Summary
Terrestrialization of planet Earth likely began more than a billion years ago with the colonization of land by bacteria, followed by eukaryotic algae much like those occupying modern soils and shallow freshwaters and the earliest embryophytes, close relatives of modern bryophytes. Colonization of land by algae and the first plants was prerequisite to the development of organic-rich soils that later supported more complex plant communities dominated by vascular plants, and the rise of land animals. Consequently, understanding terrestrialization sheds light on Earth’s early biological carbon cycling processes, which aids our understanding of global biogeochemistry in particular, and planetary science in general.
Comprehending the process and pattern of ancient terrestrialization requires both neontological and paleontological approaches. Molecular phylogenetics provides the necessary scaffold upon which terrestrialization processes can be analyzed by comparing the structures, physiologies, microbiomes, and genomes of earliest-branching lineages of modern liverworts and mosses to those of plants’ closest modern green algal relatives, the streptophyte algae (also known as charophyte algae or charophycean green algae). Such studies reveal that modern bryophytes inherited spore and body desiccation-resistance, degradation-resistant lignin-like phenolic cell wall polymers, and other physiological traits useful in terrestrial habitats from ancestral algae, indicating that such features were also traits of the earliest land plants.
Because modern algae and bryophytes possess degradation-resistant cells or tissues, artificially degrading them for comparison with enigmatic microscopic fossils has been a fruitful way to identify remains of early terrestrial photosynthesizers and thus illuminate terrestrialization patterns. Microfossils cited as evidence for terrestrial cyanobacteria occur beginning more than 1,000 million years ago in the Precambrian, as do probable remains of freshwater and terrestrial eukaryotic algae. Some microfossils obtained from 499 to 511 million year old deposits closely resemble the modern complex streptophyte alga Coleochaete when it has been cultivated subaerially, suggesting that streptophytes were able to photosynthesize on land by the Middle Cambrian. Other microfossils observed in Cambrian and early Middle Ordovician deposits may also be remains of land plants. Remains of early liverwort-like land plants are confidently known from 470 million year old mid-Ordovician deposits, as are possible fossils of early-divergent mosses. Microfossils and macrofossils that have been compared to modern liverwort and moss taxa occur in Silurian to Devonian deposits laid down before and during the first major diversification of the vascular plants in the Late Silurian to Early Devonian, 407–418 million years ago. Such evidence, together with molecular phylogenies and clock analyses, demonstrates that bryophytes and streptophyte algal relatives were the dominant eukaryotic photosynthesizers on land from about 500–400 million years ago, prior to and during the earliest stages of vascular plant evolution.
Because bryophytes and streptophyte algae produce degradation-resistant carbon that can be sequestered, thereby reducing atmospheric carbon dioxide levels, models suggest that they had significant impacts on Earth’s carbon cycle for at least 40 million years and perhaps more than 100 million years. We can thus predict that other Earth-like, habitable-zone planets may likewise experience long periods during which organisms equivalent to earthly terrestrial streptophyte algae and bryophytes impact planetary biogeochemistry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
The sum of available evidence indicates that terrestrialization of planet Earth likely began with the colonization of land by photosynthetic prokaryotes such as cyanobacteria, followed by eukaryotic algae much like those occupying modern soils and shallow freshwaters and then the earliest embryophyte plants, which were probably quite closely related to modern bryophytes. Land colonization by algae and earliest plants was prerequisite to the development of soils that supported more complex plant communities and the later rise of land animals. Understanding terrestrialization is essential to comprehending Earth’s early carbon cycling processes, which aids our understanding of modern biogeochemistry and planetary science. Comprehending the pattern of terrestrialization and the process by which photosynthesizers colonized land requires the analysis of both modern organisms and fossils, that is, both neontological and paleontological approaches (Graham 1993; Graham and Gray 2001).
This chapter begins with a brief overview of phylogenetic relationships that guide studies of terrestrialization (section “Molecular systematics provides a reasonably well-resolved framework for investigations of terrestrialization process and pattern”), continues with examples of trait evolution related to photosynthesis in early land plants and modern bryophytes and impacts on biogeochemistry that illuminate the terrestrialization process (section “Early-evolved physiological traits likely fostered the process by which streptophytes made the transition to land”), and concludes with a survey of what we currently know about the pattern of terrestrialization on Earth and the value of this information to the planetary sciences (section “Comparison of early-diverging modern photosynthesizers to Precambrian-Devonian fossils illuminates the pattern of terrestrialization”).
II. Molecular Systematics Provides a Reasonably Well-Resolved Framework for Investigations of Terrestrialization Process and Pattern
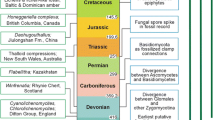
Comparative physiological and molecular analyses that inform our understanding of terrestrialization process and pattern are possible because molecular phylogenetic studies (Qiu et al. 1998, 2006, 2007; Dombrovska and Qiu 2004; Crandall-Stotler et al. 2005; Forrest et al. 2006) have identified the earliest-branching lineages of modern liverworts and mosses–which serve as models of earliest land plants–and plants’ closest modern algal relatives, the streptophyte algae (term used by Becker and Marin 2009) (Fig. 2.1).
Relationships of streptophyte algae to embryophytes. To date, molecular phylogenetic approaches have not allowed resolution of the modern streptophyte genus that is sister to embryophytes. UTC = classes Ulvophyceae, Trebouxiophyceae, Chlorophyceae, and the term Prasinophytes represents multiple classes.
Also known as the charophyte algae (Lewis and McCourt 2004) or charophycean algae (e.g. Graham et al. 2009), the streptophyte algae are a paraphyletic assemblage of green algae for which phylogenetic branching patterns are still being determined. The relatively basal positions of the unicellular flagellate Mesostigma, colonial Chlorokybus, and unbranched, filamentous Klebsormidiales seem well established, though the identity of the more complex modern streptophyte algal lineage (Charales, Coleochaetales, Desmidiales, Zygnematales, or some combination) that is sister to embryophytes continues to be debated (Turmel et al. 2007; Finet et al. 2010; Wodniok et al. 2011; Timme et al. 2012). For this reason, and because over the past several hundreds of millions of years since the divergence of embryophytes, the sister group has diverged substantially and consequently possesses traits not present in early embryophytes, comparative analyses aimed at defining the traits of earliest land plants should include representatives of multiple lineages of streptophyte algae. Streptophyte algae bequeathed diverse structural, reproductive; physiological, biochemical, and genetic traits to embryophyte descendants: (Graham 1993; Graham et al. 2000, 2004a, b). In the next section we focus on examples of photosynthesis-related physiological traits of modern streptophyte algae that (a) were likely also present in ancient relatives, (b) were likely inherited by earliest embryophytes and modern bryophytes and (c) help to explain these organisms’ past and present biogeochemical significance.
III. Early-Evolved Physiological Traits Likely Fostered the Process by Which Streptophytes Made the Transition to Land
Photosynthesis depends on the availability of (1) sufficient water as a source of reductant and as a medium from which dissolved mineral nutrients can be absorbed, (2) inorganic carbon, and (3) light. For earliest land plants, the transition from ancestral aquatic to more arid terrestrial habitats reduced accessibility to water, though CO2 and light became more available, and coping with excess light became more challenging (see Chap. 3). In addition, earliest land plants likely interacted with microbial communities in new ways. Here, we focus on (A) acquisition of desiccation-resistance as a way of coping with water insufficiency, (B) modifications of light harvesting systems, (C) changes in traits related to carbon uptake and utilization, and (D) patterns of investment of photosynthate into protective body cell wall polymers.
A. Desiccation-Tolerance Is an Early-Evolved Streptophyte Trait
Photosynthesis on land is limited by the availability of water; consequently, the history of plant evolution has involved increasingly more effective adaptation to habitats and periods of limited hydration. Many plants, including many bryophytes, display desiccation-tolerance, a collection of traits that helps organisms to resist dying when in the dry state. The pattern of occurrence of desiccation tolerance in modern bryophytes (Wood 2007) suggests that early land plants were likely also tolerant of desiccation. Though some authors have proposed that streptophyte desiccation tolerance originated in early embryophytes, recent results described next indicate that desiccation tolerance likely was present in streptophyte algal ancestors and was inherited by early land plants–thereby fostering the process of ancient terrestrialization.
