Summary
It is well known that chloroplasts move in response to changes in blue light intensity. Under low light conditions chloroplasts spread out in a so-called accumulation response and maximize light interception. Under high light they move to the anticlinal sides of cells, in a so-called avoidance reaction, minimizing light interception. In recent years tremendous progress has been made in our understanding of chloroplast movement due to a combination of new approaches and model systems. Mutant screens in Arabidopsis thaliana revealed a considerable number of new players, which modify the speed and the degree of the blue light driven movement of chloroplasts. In addition, better microscopy technologies revealed a fascinating picture of highly dynamic changes in chloroplast associated actin filaments that are essential for chloroplast movement. Our understanding has been further enhanced by studies of the gametophytes of the moss Physcomitrella patens and the fern Adiantum capillus-veneris. Using a microbeam that illuminates part of a cell, these microscopy studies gave insights into differences and similarities in photoreception and the mechanics of chloroplast movement comparing angiosperms and cryptogams. In addition by studying the behavior of individual chloroplasts within cells, information was gained on the speed and duration with which light signal information travels. Despite advances on the molecular level, our understanding of the species-specific variability and ecological importance of chloroplast movement is still rudimentary. This review will give an overview of our current understanding of chloroplast movement and will point out similarities and differences in behavior among higher plants, ferns and bryophytes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Photosynthesis is of central importance to all plants, but light which drives photosynthesis is one of the most challenging and variable environmental factors that plants have to content with. In environments such as the understory, plants are limited by light and need to maximize light interception, while canopy leaves have to protect themselves from excess light and the danger of photoinhibition. In addition, light intensities can vary greatly within minutes, which poses great challenges if plants are to optimize their photosynthetic behavior. Not surprisingly, plants have evolved a wide range of sophisticated mechanisms that allow them to deal with ever changing light intensities. Those mechanisms range from acclimation via altered gene expression to physiological processes that act on a time scale of minutes (Li et al. 2009). One such mechanism is the ability of plants to move their chloroplasts into regions of more desirable light intensities within minutes. Under low light intensities chloroplasts spread out within a cell in a so-called accumulation response, thereby maximizing light interception (Zurzycki 1955), while under high light they move to the anticlinal cell walls in a so-called avoidance response thereby minimizing the exposure to light and the likelihood of photoinhibition (Kasahara et al. 2002; Königer et al. 2008). This ability of chloroplasts to move within cells was first documented over a century ago in studies on algae, mosses, ferns and higher plants (Senn 1908). For an example, showing the chloroplast distribution in the model species Arabidopsis thaliana, Adiantum capillus-veneris and Physcomitrella patens see Fig. 8.1. Clever experimental set-ups laid the groundwork for our understanding of the phenomenon, but it has been only during the past 10 years that some of the key players involved in chloroplast movement and anchoring have been discovered (for reviews see Wada et al. 2003; Takagi et al. 2009).
Chloroplast distribution in three model species under low and high light intensities. In A. thaliana chloroplasts assume very clear accumulation and avoidance positions, while changes in chloroplast positioning are not as obvious in the other two species.
The chloroplast positioning on the adaxial (a, c) and abaxial (b, d) leaf sides of Arabidopsis thaliana and Adiantum capillus-veneris after a 1 h exposure of leaves to white light of 1 (a, b) or 1,000 μmol photons m−2 s−1 (c, d). For Physcomitrella patens leaflets and protonemata (cultured on plates) were exposed for a 1 h to white light of 1 (e1−3) or 1,000 μmol photons m−2 s−1 (f1−3). Samples were fixed in 2 % glutaraldehyde and chlorophyll a fluorescence was used to image the chloroplasts. Confocal images are maximum projections of optical sections spaced at 1 μm. Scale bar = 200 μm.
II. Photoreceptors
Significant progress has been made characterizing the key components involved in perceiving the light signals that induce chloroplast movement in various species. Blue light exclusively induces accumulation and avoidance movements in all terrestrial higher plants, most fern (e.g., Pteris vittata, Pteris cretica, Adiantum caudatum, Adiantum diaphanum, Cyrtomium fortunei, Microsorum pustulatum) and moss species (e.g., Funaria hygrometrica, Ceratodon purpureus) studied so far (Zurzycki 1967; Inoue and Shibata 1974; Kadota et al. 1989; Kagawa et al. 1997; Augustynowicz and Gabryś 1999; Königer and Bollinger 2012). In these species blue light is perceived by phototropin1 and phototropin2, plasma membrane-associated serine/threonine protein kinases that undergo autophosphorylation in response to blue light. Both phototropins contain two LOV domains, which sense light through the cofactor flavin mononucleotide. Light stimulation leads to the autophosphorylation of the kinase domain, which then phosphorylates other yet unknown targets (Kagawa et al. 2001; Jarillo et al. 2001; Christie 2007). In Arabidopsis thaliana the accumulation response is triggered by signals from phot1 and phot2, which operate redundantly, but through distinct pathways. The phot2 mediated avoidance response overrides the phot1 mediated accumulation response under high light intensities. The dark positioning of chloroplasts is also controlled by phot2 (Kagawa et al. 2001; Sakai et al. 2001; Suetsugu et al. 2005a; Luesse et al. 2010). A study on the distinct functions of the various regions and domains of the phototropin receptors showed that in A. thaliana the N-terminal end mainly determines the light sensitivity of the phototropins, while the specific combination of the N- and C-terminal regions of phot1 suppresses the avoidance response. Part of the N-terminus of phot2 is required for the proper dark positioning of chloroplasts (Aihara et al. 2008). Using a GFP-phot1 fusion in A. thaliana showed that phot1 localizes to the plasma membrane regions of leaf epidermal and mesophyll cells in the dark (Sakamoto and Briggs 2002), but moves to unidentified cytosolic structures in the light in a response that is dependent on phot1 phosphorylation status and kinase activation (Kaiserli et al. 2009; Sullivan et al. 2010). Phot2 localizes mainly to the plasma membrane in the dark, but a fraction of the receptor pool moves to the Golgi apparatus in response to blue light (Kong et al. 2006).
The phototropins and their functions are well conserved among higher plants, ferns and mosses (Christie 2007; Suetsugu and Wada 2007). There are however, a few plant species in which chloroplast movement is induced by red light in addition to blue light. For example, in the aquatic monocot Vallisneria gigantea chloroplasts in the epidermis move into an accumulation position in response to dim red light, while they move to an avoidance position in response to elevated red and strong blue light (Izutani et al. 1990; Dong et al. 1995; Takagi 2003). The accumulation response of chloroplasts in these epidermal cells is affected by illumination with far-red light, pointing towards a phytochrome-dependent mechanism. In addition, DCMU, an inhibitor of photosynthesis adversely affected the ability of chloroplasts to reach the accumulation position (Takagi 2003). In gametophytes and sporophytes of some fern species such as Adiantum capillus-veneris and Dryopteris sparsa both blue and red light can cause chloroplasts to move (Yatsuhashi et al. 1985; Yatsuhashi and Kobayashi 1993; Augustynowicz and Gabryś 1999). Blue light induced chloroplast avoidance movement in A. capillus-veneris was observed under ten-fold lower blue light intensities than under red light (Yatsuhashi et al. 1985). In addition to phot1 and phot2, A. capillus-veneris has a chimeric photoreceptor (NEOCHROME1) made up of the chromophore-binding domain of phytochrome3 and nearly full-length phot1. Neochrome functions both as a red and a blue light receptor. Interestingly phot1 and NEO1 are responsible for the accumulation response, while phot2 is responsible exclusively for the avoidance response and proper dark positioning (Nozue et al. 1998; Kagawa et al. 2004; Suetsugu et al. 2005b; Tsuboi et al. 2009). NEO1-like sequences have been found also in some other polypodiaceous ferns (Dryopteris filix-max, Hypolepis punctata, Onoclea sensibilis), but not in more primitive ferns (Osmunda japonica, Lygodium japonicum) (Kawai et al. 2003; Suetsugu et al. 2005b).
