Abstract
Laparoscopic Heller myotomy has proved to be superior to endoscopic techniques in improving symptoms and duration of effect for achalasia (Campos et al., Ann Surg 249:45–57, 2009). The addition of an anterior fundoplication decreases symptoms of acid reflux without exacerbating or promoting dysphagia (Rebecchi et al., Ann Surg 248:1023–1030, 2008; Tapper et al., Am Surg 74:626–634, 2008). It has also been proved that laparoscopic hiatus hernioplasty and Nissen fundoplication is superior to open surgery in medical cost and postoperative complications with similar anti-reflux effect (Siddiqui et al., Pediatr Surg Int 27:359–366, 2011; Ostlie et al., J Laparoendosc Adv Surg Tech A 17:493–496, 2007).
Recent publications confirm the rapid increase in the use of SILS for a wide range of laparoscopic procedures (Fan et al., Surg Innov 18:185–188, 2011; Fan et al., J Laparoendosc Adv Surg Tech A 21:243–247, 2011; Joseph et al., Am Surg 78:119–124, 2012). However, application of SILS technique in Heller myotomy and Dor fundoplication or hiatus hernioplasty and Nissen fundoplication is still in its infancy due to its technical difficulties (Huang et al., Obes Surg 19:1711–1715, 2009; Sakaguchi et al., Surg Endosc 22:2532–2534, 2008; Gianni et al., Obes Surg 22:190–191, 2012). So far, we have performed single incision laparoscopic fundoplication in more than ten cases with good results (Fan et al., J Laparoendosc Adv Surg Tech A 23:356–360, 2013).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Laparoscopic Hiatus Hernioplasty and Nissen Fundoplication
2.1.1 Indications and Case Selection
-
1.
Patients with type II/III/IV esophageal hiatal hernia.
-
2.
Patients with type I esophageal hiatal hernia complicating with esophageal reflux, but medication is ineffective.
-
3.
Patients complicating with severe peptic esophagitis, hemorrhage, recurrent aspiration pneumonia, etc.
2.1.2 Contraindications
-
1.
Absolute contraindications
-
(a)
severe cardiorespiratory dysfunction.
-
(b)
inability to tolerate general anesthesia or pneumoperitoneum.
-
(c)
refractory coagulopathy.
-
(a)
-
2.
Relative contraindications
-
(a)
extensive previous surgery, or previous peritonitis leading to severe adhesion.
-
(b)
morbid obesity.
-
(c)
pregnancy (during first and third trimesters).
-
(a)
2.1.3 Major Instruments or Energy Sources
-
1.
Laparoscopy System.
-
2.
Holding forceps, non-traumatic intestinal clamps, needle holder etc.
-
3.
Harmonic scalpel.
2.1.4 Team Setup, Anesthesia and Position
The procedures were performed under general anesthesia with the patients in the supine position with legs parted, left shoulder raised 15–20°. The viewing monitor was placed above the patient’s left shoulder, with the surgeon standing between the patient’s legs and the camera operator on the patient’s right side (Fig. 2.1).

Fig. 2.1
2.1.5 Key Steps
-
1.
Establishment of Pneumoperitoneum and Placement of Trocars.
-
2.
Abdominal Exploration.
-
3.
Division of lower esophagus and bilateral crura.
-
4.
Division of the cardia and fundus of the stomach.
-
5.
Hernioplasty.
-
6.
Fundoplication.
-
7.
Placement of the suction drainage.
2.1.6 Surgical Techniques
-
1.
Establishment of Pneumoperitoneum and Placement of Trocars.
-
2.
Abdominal Exploration.
The fundus of the stomach was retracted to expose the hiatus and the degree of hiatus hernia was judged (Fig. 2.2).

Fig. 2.2
-
3.
Division of lower esophagus and bilateral crura.
The right crura were retracted laterally with a roticulated grasper, and peritoneum was incised with Harmonic scalpel. The hernia sac was dissected and totally mobilized in a standard fashion from right to left. Then, the gastrosplenic ligament was divided. The esophagus was retracted upwards, thus exposing the posterior crus of the diaphragm (Fig. 2.3a–o).