Though many modern streptophyte algal species occupy only or mainly freshwaters, representatives of several morphologically simple streptophyte orders (Chlorokybales, Klebsormidiales, Zygnematales, Desmidiales) commonly occur in terrestrial habitats (Graham et al. 2009). Here, they display desiccation-tolerance and so foster photosynthesis in subaerial (“under air”) environments. The early-diverging species Chlorokybus atmophyticus has been isolated or identified from terrestrial habitats (Škaloud 2009), and Klebsormidiales are known for desiccation tolerance (Elster et al. 2008). Klebsormidium crenulatum, for example, shows full photosynthetic recovery after desiccation periods as long as 7 days (Karsten et al. 2010), using the osmolyte sucrose to cope with osmotic stress (Nagao et al. 2008). Certain unicellular desmidialeans have been reported to survive in dry soil for up to 3 months (Brook and Williamson 1998), and some zygnemataleans likewise are known to be desiccation tolerant (Holtzinger et al. 2010). Similar behavior is characteristic of some species of the chlorophyte clade, including members of Chlorophyceae and Trebouxiophyceae (Gray et al. 2007).
When grown in subaerial conditions such as on the surface of quartz sand, two species of the morphologically complex genus Coleochaete maintain intact green cells when air-dry for months, and (like K. crenulatum cited above) grow and asexually reproduce normally when moistened after having been air-dried for a week. These observations indicate that Coleochaete and earlier-diverging relatives possess the genetic potential for desiccation tolerance, indicating that ancient morphologically and reproductively complex streptophyte algae should have had the mechanisms to repair photosynthesis upon recovery and reproduce on land (Graham et al. 2012).
B. The Evolution of Distinctive Light-Harvesting Pigment-Protein Complexes May Have Accompanied the Streptophyte Transition to Land
Genome sequence projects have allowed comparisons of the photosynthetic light-harvesting complexes (LHCs) of higher plants with those of some green algae, revealing differences. Although prasinophyte and chlorophyte green algae and embryophytes (represented by Arabidopsis) display a number of homologous LHC genes, LHC1-like genes of these green algae show little homology to those of land plants, and homologs for higher plant Ll818 and some other genes have not been found in chlorophyte or prasinophyte genomes (Koziol et al. 2007). As noted earlier, several streptophyte algal species can be found in terrestrial locales, and many others inhabit quite shallow, nearshore freshwaters where they are exposed to high irradiance. To better understand the impacts of possible changes in light harvesting and photoprotection during the process of terrestrialization, it will be important to compare the LHCs of streptophyte algae to those of early-diverging lineages of bryophytes, aided by expressed sequence data being acquired for charophyceans and forthcoming genome sequences for the liverwort Marchantia and the early-diverging moss Sphagnum, as well as genomic information currently available for the more derived moss Physcomitrella (see Chap. 11). For example, the Physcomitrella genome includes sequence information for the antenna polypeptides Lhcb3 and Lhcb6. These proteins, as well as occurrence of an ortholog of PsbS and evidence for non-photochemical quenching, represent features hypothesized to be terrestrial adaptations (Alboresi et al. 2008).
C. Streptophyte Algae Bequeathed Carbon Acquisition Versatility to Embryophyte Descendants
The vast majority of modern streptophyte algae and bryophytes occupy freshwater or moist terrestrial habitats, suggesting that terrestrialization likely occurred by the transition from freshwater (rather than saline) habitats to land (Becker and Marin 2009). Carbon dioxide, the raw material for carbon fixation, can limit photosynthesis of algae and bryophytes that grow submerged in freshwaters when pH is low and the dominant photosynthesizers primarily use CO2 as an inorganic carbon source. Such conditions prevail, for example, in modern humic lakes where desmidialean and zygematalean algae and peatmosses are diverse and abundant (see Chap. 13). Consequently, Graham (1993) and Graham and Gray (2001) proposed that CO2-limitation was a major selective force driving streptophyte ecological transition from (1) inorganic C-limited deeper freshwater habitats to (2) shallower and more turbulent nearshore freshwaters richer in dissolved CO2 to (3) the wave-splashed and unpredictably arid shores of freshwater ponds, lakes or streams. Atmospheric CO2 has a much greater diffusivity in subaerial habitats (104 higher) than in water, and is thought to have been particularly abundant in pre-Carboniferous times (Berner 1997), including the Cambrian-Ordovician period commonly associated with the rise of pre-vascular land vegetation. Even so, the occurrence in modern streptophyte algae of additional carbon acquisition strategies suggests that earliest land plants and their direct ancestors may have also possessed flexibility in obtaining carbon, such as the ability to use bicarbonate in addition to CO2 as an inorganic C source and capacity to utilize exogenous organic carbon.
1. Use of Bicarbonate as a Source of Dissolved Inorganic C
Streptophyte algae that occur in higher pH aquatic systems are known to produce intracellular and extracellular (periplasmic) carbonic anhydrases (CAs) that interconvert CO2 and bicarbonate, thereby maintaining equilibrium levels of CO2 for rubisco (Arancibia-Avila et al. 2000, 2001). These carbon-concentration systems endow plant relatives with considerable flexibility in meeting challenges of inorganic carbon availability. For example, a strain of Mougeotia isolated from nuisance growths that form in acidic lakes but are also capable of growth in more alkaline waters appears to upregulate external CA activity when grown at pH 8, by comparison to growth at pH 5 (Arancibia-Avila et al. 2000). This change allows the alga to utilize bicarbonate, which is considerably more abundant at pH 8 than pH 5.
2. Origin of Beta-Type Carbonic Anhydrases
Immunolocalization analyses indicate that at least one species of Chara possesses a dispersed beta-type chloroplast stromal CA that is otherwise not known to occur in eukaryotes other than land plants. The apparent absence from a Mougeotia strain of beta-CA, whose encoding genes bear no sequence similarity to genes for more widely-distributed alpha-type periplasmic or thylakoidal CAs, suggests that the beta-type CA appeared in streptophyte algae after the divergence of Zygnematales and was inherited by earliest land plants (Arancibia-Avila et al. 2001).
During their evolutionary history, streptophytes have transitioned from a condition in which the carbon fixation enzyme rubisco is aggregated into intraplastidal spheres known as pyrenoids (the case for most streptophyte algae) to dispersal of rubisco throughout the chloroplast stroma (the case for most embryophytes). The acquisition of beta-type CA allows embryophytes to increase the degree of spatial association between CO2-releasing CA and CO2-binding rubisco. Such a change would have allowed streptophytes to transition from one or two larger, pyrenoid-bearing plastids to multiple, smaller, pyrenoidless plastids that can be rapidly repositioned for maximum light absorption or photoprotection (see Chap. 8). The presence of single, large pyrenoid-containing plastids in the cells of many streptophyte algae as well as early-diverging hornworts indicates that the transition to many, small, pyrenoidless plastids occurred more than once during early plant diversification and thus does not necessarily mark plants’ closest algal relatives.
3. Mixotrophy
Several species of streptophyte algae and early-diverging bryophytes have been demonstrated capable of mixotrophy, the uptake and utilization of exogenous organic compounds (in addition to those produced endogenously by photosynthetic carbon fixation) (Graham et al. 1994, 2010a, b). These results suggest that mixotrophy is an early-evolved streptophyte trait that likely characterized earliest land plants, providing several possible advantages during the terrestrialization process. Absorbed sugars could be used as a substrate for cellulose biosynthesis, to cope with: (1) periods of reduced photosynthesis such as occur during drought, photoinhibition, or as the result of mineral nutrient deficiencies; and (2) to recover organic exudations. In addition, mixotrophy has been proposed to subsidize the energy costs of producing degradation-resistant cell wall polymers similar to lignin, which are discussed next.
