Abstract
Endemics are not incidentally or uniformly distributed around the world. We describe and analyse general distribution patterns of endemic vascular plants in different climate zones, ecoregions, and altitudes. We distinguish endemism of vascular plants related to mainland, continental and oceanic islands and different physiognomic habitat types.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Latitudinal, Longitudinal, Radial and Altitudinal Gradients in Endemism
The overall taxonomic diversity of vascular plants is low towards high latitudes and high towards the tropics. This latitudinal gradient is, in general, also true for endemic vascular plant taxa. A large number of processes which might be responsible for latitudinal gradients of species diversity have been discussed and tested. These include processes such as productivity, competition or mutualism, as well as dispersal, genetical isolation, geographical separation, speciation, survival and extinction processes (Jansson 2003; Gaston 1996; Pianka 1966). Thus, it is clear that environmental heterogeneity and contemporary and historical climate parameters are important factors explaining regional to continental patterns of diversity, independent of the fact that the underlying processes are not yet fully understood.
In contrast to latitudinal or altitudinal gradients, longitudinal and radial gradients of species diversity (cf. Huston 1994) are often not recognised or non-existent. However, hardly any biodiversity hotspots and endemic-rich regions are located far from the sea. Exceptions to this rule are found in Asia (Caucasus, Himalaya and SW China; cf. Huang et al. 2011a, b; Mittermeier et al. 2005; Davis et al. 1994, 1995, 1997).
The inverse richness gradients of endemism from the coasts to the central parts of the continents often reflect a climatic gradient of decreasing humidity and increasing amplitude between summer and winter temperature.
Latitudinal and altitudinal patterns and gradients have often been compared with regard to ecology and biodiversity. The richness of all vascular plant taxa declines with latitude and altitude as well, and biogeographers have long recognised that the altitudinal gradient of biodiversity and climate mirrors the latitudinal gradient (Körner 2000). Stevens (1992) has shown that total maximum species diversity along an altitudinal gradient is often found towards the lower end of the elevation. This finding is compatible with the view that tropical climates harbour the highest biodiversity.
However, the mirror does not work perfectly (see Körner 2000 also for differences of latitudinal and altitudinal richness patterns). The alpine zone in mountain areas of the tropics is climatically different from arctic regions in the North or South because, for example, the amplitudes of temperature, light, vegetation period and also precipitation can differ remarkably if one compares average winter and summer or night and day values in these regions. One of the biologically most important differences between the climate of tropical alpine zones and arctic regions is that the tropics have warm days, cold nights and long vegetation periods whereas the winter of arctic regions is cold and long and the vegetation period is short.
In many temperate, subtropical and tropical regions endemism peaks at mid-altitudes; this does not reflect the altitudinal distribution of vascular plants in total which, in general, shows highest diversity at the lower end of the altitudinal gradient.
The interval of maximum endemic species richness – in absolute numbers – in Nepal is between 3,800 and 4,200 m (Vetaas and Grytnes 2002), in SW China 1,000–2,000 m (Huang et al. 2011a, b), in Taiwan 0–1,500 m (Hsieh 2002), in Peru 2,500–3,000 m (Van der Werff and Consiglio 2004), and in Ecuador 1,300–2,500 m (assessment of the list given in Valencia et al. 2000). Endemics of Corsica, in the Mediterranean, are also concentrated at intermediate levels of elevation between 1,000 and 1,800 m (see figure 48 in Hobohm 2000a). In Europe, endemics of rocks and screes are concentrated between 500 and 1,800 m, grassland endemics peak between 550 and 2,100 m, endemics of scrub and heath formations between 200 and 1,550 m, forest endemics between 300 and 1,500 m, endemics of coastal and saline habitats, arable land, ruderal habitats and settlements at altitudes between 0 and 1,200 m, endemics connected to freshwater habitats, mires, swamps, fens and bogs between 100 and 1,750 m (Hobohm and Bruchmann 2009).