Both red and blue light also can induce chloroplast movement in protonemal cells of the moss Physcomitrella patents (Kadota et al. 2000), as long as the cells were cultured in red, not white light (Kadota et al. 2000). P. patens has a complex set of receptors with four phototropins (photA1, photA2, photB1, photB2) which are responsible for the blue light induced chloroplast movement. Interestingly, both photA and photB groups are involved in the avoidance response. The primary photoreceptor for the red light induced chloroplast movement is phytochrome, but phototropins may act downstream since the triple phototropin mutant photA2photB1photB2 showed reduced red light induced chloroplast movement. No neochrome-like protein has been found in the mosses P. patens and Ceratodon purpureus (Kasahara et al. 2004; Suetsugu et al. 2005b). In P. patens the canonical PHY1-3, which are localized in the cytoplasm, are involved in the avoidance response in protoplasts (Uenaka and Kadota 2007), while PHY4 seems to be involved in the avoidance response in protonemal cells (Mittmann et al. 2004). It is not clear why these mutant studies showed conflicting results. Interestingly, the intensities at which the accumulation and avoidance responses occurred were different depending on the light quality (Kadota et al. 2000).
Little is known about the signaling pathways involved in phototropin mediated chloroplast movement. However, it is clear that blue light leads to characteristic changes in internal calcium concentrations in species ranging from higher plants and ferns to mosses. Insights into the role of calcium were gained using a variety of approaches ranging from inhibiting different types of calcium channels with chemicals, measuring calcium channel activities, and determining localized calcium levels with aequorin Ca2+ reporter systems. The details that are emerging show calcium changes to be species specific. For example, in A. thaliana phot1 and phot2 mediated the blue light induced activation of Ca2+ channels in the plasma membrane of mesophyll protoplasts, which in turn led to specific transient increases in cytoplasmic Ca2+ levels. Phot2 also induced the release of Ca2+ from internal storage compartments (Baum et al. 1999; Harada et al. 2003; Stoelzle et al. 2003). In contrast, the influx of external Ca2+ seemed unimportant for blue light induced chloroplast movement in the aquatic angiosperm Lemna trisulca (Tlalka and Gabryś 1993; Tlalka and Fricker 1999) and the fern A. capillus-veneris (Sato et al. 2001a, b). In the moss P. patens external calcium influx plays an important role in chloroplast movement under blue, but not red light conditions (Russell et al. 1998). In some cases it was difficult to pinpoint if changes of calcium concentration were early or late components of the phototropin signaling pathways, but at least in a few species Ca2+ seems to be acting downstream of pathways that involve phosphoinositide-3-kinases, as experiments with wortmannin in Nicotiana tabacum (Anielska-Mazur et al. 2009) and Lemna trisulca (Grabalska and Malec 2004) showed. These results suggested that the directionality of chloroplast movement was not influenced by calcium, but by the phosphoinositides. Ca2+ may affect chloroplast movement through its influence on actin filament integrity or motor molecule activity (Kadota and Wada 1992b; Grabalska and Malec 2004; Anielska-Mazur et al. 2009).
Studies using microbeams that illuminate only a small fraction of an individual cell in A. thaliana or A. capillus-veneris have shown that whatever the signaling cascade, the light signal does not travel to neighboring cells. Interestingly chloroplasts that were positioned in the area that was illuminated with the high light microbeam, moved away from the light, while those outside of the microbeam moved towards it but stopped before entering the high light area. This indicates that there may exist a gradient in signaling molecules that can reach chloroplasts at a fair distance, but when chloroplasts get close to excessively high light they stop moving. Studies in which only a brief pulse of light was given indicated that high light signals lasted for a shorter period of time (three-fold difference) than low intensity signals and hence could induce movement on different time scales after the microbeam was turned off (Kagawa and Wada 1994, 1999). The distances from which chloroplasts could be attracted towards the light were greater the higher the light intensity of the microbeam (Kagawa and Wada 1996).
III. The Role of the Cytoskeleton
It has been long suggested that chloroplasts in higher plants, ferns and mosses move along actin cables. Numerous studies have shown that chloroplasts are surrounded by a basket of actin filaments, or honeycomb-like actin structures (e.g., in A. thaliana, Nicotiana tabacum, Spinacia oleraceae, Vallisneria gigantea, A. capillus-veneris, Selaginella helvetica, P. patens) and that they are localized in close proximity to larger actin cables that transverse the cells. In addition there is evidence in many species that actin polymerization inhibitors prevent chloroplast movement (Cox et al. 1987; Kadota and Wada 1992b; Dong et al. 1996; Kandasamy and Meagher 1999; Sato et al. 2001a, b; Takagi 2003; Kumatani et al. 2006; Anielska-Mazur et al. 2009). Several studies also documented a reorganization of the actin cytoskeleton in response to strong light. For example, in the epidermal cells of the aquatic monocot Vallisneria gigantea, blue light led to the reorganization of the actin cytoskeleton. Thick bundles that surrounded and anchored the chloroplasts in the dark, disappeared under strong blue light, and instead straight, aggregated actin bundles appeared (Sakurai et al. 2005). Under weak red light actin cables formed honeycomb like structures, which trapped the chloroplasts of V. gigantea (Takagi 2003). In A. thaliana red light had more significant effects on the F-actin filaments than blue light and high light intensities appeared to lead to more fragile actin filaments (Krzeszowiec et al. 2007). In Nicotiana tabacum, leaves exposure to strong red or blue light led to diffuser and wider actin cables. However, these changes did not correlate with the directionality of chloroplast movement (Anielska-Mazur et al. 2009). In the fern A. capillus-veneris it had been shown that arrays of actin filaments appeared when chloroplasts reached their final destination, while they disappeared before movement started (Kadota and Wada 1992b). Using GFP-mTalin constructs that allowed the visualization of very fine actin filaments in combination with a microcopy system in which part of a cell could be illuminated with a microbeam to induce chloroplast movement, finally led to a significant breakthrough. A. thaliana chloroplasts were shown to be surrounded by very small actin filaments covering the entire surface of the chloroplasts when they were anchored to the plasma membrane. When high intensity blue light was shining on them, these filaments first disappeared and then formed on the leading edge of the chloroplasts, meaning on the side facing the direction of chloroplast movement. Hence, chloroplasts did not utilize preexisting or newly formed large cables, but depended on small, newly polymerized actin filaments that formed on the leading edge of chloroplasts (cp-actin filaments) in response to blue light in a phototropin dependent fashion (Kadota et al. 2009).
In the moss P. patens chloroplast movement depends both on microtubules and microfilaments. In the absence of light, chloroplasts moved quickly back and forth along microtubules in a longitudinal direction, while actin cables allowed for slow movement in any direction. Red light induced movement via the photoreceptor phytochrome occurred only along microtubules, while blue-light induced movement could take place either along microtubules or actin filaments. Interestingly, red light caused chloroplasts to move in a fairly inefficient way towards their final destination, while blue light caused chloroplasts to move along the shortest way. This specific system may be an intermediate between the algal motility system, which relies mainly on microtubules and that of higher plants, which relies exclusively on microfilaments (Sato et al. 2001b). A recent study using GFP-labeled actin and microtubules revealed that irradiation with a blue microbeam induced changes in actin filaments but not microtubules. High blue light intensities led to the disappearance of actin filaments in the high light area, while low and high blue light led to the appearance of actin filaments in the areas to which the chloroplasts migrated. These short actin filaments (cp-actin) seemed to emerge from the center part of chloroplasts and soon extended to the area of the chloroplast surface facing the plasma membrane. Just like in A. thaliana these cp-actin filaments seemed to form in many cases on that side of the chloroplasts that was leading the directional movement. In contrast to A. thaliana the cp-actin filaments in P. patens were not present in the dark, hence they may not be involved in anchoring the chloroplasts under these conditions (Yamashita et al. 2011). For an overview of our understanding of the light receptors and the cytoskeletal elements in movement in a range of species see Table 8.1 and Fig. 8.2.