D. Sporopollenin and Lignin-Like Vegetative Cell Wall Components Originated in Streptophyte Algae and Were Inherited by Earliest Land Plants, Influencing Their Carbon Cycle Impacts
Together, photosynthesis and uptake of organic carbon that might be available provide the organic resources needed by modern streptophyte algae and bryophytes to metabolize, grow, and reproduce, and likely also reflect the condition of earliest land plants. Sporopollenin and lignin-like phenolic wall polymers are examples of metabolic products known to enhance the survival of these organisms, but involve long biosynthetic pathways with high metabolic costs. Consequently, modern streptophyte algae and bryophytes, modeling earliest land plants, deploy sporopollenin and lignin-like wall polymers judiciously, in cells or tissues of greatest effectiveness.
1. Sporopollenin
The origin of sporopollenin-encased spores was a key embryophyte innovation allowing dispersal in dry air (Graham et al. 2004a). Some investigators have recently suggested that sporopollenin-walled spores might have arisen prior to the multicellular diploid (sporophyte) generation or its developmental precursor–the embryo (Taylor and Strother 2009; Brown and Lemmon 2011). Material similar in chemistry and ultrastructural appearance to sporopollen occurs as an inner zygote cell wall layer in Coleochaete (Delwiche et al. 1989) and other later-branching streptophyte algae, thereby indicating the origin of sporopollenin during streptophyte algal diversification, prior to the origin of embryophyte spores (Graham et al. 2004a). Streptophyte algal and bryophyte wall sporopollenin displays characteristic autofluorescence in violet or UV excitation, a property indicating presence of phenolic groups. Although the precise chemical composition of sporopollenin is uncertain, this material is regarded as the most degradation-resistant known biopolymer, helping to explain its protective role during the dispersal of streptophyte algal zygotes and embryophyte spores.
2. Lignin-Like Vegetative Cell Wall Polymers
In addition to sporopollenin, streptophyte algae appear to have bequeathed to earliest land plants and modern bryophytes a biochemically-distinct but likewise degradation-resistant, phenol-containing biopolymer that is deposited in the cell walls of vegetative body cells, rather than zygotes or spores. Gunnison and Alexander (1975a, b) were the first to discover that vegetative cell walls of some streptophyte algae possess the potential to resist microbial degradation in aquatic sediments, demonstrating the presence of “lignin-like” phenolics in decay-resistant cell walls of the desmidialean genus Staurastrum. Delwiche et al. (1989) subsequently revealed the occurrence of chemically similar, hydrolysis-resistant wall compounds in walls of Coleochaete vegetative cells positioned nearby zygotes, and also demonstrated that such wall compounds displayed specific fluorescence properties identical to those of vascular plant lignin, and were chemically, positionally, and ultrastructurally distinct from sporopollenin. Building on this work, Kroken et al. (1996) provided evidence that phenolic wall materials having lignin-like autofluorescence properties appear to confer hydrolysis-resistance upon positionally-equivalent tissues of bryophytes, namely specialized placental tissues located at the N/2 N interface. In the same project, a survey of the production of lignin-like phenolic wall polymers by representative streptophyte algae and bryophytes indicated that such materials likely evolved after the divergence of Klebsormidiales, and before the divergence of desmids such as Staurastrum, and were inherited by earliest land plants. The Kroken et al. (1996) survey also indicated that liverworts and mosses have commonly deployed phenolic materials in additional specialized tissues where degradation resistance is advantageous. Examples include the lower epidermis and rhizoid outgrowths (which lie in direct contact with substrate microbes) and sporangial epidermis, i.e. the capsule wall of the sporophyte generation, providing protection for developing spores. These evolutionary changes reflect differential regulatory control over the location of phenolic deposition in the bodies of different bryophyte species, thereby affecting the extent to which bodies and tissues are able to resist microbial and chemical degradation.
Recent application of thioacidolysis methodology to the liverwort Marchantia and the moss Physcomitrella has revealed the occurrence of lignin-specific monomers in these bryophyte model systems (as well as vascular plants and certain tested seaweeds) (Espiñeira et al. 2010). These findings help to explain the degradation resistance of liverwort and moss sporangial epidermis (Kroken et al. 1996), the Polytrichum calyptra (Kodner and Graham 2001), and the lower epidermis and rhizoids of Marchantia, as well as these materials’ close resemblance to particular cellular sheets and tube microfossils (Graham et al. 2004b) and Late Silurian to Late Devonian (420–370 million years ago) macrofossils of variable size known as Prototaxites. In the past, the latter have been interpreted in diverse ways, but were more recently suggested to represent rolled and slumped degradation-resistant lower body remains of Marchantia-like liverwort mats, which are known to occur in large dimensions both today and in the fossil record (Graham et al. 2010b, c).
Interpretation of at least some of the “dispersed cuticle” and tubular microfossils as liverwort or moss epidermal tissues explains why the cell sheet microfossils are invariably monostromatic (one cell layer thick), and why these particular tissues have survived degradation long enough to fossilize. The selective deposition of phenolic polymers into cell walls of some but not all bryophyte tissues explains why certain cells or tissues survive degradation and thus fossilize better than others. This suggests a testable explanation for the lack to date of intact body fossils of earliest bryophyte-like land plants, which are known only from spores and other microfossils. Results available to date (Graham et al. 2010a) predict that artificial degradation of a suite of modern bryophyte representatives chosen on the basis of key phylogenetic position and/or ecological criteria would reveal a pattern of adaptation to terrestrial stressors by increased production of protective lignin-like wall polymers.
Because there is cellular sheet (“dispersed cuticle”) evidence for the existence of Sphagnum-like mosses as early as the mid-Ordovician (Kroken et al. 1996). Graham et al. 2004a generated quantitative estimates of degradation-resistant biomass for three modern, but relatively early-branching moss lineages (including Sphagnum) in order to model carbon sequestration for a conservatively-estimated 40 million year period prior to end-Ordovician glaciations and the subsequent rise of vascular plants. Such calculations indicate that bryophyte-like land plants played an important role in Earth’s carbon cycle and played other key ecological roles for at least tens of millions of years prior to the rise of vascular plants, as bryophytes do today (Turetsky 2003).
IV. Comparison of Early-Diverging Modern Photosynthesizers to Precambrian-Devonian Fossils Illuminates the Pattern of Terrestrialization
Because modern algae (cyanobacteria and photosynthetic protists) and modern bryophytes possess degradation-resistant components, artificially degrading them for comparison with enigmatic microscopic fossils (microfossils) has proven to be a fruitful way to identify early terrestrial photosynthesizers and understand their structure. Artificial degradation allows investigators to identify the most hydrolysis-resistant and thus potentially preservable parts of modern algae and plants for comparison to ancient microfossils (Gensel 2008). This section describes how comparative studies of fossils and modern representatives of ancient lineages provide insight into photosynthetic and other characteristics of earliest terrestrial algae and land plants, beginning with the cyanobacteria.
A. Cyanobacteria Were Likely Earth’s First Terrestrial Photosynthesizers
Earth’s earliest terrestrial surfaces were almost certainly devoid of organic-rich soils typical of modern times, but rather consisted of rocky or sandy substrates, the latter generated by the weathering actions of wind and water. Geological features cited as evidence for terrestrial cyanobacteria have been described from the Precambrian. Horodyski and Knauth (1994) interpreted beads and cylinders of the iron oxide mineral hematite found in a 1.2 billion year old paleokarst as mineralized cyanobacteria. Prave (2002) cited 1.0 billion year old Scottish Torridonian sedimentary features similar to those associated with modern microbial mats subject to periodic air exposure as indirect evidence for terrestrial cyanobacteria. Strother et al. (2011) report cyanobacterial sheaths and Halothece-like cells recovered from the same deposits by acid maceration. Though Cambrian and Ordovician records of terrestrial cyanobacteria have yet to appear, Early Silurian (Llandovery) microfossils have been interpreted as terrestrial cyanobacteria (Tomescu et al. 2009).