We assume that the higher altitude of maximum endemism in contrast to total taxonomic diversity is related to both the stricter separation/isolation of the high mountain zones and the higher total species diversity at lower altitudes which is the basis for evolution and dispersal (cf. Hobohm 2008b; Vetaas and Grytnes 2002). Interestingly, in continental SW China and in Taiwan (Huang et al. 2011a, b; Hsieh 2002) the number of endemics and the total number of vascular plant taxa seem to peak at the same altitudinal zone in the lowlands, whereas in most other regions endemism peaks at higher elevations than overall diversity.
The number of endemics found in an altitudinal zone is also a function of the area and climate of each zone and depends on maximum elevation. These factors might explain the differences in the maxima in the two neighbouring countries Ecuador and Peru. The coastal zone of Ecuador is relatively large relative to the whole size of the country. This zone is part of the Tumbes-Chocó-Magdalena Hotspot (Mittermeier et al. 2005) and very rich in endemics. The altitude of maximum endemism in Peru is more elevated because the coastal zone is small, extremely dry and relatively poor in endemics (Van der Werff and Consiglio 2004).
The rate of endemism (as a percentage of all taxa) in a mountain range normally increases continuously with altitude. This finding is not in conflict with the patterns discussed above showing that absolute numbers of endemics peak at intermediate or lower altitudes in most cases. The increasing rates in high mountain areas are comparable with values for oceanic islands which are highest far distant from the mainland (Fig. 5.1). However, the altitudinal gradient of the proportion of endemics (in %) is normally not mirrored by a latitudinal gradient (Hsieh 2002; Dhar 2002; Vetaas and Grytnes 2002; Hobohm 2000a; Talbot et al. 1999).
Stebbins and Major (1965: 16) analysed the endemic flora of California and concluded that
“…a high degree of endemism is found in those regions having a great variety of plant habitats.”
Hendrych (1982) examined the diversity of endemic plant species in Europe. He concluded that endemics are concentrated in high mountain areas such as the Alps, Sierra Nevada and Greater Caucasus, which are characterised by high habitat diversity.
At regional to continental scales, the diversities of higher plant species and endemic species increase with increasing habitat diversity. Areas with high habitat diversity generally also have high species diversity, although this is not always the case (cf. Cowling and Lombard 2002; Trinder-Smith et al. 1996; Huston 1994).
The main reasons are positive effects such as the number of speciation processes caused by a greater species pool and the avoidance of negative influences, including anthropogenic impacts, which are more effective in habitat-poor than habitat-rich environments. It is more likely that suitable habitats will remain in habitat-rich as compared to habitat-poor regions.
Habitat diversity is not an obligatory precondition for the coexistence of (endemic) species under stable ecological conditions. This finding does not question the positive effects of spatial heterogeneity for endemism. Spatial heterogeneity might be primarily important for the survival of species under changing climate conditions.
On the other hand, it is very easy to find high-elevation and habitat-rich ecoregions with relatively poor endemism. For example, mountain areas in northern North America, South Chile and Argentina, Norway, Central Australia, and the Cape Verde Islands are such regions. All these areas have been influenced by strong climate change, the northern and southern territories by Pleistocene glaciation cycles, Central Australia and the Cape Verde Islands by several dramatic cycles of wet and dry periods during the Quaternary (Crisp et al. 2001; Brochmann et al. 1997; Hendrych 1982).
Size of area, which is a factor often strongly correlated with the diversity of (endemic) species (see e.g. discussion in Körner 2000; Rosenzweig 1995a, b), has similar effects. According to Rosenzweig’s area hypothesis the size of geographic area is responsible for the latitudinal gradient of species diversity. This hypothesis is based on the fact that the tropics, as the largest zone and having a continuous cover stretching into the northern and the southern hemisphere, can host species with larger range sizes, resulting in higher speciation rates and lower extinction rates (cf. Rohde 1992; Rapoport 1982). But, as the discussion of Chown, Gaston and Storch (Chown and Gaston 2000; Storch 2000) has shown, too little is currently known about range sizes of species in a given latitudinal gradient or in a humidity gradient. And actually, such gradients may divide the tropics into a larger number of different wet to dry ecozones than higher latitude areas (e.g. Bailey 1998). We are not aware of any studies that have tried to examine range size differences of vascular plant taxa in relation to area size of different climate zones or ecoregions.