Fig. 2.3
-
4.
Division of the cardia and fundus of the stomach.
Then, the gastrocolic and gastrosplenic ligament was divided to free the fundus of the stomach (Fig. 2.4a–j).
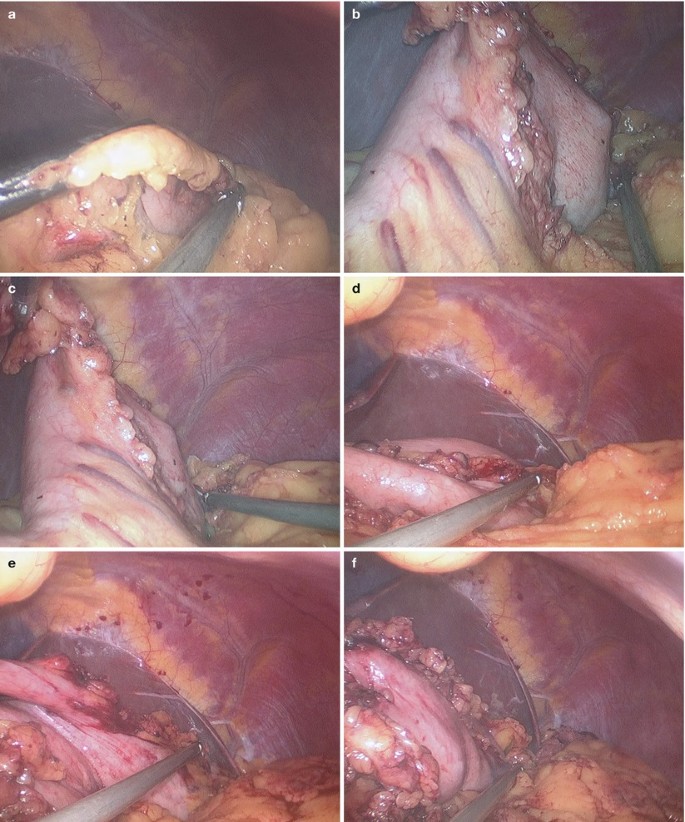

Fig. 2.4
-
5.
Hernioplasty.
After completion of dissection and removal of the whole hernia sac, a gauze strip was introduced into the abdomen and placed behind the esophagus to encircle the esophagus thus exposing the posterior crus of the diaphragm. Prolene (polypropylene) suture w/wo needle was used to close the hiatus to 0.5–1.0 cm (Fig. 2.5a–l).


Fig. 2.5
-
6.
Fundoplication.
The fundus was then brought behind the esophagus creating a 360° wrap. Nissen fundoplication was created the same as conventional laparoscopic multiple-incision procedure. The sutures were tied using the intracorporeal technique. The length of the wrap was approximately 3–4 cm (Fig. 2.6a–j).


Fig. 2.6
-
7.
Placement of the suction drainage.
After adequate flushing of the operative field, a closed suction drain was placed into the surgical field through the umbilical incision (Fig. 2.7a–d).