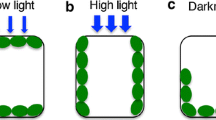
Overview of factors driving chloroplast movement in three model species. In Arabidopsis thaliana blue light drives the accumulation response through phot1 and phot2, and the avoidance response through phot2. Through a signaling cascade that is not yet indentified cp-actin filaments form on the leading edge of chloroplasts, allowing chloroplasts to be pulled either into or out of the light. In the dark chloroplasts are anchored via cp-actin filaments to the plasma membrane. A similar model is proposed for Adiantum capillus-veneris, however in this species red and blue light can also induce an accumulation response through the photoreceptor neo1. In Physcomitrella patens the situation is more complicated and less well understood. Blue light causes an accumulation response in protonemal cells via photA and photB through the biased formation of cp-actin filaments. In addition blue light and red light (via phytochrome) can induce chloroplast movement along microtubules. Chloroplasts are not anchored in the dark, but move along microtubules and possibly actin filaments.
It is not known how the force is generated that allows the chloroplasts to move, as there is contradictory evidence for the involvement of myosins. While some, but not all inhibitor studies pointed towards a role of myosin in the accumulation response of higher plants and ferns (Liebe and Menzel 1995; Paves and Truve 2007), there had been limited success localizing specific myosins to the chloroplasts of higher plants (Malec et al. 1996; Wang and Pesacreta 2004; Reisen and Hanson 2007) and none of the myosin mutant lines in higher plants investigated so far have shown any deficits in chloroplast movement (Peremyslov et al. 2008). A recent study which used transient RNA silencing and YFP::myosin XI fusions in tobacco plants found evidence of myosin XI-F involvement in chloroplast dark positioning (Sattarzadeh et al. 2009). This indicates that while myosins may be involved in chloroplast movement, there is either considerable redundancy between the members of this large gene family found in plants and/or they are only partially responsible for the movement of chloroplasts. There is also evidence that myosins change their localization in a blue-light and phot2 dependent fashion in A. thaliana. Under weak blue light antibodies detected the presence of myosin associated with the chloroplast envelope, while in strong light very few patches of myosin could be detected on the chloroplasts (Krzeszowiec and Gabryś 2007). Hence myosin relocation may be essential in chloroplast movement and may play a role in signaling rather than the movement itself. Alternatively, myosins may only be involved in certain circumstances such as in anchoring of chloroplasts in the dark and positioning them under low light, but not in the avoidance response. Clearly more work is needed to elucidate the precise role of myosin in chloroplast movement and its behavior across various species.
An important player in the actin mediated chloroplast movement and anchoring to the plasma membrane is the protein CHUP1 (chloroplast unusual positioning), which localizes to the outer chloroplast envelope via its hydrophobic N-terminus. CHUP1 contains a coiled-coil domain, an actin-binding domain that allows it to interact with G- and F-actin, a proline-rich motif and two leucin-zipper domains (Oikawa et al. 2003; Oikawa et al. 2008). CHUP1 cannot polymerize G-actin, but interacts with the actin-binding protein profilin (Schmidt von Braun and Schleiff 2008a, b). Recent evidence suggests that the leucine zipper motifs in the N- and C-terminal regions of CHUP1 are important for an intramolecular fold that may help to bring the actin- and profilin-binding domains together (Lehmann et al. 2011). Three other proteins have been shown to affect chloroplast movement by influencing cp-actin filament formation: Two kinesin-like proteins, KAC1 and KAC2, with a C-terminus that can interact with F-actin are crucial for chloroplast movement and anchoring and seem to be involved in the generation or stability of cp-actin filaments (Suetsugu et al. 2010a, b). THRUMIN1, an actin-bundling protein that localizes to the plasma membrane in a light- and phototropin-dependent fashion, plays an important role in chloroplast movement under low and high light intensities (Whippo et al. 2011). More studies are needed to investigate how KAC1, KAC2, CHUP1 and THRUMIN1 interact and if they play similarly important roles in other species. So far CHUP1 orthologues have been reported in Zea mays and Eleusine coracana (Kobayashi et al. 2009), but no mutants are available as of yet to test if they are functionally equivalent.
Not only is it important to move chloroplasts into the correct position to optimize light interception, it is equally important to ensure the anchoring of chloroplasts once they have reached the appropriate position. It has been shown in A. thaliana that chloroplasts seem to be anchored to the plasma membrane through CHUP1 and actin cables, as chloroplasts cluster together in chup1 mutants or after application of actin de-polymerization agents such as cytochalasin B. If chloroplasts were not anchored cytoplasmic streaming would displace them (Takagi et al. 2009). Chloroplast anchoring plays an especially important for chloroplasts in the bundle sheath cells of C4 plants such as Eleusine coracana, where they are organized in a centripetal fashion, supposedly to allow for the efficient exchange of metabolites between mesophyll and bundle sheath cells. In young leaves chloroplasts are distributed evenly along the cell walls and only achieve the centripetal arrangement as the leaves mature (Miyake and Yamamoto 1987; Miyake and Nakamura 1993). The actin cytoskeleton and cytosolic protein synthesis seem to be crucial for chloroplast movement and anchoring after disturbance (through centrifugation). Interestingly, the bundle sheath chloroplasts do not move in response to changes in blue light (Kobayashi et al. 2009).
IV. Chloroplast Movement Speed
Several methods have been employed to characterize chloroplast movement behavior in plants. On a leaf level one can determine the changes in transmission to red light through the leaf in response to various blue light intensity. If the chloroplasts within the cells are spread out in a typical accumulation response then the transmission value will be low, as most of the light will be absorbed by the chloroplasts. On the other hand, if the chloroplasts are arranged along the anticlinal cell walls, as is typical in an avoidance response, the transmission through the leaf will be high (Walczak and Gabryś 1980; DeBlasio et al. 2005; Berg et al. 2006). One can determine the speed of movement as a change in transmission per unit time when the light intensity is changed and chloroplasts move in order to achieve a more favorable positioning (Königer and Bollinger 2012). For an example showing transmission changes in the model organisms A. thaliana, A. capillus-veneris and P. patens see Fig. 8.3. Alternatively, one can follow the movement of individual chloroplasts within a cell that is partially illuminated by a microbeam that induces chloroplasts to move (e.g., Kadota and Wada 1992a, 1999). Both methods have been used to characterize the behavior of various species and mutants under control and experimental conditions.
Chloroplast movement behavior in the model species of higher plants, ferns and mosses, measured as the percentage of red light transmission through leaves or pieces of moss. Plants were dark-adapted overnight before leaves or pieces of moss (leaflets and protonemata grown on agar plates) were placed in a photometer measuring the red light transmission under increasing blue light intensities (1 h exposures to 0, 0.1, 40 and 100 μmol photons m−2 s−1). While Arabidopsis thaliana showed both strong and fast accumulation and avoidance responses, Adiantum capillus-veneris showed no accumulation response and a slow avoidance response. No net change in transmission could be observed in Physcomitrella patens.