However, area as a driver of biological processes means very little (cf. Ricklefs and Lovette 1999; Buckley 1985; MacArthur and Wilson 1967; Abbott 1977). But, in the context of negative influences on biodiversity in combination with a variety of environmental conditions area becomes important for the probability of the survival of individuals and populations. Survival of the biota in large regions under changing conditions depends on having a sufficient number of patches so that changes in one patch will not affect other patches, and consequently there is only little or no net change over a large area. For example, almost all the mid- and high altitudes of the Alps were covered by Pleistocene glaciers. But, the Alps harbour many endemic vascular plants which survived in the valleys or in the foothills of the Alps close to the Mediterranean Basin. Stability at this scale is a stochastic phenomenon where local and regional processes such as catastrophes, gap dynamics and successions are involved, and is different from the deterministic mathematical equilibrium of the competitive exclusion principle.
2 Oceanic Islands, Continental Islands and Mainland Regions
Oceanic islands are defined by their origin and development within the marine environment. When an oceanic island rises above sea level, colonisation from other islands or mainland regions can begin. All plants which are endemic to an oceanic island must have been developed and evolved in three steps: (i) dispersal of ancestors, which in the case of a great distance is an improbable event, (ii) establishment of a founder-population which normally involves finding adequate ecological conditions and overcoming a genetic bottleneck, and (iii) speciation processes on the basis of a reduced genepool and genetic drift in an environment which is more or less different from the original habitat. Because of reduced dispersal across the sea, oceanic islands are often relatively poor in total species numbers. On the other hand, isolation favours speciation. Thus, the percentage of endemics can be high.
In contrast, continental islands were by definition part of the mainland before they became separated. When they first became islands they were normally already covered by vegetation. This is the reason why continental islands do not normally reach high rates of endemism. Because of the larger species pool the conditions for increasing endemism are favourable compared to oceanic islands, and continental islands, in general, are not poorer in endemic vascular plant taxa than oceanic islands.
Unlike islands, mainland regions do not have clear natural borders, such as a coastline, everywhere. Biogeographical information about countries such as Austria or Switzerland is often much better than information related to mountain ranges which belong to two or several countries, such as the Alps. This is the reason why terrestrial ecoregions are often analysed with a strong focus on countries.
3 Habitats
Endemic vascular plants are found in diverse habitat types in almost all tropical, subtropical, temperate, boreal and arctic climate zones of the world. Tables 5.1, 5.2, 5.3, 5.4, and 5.5 present examples of regions with the respective numbers of endemics and information about the main physiognomic habitat types to be found there. Many of the examples show a relatively high total number of endemic species. This might be a result of the major scientific effort, especially in species-rich areas, and overestimations due to taxonomic double, or even triple, identity or to the revised taxonomic status of species (Kier et al. 2005; Yena 2007). We assume that the scientific effort into biogeography in general is greater where biodiversity is high or unique.
Most regions and landscapes with endemic vascular plants represent more than a single group of physiognomic habitats. Furthermore, many endemics occur in more than a single vegetation unit. Thus, it is very often difficult to determine how many endemics are related to a particular habitat type. Even those scientists who have expert knowledge of a certain region, such as a biodiversity hotspot or an ecoregion, might find it difficult to estimate where endemics are concentrated or which habitat type harbours most endemics.
We were only able to obtain information for a few regions on both the relationship between endemism and habitat type and the importance (size) of different habitat types within the region. These regions are e.g. Europe, Cape Floristic Region, Madagascar, New Caledonia, and New Zealand (cf. Tables 5.1, 5.2, 5.3, 5.4, and 5.5).
In Europe, most endemics are found in habitats with rocks and screes or in grasslands and scrub which together cover much smaller areas than cropland, forest or urban habitats. Most endemics in Europe are basiphytes or indifferent to soil-pH, but acidic substrates are dominant throughout the continent; acidic substrates harbour fewer endemics (Ewald 2003; Hobohm 2008a).
The predominant habitat in the Cape Floristic Region is fynbos. This habitat type harbours the largest number of endemics, almost all of which grow on nutrient-poor and acidic soils.
In New Caledonia, the majority of endemics inhabit wet evergreen forest which nowadays has been reduced to a fifth of its former range. A further large proportion of the endemics is found in the maquis vegetation which covers a third of the archipelago’s area. Endemism on this archipelago is much higher on ultrabasic substrates than elsewhere.