Such paleobiological observations are consistent with the widespread occurrence of cyanobacteria that are tolerant of high irradiance and drought in modern environments ranging from extremely cold dry valleys of Antarctica to deserts of varying aridity conditions (Hughes and Lawley 2003; see also review of terrestrial algae in Graham et al. (2009) and Chapter 16). In such locales, cyanobacteria can occur at the soil surface (e.g. Hu et al. 2003) or beneath or within translucent rocks such as quartzite, sandstone, or granite. Such rocks transmit sufficient light for photosynthesis and reduce the evaporation of moisture needed for active metabolism. For example, at a study site in the US Mojave Desert (California), all quartz pebbles thinner than 25 mm provided favorable light and moisture conditions for cyanobacteria growing beneath (Schlesinger et al. 2003). Likewise, the cyanobacterium Chroococcidiopsis was commonly found in hot and cold deserts of China associated with larger rocks in quartz stony pavements (Warren-Rhodes et al. 2007). Water amounts sufficient for at least some periods of active photosynthesis and growth are necessary for cyanobacterial survival. For example, in the hyperarid Atacama Desert of Chile, the lower precipitation limit allowing photosynthesis by cyanobacterial communities is 5 mm annually (Cockell et al. 2008).
Cyanobacteria of diverse types have adapted to modern terrestrial habitats (Fig. 2.2) and it is likely that many of their adaptive features are ancient, present in Precambrian representatives. Most cyanobacteria generate extracellular polysaccharide sheaths, which can aid in water absorption and retention and provide other functions useful in coping with terrestrial stresses (reviewed in Graham et al. 2009). For example, a number of cyanobacteria possess UV-protective sheath pigments (Sinha and Häder 2007) and mycosporine-like amino acids provide protection from UV radiation and diverse oxidative stresses (Oren and Gunde-Cimerman 2007; Sinha and Häder 2007). Intracellular carotenoids provide photoprotection in diverse cyanobacteria (Lakatos et al. 2001; Kirilovsky 2010). Other species display exceptional tolerance of their cellular components to severe water loss (Potts 1999). Cyanobacteria are also known to produce antibiotic compounds (e.g. Jaiswal et al. 2008), thereby slowing growth of degradative microorganisms.
B. Cyanobacterial and Other Microbial Associations Aid Bryophyte Photosynthesis
The geological evidence for early appearance of terrestrial cyanobacteria together with the physiological ability of many modern representatives to tolerate harsh environmental conditions suggests that cyanobacteria had colonized land well before the first land plants appeared. Consequently, well-established terrestrial cyanobacteria may have competed for resources with earliest plants, but likely also became associated with early plants in mutually beneficial partnerships. Cyanobacterial partners could contribute combined nitrogen generated by nitrogen-fixation metabolism (unique to certain prokaryotes), helping plants to cope with nutrient-poor substrates. Cyanobacterial partnerships also have the potential to provide protective benefits (water-holding mucilage, sunscreens, antibiotics). The potential value of cyanobacterial partners to early land plants is illustrated by symbiotic associations that have been documented for modern early-diverging liverworts and other bryophytes (Basilier 1980; West and Adams 1997; Mitchell et al. 2003; Gentili et al. 2005; Houle et al. 2006; Nilsson et al. 2006; Villarreal and Renzaglia 2006; Adams and Duggan 2008; Rikkinen and Vertanen 2008; Zackrisson et al. 2009).
Streptophyte algae commonly display communities with heterotrophic bacteria that live on algal cell walls or within extensive mucilaginous extracellular matrices generated by the algae (Fisher and Wilcox 1996; Fisher et al. 1998) (Fig. 2.3). Though little is currently known about their composition and function, such microbiomes are hypothesized to receive metabolizable exudates such as glycolate from the algae and contribute fixed nitrogen or vitamins to algal hosts. Heterotrophic prokaryotes also known to partner with bryophytes (Basile et al. 1969; Costa et al. 2001; Dedysh et al. 2002, 2006; Raghoebarsing et al. 2005; Opelt et al. 2007; Chen et al. 2008) can play important roles in carbon cycling (see Chap. 13).
TEM of the lower epidermis of a marchantialean liverwort and associated glomalean hyphae. Cells of the liverwort ventral surface (upper left corner) possess distinctively electron-dense cell walls, thought to reflect the presence of resistant phenolic polymers. Nearby sections of rhizoids reveal that compression resistance is a feature of these long, narrow cells bearing cell wall ingrowths. The smaller diameter, denser-walled sections are of the hyphae of glomalean fungi that are commonly associated with the lower epidermis of this liverwort. Scale bar = 6 μm.
Mycorrhizal-like fungal associations (Fig. 2.4), which occur widely in modern bryophytes, are suggested by several experts to have been critical to the success of early plants on land (Read et al. 2000; Bidartondo et al. 2002; Russell and Bulman 2004; Duckett et al. 2006; Wang and Qiu 2006; Ligrone et al. 2007; Pressel et al. 2008; Bidartondo and Duckett 2010; Bonfante and Selosse 2010). Specific genes known to be critical to the development of embryophyte-fungal symbioses are likely to have been features of early land plants (Wang et al. 2010).
As some experts have noted, at least some fungal-bryophyte associations may be of relatively recent origin, though certain fossil evidence supports the concept that bryophyte-fungal associations are ancient. The microfossil Palaeoglomus grayi–similar to modern glomaleans that today are not known to live independently from plant hosts and provide many modern plant species with mineral nutrients–occurs in mid-Ordovician deposits (Redeker et al. 2000, 2002). By fostering essential mineral acquisition, such beneficial microbial associations likely fostered earliest plant photosynthesis.
C. Microfossils Indicate That Freshwater and/or Terrestrial Eukaryotic Algae Were Present in the Precambrian
Geochemical features have also provided indirect evidence for the occurrence of terrestrial eukaryotes in the Precambrian. For example, an increasing trend in smectitic clays seen in the rock record beginning around 850 Ma, presumably caused by retention of acidic water at the surfaces of weathering rocks, has been interpreted as the consequence of a primitive land biota including eukaryotes capable of secreting organic acids (Kennedy et al. 2006). Carbon isotopic data likewise indicate presence of terrestrial photosynthesizers in the late Precambrian (Knauth and Kennedy 2009). More recently, ancient lacustrine deposits from the 1.0 Ga Torridon Group (NW Scotland) were found to contain diverse assemblages of microfossils and structural organic fragments. In addition to previously-mentioned forms interpreted as prokaryotes, a diverse array of more complex structures mostly interpreted as eukaryotic were found (Strother et al. 2011). These shale deposits accumulated in lakes, but were frequently subaerially exposed as evidenced by numerous levels exhibiting desiccation cracks and possible raindrops. The dominant lifeforms are simple spherical cells and cell clusters (Zhang 1982; Brasier 2009), but more complex remains include forms with internal bodies, external processes and even flask-shaped and thalloid organisms (Strother et al. 2011). Some of these colonial or coenobial growth forms resemble those of modern eukaryotic algae. While many of the producers probably inhabited the water column, at least some were likely transported into the lake from surrounding streams, and some may even have derived from the land surface before being flushed into the river/lake. In addition, certain microfossil remains from Precambrian (Mesoproterozoic) to early Cambrian sites are said to resemble the modern unicellular freshwater green, zygnematealean algal genus Spirotaenia (Leiming et al. 2005; Leiming and Xunlai 2007).
These fossil observations are consistent with observations that diverse species of extant eukaryotic algae occupy a wide range of terrestrial and even arid habitats (Flechtner et al. 2008; Büdel et al. 2009) and display traits that aid in coping with high irradiance and desiccation (reviewed in Chapter 23 of Graham et al. 2009). Some green algae possess similar mechanisms to those found in cyanobacteria for dealing with high light stress, including the use of carotenoids and MAAs for photoprotection. The cellular mechanisms used by green algae for vegetative desiccation tolerance are not well studied, although the ability of vegetative cells to tolerate desiccation is phylogenetically widespread (e.g., Gray et al. 2007; Luttge and Büdel 2010).
