship is complex. We know that the differences in survival become apparent after 4 years and that adults appear to relapse often at later stages, but the difficult part is finding out why. Adults are more likely to be affected by the SHH-subtype which has a worse prognosis. Children may be more likely to complete chemotherapy protocols and their tumors progress/recur earlier. But there are many factors that speak in favor of adult tumors having better prognosis. Therefore, while the relationship is complex, new information on the genomics is emerging. Thus, to find out if there is truly a relationship between age and survival, we must first be able to control for the imbalances in the independant predictors of survival.
References
Allen J, Donahue B, Mehta M, Miller DC et al (2009) A phase II study of preradiotherapy chemotherapy followed by hyperfractionated radiotherapy for newly diagnosed high-risk medulloblastoma/primitive neuroectodermal tumor: a report from the Children’s Oncology Group (CCG 9931). Int J Radiat Oncol Biol Phys 74:1006–1011
Bloom HJ, Bessell EM (1990) Medulloblastoma in adults: a review of 47 patients treated between 1952 and 1981. Int J Radiat Oncol Biol Phys 18:763–772
Brandes AA, Franceschi E, Tosoni A, Blatt V et al (2007) Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer 110:2035–2041
Dickman PW, Sloggett A, Hills M, Hakulinen T (2004) Regression models for relative survival. Stat Med 23:51–64
Evans AE, Jenkin RD, Sposto R, Ortega JA et al (1990) The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg 72:572–582
Giordana MT, Cavalla P, Dutto A, Borsotti L et al (1997) Is medulloblastoma the same tumor in children and adults? J Neurooncol 35:169–176
Greenberg HS, Chamberlain MC, Glantz MJ, Wang S (2001) Adult medulloblastoma: multiagent chemotherapy. Neuro Oncol 3:29–34
Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10:37–48
Hernan MA (2010) The hazards of hazard ratios. Epidemiology 21:13–15
Jones DT, Jager N, Kool M, Zichner T et al (2012) Dissecting the genomic complexity underlying medulloblastoma. Nature 488:100–105
Khalil EM (2008) Treatment results of adults and children with medulloblastoma NCI, Cairo University experience. J Egypt Natl Canc Inst 20:175–186
Kool M, Korshunov A, Remke M, Jones DT et al (2012) Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123:473–484
Kortmann RD, Kuhl J, Timmermann B, Mittler U et al (2000) Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ’91. Int J Radiat Oncol Biol Phys 46:269–279
Packer RJ, Sutton LN, Elterman R, Lange B et al (1994) Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg 81:690–698
Packer RJ, Gajjar A, Vezina G, Rorke-Adams L et al (2006) Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24:4202–4208
Padovani L, Sunyach MP, Perol D, Mercier C et al (2007) Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys 68:433–440
Parsons DW, Li M, Zhang X, Jones S et al (2010) The genetic landscape of the childhood cancer medulloblastoma. Science, pp 435–439
Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM et al (2002) Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415:436–442
Rutkowski S, Bode U, Deinlein F, Ottensmeier H et al (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352:978–986
Rutkowski S, von Hoff K, Emser A, Zwiener I et al (2010) Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 28:4961–4968
Sarkar C, Pramanik P, Karak AK, Mukhopadhyay P et al (2002) Are childhood and adult medulloblastomas different? A comparative study of clinicopathological features, proliferation index and apoptotic index. J Neurooncol 59:49–61
Singer JD, Willett JB (1993) It’s about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Stat 18:40
Smoll NR (2012) Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer 118:1313–1322
Smoll NR, Drummond KJ (2012) The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 19:1541–1544
Smoll NR, Gautschi OP, Schatlo B, Schaller K et al (2012) Relative survival of patients with supratentorial low-grade gliomas. Neuro Oncol 14:1062–1069
Zeltzer PM, Boyett JM, Finlay JL, Albright AL et al (1999) Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol 17:832–845
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Smoll, N. (2014). Medulloblastomas: Survival Differences Between Children and Adults. In: Hayat, M. (eds) Tumors of the Central Nervous System, Volume 12. Tumors of the Central Nervous System, vol 12. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7217-5_10
Download citation
DOI: https://doi.org/10.1007/978-94-007-7217-5_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7216-8
Online ISBN: 978-94-007-7217-5
eBook Packages: MedicineMedicine (R0)