A study comparing four fern species (Pteris cretica, Adiantum caudatum, Adiantum diaphanum, Adiantum capillus-veneris) found considerable differences in overall movement speeds as determined by changes in transmission values per unit time. Since the two species that exhibited the fastest speed came from environments with variable light intensities, the authors speculated that environmental flexibility rather than growth light conditions determine chloroplast movement speed (Augustynowicz and Gabryś 1999). However, a more recent study that included ten species ranging from ferns to monocots and eudicots found no support for this idea. In this study the plants that preferred higher light intensities during growth exhibited on average higher speeds of movement during both accumulation and avoidance responses than those that preferred shade environments (Königer and Bollinger 2012). Clearly the question regarding the ecological pressures that lead to the selection of different chloroplast movement speeds needs further elucidation.
Among the higher plant and fern species that have been investigated by measuring transmission changes through the leaves, the speed during the avoidance response was consistently about three-times faster than the accumulation response (Augustynowicz and Gabryś 1999; Königer and Bollinger 2012). Studies in A. thaliana indicate that reasons for the differences in speed seem to be related mainly to factors that influence the formation of cp-actin filaments. Both the accumulation and the avoidance speeds of individual chloroplasts correlated with the difference in amounts of cp-actin filaments comparing the front and rear ends of chloroplasts: the larger the difference, the faster the chloroplasts moved. Increasing light intensities also led to increasing movement speeds through this mechanism (Kadota et al. 2009). Mutant screens in A. thaliana identified four proteins (PMI2, WEB1, KAC1 and PHOT2), which all modified the speed of movement via their effects on cp-actin filaments. PMI2 (plastid movement impaired 2), a protein with a long coiled-coil domain (Luesse et al. 2006), interacts with WEB1 (weak chloroplast movement under blue light 1) in the cytosol. A mutation in either protein impaired both the accumulation and the avoidance speeds of individual chloroplasts (Kodama et al. 2010). The kinesin-like protein KAC1 was shown to be essential for rapid avoidance speeds also through its effects on the dynamics of cp-actin filaments (Suetsugu et al. 2010a, b). It is not known if mutations in the sequences or different protein concentrations of WEB1, PMI2 and KAC1 can explain the variation in chloroplast movement speed observed between different species, however in A. thaliana it has been shown that PHOT2 has a concentration-dependent effect on movement speed. Heterozygous PHOT2/phot2 mutant plants moved their chloroplasts at half the speed as wild type A. thaliana (Kagawa and Wada 2004), and PHOT2 overexpressor lines showed increasing speed with elevated PHOT2 concentrations, but saturated at PHOT2 concentrations more than five-times higher than those of wild-type (Kimura and Kagawa 2009). Further evidence for the involvement of PHOT2 comes from a study that showed that prolonged exposure to sucrose or glucose reduced the speed of accumulation and avoidance movement in A. thaliana and Lemmna trisulca. This effect was less severe in PHOT2 overexpressors than in wild-type, pointing towards the involvement of the phot2-signaling pathway (Banaś and Gabryś 2007).
Microbeam studies have greatly enhanced our understanding of the behavior of individual chloroplasts, but are mostly limited to organisms with a single layer of cells like gametophytes. In higher plants such as A. thaliana it is possible to use a microbeam, but since the light has to traverse the epidermis, the beam is not as focused as when applied to gametophytes and A. thaliana chloroplasts are not as sensitive to increases in blue light in this system. In general, chloroplasts in all species moved away from the area illuminated by a strong blue light microbeam, but those situated outside of the beam moved towards it without entering it (e.g., Kagawa and Wada 2000; Sato et al. 2001b). The speed with which individual chloroplasts move seemed comparable across species, as individual chloroplasts in A. thaliana moved along actin cables at about the same speed as those of P. patens and A. capillus-veneris (Sato et al. 2003). In A. thaliana and A. capillus-veneris the velocity of individual chloroplasts during an avoidance response increased with increasing blue light intensities, while the speed during the accumulation response was unaffected by light intensities. As a consequence the avoidance movement was faster than the accumulation movement (Kagawa and Wada 2004; Tsuboi and Wada 2010b). In contrast, light intensity had no effect on speed in P. patens, resulting in similar speeds when comparing accumulation and avoidance responses (Sato et al. 2001b). The specific behavior of individual chloroplasts and the influence of light quality was also species specific. In A. thaliana it was necessary to apply background red light illumination, which increased cytoplasmic motility, to achieve significant blue light induced chloroplast movement during microbeam irradiation (Kagawa and Wada 2000). In A. capillus-veneris the speed of movement of individual chloroplasts was the same in red and blue light, but the further the chloroplasts were away from the microbeam the faster they moved towards it (Kagawa and Wada 1996; Tsuboi and Wada 2010a). In the moss P. patens fast chloroplast movement was observed along microtubules and slower movement along actin cables (Sato et al. 2000). With the recent breakthroughs in microscopy technology, it should be possible to further investigate the dynamic changes in cp-actin filaments in different species and under different light qualities and quantities.
V. Degrees of Movement
By determining the transmission levels at maximum accumulation and avoidance relative to the dark values one can quantify the degree or amplitude of movement in a given species. It is necessary to normalize transmission levels relative to the dark level, since chloroplast arrangements in dark-adapted leaves vary greatly among species, as does leaf thickness. For example, in dark-adapted leaves of Tradescantia, chloroplasts distribute themselves evenly along all cell walls (Zurzycki 1980), while in A. thaliana they assume a position similar to the avoidance response in the palisade cells, but a position similar to an accumulation response in the spongy mesophyll cells (Berg et al. 2006). Dark level transmission values are species specific (Königer and Bollinger 2012) and are influenced by the light conditions during growth. For example, A. thaliana leaves exhibited dark transmission values nearly twice as high when grown under very low versus high light due to differences in chloroplast positioning and leaf thickness (Trojan and Gabryś 1996). Mutant screen in A. thaliana have identified two proteins, namely JAC1 (J-domain accumulation response 1) and PHOT2, as important players in the proper dark positioning of mesophyll chloroplasts, but it is not clear how they mediate their effects (Suetsugu et al. 2005a). In dark-adapted A. capillus-veneris and P. patens protonemal cells, the chloroplasts are spread out evenly along the entire cell periphery (Sato et al. 2001b; Kadota and Wada 1992a), in prothallial cells of A. capillus-veneris the chloroplasts are localized along the anticlinal wall excluding the upper surface (Kagawa and Wada 1999), and in sporophytes the chloroplasts are randomly distributed within the cells (Kawai et al. 2003). It is interesting that the dark positioning in A. capillus-veneris was so distinctly different depending on the developmental state of the plant. Interestingly, in the protonemal cells of P. patens chloroplasts were not anchored to the plasma membrane in the dark, but exhibited a back and forth motion along the longitudinal axis probably along microtubules (Sato et al. 2001b). Is not known if the same proteins are responsible for the dark positioning in ferns and mosses as in A. thaliana and if the developmental state also changes chloroplast distribution in mosses. Clearly, more research is needed to understand the physiological importance of distinct dark positions in various species and to identify the components that determine the proper dark positioning in various species.
Species also differ greatly with regard to the degree of their accumulation and avoidance responses. In general, the species-specific variations observed in the avoidance response were smaller than those in the accumulation response. Interestingly, growth light preferences seemed to influence the degree of accumulation responses. For example, while some shade plants such as the fern Cyrtomium fortunei, and the monocot Alocasia odora showed barely a decrease in transmission after the change from dark to low blue light, sun plants such as Taraxacum officinale and Digitaria sanguinalis showed very distinct accumulation responses (Königer and Bollinger 2012). It is not well understood how various molecular factors influence the amplitude of accumulation and avoidance responses. Interestingly some proteins only influence the accumulation or the avoidance response, while others influence both. The amplitude of the accumulation response was adversely affected by mutations in JAC1 (Suetsugu et al. 2010a, b), the amplitude of the avoidance response was reduced by knockouts of PMI2 (Luesse et al. 2006; Kodama et al. 2010), while THRUMIN1, PMI1, and WEB1 influenced both the degree of the accumulation and the avoidance response (DeBlasio et al. 2005; Kodama et al. 2010; Whippo et al. 2011). Taken together these results point towards separate mechanisms and signaling pathways for dark positioning, accumulation and avoidance responses. Careful studies on chloroplast movement in A. capillus-veneris prothallial cells supported this idea of separate signaling pathways as it was shown that the red light signal was transferred over longer distances the higher the light intensity (Kagawa and Wada 1996). For blue light the signals were transferred over longer distances and lasted longer for low light intensities than for the high intensities (Kagawa and Wada 1994, 1999).