Fig. 2.7
2.1.7 Tips and Tricks
-
1.
To achieve adequate retraction of the liver, without resorting to the standard trocar placement, some methods have been invented to overcome this obstacle, such as a Jackson–Pratt drainage penetrated with a 2-0 Prolene and fixed with Kelly clamps at the skin, a Penrose drain tied with two silk sutures of 10 cm in length and fixed at the skin or utilizing a 15-cm Veress needle, etc. As described, additional puncture of the abdominal wall or incision is needed during the techniques mentioned above. In our procedure, adequate retraction of the liver was achieved by binding the lateral left lobe of the liver to the diaphragm with Cyanoacrylate glue.
-
2.
The dissection should start with the division of Leimer ligament. The right crura was retracted laterally with a grasper, and peritoneum was incised with harmonic scalpel.
-
3.
The hernia sac should be dissected and totally mobilized by using harmonic ace in a standard fashion from right to left. Esophagus was further mobilized in the thorax, and the gastrosplenic ligament was divided.
2.2 Laparoscopic Heller Myotomy and Dor Fundoplication
2.2.1 Indications and Case Selection
-
1.
Patients with moderate or severe achalasia of cardia.
-
2.
Patients with achalasia of cardia, but it’s ineffective when treated by bouginage of esophagus.
-
3.
Partial fundoplication is indicated for isolated hypertensive lower esophageal sphincter, while Nissen fundoplication should be performed in patients with hypertensive lower esophageal sphincter with GERD/type III hiatal hernia.
2.2.2 Contraindications
-
1.
Absolute contraindications
-
(a)
severe cardiorespiratory dysfunction.
-
(b)
inability to tolerate general anesthesia or pneumoperitoneum.
-
(c)
refractory coagulopathy.
-
(d)
complicated with esophageal malignancies, or documented diffuse esophageal spasm.
-
(a)
-
2.
Relative contraindications
-
(a)
extensive previous surgery, or previous peritonitis leading to severe adhesion.
-
(b)
morbid obesity.
-
(c)
pregnancy (during first and third trimesters).
-
(a)
2.2.3 Major Instruments or Energy Sources
-
1.
Laparoscopy System.
-
2.
Holding forceps, non-traumatic intestinal clamps, needle holder etc.
-
3.
Harmonic scalpel.
2.2.4 Team Setup, Anesthesia and Position
The same as Sect. 2.1.4.
2.2.5 Key Steps
-
1.
Establishment of Pneumoperitoneum and Placement of Trocars.
-
2.
Abdominal Exploration.
-
3.
Division of lower esophagus and bilateral cruras.
-
4.
Division of the cardia and fundus of the stomach.
-
5.
Hernioplasty.
-
6.
Fundoplication.
-
7.
Placement of the suction drainage.
2.2.6 Surgical Techniques
-
1.
Establishment of Pneumoperitoneum and Placement of Trocars.
-
2.
Abdominal Exploration.
The gastroesophageal junction was retracted to expose the cardia and fundus of the stomach. Careful checking for adhesions and ruling out other factors of stenosis were performed (Fig. 2.8a, b).

Fig. 2.8
-
3.
Division of bilateral cruras, lower esophagus and cardia of stomach.
The right crura was retracted laterally with a roticulated grasper, and peritoneum was incised with Harmonic scalpel to expose the right margin of the abdominal segment of the esophagus (Fig. 2.9a–f).

Fig. 2.9
The gastrocolic and gastrosplenic ligament were opened by using the harmonic scalpel to mobilize the fundus of the stomach until the fundus could be folded 180° in front of the esophagus. Continuous mobilization of the left crura was performed to fully free the abdominal segment of the esophagus (Fig. 2.10a–h).


Fig. 2.10
The stomach was retracted downward to expose the esophagus. The adipose tissues on the anterior wall of esophagus and cardia were cleared by using the harmonic scalpel (Fig. 2.11a–c).

Fig. 2.11
-
4.
Heller myotomy.
The esophageal–gastric junction was identified by intraoperative gastroscope. The myotomy was to be 7 cm long, extending 2 cm to the gastric wall, using the Harmonic scalpel. Intraoperative gastroscope was used to assess the adequacy of the myotomy and to detect any esophageal mucosal tears (Fig. 2.12a–k).


Fig. 2.12
-
5.
Dor fundoplication.
The fundus was then brought in front of the esophagus creating a 180° wrap. Dor fundoplication was created the same as conventional laparoscopic multiple-incision procedure. The sutures were tied using the intracorporeal technique (Fig. 2.13a–p).



Fig. 2.13
-
6.
Placement of the suction drainage.
After adequate flushing of the operative field, a closed suction drain was placed into the surgical field through the umbilical incision (Fig. 2.14a, b).