D. Fossil Evidence Suggests That Streptophyte Algae Were Established on Land by the Middle Cambrian
As previously noted, like modern bryophytes, later-diverging streptophyte algae such as Coleochaete are known to be capable of producing degradation-resistant cell walls (Kroken et al. 1996). It has recently been observed that when terrestrially-grown Coleochaete has been subjected to artificial degradation, the remains closely resemble certain Middle Cambrian (499–511 million year old) microfossils (Graham et al. 2012), as well as some of the microfossil remains recovered from Early Middle Ordovician sediments (see figure 2h of Rubinstein et al. 2010). Such observations suggest that streptophytes had become terrestrial photosynthesizers by the Middle Cambrian and that terrestrial streptophyte algae continued to compete successfully with other terrestrial photosynthesizers well into the Ordovician.
E. Some Experts Think That Early Land Plants Had Evolved by the Middle Cambrian, Though the Concept Is Controversial
The most ancient remains that have been linked with earliest land plants are Cambrian-Early Devonian microscopic fossils that include: (A) spores and spore-like objects, (B) small tubes of various types, and (C) sheets of cells often referred to as “dispersed cuticle” (Gensel et al. 1991). Many of the latter structures are clearly not homologous to the cuticles of vascular plant shoots (non-cellular, superficial layers of cutin and wax), or to the chitin and protein cuticles (exoskeletons) of arthropods, and thus are better termed cellular sheets.
Identifying the sources of microfossils is important in comprehending early terrestrialization because larger pieces of earliest land plants, visible with the unaided eye and thus known as macrofossils, have so far not been identified from Cambrian through Ordovician deposits. This lack has been attributed to climatic conditions, occurrence of early plants in places where remains were not readily transported, or absence of degradation-resistant material other than sporopollenin-coated spores (reviewed by Gensel 2008). However, if earliest land plants resembled modern, early-diverging bryophytes, as suggested by molecular systematic data, they likely produced some degradation-resistant materials that could have survived as fragmentary remains (Kroken et al. 1996; Graham and Gray 2001; Kodner and Graham 2001; Graham et al. 2004a, b). Except under unusual environmental conditions, only degradation-resistant cells and tissues are likely to survive transport from the site of death to the site of deposition, burial in aquatic sediments, and fossilization as compressions, impressions, or petrifactions (Gensel 2008).
Tantalizing organic fragments – scraps of cuticle, wefts of tubes and sheets of cells/spores – are known from a number of Middle Cambrian – Early Ordovician deposits in North America. These may be early terrestrial multicellular autotrophs that disarticulated before becoming fossils. Given the half billion years between these organisms – whether algae or plants or something in between – and extant bryophytes, it is not surprising that the remains are enigmatic. One thing is certain however; something living in the Middle Cambrian produced spores with thick, multilayered walls, more robust than that produced by any extant alga, and nearly indistinguishable from some relatively advanced liverworts. Whether bryophytes had evolved even earlier than has been recognized, or thick, multilayered spore walls were among the first features to evolve among terrestrial algae is still an open question. Microfossils cited as remains of land plants also occur in early Middle Ordovician (473–471 million years ago) (Rubinstein et al. 2010).
The concept that the embryophyte clade originated in Cambrian or even Precambrian times is supported by some molecular clock estimates, which place the split between streptophyte algae and embryophytes at more than 700 million years ago (Hedges et al. 2006; Zimmer et al. 2007; Clarke et al. 2011). This timing is substantially earlier than the mid-Ordovician (about 460 million year) period commonly cited for the origin of land plants (e.g. Sanderson 2003). However, molecular clock methods are constantly improving, genome projects are contributing many new sequences, and additional fossils are being found and identified. Consequently, it may eventually be possible to reconcile embryophyte divergence dates that result from molecular and fossil approaches, as has recently been accomplished for the divergence of marsupial and placental mammals (Luo et al. 2011).
F. Microfossil and Macrofossil Evidence Indicates the Widespread Occurrence of Early Liverwort-Like and Moss-Like Land Plants by Mid-Ordovician Times, Extending into the Silurian and Devonian
Microfossils that have been confidently identified as remains of early liverwort-like land plants existed by the mid-Ordovician (Strother et al. 1996; Wellman 2010). Certain modern liverworts are known to disperse their spores in groups of four attached meiotic products, thereby resembling intriguing fossil spore tetrads known from the Middle Ordovician and later times (Gray 1985; Graham and Gray 2001). In pioneering the ultrastructural comparative analysis approach to the study of ancient and modern spore walls, Taylor (1995) demonstrated that certain spores dispersed in pairs (dyads) were similar in cellular detail to those of modern liverworts. Spores lacking a Y-shaped trilete mark (characteristic of dispersed spores of vascular plants) were found within sporangia of Lower Devonian plants having certain bryophyte features (Edwards et al. 1999). Aggregates of fossil spores enclosed in resistant sporangial epidermal tissue (Wellman et al. 2003) suggested affinity with modern liverworts, which had previously been demonstrated to produce degradation-resistant sporangia (Kroken et al. 1996). Fourier transform infrared (FTIR) spectroscopy analysis shows that Silurian spores of types putatively identified as the reproductive dispersal units of early embryophytes are chemically similar to the trilete single spores characteristically dispersed by vascular land plants (Steemans et al. 2010).
Evidence that early mosses existed by the Ordovician is provided by 460 million year old cellular sheet microfossils (Gray et al. 1982) that: (1) closely resemble in cellular pattern and cell dimensions degradation-resistant sporangial epidermis produced by the early-divergent moss genus Sphagnum (Kroken et al. 1996), and (2) have not been otherwise classified. The observation that artificially degraded gametophytic cells that conspicuously cap sporophytes of modern Polytrichum mosses, known as calyptrae, closely resemble particular branched tube microfossils having unusually thick (8.5 μm) cell walls known from Silurian-Devonian sites (Kodner and Graham 2001 and articles referenced therein) provides evidence for the occurrence of somewhat more derived mosses by this time.
As earlier noted, lignin has been found in the complex thalloid liverwort Marchantia, explaining degradation resistance of the lower epidermis and rhizoids of Marchantia, and these materials’ close resemblance to particular porose cellular sheets and tube microfossils (Graham et al. 2004b), as well as Late Silurian to Late Devonian (420–370 million years ago) macrofossils of variable size known as Prototaxites (Graham et al. 2010b, c). Macrofossils that have been compared to modern derived liverworts are known from the Middle Devonian (Metzgeriothallus sharonae) (Hernick et al. 2008) and lowermost Upper Devonian (Hepaticites devonicus) (Hueber 1961). The occurrence of confidently-identified macrofossils of derived liverwort lineages in these Devonian samples increases the likelihood that earlier-diverging liverworts existed considerably earlier.
Microfossil evidence indicates that early vascular land plants were likely present in the Ordovician, though were rare (Steemans et al. 2009). Vascular plant diversification, possibly delayed by Late Ordovician glaciations, did not proceed rapidly until the Late Silurian to Early Devonian, 407–418 million years ago (Gensel 2008). Consequently, Middle Cambrian (499–511 million years ago) fossil evidence for terrestrial Coleochaete-like streptophyte algae (Graham et al. 2012) and Ordovician evidence for liverwort-like early land plants (e.g., Wellman et al. 2003) indicate that non-vascular terrestrial streptophytes were successful for more than 100 million years prior to the rise of vascular plants and in addition left descendants through at least five major species extinction events.
A similar extremely long period of terrestrial domination by analogs of Earth’s terrestrial streptophyte algae and bryophytes may generally characterize the development of planets similar to Earth that are located in habitable zones of other suns. Increasingly better-developed spectroscopic techniques are allowing astrobiologists to infer the properties of extrasolar planets (reviewed by Baraffe et al. 2010; Kaltenegger et al. 2010). Future planetary surveys may reveal spectroscopic evidence for terrestrialization, such as red reflectance suggesting chlorophyll and IR evidence for volatiles such as isoprene, the latter produced by Earth mosses (as well as some other plants) as an adaptation to terrestrial stressors (Hanson et al. 1999).
V. Perspective
-
Understanding Earth terrestrialization is important in comprehending the origin of modern ecosystems, and requires analyses of modern bryophytes and their closest algal relatives as well as Precambrian-Devonian fossil remains attributed to earliest land plants, which are largely fragmentary. Artificial degradation of modern bryophytes and algae allows comparison of the remains with enigmatic microfossil fragments, aiding fossil classification.