On Madagascar, most endemics are woody plants that inhabit forest and woodland. The majority inhabit tropical and subtropical humid forest. Many others inhabit more or less dry woodlands and thickets. Today, secondary grassland and wooded grassland cover most of the island. These open vegetation types are extremely poor in endemics.
In New Zealand, the majority of the endemics are found in alpine habitats although low and mid-altitude habitats are dominant in the landscape. Forest, woody scrub, wet and aquatic habitats also house many endemics in New Zealand.
In the following we group habitats according to Davies et al. (2004) or Song and Xu (2003), respectively.
3.1 Coastal and Saline Habitats
Many coastal and saline habitats that extend in narrow strips along coasts or shorelines represent very old arrays of environmental conditions. The abiotic factors of certain regions in this transition zone might be as old as the ocean. However, marine currents, winds and migrating birds support dispersal processes which lead to a low likelihood of genetic isolation.
At a regional scale, the database for endemism in these habitats is largely fragmentary.
Analyses of the European endemic flora show that at least 450 endemic species and subspecies are restricted to coastal habitats (Hobohm 2008a; Hobohm and Bruchmann 2009, Hobohm, database EvaplantE in progress).
Van der Maarel and Van der Maarel-Versluys (1996) found that many of the coastal endemics are not necessarily restricted to coastal habitats but that the latter represent their optimal habitat. They found that about 30 % of the endemic species are dune and beach species and another 30 % are species of maritime rocks. It should be noted that this distribution pattern varies greatly, depending on the different coastal regions of Europe, as the coastlines extend from Arctic and Subarctic to Mediterranean regions (further analyses in Van der Maarel and Van der Maarel-Versluys 1996).
The Eastern mangroves of the Indian, Indo-Pacific and Pacific Ocean on the coasts of Indonesia, Madagascar, Southeast Asia and North Australia are more species-rich than western mangroves on the Atlantic Ocean coasts of Africa and America. This might also be the case for endemic species (Schroeder 1998; Wikramanayake et al. 2002). However, at present we can only speculate on this, and further research and data are indispensable.
3.2 Inland Waters, Mires, Bogs and Fens
3.2.1 Overview
Only few comprehensive analyses on endemic vascular plant taxa deal with lakes, rivers, bogs, mires, swamps, or other aquatic habitats. These are, for example, associated with the vernal pools of California, wetlands, mires, bogs, fens and swamps of Europe, aquatic and semiaquatic vegetation on Madagascar and New Zealand, and swamps and other freshwater habitats of the Nile Delta in Egypt (Bruchmann 2011; Hobohm and Bruchmann 2009; McGinley 2008h; Hobohm 2008b; Lazar 2004; Gautier and Goodman 2003; Ranarijaona in Goodman and Benstead 2003; McGlone et al. 2001; Ferry et al. 1999; Davis et al. 1994, 1995, 1997). Information about single endemic plants and their (aquatic or wet) habitats can be found on a number of websites (e.g. IUCN Red List data, www.iucnredlist.org) and many regional floras.
Only a few regions show moderate or high endemism associated with water bodies or wetlands. The vernal pools of California harbour c. 140 endemics in ephemeral freshwater communities and related vegetation types (Lazar 2004; Keeler-Wolf et al. 1998; Zedler 1990; Holland and Jain 1988).
In Baja California only few taxa are associated with water habitats or wetlands, e.g. the palm Brachea armata which is found in permanent oases (Vanderplank in lit.).
In the swamp habitats and other freshwater wetlands of the Nile Delta in Egypt McGinley (2008f) identified 8 endemic species (of a total of 553 plant species).