Fig. 2.14
2.2.7 Tips and Tricks
-
1.
The patient should be placed in the supine position with the legs straddled. The surgeon stood between the legs, and the first assistant and the cameraman (second assistant) stood on the right and left side of the patient, respectively.
-
2.
The patient should be placed in an anti-Trendelenburg position and rotated to the right.
-
3.
To achieve adequate retraction of the liver, we binded the lateral left lobe of the liver to the diaphragm using Cyanoacrylate glue as mentioned above.
-
4.
All short gastric vessels were divided to relieve the tension from the wrap.
-
5.
Heller myotomy was performed by incising approximately 6 cm of the abdominal esophagus and 2 cm of the gastric wall to relieve the obstruction.
-
6.
After myotomy, endoscopy confirmed that the esophageal-gastric junction was sufficiently expanded, and the endoscope could be inserted into the stomach without resistance. Leakage of the endoscopic supply of air, which is a sign of mucosal tear, was not identified.
-
7.
Dor fundoplication was subsequently performed. Several sutures with a polypropylene thread to cover the exposed submucosa were used to fix the edge of the esophageal sphincter and fundic wrap.
-
8.
Finally, endoscopy confirmed the absence of large aperture and stenosis at the junction.
2.3 Complications Analysis and Management
2.3.1 Haemorrhage
Bleeding in this procedure is rare and a greater risk of bleeding due to blunt dissection. The small arterial branches that feed the esophagus directly from the aorta or bronchial arteries are rarely the source of major haemorrhage. The greater risk is the violent tearing of venous structures, such as the azygous vein. Ultrasonic scalpel can directly cut off the vessels less than 1.5 mm in diameter. For the larger vessels, Ligasure or firing stapler is necessary for safe and effective hemostasis. Before the end of surgery, haemorrhage should be examined carefully, because there is also a risk of delayed bleeding from the short branches that feed the esophagus directly from the aorta, the short gastric vessels, or from an unrecognized splenic injury.
2.3.2 Esophageal Perforation
For the patient with severe esophagitis or obvious adhesions caused by huge hiatal hernias, esophagus can be damaged during the dissection of esophageal hiatus. Intraoperative repair under laparoscopy is feasible and the incidence of perforation is about 0.1 %. If the drainage is adequate and leak area is limited, fasting and persistent gastrointestinal decompression can be used for postoperative esophageal perforation. Surgery is used for invalid conservative treatment.
2.3.3 Spleen Damage
To avoid postoperative dysphagia caused by tight gastric fundus wrap, adequately dissection of gastric fundus is necessary. When dissecting the short gastric arteries, violently tearing or blindly cutting can induce capsular tears of the spleen. Most of these tears can be controlled with tamponade or electrocautery, but sometimes splenectomy is inevitable.
2.3.4 Esophageal Leak
Because only esophageal mucous layer of lower esophagus and anterior wall of cardia is kept in Heller myotomy, so this part is thin and breakable. Perforation can be induced by intraoperative ultrasonic scalpel thermal damage, which leads to delayed esophageal leak. The major manifestations are postoperative severe abdominal pain, fever and obvious peritonitis. An unrecognized, untreated esophageal perforation is deemed to be uniformly fatal. So, it is important to distinguish the muscular layer when using the ultrasonic scalpel to dissect the esophagus. It is necessary to put the clamping arm of ultrasonic scalpel into the inferior part of dissected muscular layer and keep the working (cutting) arm on the muscular layer surface, cut off the layer under direct vision. Intraoperative gastroscope is not only useful to verify the definite narrow part, but to distinguish perforation. The primary repair of a perforated esophagus without reinforcement is usually inadequate and prone to dehiscence. Therefore the gastric fundus wrap in the fundoplication is advocated to reinforce the primary repair.
2.3.5 Dysphagia
Postoperative dysphagia is the most common complication. The incidence is 17 % at the early stage and can decrease to 4 % after a period of training. Postoperative mucous layer edema may play an important part. If Nissen procedure makes a tight gastric fundus wrap, which may induce an esophageal stenosis, Toupet procedure is alternative. If the dysphagia is not released after 3–4 weeks postoperatively, balloon dilation is an advantage approach with the advantage of non-reoperation.