-
The sum of available molecular and microfossil evidence indicates that earliest land plants evolved from streptophyte algae that had accumulated physiological and other preadaptations relevant to land colonization, but the order of early embryophyte-specific trait acquisition has not as yet been well defined.
-
Available molecular and microfossil evidence does not yet allow confident determination of the time when earliest land plants appeared. Some molecular clock and controversial microfossil data argue for a Precambrian (700 million years ago) to Middle Cambrian (500 million years ago) emergence time. Widely accepted microfossils indicate that liverwort-like early land plants existed by the Middle Ordovician (470 million years ago). Some microfossils indicate the presence of early-diverging mosses by the Mid-Ordovician and later-diverging mosses by the Silurian. Certain microfossil and macrofossils suggest the existence of Marchantia-like liverworts by the Late Silurian, and non-controversial macrofossils show that liverworts similar to those of other modern species existed by the Middle Devonian. Existing molecular systematic analyses and an increasing body of fossil evidence indicates that modern liverworts and mosses are not the descendants of degenerate vascular land plants.
-
Models of carbon sequestration by early bryophyte-like land plants, constructed from data obtained from modern early-divergent bryophytes, indicate that prevascular land plants influenced Earth’s carbon cycle for tens of millions of years before vascular plants became common, and help to explain how modern liverworts and mosses are biogeochemically significant. These results, together with microfossil evidence for earliest terrestrial streptophytes, suggest that Earth-like extrasolar planets that occupy habitable zones may likewise display an extended period of terrestrial carbon cycle dominance by streptophyte algae and bryophyte-like early land plants.
References
Adams DG, Duggan PS (2008) Cyanobacteria-bryophyte symbioses. J Exp Bot 59:1047–1058
Alboresi A, Caffarri S, Nogue F, Bassi R, Morosinotto T (2008) In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: identification of subunits which evolved upon land adaptation. PLoS One 3:e2033
Arancibia-Avila P, Coleman JR, Russin WA, Wilcox LW, Graham LE (2000) Effects of pH on cell morphology and carbonic anhydrase activity and localization in bloom-forming Mougeotia (Chlorophyta, Charophyceae). Can J Bot 78:1206–1214
Arancibia-Avila PA, Coleman JR, Russin WA, Graham JM, Graham LE (2001) Carbonic anhydrase localization in charophycean green algae: ecological and evolutionary significance. Int J Plant Sci 162:127–135
Baraffe I, Chabrier G, Barman T (2010) The physical properties of extra-solar planets. Rep Prog Phys 73:1–30
Basile DV, Slade LL, Corpe WA (1969) An association between a bacterium and a liverwort, Scapania nemorosa. J Torrey Bot Soc 96:711–714
Basilier K (1980) Fixation and uptake of nitrogen in Sphagnum blue-green algal associations. Oikos 34:239–242
Becker B, Marin B (2009) Streptophyte algae and the origin of embryophytes. Ann Bot 103:999–1004
Berner RA (1997) The rise of land plants and their effect on weathering and CO2. Science 276:544–546
Bidartondo MI, Duckett JG (2010) Conservative ecological and evolutionary patterns in liverwort-fungal symbioses. Proc R Soc B Biol Sci 277:485–492
Bidartondo MI, Bruns TD, Weiss M, Sérgio C, Read DJ (2002) Specialized cheating of the ectomycorrhizal symbiosis by an epiparasitic liverwort. Proc R Soc Biol Sci Ser B 270:835–842
Bonfante P, Selosse M-A (2010) A glimpse into the past of land plants and of their mycorrhizal affairs: from fossils to evo-devo. New Phytol 186:267–270
Brasier MD (2009) Darwin’s lost world: the hidden history of animal life. Oxford University Press, Oxford
Brook AJ, Williamson DB (1998) The survival of desmids on the drying mud of a small lake. In: Round FE (ed) Algae and the aquatic environment. BioPress, Bristol, pp 185–196
Brown RC, Lemmon BE (2011) Spores before sporophytes: hypothesizing the origin of sporogenesis at the algal-plant transition. New Phytol 190:875–881
Büdel B, Darienko T, Deutschewitz K, Dojani S, Friedl T, Mohr KI, Salisch M, Reisser R, Weber B (2009) Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb Ecol 57:229–247
Chen Y, Dumont MG, McNamara NP, Chamberlain PM, Bodrossy L, Stralis-Pavese N, Murrell JC (2008) Diversity of the active methanotrophic community in acidic peatlands as assessed by mRNA and SIP-PFLA analyses. Environ Microb 10:446–459
Clarke JT, Warnock RCM, Donoghue PCJ (2011) Establishing a time-scale for plant evolution. New Phytol 192:266–301
Cockell CS, McKay CP, Warren-Rhodes K, Horneck G (2008) Ultraviolet radiation-induced limitation to epilithic microbial growth in arid deserts – dosimetric experiments in the hyperarid core of the Atacama desert. J Photochem Photobiol B 90:79–87
Costa J-L, Paulsrud P, Rikkinen J, Lindblad P (2001) Genetic diversity of Nostoc symbionts endophytically associated with two bryophyte species. Appl Environ Microbiol 67:4393–4396
Crandall-Stotler BJ, Forrest L, Stotler RE (2005) Evolutionary trends in the simple thalloid liverworts (Marchantiophyta, Junnermanniopsida subclass Metzgeriidae). Taxon 54:299–316
Dedysh SN, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Liesack W, Tiedje JM (2002) Methylocapsa acidiphila gen. nov., sp. nov., a novel methane-oxidizing and dinitrogen-fixing acidophilic bacterium from Sphagnum bog. Int J Syst Evol Microbiol 52:251–261
Dedysh SN, Pankratov TA, Belova SE, Kulichevskaya IS, Liesack W (2006) Phylogenetic analysis and in situ identification of bacteria community composition in an acidic Sphagnum peat bog. Appl Environ Microbiol 72:2110–2117
Delwiche CF, Graham LE, Thomson N (1989) Lignin-like compounds and sporopollenin in Coleochaete, an algal model for land plant ancestry. Science 245:399–401
Dombrovska O, Qiu Y-L (2004) Distribution of introns in the mitochondrial gene nad1 in land plants: phylogenetic and molecular evolutionary implications. Mol Phylogenet Evol 32:246–263
Duckett JG, Carafa A, Ligrone R (2006) A highly-differentiated glomeromycotean association with the mucilage-secreting, primitive antipodean liverwort Treubia (Treubiaceae): clues to the origins of mycorrhizas. Am J Bot 93:797–813
Edwards D, Wellman CH, Axe L (1999) Tetrads in sporangia and spore masses from the Upper Silurian and Lower Devonian of the Welsh Borderland. Bot J Linn Soc 130:111–115
Elster JD, Peter D, L’ubomir K, Lucia V, Katarina Š, Antonio BP (2008) Freezing and desiccation injury resistance in the filamentous green alga Klebsormidium from the Antarctic, Arctic and Slovakia. Biologia 63:843–851
Espiñeira JM, Uzal EN, Gómez Ros LV, Carrión JS, Merino F, Ros Barceló A, Pomar F (2010) Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol 13:59–68
Finet C, Timme RE, Delwiche CF, Marlétaz F (2010) Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Curr Biol 20:1–6
Fisher MM, Wilcox LW (1996) Desmid-bacterial associations in Sphagnum-dominated Wisconsin peatlands. J Phycol 32:543–549
Fisher MM, Wilcox LW, Graham LE (1998) Molecular characterization of epiphytic bacterial communities on charophycean green algae. Appl Environ Microbiol 64:4384–4389
Flechtner VR, Johansen JR, Belnap J (2008) The biological soil crusts of the San Nicolas Island: enigmatic algae from a geographically isolated ecosystem. West N Am Nat 68:405–436