VI. Effects of Other Environmental Factors on Chloroplast Positioning
Certainly light seems to be the most important factor inducing chloroplast movement through the phototropin, neochrome or phytochrome pathways. However, several other environmental stressors can modify or induce chloroplast movement, at least in some species. These studies clearly show that our picture of light induced chloroplast movement is too simplistic. The effects of other environmental factors and drastic differences in the behavior of various species need to be included in our models of chloroplast movement and may yield helpful information on the signaling pathways that trigger movement.
For example, chloroplast movement in the epidermal cells of Vallisneria gigantea is known to be red light induced, in a phytochrome-mediated manner, but red light also acts through its effects on photosynthesis in some unknown way (Dong et al. 1995, 1996). A recent study in a different system, namely the prothallial cells of A. capillus-veneris, also showed that red light induced chloroplast movement through its effects on photosynthesis in neo1 mutants, which could be eliminated by treating the cells with inhibitors of photosynthesis (Sugiyama and Kadota 2011). It is unclear how these light receptor independent pathways mediate their effects, but possibly photosynthetic rates affect Ca2+ concentrations outside of the chloroplasts. Alternatively depending on the amount of light, zeaxanthin concentrations within the chloroplasts change (Demmig-Adams and Adams III 2006) and may modulate chloroplast movement behavior (Tlalka et al. 1999).
In addition to light, low temperatures have been shown to induce chloroplast movement in a variety of species ranging from higher plants to ferns. For example temperatures below 10 °C induced an avoidance movement in prothallial cells of A. capillus-veneris. Interestingly the movement was enhanced by high light and not observed in phot2 indicating that temperature also mediated its effect through phototropin (Kodama et al. 2008). Low temperatures affected chloroplast movement in the tropical evergreen higher plant Tradescantia albiflora and the conifer Taxus cuspidata, but not in herbaceous plants such as A. thaliana, Nicotiana tabacum, Viola odorata and Taraxacum officinale. Low temperature induced chloroplast positioning was further observed in several evergreen ferns (Pteris cretica, Pteris vittata, Crepidomanes amabile, Hymenophyllum wrighii), but not in summer-green ferns (Lygodium japonicum, Pteridium aquilinum; Haberlandt 1876; Gabryś and Konopacka 1980; Tanaka 2007; Kodama et al. 2008). Hence temperature induced movement may be a mechanism important for plants with overwintering leaves or plants that need to protect their chloroplasts from photoinhibition induced by a combination of unfavorable temperatures and high light.
In addition to cold temperatures, water stress also seems to affect chloroplast positioning in the mesophyll cells of some C4 plants. Studies in Eleusine coracana and Zea mays showed that mesophyll chloroplasts aggregated in response to severe drought or after application of the water stress hormone ABA in the presence of moderate blue light (Yamada et al. 2009; Maai et al. 2011). Similarly, high light stress in combination with water stress or a treatment with ABA induced clumping of chloroplasts in the CAM plants Zygocactus truncatus, Kalanchoe fedtschenkoi and K. blossfeldiana (Kondo et al. 2004). ABA also influenced the chloroplast positioning in guard cells of the C3 plant A. thaliana, causing them to cluster in the center of the guard cells (Königer et al. 2010). Clumping of chloroplasts has been also observed in the submerged seagrass Halophila stipulacea, but was certainly not caused by water stress (Sharon and Beer 2008).
Given that various environmental stressors can influence chloroplast positioning it is not surprising that hydrogen peroxide, a reactive oxygen species that is formed in response to stress, can affect chloroplast movement. Studies in A. thaliana showed that elevated levels of hydrogen peroxide induce an avoidance response at lower light intensities and caused an increased degree of avoidance movement. The increase in hydrogen peroxide was PHOT2 dependent and DCMU, an inhibitor of the photosynthetic electron transport chain, prevented in part the blue light induced generation of hydrogen peroxide (Wen et al. 2008). In contrast, in the C4 plants Eleusine coracana and Zea mays, application of hydrogen peroxide did not alter the chloroplast distribution in mesophyll cells and did not induce an aggregation of chloroplasts in the dark (Maai et al. 2011).
In the bryophyte P. patens and the fern A. capillus-veneris chloroplast movement can also be induced by mechanical stress e.g., by touching the protenemal cells with a microcapillary. In A. capillus-veneris and P. patens this response is dependent on Ca2+ influx via the plasma membrane and the mechano-movement is dominant over light induced chloroplast movement. Interestingly in other respects there are species-specific differences: in the mosses P. patens, Ceratodon purpureus and Marchantia polymorpha chloroplasts move towards the stimulus, while they move away from the stimulus in the ferns A. capillus-veneris, Dryopteris filix-mas, Onoclea sensibilis, and Matteucia struthiopteris. Chloroplasts also move along different systems with mosses using microtubules, while ferns employ actin for the mechano-relocation (Sato et al. 1999, 2001a, b, 2003).
VII. Chloroplast Movement in Different Cellular Locations
Nearly all studies on chloroplast movement of terrestrial higher plants focus on the behavior of chloroplasts in palisade mesophyll cells, however several studies show the behavior of chloroplasts is greatly influenced by their cellular location.
For example, when leaves of a wide range of species were illuminated with high light, the chloroplasts in the cells on the adaxial and abaxial leaf surfaces did not always behave in a uniform way. In some species, such as A. thaliana, chloroplasts in both palisade and spongy mesophyll cells responded by retracting to the anticlinal walls, but in other species such as Taraxacum officinale and Eichhornia crassipes, only the palisade cell chloroplasts showed an avoidance response. In the shade plant Hosta, the chloroplasts in the cells on the lower leaf surface even spread out more in high light than low light (Königer and Bollinger 2012).
Even more extreme is the situation in C4 plants in which the chloroplasts in the mesophyll and bundle sheath cells exhibit vastly different behavior. Chloroplasts in bundle sheath cells are either centrifugally or centripetally arranged and do not move in response to light, while those in the mesophyll do. In some of the C4 species investigated, like Zea mays, mesophyll chloroplasts behave similarly to those in C3 species by exhibiting accumulation and avoidance responses. However, in Eleusine coracana mesophyll chloroplasts behaved differently in that they moved during an avoidance response mainly towards the anticlinal cell walls close to the bundle sheath cells. They also moved very slowly, over the course of hours rather than minutes, and only did so in response to light intensities higher than full sunlight. When environmental stressors such as water stress acted on C4 plants in addition to the high light stress then an aggregation of the mesophyll chloroplasts was observed (Yamada et al. 2009).
Chloroplasts in submerged aquatic plants such as Vallisneria gigantea and Halophila stipulacea are present in epidermal cells and move in response to light (Takagi 2003; Sharon and Beer 2008). Chloroplast movement in the epidermis has also been observed in a couple of fern species (Königer and Bollinger 2012) and in the guard cells of A. thaliana where chloroplasts moved horizontally towards the pore under high light conditions and exhibited a behavior that was in part similar to an avoidance response, but clearly had other qualities (Königer et al. 2010).
As mentioned earlier the dark positioning of chloroplasts in A. capillus-veneris was different in protonemal cells than prothallial cells than sporophytes (Kadota and Wada 1992a; Kagawa and Wada 1999; Kawai et al. 2003). Clearly, the question of how the cellular environment mediates its effects on chloroplast positioning needs to be addressed.