2.3.6 Postoperative Stenosis Does Not Alleviate
Most dysphagia can be alleviated postoperatively, but some will be not. Non-simple achalasia such as malignant esophageal lesion or diffuse esophageal spasm is not differentiated before the operation. Intraoperative cutting off area of muscular layer includes the entire stenosis part. Inadequate cutting off area can induce postoperative stenosis. Intraoperative gastroscope is significant to inspect the effects of incision and effectively avoid missing stenosis lesion. Postoperative cicatricial contraction and mucous layer edema also can induce dysphagia, and balloon dilation is recommended in these conditions with the advantage of a low rate of complication and non-reoperation.
2.3.7 Gastric Acid Reflux
Because the anti-reflux function of cardia and lower esophagus loses, gastric acid reflux is the most common postoperative complication. The introduction of gastric fundus wrap in the fundoplication can improve the manifestation caused by gastric acid reflux. But according to our observation, though the reflux symptoms can be released to a certain extent, but it can’t be avoided completely.
2.3.8 Other Complications
Pyothorax, pneumothorax, subcutaneous emphysema and mediastinal emphysema are some rare complications of laparoscopic hiatus hernioplasty. But the incidence of these complications has no difference between laparoscopic and open procedure.
References
Campos G, Vittinghoff E, Rabl C, Takata M, Gadenstatter M, Lin F, Ciovica R. Endoscopic and surgical treatments of achalasia: a systemic review and meta-analysis. Ann Surg. 2009;249:45–57.
Rebecchi F, Giaccone C, Farinella E, Campaci R, Morino M. Randomized controlled trial of laparoscopic Heller myotomy plus Dor fundoplication versus Nissen fundoplication for achalasia: long-term results. Ann Surg. 2008;248:1023–30.
Tapper D, Morton C, Kraemer E, Villadolid D, Ross S, Cowgill S, Rosemurgy A. Does concomitant anterior fundoplication promote dysphasia after laparoscopic Heller myotomy? Am Surg. 2008;74:626–34.
Siddiqui MR, Abdulaal Y, Nisar A, Ali H, Hasan F. A meta-analysis of outcomes after open and laparoscopic Nissen’s fundoplication for gastro-oesophageal reflux disease in children. Pediatr Surg Int. 2011;27:359–66.
Ostlie DJ, St Peter SD, Snyder CL, Sharp RJ, Andrews WS, Holcomb 3rd GW. A financial analysis of pediatric laparoscopic versus open fundoplication. J Laparoendosc Adv Surg Tech A. 2007;17:493–6.
Fan Y, Wu SD, Siwo EA. Emergency transumbilical single-incision laparoscopic splenectomy for the treatment of traumatic rupture of the spleen: report of the first case and literature review. Surg Innov. 2011;18:185–8.
Fan Y, Wu SD, Kong J. Single-port access transaxillary totally endoscopic thyroidectomy: a new approach for minimally invasive thyroid operation. J Laparoendosc Adv Surg Tech A. 2011;21:243–7.
Joseph M, Phillips M, Rupp CC. Single-incision laparoscopic cholecystectomy: a combined analysis of resident and attending learning curves at a single institution. Am Surg. 2012;78:119–24.
Huang CK, Houng JY, Chiang CJ, Chen YS, Lee PH. Single incision transumbilical laparoscopic Roux-en-Y gastric bypass: a first case report. Obes Surg. 2009;19:1711–5.
Sakaguchi Y, Ikeda O, Toh Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, Ohga T, Adachi E, Okamura T. New technique for the retraction of the liver in laparoscopic gastrectomy. Surg Endosc. 2008;22:2532–4.
Gianni S, De Luca M, Oscar B, Andrea C, Luca B, David A, Franco F. Veress needle: a simple liver retraction technique for lap band positioning in (single incision laparoscopic technique) SILS. Obes Surg. 2012;22:190–1.
Fan Y, Wu SD, Kong J, Su Y, Tian Y. Transumbilical single-incision laparoscopic fundoplication: a new technique for liver retraction using cyanoacrylate. J Laparoendosc Adv Surg Tech A. 2013;23:356–60.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wu, S., Fan, Y., Tian, Y. (2013). Esophageal Surgery. In: Wu, S., Fan, Y., Tian, Y. (eds) Atlas of Single-Incision Laparoscopic Operations in General Surgery. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6955-7_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-6955-7_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6954-0
Online ISBN: 978-94-007-6955-7
eBook Packages: MedicineMedicine (R0)