Forrest LL, Davis EC, Long DG, Crandall-Stotler BJ, Clark A, Hollingsworth ML (2006) Unraveling the evolutionary history of the liverworts (Marchantiophyta): multiple taxa, genomes and analyses. Bryologist 109:304–334
Gensel PG (2008) The earliest land plants. Annu Rev Ecol Evol Syst 39:459–477
Gensel PG, Johnson NG, Strother PK (1991) Early land plant debris (Hooker’s “waifs and strays”?). Palaios 5:520–547
Gentili F, Nilsson M-C, Zackrisson O, DeLuca TH, Sellstedt A (2005) Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J Exp Bot 56:3121–3127
Graham LE (1993) The origin of land plants. Wiley, New York
Graham LE, Gray J (2001) The origin, morphology and ecophysiology of early embryophytes: neontological and palaeontological perspectives. In: Gensel P, Edwards D (eds) Plants invade the land. Columbia University Press, New York, pp 140–156
Graham LE, Graham JM, Russin WA, Chesnick JM (1994) Occurrence and phylogenetic significance of glucose utilization by charophycean algae: glucose enhancement of growth in Coleochaete orbicularis. Am J Bot 81:423–432
Graham LE, Cook ME, Busse JS (2000) The origin of plants: body plan changes contributing to a major evolutionary radiation. Proc Natl Acad Sci USA 97:4535–4540
Graham LE, Kodner RG, Fisher MM, Graham JM, Wilcox LW, Hackney JM, Obst J, Bilkey PC, Hanson DT, Cook ME (2004a) Early land plant adaptations to terrestrial stress: a focus on phenolics. In: Hemsley A, Poole I (eds) Evolution of plant physiology. Academic, New York, pp 155–170
Graham LE, Wilcox LW, Cook ME, Gensel PG (2004b) Resistant tissues of marchantioid liverworts resemble enigmatic Early Paleozoic microfossils. Proc Natl Acad Sci USA 101:11025–11029
Graham LE, Graham JM, Wilcox LW (2009) Algae. Benjamin Cummings/Pearson, San Francisco
Graham LE, Kim E, Arancibia-Avila P, Graham JM, Wilcox LW (2010a) Evolutionary and ecophysiological significance of sugar utilization by the peatmoss Sphagnum compactum (Sphagnaceae) and the common charophycean associates Cylindrocystis brebissonii and Mougeotia sp. (Zygnemataceae). Am J Bot 97:1485–1491
Graham LE, Cook ME, Hanson DT, Pigg KB, Graham JM (2010b) Structural, physiological, and stable carbon isotopic evidence that the enigmatic Paleozoic fossil Prototaxites formed from rolled liverwort mats. Am J Bot 97:1–8
Graham LE, Cook ME, Hanson DT, Pigg KB, Graham JM (2010c) Rolled liverwort mats explain major Prototaxites features: response to commentaries. Am J Bot 97:1079–1086
Graham LE, Arancibia-Avila P, Taylor WA, Strother PK, Cook ME (2012) Aeroterrestrial Coleochaete (Streptophyta, Coleochaetales) models early plant adaptation to land. Am J Bot 99:130–144
Gray J (1985) The microfossil record of early land plants: advances in understanding of early terrestrialization, 1970–1984. Philos Trans R Soc Lond B Biol Sci 309:167–195
Gray J, Massa D, Boucot AJ (1982) Caradocian microfossils from Libya. Geology 10:197–201
Gray DW, Lewis LA, Cardon ZG (2007) Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant Cell Environ 30:1240–1255
Gunnison D, Alexander M (1975a) Resistance and susceptibility to decomposition by natural microbial communities. Limnol Oceanogr 20:64–70
Gunnison D, Alexander M (1975b) Basis for the resistance of several algae to microbial decomposition. Appl Microbiol Biotechnol 29:729–738
Hanson DT, Swanson S, Graham LE, Sharkey TD (1999) Evolutionary significance of isoprene emission from mosses. Am J Bot 86:634–639
Hedges SB, Battistuzzi U, Blair JE (2006) Chapter 7. Molecular timescale of evolution in the Proterozoic. In: Xiao S, Kaufman AJ (eds) Neoproterozoic geology and paleobiology. Springer, New York
Hernick LVA, Landing E, Bartowski KE (2008) Earth’s oldest liverworts – Metzgeriothallus sharonae sp. nov. from the Middle Devonian (Givetian) of eastern New York, USA. Rev Palaeobot Palynol 148:154–162
Holtzinger A, Tschaikner A, Remias D (2010) Cytoarchitecture of the desiccation-tolerant green alga Zygogonium erecitorum. Protoplasma 243:15–24
Horodyski RJ, Knauth LP (1994) Life on land in the Precambrian. Science 263:494–498
Houle D, Gauthier SB, Paquet S, Planas D, Warren A (2006) Identification of two genera of N2-fixing cyanobacteria growing on three feather moss species in boreal forests of Quebec-Canada. Can J Bot 84:1025–1029
Hu CX, Zhang DL, Huang ZB, Liu YD (2003) The vertical microdistribution of cyanobacteria and green algae within desert crusts and the development of the algal crusts. Plant Soil 257:97–111
Hueber FM (1961) Hepaticites devonicus a new fossil liverwort from the Devonian of New York. Ann Mo Bot Gard 48:125–131
Hughes KA, Lawley B (2003) A novel Antarctic microbial endolithic community within gypsum crusts. Environ Microbiol 5:555–565
Jaiswal P, Singh PK, Prasanna R (2008) Cyanobacterial bioactive molecules – an overview of their toxic properties. Can J Microbiol 54:701–717
Kaltenegger L, Selsis F, Fridlund M, Lammer H, Beichman C, Danchi W, Eiroa C, Henning T, Herbst T, Léger A, Liseau R, Lunine J, Paresca F, Penny A, Quirrenbach A, Röttgering H, Schneider J, Stam D, Tinetti G, White GJ (2010) Deciphering spectral fingerprints of habitable exoplanets. Astrobiology 10:89–102
Karsten U, Lütz C, Holzinger A (2010) Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Charophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J Phycol 46:1187–1197
Kennedy M, Droser M, Mayer LM, Pevear D, Mrofka D (2006) Late Precambrian oxygenation; inception of the clay mineral factory. Science 311:1446–1449
Kirilovsky D (2010) The photoactive orange carotenoid protein and photoprotection in cyanobacteria. In: Hallenbeck PC (ed) Recent advances in phototrophic prokaryotes. Springer, Philadelphia, pp 139–159
Knauth LP, Kennedy MJ (2009) The late Precambrian greening of the earth. Nature 460:728–732
Kodner RB, Graham LE (2001) High-temperature, acid-hydrolyzed remains of Polytrichum (Musci, Polytrichaceae) resemble enigmatic Silurian-Devonian tubular microfossils. Am J Bot 88:462–466
Koziol AG, Borza T, Ishida K-I, Keeling P, Lee RW, Durnford DG (2007) Tracing the evolution of the light-harvesting antennae in chlorophyll a/b-containing organisms. Plant Physiol 143:1802–1816
Kroken SB, Graham LE, Cook ME (1996) Occurrence and evolutionary significance of resistant cell walls in charophytes and bryophytes. Am J Bot 83:1241–1254
Lakatos M, Bilger W, Büdel B (2001) Carotenoid composition of terrestrial cyanobacteria: response to natural light conditions in open rock habitats in Venezuela. Eur J Phycol 36:367–375
Leiming Y, Xunlai Y (2007) Radiation of Meso-Neoproterozoic and early Cambrian protists inferred from the microfossil record of China. Palaeogeogr Palaeoclimatol Palaeoecol 254:350–361
Leiming Y, Xunlai Y, Fanwei M, Jie H (2005) Protists of the Upper Mesoproterozoic Ruyang group in Shanxi Province, China. Precambrian Res 141:49–66
Lewis LA, McCourt RM (2004) Green algae and the origin of land plants. Am J Bot 91:1535–1556
Ligrone R, Carafa A, Lumini E, Bianciotto V, Bonfante P, Duckett JG (2007) Glomeromycotean associations in liverworts: a molecular, cellular, and taxonomic analysis. Am J Bot 94:1756–1777
Luo Z-X, Yuan CX, Meng Q-J, Ji Q (2011) A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476:442–445
Luttge U, Büdel B (2010) Resurrection kinetics of photosynthesis in desiccation-tolerant terrestrial green algae. Plant Biol 12:437–444