VIII. Ecological Importance
It has long been suggested that chloroplast movement serves as a means to optimize light interception (Zurzycki 1955) and the amazing ability of species to fine-tune their chloroplast positioning even after small changes in light intensity certainly speaks to its importance (Gorton et al. 1999; Williams et al. 2003; Königer and Bollinger 2012). Particular importance has been given to the avoidance response as a photoprotective mechanism under conditions of excess light. Several studies provide clear evidence for this in A. thaliana, as phot2 and chup1 plants were shown to be more sensitive to high light stress treatments than WT and phot1 plants (Kasahara et al. 2002; Sztatelman et al. 2010; Königer and Bollinger 2012).
However, little is known about how different movement behaviors affect stress tolerance across different species. As discussed earlier, species vary greatly in their chloroplast movement behavior in terms of light qualities that trigger it, the cytoskeletal elements that provide the tracks, and especially the degree and speed with which their chloroplasts move. Only a few studies have compared species with regard to their chloroplast movement behavior and their stress tolerance. A comparison of a wide range of species including ferns, monocots and eudicots showed that there was no correlation between the speed or the degree of chloroplast avoidance responses and high light stress tolerance of these species (Königer and Bollinger 2012). Clearly, plants utilize various mechanisms to deal with high light stress and an analysis of the relative importance of chloroplast movement is complicated by the extent to which other photoprotective mechanisms are employed. Interestingly, two studies indicated that the avoidance movement probably is more important for shade than sun plants. For example, the shade plant T. albicans exhibited greater high light stress tolerance than the sun plant P. sativum due its superior chloroplast movement behavior despite a lower ability to utilize light for photosynthesis and to repair damage to the D1 protein (Park et al. 1996). Another study showed that on average sun plants showed a lower degree of avoidance response than shade acclimated plants (Königer and Bollinger 2012). Maybe chloroplast movement is not as important for most sun loving species, since they have higher photosynthetic capacities and a larger potential for non-photochemical dissipation of excess light via a zeaxanthin-dependent mechanism (Königer et al. 1995; Demmig-Adams 1998).
It has been suggested that the avoidance response may not only be important for minimizing high light stress, but that it allows light to penetrate deeper into leaves thereby increasing photosynthesis in more light-limited tissue layers (Brugnoli and Björkman 1992; Terashima and Hikosaka 1995; Gorton et al. 1999). This is certainly possible, as in many species investigated few chloroplasts were found in the periclinal position of the cells on the adaxial leaf surface under high light intensities, allowing more light to reach the chloroplasts in the layer below under high light intensities (Königer and Bollinger 2012).
Chloroplasts need not only light but also CO2 for photosynthesis and hence there has been interest in understanding if chloroplasts also position themselves to influence leaf mesophyll conductance for CO2. In moderate to high light chloroplasts move to the anticlinal cell walls, hence closer to intercellular airspaces where CO2 concentrations are supposedly higher (Terashima and Hikosaka 1995). Studies in Alocasia brisbanensis, and A. thaliana wild-type and mutant plants indicated that when the chloroplasts were in their avoidance response they were not enhancing or in some cases even limiting CO2 diffusion within the leaf. This was the consequence of the fact that this position on the anticlinal cell walls reduced the surface area of chloroplasts bordering intercellular airspaces and hence decreased internal conductance for CO2 through the mesophyll, which in turn limited photosynthesis. This reduction in conductance was not observed in phot2 mutants, which do not assume an avoidance positioning. However, the conductance was always low in chup1 plants because of their clustered arrangement (Gorton et al. 2003; Tholen et al. 2008). However, it seems that the reduction in conductance after blue light irradiation was not exclusively caused by the avoidance response since part of it still occurred after treatment with cytochalasin B which inhibits chloroplast movement, and since the kinetics with which conductance changed and chloroplasts moved did not match up (Loreto et al. 2009).
IX. Conclusions
The last decade has brought exciting new insights into the mechanism and importance of chloroplast movement. Mutant screens have revealed some of the proteins that are involved in chloroplast movement and anchoring, while other technological advances have allowed us to study the behavior of individual chloroplasts in more detail. Most of this work has been done in the model species A. thaliana, A. capillus-veneris and P. patens. It will be crucial to continue working on these model species in order to gain a better understanding of how the different players that have been identified interact. In addition we will need a broader approach comparing mechanisms and the importance of chloroplast movement behavior in a range of species in order to understand the ecological importance of this behavior.
References
Aihara Y, Tabata R, Suzuki T, Shimazaki K-I, Nagatani A (2008) Molecular basis of the functional specificities of phototropin 1 and 2. Plant J 56:364–375
Anielska-Mazur A, Bernaś T, Gabryś H (2009) In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses. BMC Plant Biol 9:64
Augustynowicz J, Gabryś H (1999) Chloroplast movements in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant Cell Environ 22:1239–1248
Banaś AK, Gabryś H (2007) Influence of sugars on blue light-induced chloroplast relocations. Plant Signal Behav 2(4):221–230
Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 96:13554–13559
Berg R, Königer M, Schjeide B-M, Dikmak G, Kohler S, Harris GC (2006) A simple low-cost microcontroller-based photometric instrument for monitoring chloroplast movement. Photosynth Res 87:303–311
Brugnoli E, Björkman O (1992) Chloroplast movements in leaves: influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to ΔpH and zeaxanthin formation. Photosynth Res 32:23–35
Christie JM (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58:21–45
Cox G, Hawes CR, Van Der Lubbe L, Juniper BE (1987) High-voltage electron microscopy of whole, critical-point dried plant cells 2. Cytoskeletal structures and plastid motility in Selaginella. Protoplasma 140(2–3):173–186
DeBlasio SL, Luesse DL, Hangarter RP (2005) A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiol 139:101–114
Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39(5):474–482
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
Dong X-J, Takagi S, Nagai R (1995) Regulation of the orientation movement of chloroplasts in epidermal cells of Vallisneria: cooperation of phytochrome with photosynthetic pigment under low-fluence-rate light. Planta 197:257–263
Dong X-J, Ryu J-H, Takagi S, Nagai R (1996) Dynamic changes in the organization of microfilaments associated with the photocontrolled motility of chloroplasts in epidermal cells of Vallisneria. Protoplasma 195:18–24
Gabryś H, Konopacka M (1980) The effect of temperature on chloroplast phototranslocations in Tradescantia albiflora leaves. Acta Physiol Plant 2(4):291–297
Gorton HL, Williams WE, Vogelmann TC (1999) Chloroplast movement in Alocasia macrorrhiza. Physiol Plant 106:421–428
Gorton HL, Herbert SK, Vogelmann TC (2003) Photoacoustic analysis indicates that chloroplast movement does not alter liquid-phase CO2 diffusion in leaves of Alocasia brisbanensis. Plant Physiol 132:1529–1539
Grabalska M, Malec P (2004) Blue light-induced chloroplast reorientations in Lemna trisulca L. (duckweed) are controlled by two separable cellular mechanisms as suggested by different sensitivity to wortmannin. Photochem Photobiol 79(4):343–348
Haberlandt G (1876) Über den Einfluss des Frostes auf the Chlorophyllkörner. Österreichische Botanische Zeitschrift 26:249–255
Harada A, Sakai T, Okada K (2003) Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100(14):8583–8588
Inoue Y, Shibata K (1974) Comparative examination of terrestrial plant leaves in terms of light-induced absorption changes due to chloroplast rearrangements. Plant Cell Physiol 15(4):717–721
Izutani Y, Takagi S, Nagai R (1990) Orientation movements of chloroplasts in Vallisneria epidermal cells: different effects of light at low-and high-fluence rate. Photochem Photobiol 51:105–111
Jarillo JA, Gabryś H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410:952–954
Kadota A, Wada M (1992a) Photoorientation of chloroplasts in protonemal cells of the fern Adiantum as analyzed by use of a video-tracking system. Bot Mag 105:265–279
Kadota A, Wada M (1992b) Photoinduction of formation of circular structures by microfilaments on chloroplasts during intracellular orientation in protonemal cells of the fern Adiantum capillus-veneris. Protoplasma 167:97–107
Kadota A, Kohyama I, Wada M (1989) Polarotropism and photomovement of chloroplasts in the protonemata of the ferns Pteris and Adiantum. Evidence for the possible lack of dichroic phytochrome in Pteris. Plant Cell Physiol 30(4):523–531
Kadota A, Sato Y, Wada M (2000) Intracellular chloroplast photorelocation in the moss Physcomitrella patens is mediated by phytochrome as well as a blue-light receptor. Planta 210:932–937
Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106(31):13106–13111
Kagawa T, Wada M (1994) Brief irradiation with red or blue light induces orientational movement of chloroplasts in dark-adapted prothallial cells of the fern Adiantum. J Plant Res 107:389–398
Kagawa T, Wada M (1996) Phytochrome- and blue light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta 198:488–493
Kagawa T, Wada M (1999) Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiol 119:917–923
Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41(1):84–93
Kagawa T, Wada M (2004) Velocity of chloroplast avoidance movement is fluence rate dependent. Photochem Photobiol Sci 3:592–595
Kagawa T, Lamparter T, Hartman E, Wada M (1997) Phytochrome-mediated branch formation in protonemata of the moss Ceratodon purpureus. J Plant Res 110:363–370
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291:2138–2141
Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M (2004) Function analysis of Phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol 45(4):416–426
Kaiserli E, Sullivan S, Jones MA, Feeney KA, Christie JM (2009) Domain swapping to assess the mechanistic basis of Arabidopsis phototropin1 receptor kinase activation and endocytosis by blue light. Plant Cell 21:3226–3244
Kandasamy MK, Meagher RB (1999) Actin-organelle interaction: association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskeleton 44:110–118
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kasahara M, Kagawa T, Sato Y, Kiyosue T, Wada M (2004) Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol 135:1388–1397
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imalzumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290
Kimura M, Kagawa T (2009) Blue light-induced chloroplast avoidance and phototropic responses exhibit distinct dose dependency of PHOTOTROPIN2 in Arabidopsis thaliana. Photochem Photobiol 85(5):1260–1264
Kobayashi H, Yamada M, Taniguchi M, Kawasaki M, Sugiyama T, Miyake H (2009) Differential positioning of C4 mesophyll and bundle sheath chloroplasts: recovery of chloroplast positioning requires the actomyosin system. Plant Cell Physiol 50(1):129–140
Kodama Y, Tsuboi H, Kagawa T, Wada M (2008) Low temperature-induced chloroplast relocation mediated by a blue light receptor, phototropin 2, in fern gametophytes. J Plant Res 121:441–448
Kodama Y, Suetsugu N, Kong S-G, Wada M (2010) Two interacting coiled-coil proteins, WEB1 and PMI2, maintain the chloroplast photorelocation movement velocity in Arabidopsis. Proc Natl Acad Sci USA 107(45):19591–19596
Kondo A, Kaikawa J, Funaguma T, Ueno O (2004) Clumping and dispersal of chloroplasts in succulent plants. Planta 219:500–506
Kong S-G, Suzuki T, Tamura K, Mochizuki N, Hara-Nishimura I, Nagatani A (2006) Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J 45(6):994–1005
Königer M, Bollinger N (2012) Chloroplast movement behavior varies widely among species and does not correlate with high light stress tolerance. Planta 236(2):411–426
Königer M, Harris GC, Virgo A, Winter K (1995) Xanthophyll-cycle pigments and photosynthetic capacity in tropical forest species: a comparative field study on canopy, gap and understory plants. Oecologia 104:280–290
Königer M, Delamaide JA, Marlow ED, Harris GC (2008) Arabidopsis thaliana leaves with altered chloroplast numbers and chloroplast movement exhibit impaired adjustments to both low and high light. J Exp Bot 59(9):2285–2297
Königer M, Jessen B, Yang R, Sittler D, Harris GC (2010) Light, genotype, and abscisic acid affect chloroplast positioning in guard cells of Arabidopsis thaliana leaves in distinct ways. Photosynth Res 105(3):213–227
Krzeszowiec W, Gabryś H (2007) Phototropin mediated relocation of myosins in Arabidopsis thaliana. Plant Signal Behav 2(5):333–336
Krzeszowiec W, Rajwa B, Dobrucki J, Gabryś H (2007) Actin cytoskeleton in Arabidopsis thaliana under blue and red light. Biol Cell 99:251–260
Kumatani T, Sakurai-Ozato N, Miyawaki N, Yokota E, Shimmen T, Terashima I, Takagi S (2006) Possible association of actin filaments with chloroplasts of spinach mesophyll cells in vivo and in vitro. Protoplasma 229:45–52
Lehmann P, Bohnsack MT, Schleiff E (2011) The functional domains of the chloroplast unusual positioning protein 1. Plant Sci 180:650–654
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Liebe S, Menzel D (1995) Actomyosin-based motility of endoplasmic reticulum and chloroplasts in Vallisneria mesophyll cells. Biol Cell 85:207–222
Loreto F, Tsonev T, Centritto M (2009) The impact of blue light on leaf mesophyll conductance. J Exp Bot 60(8):2283–2290
Luesse DR, DeBlasio SL, Hangarter RP (2006) Plastid movement impaired 2, a new gene involved in normal blue-light-induced chloroplast movements in Arabidopsis. Plant Physiol 141:1328–1337
Luesse DR, DeBlasio SL, Hangarter RP (2010) Integration of phot1, phot2, and phyB signalling in light-induced chloroplast movements. J Exp Bot 61(15):4387–4397
Maai E, Shimada S, Yamada M, Sugiyama T, Miyake H, Taniguchi M (2011) The avoidance and aggregative movements of mesophyll chloroplasts in C4 monocots in response to blue light and abscisic acid. J Exp Bot 62(9):3213–3221
Malec P, Rinaldi RA, Gabryś H (1996) Light-induced chloroplast movements in Lemna trisulca. Identification of the motile system. Plant Sci 120:127–137
Mittmann F, Brücker G, Zeidler M, Repp A, Abts T, Hartmann E, Hughes J (2004) Targeted knockout in Physcomitrella reveals direct actions of phytochrome in the cytoplasm. Proc Natl Acad Sci USA 101(38):13939–13944
Miyake H, Nakamura M (1993) Some factors concerning the centripetal disposition of bundle sheath chloroplasts during the leaf development of Eleusine coracana. Ann Bot 72:205–211
Miyake H, Yamamoto Y (1987) Centripetal disposition of bundle sheath chloroplasts during the leaf development of Eleusine coracana. Ann Bot 60:641–647
Nozue K, Kanegae T, Imaizumi T, Fukuda S, Okamoto H, Yeh K-C, Lagarias JC, Wada M (1998) A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc Natl Acad Sci USA 95:15826–15830
Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanegae T, Niwa Y, Kadota A, Wada M (2003) Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell 15:2805–2815
Oikawa K, Yamasato A, Kong S-G, Kasahara M, Nakai M, Takahashi F, Ogura Y, Kagawa T, Wada M (2008) Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol 148:829–842