Mitchell EAD, Gilbert D, Buttler A, Amblard C, Grosvernier P, Gobat J-M (2003) Structure of microbial communities in Sphagnum peatlands and effect of atmospheric carbon dioxide enrichment. Microb Ecol 46:187–199
Nagao MK, Matsui K, Uemura M (2008) Klebsormidium flaccidum, a streptophyte algae green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ 31:872–875
Nilsson M, Rasmussen U, Bergman B (2006) Cyanobacterial chemotaxis to extracts of host and nonhost plants. FEMS Microbial Ecol 55:382–390
Opelt K, Chobot V, Hadacek F, Schönmann S, Eberl L, Berg G (2007) Investigations of the structure and function of bacterial communities associated with Sphagnum mosses. Environ Microbiol 9:2795–2809
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269:1–10
Potts M (1999) Desiccation tolerance in cyanobacteria. Eur J Phycol 34:319–328
Prave AR (2002) Life on land in the Proterozoic: evidence from the Torridonian rocks of northwest Scotland. Geology 30:811–814
Pressel S, Ligrone R, Duckett JG, Davis EC (2008) A novel ascomycetous endophytic association in the rhizoids of the leafy liverwort family Schistochilaceae (Jungermanniidae, Hepaticopsida). Am J Bot 95:531–541
Qiu Y-L, Cho Y, Cox JC, Palmer JD (1998) The gain of three mitochondrial introns identifies liverworts as the earliest land plants. Nature 394:671–674
Qiu Y-L, Li L, Bin W, Chen Z, Knoop V, Groth-Malonek M, Dombrovska O, Lee J, Kent L, Rest J, Estabrook GF, Hendry TA, Taylor DW, Testa CM, Ambros M, Crandall-Stotler B, Duff RJ, Stech M, Frey W, Quandt D, Davis CC (2006) The deepest divergences in land plants inferred from phylogenomic evidence. Proc Natl Acad Sci USA 103:15511–15516
Qiu Y-L, Li L, Wang B, Chen Z, Dombrovska O, Lee J, Kent L, Li R, Jobson RW, Hendry TA, Taylor DW, Testa CM, Ambros M (2007) A nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. Int J Plant Sci 168:691–708
Raghoebarsing AA, Smolders AJP, Schmid MC, Riipstra WIC, Wolters-Arts M, Derksen J, Jetten MSM, Schouten S, Damsté S, Lamers LPM, Roelofs JGM, Op den Camp HJM, Strous M (2005) Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436:1153–1156
Read DJ, Duckett JG, Francis R, Ligrone R, Russell A (2000) Symbiotic fungal associations in ‘lower’ land plants. Philos Trans R Soc Lond B Biol Sci 355:815–831
Redeker D, Kodner R, Graham LE (2000) Glomalean fungi from the Ordovician. Science 289:1920–1921
Redeker D, Kodner R, Graham LE (2002) Palaeoglomus grayi from the Ordovician. Mycotaxon 84:33–37
Rikkinen J, Vertanen V (2008) Genetic diversity in cyanobacterial symbionts of thalloid bryophytes. J Exp Bot 59:1013–1021
Rubinstein CV, Gerrienne P, de la Puente GS, Astini RA, Steemans P (2010) Early Middle Ordovician evidence for land plants in Argentina (Eastern Gondwana). New Phytol 188:365–369
Russell J, Bulman S (2004) The liverwort Marchantia foliacea forms a specialized symbiosis with arbuscular mycorrhizal fungi in the genus Glomus. New Phytol 165:567–569
Sanderson MJ (2003) Molecular data from 27 proteins do not support a Precambrian origin of land plants. Am J Bot 90:954–956
Schlesinger WH, Pippen JS, Wallenstein MD, Hofmockel KS, Klepeis DM, Mahall BE (2003) Community composition and photosynthesis by photoautotrophs under quartz pebbles, southern Mohave desert. Ecology 84:3222–3231
Sinha RP, Häder D-P (2007) UV-protectants in cyanobacteria. Plant Sci 174:278–289
Škaloud P (2009) Species composition and diversity of aero-terrestrial algae and cyanobacteria of the Boreč Hill ventaroles. Fottea 9:65–80
Steemans P, Le Hérissé A, Melvin J, Miller MA, Paris F, Verniers J, Wellman CH (2009) Origin and radiation of the earliest vascular land plants. Science 324:353
Steemans P, Lepot K, Marshall CP, Le Hérissé A, Javaux EJ (2010) FTIR characterization of the chemical composition of Silurian miospores (cryptospores and trilete spores) from Gotland, Sweden. Rev Palaeobot Palynol 162:577–590
Strother PK, Al-Hajri S, Traverse A (1996) New evidence for land plants from the lower Middle Ordovician of Saudi Arabia. Geology 24:55–58
Strother PK, Battison L, Brasier MD, Wellman CH (2011) Earth’s earliest non-marine eukaryotes. Nature 473:505–509
Taylor WA (1995) Spores in earliest land plants. Nature 373:391–392
Taylor WA, Strother PK (2009) Ultrastructure, morphology, and topology of Cambrian palynomorphs from the Lone Rock Formation, Wisconsin, USA. Rev Palaeobot Palynol 153:296–309
Timme RE, Bachvaroff TR, Delwiche CF (2012) Broad phylogenomic sampling and the sister lineage of land plants. PLoS One 7(1):e29696
Tomescu AMF, Rothwell GW, Honeggar R (2009) A new genus and species of filamentous microfossil of cyanobacterial affinity from Early Silurian fluvial environments (lower Massanutten Sandstone, Virginia, USA). Bot J Linn Soc 160:284–289
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409
Turmel M, Pombert J-F, Charlebois P, Otis C, Lemieux C (2007) The green algal ancestry of land plants as revealed by the chloroplast genome. Int J Plant Sci 168:679–689
Villarreal JCA, Renzaglia KS (2006) Structure and development of Nostoc strands in Leiosporoceros dussii (Anthocerotophyta): a novel symbiosis in land plants. Am J Bot 93:693–705
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Wang B, Yeun LH, Xue J-Y, Liu Y, Ané J-M, Qiu Y-L (2010) Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol 186:514–525
Warren-Rhodes KA, Rhodes KL, Boyle LN, Pointing SB, Chen Y, Liu S, Zhuo P, McKay CP (2007) Cyanobacterial ecology across environmental gradients and spatial scales in China’s hot and cold deserts. FEMS Microbiol Ecol 61:470–482
Wellman CH (2010) The invasion of the land by plants: when and where? New Phytol 188:306–309
Wellman CH, Osterloff PL, Mohiuddin U (2003) Fragments of the earliest land plants. Nature 425:282–285
West NJ, Adams DG (1997) Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site. Appl Environ Microbiol 63:4479–4484
Wodniok S, Brinkmann H, Glöckner G, Heidel AJ, Phillippe H, Melkonian M, Becker B (2011) Origin of land plants: do conjugating green algae hold the key? BMC Evol Biol 11:104–111
Wood AJ (2007) The nature and distribution of vegetative desiccation-tolerance in hornworts, liverworts, and mosses. Bryologist 110:163–177
Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A (2009) Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia 160:309–319
Zhang Z (1982) Upper Proterozoic microfossils from the Summer Isles, NW Scotland. Palaeontology 25:443–460
Zimmer A, Lang D, Richardt S, Frank W, Reski R, Rensing SA (2007) Dating the early evolution of plants: detection and molecular clock analyses of orthologs. Mol Genet Genom 278:393–402
Acknowledgements
Sarah Friedrich provided assistance with illustrations. We thank Dr. James Graham and Christopher Cardona-Correa for helpful discussions. Grant support from the US National Science Foundation (NSF) and the United Kingdom Natural Environment Research Council (NERC) is much appreciated.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Graham, L., Lewis, L.A., Taylor, W., Wellman, C., Cook, M. (2014). Early Terrestrialization: Transition from Algal to Bryophyte Grade. In: Hanson, D., Rice, S. (eds) Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration, vol 37. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6988-5_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-6988-5_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6987-8
Online ISBN: 978-94-007-6988-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)