Park Y-I, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111:867–875
Paves H, Truve E (2007) Myosin inhibitors block accumulation movement of chloroplasts in Arabidopsis thaliana leaf cells. Protoplasma 230:165–169
Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV (2008) Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146:1109–1116
Reisen D, Hanson MR (2007) Association of six YFP-myosin XI-tail fusions with mobile plant cell organelles. BMC Plant Biol 7:6
Russell AJ, Cove DJ, Trewavas AJ, Wang TL (1998) Blue light but not red light induces a calcium transient in the moss Physcomitrella patens (Hedw) B, S & G. Planta 206:278–283
Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98(12):6969–6974
Sakamoto K, Briggs WR (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14:1723–1735
Sakurai N, Domoto K, Takagi S (2005) Blue-light-induced reorganization of the actin cytoskeleton and the avoidance response of chloroplasts in epidermal cells of Vallisneria gigantea. Planta 221:66–74
Sato Y, Kadota A, Wada M (1999) Mechanically induced avoidance response of chloroplasts in fern protonemal cells. Plant Physiol 121:37–44
Sato Y, Wada M, Kadota A (2001a) External Ca2+ is essential for chloroplast movement induced by mechanical stimulation but not by light stimulation. Plant Physiol 127:497–504
Sato Y, Wada M, Kadota A (2001b) Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J Cell Sci 114:269–279
Sato Y, Kadota A, Wada M (2003) Chloroplast movement: dissection of events downstream of photo- and mechano-perception. J Plant Res 116:1–5
Sattarzadeh A, Krahmer J, Germain AD, Hanson MR (2009) A myosin XI tail domain homologous to the yeast myosin vacuole-binding domain interacts with plastids and stromules in Nicotiana benthamiana. Mol Plant 2(6):1351–1358
Schmidt von Braun S, Schleiff E (2008a) Moving the Green. CHUP1 and chloroplast movement – an obvious relationship? Plant Signal Behav 3:488–489
Schmidt von Braun S, Schleiff E (2008b) The chloroplast outer membrane protein CHUP1 interacts with actin and profilin. Planta 227:1151–1159
Senn G (1908) Die Gestalts- und Lageveränderung der pflanzenchromatophoren. Engelmann, Leipzig
Sharon Y, Beer S (2008) Diurnal movements of chloroplasts in Halophila stipulacea and their effect on PAM fluorometric measurements of photosynthetic rates. Aquat Bot 88:273–276
Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100(3):1456–1461
Suetsugu N, Wada M (2007) Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol Chem 388:927–935
Suetsugu N, Kagawa T, Wada M (2005a) An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol 139:151–162
Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005b) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102:13705–13709
Suetsugu N, Takano A, Kohda D, Wada M (2010a) Structure and activity of JAC1 J-domain implicate the involvement of the cochaperone activity with HSC70 in chloroplast photorelocation movement. Plant Signal Behav 5(12):1602–1606
Suetsugu N, Yamada N, Kagawa T, Yonekura H, Uyeda TQP, Kadota A, Wada M (2010b) Two kinesin-like proteins mediate actin-based chloroplast movement in Arabidopsis thaliana. Proc Natl Acad Sci USA 107(19):8860–8865
Sugiyama Y, Kadota A (2011) Photosynthesis-dependent but neochrome1-independent light positioning of chloroplasts and nuclei in the fern Adiantum capillus-veneris. Plant Physiol 155:1205–1213
Sullivan S, Kaiserli E, Tseng T-S, Christie JM (2010) Subcellular localization and turnover of Arabidopsis phototropin 1. Plant Signal Behav 5(2):184–186
Sztatelman O, Waloszek A, Banas AK, Gabryś H (2010) Photoprotective function of chloroplast avoidance movement: in vivo chlorophyll fluorescence study. J Plant Physiol 167:709–716
Takagi S (2003) Actin-based photo-orientation movement of chloroplasts in plant cells. J Exp Biol 206:1963–1969
Takagi S, Takamatsu H, Sakurai-Ozato N (2009) Chloroplast anchoring: its implications for the regulation of intracellular chloroplast distribution. J Exp Bot 60(12):3301–3310
Tanaka A (2007) Photosynthetic activity in winter needles of the evergreen tree Taxus cuspidata at low temperatures. Tree Physiol 27:641–648
Terashima I, Hikosaka K (1995) Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ 18:1111–1128
Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I (2008) The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant Cell Environ 31:1688–1700
Tlalka M, Fricker M (1999) The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. Plant J 20(4):461–473
Tlalka M, Gabryś H (1993) Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L. Planta 189:491–498
Tlalka M, Runquist M, Fricker M (1999) Light perception and the role of the xanthophyll cycle in blue-light-dependent chloroplast movements in Lemna trisulca L. Plant J 20(4):447–459
Trojan A, Gabryś H (1996) Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol 111:419–425
Tsuboi H, Wada M (2010a) Speed of signal transfer in the chloroplast accumulation response. J Plant Res 123(3):381–390
Tsuboi H, Wada M (2010b) The speed of intracellular signal transfer for chloroplast movement. Plant Signal Behav 5(4):433–435
Tsuboi H, Yamashita H, Wada M (2009) Chloroplasts do not have a polarity for light-induced accumulation movement. J Plant Res 122:131–140
Uenaka H, Kadota A (2007) Functional analyses of the Physcomitrella patens phytochromes in regulating chloroplast avoidance movement. Plant J 51:1050–1061
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Walczak T, Gabryś H (1980) New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica 14:65–72
Wang Z, Pesacreta TC (2004) A subclass of myosin XI is associated with mitochondria, plastids and the molecular chaperone subunit TCP-1a in maize. Cell Motil Cytoskeleton 57:218–232
Wen F, Xing D, Zhang L (2008) Hydrogen peroxide is involved in high blue light-induced chloroplast avoidance movements in Arabidopsis. J Exp Biol 59(10):2891–2901
Whippo CW, Khurana P, Davis PA, DeBlasio SL, DeSloover D, Staiger CJ, Hangarter RP (2011) THRUMIN1 is a light-regulated actin-bundling protein involved in chloroplast motility. Curr Biol 21:59–64
Williams WE, Gorton HL, Witiak SM (2003) Chloroplast movements in the field. Plant Cell Environ 26:2005–2014
Yamada M, Kawasaki M, Sugiyama T, Miyake H, Taniguchi M (2009) Differential positioning of C4 mesophyll and bundle sheath chloroplasts: aggregative movement of C4 mesophyll chloroplasts in response to environmental stresses. Plant Cell Physiol 50(10):1736–1749
Yamashita H, Sato Y, Kanegae T, Kagawa T, Wada M, Kadota A (2011) Chloroplast actin filaments organize meshwork on the photorelocated chloroplasts in the moss Physcomitrella patens. Planta 233(2):357–368
Yatsuhashi H, Kobayashi H (1993) Dual involvement of phytochrome in light-oriented chloroplast movement in Dryopteris sparsa protonemata. J Photochem Photobiol B 19:25–31
Yatsuhashi H, Kadota A, Wada M (1985) Blue- red-light action in photoorientation of chloroplasts in Adiantum protonemata. Planta 165:43–50
Zurzycki J (1955) Chloroplast arrangement as a factor in photosynthesis. Acta Soc Bot Pol 24:27–63
Zurzycki J (1967) Properties and localization of the photoreceptor active in displacements of chloroplasts in Funaria hygrometrica. I. Action spectrum. Acta Soc Bot Pol 36:133–142
Zurzycki J (1980) Blue light-induced intracellular movements. In: Senger H (ed) The Blue light syndrome. Springer, New York, pp 50–68
Acknowledgements
I would like to thank Wellesley College for granting me a sabbatical leave and Prof. Schleiff for providing me with a ‘home’ in his lab to think and write.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Königer, M. (2014). Chloroplast Movement in Higher Plants, Ferns and Bryophytes: A Comparative Point of View. In: Hanson, D., Rice, S. (eds) Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration, vol 37. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6988-5_8
Download citation
DOI: https://doi.org/10.1007/978-94-007-6988-5_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6987-8
Online ISBN: 978-94-007-6988-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)