According to Ranarijaona (in Goodman and Benstead 2003: 251) c. 128 aquatic and semiaquatic vascular plant taxa are endemic to Madagascar. This number is relatively low compared to the total number of endemics on the island (8,000–10,000; see Gautier and Goodman 2003). Ferry et al. (1999) suggest that the low number of endemic aquatic plants on Madagascar may be a result of Quaternary climate fluctuations; during dry periods, freshwater and semi-aquatic habitats were essentially dry, eliminating local floras which depended on wet conditions. The small total area of water bodies and wetlands and the fact that lakes and running waters are normally relatively young and discrete units are not the best conditions for promoting endemism. Compared to other regions in the world the percentage of wetland inhabitants that are endemic to Madagascar is high. Of the 338 Malagasy vascular plants that belong to the flora of aquatic and semiaquatic vegetation, 128 (or 38 %) are endemics. The relatively high ratio can be explained by the fact that Madagascar is most likely the oldest island in the world and separated from the mainland of Africa about 165 million years ago (Goodman and Benstead 2003).
For New Zealand a figure of 264 endemic species is given for wetlands (McGlone et al. 2001). The relatively high number for the much smaller areas of New Zealand (269,000 km2) compared to Europe (275 endemic taxa, 10,500,000 km2) or Madagascar (128 endemic taxa, 587,000 km2) might be explained by less marked effects of climate fluctuations, higher precipitation rates, and the higher proportion of wetland areas in New Zealand in general (De Lange et al. 2006, 2009; Connor 2002; Davis et al. 1994, 1995).
We assume that the global proportion of endemics in wetlands and water bodies is indeed very low (Photos 5.1, 5.2, and 5.3).
In the following we give an overview of the endemics which are associated with the wetlands of Europe.
3.2.2 Wet Habitats of Europe
We here present an analysis based on an earlier publication (Hobohm and Bruchmann2011). The improvement of the list of endemics and discussions with colleagues resulted in a few changes. However, the main result of the earlier publication remain valid.
3.2.2.1 Analysis of Endemism Associated with Wet Habitats in Europe
The area covered here is Europe as defined in Fontaine et al. (2007). We divided Europe into 42 geographical units representing islands or groups of islands, nations, groups of small nations or, in the case of the former Soviet Union, parts of a nation (cf. Bruchmann 2011; Hobohm and Bruchmann 2009; Tutin et al. 1968–1993, see Fig. 5.2). The details are given in Bruchmann (2011).
The data base EvaplantE (cf. Bruchmann 2011; Bruchmann and Hobohm 2010; Hobohm and Bruchmann 2009; Hobohm 2008a) contains information about most endemic vascular plant species or subspecies of Europe. The version EvaplantE 11/2012 shows 6,244 vascular plant taxa as endemics of Europe.
The taxonomic status and our species concept is primarily based on Flora Europaea (Tutin et al. 1968–1993) plus floras and lists of the Canary Islands, the Madeira archipelago and Cyprus (Borges et al. 2008; Izquierdo et al. 2004; Press and Short 1994; Meikle 1977, 1985). Many other regional and national floras were also used to gather information about habitats, ecological conditions, altitudes, and so on (cf. Bruchmann 2011; Hobohm 2008a, b; Kliment 1999).
In general, biogeographical analyses based on field data of plant compositions show some degree of bias. An imbalance in the biogeographical data arises as a result of geographically different perceptions and activities in the field and from the use of different taxonomies (different floras). We tried to minimise this problem by using a broad species concept and international floras and checklists, such as Flora Europaea (Tutin et al. 1968–1993, reprints 1996) and Euro + Med plantbase (cf. e.g. Greuter and Raab-Straube 2012) as primary sources.
We filtered and analysed the recent version of the data base (EvaplantE; version 11/2012) with a focus on European endemic vascular plants occurring under wet conditions or in the succession stages following inundation. We excluded taxa which primarily inhabit coastal habitats such as saltmarshes or rocky habitats near the sea, taxa that only exceptionally occur in wet or inundated habitats, and apomictic microspecies (Alchemilla, Taraxacum, Ranunculus auricomus). Recently, we excluded Marsilea azorica which is conspecific with M. hirsuta from Australia. Thus, this species was reclassified from endemic to neophytic (Schaefer et al. 2011).
Additionally, we verified or corrected the nomenclature and geographical distribution for most taxa on our list using the Euro+Med plantbase on the internet (Euro+Med 2006–2011). In a few cases, this procedure had the effect that taxa changed their designation from endemic to subendemic because they have since been found in North African countries, such as Morocco, Algeria, Tunesia, or in parts of western Asia. These taxa were also eliminated from the list.
The analysis is founded on absolute numbers of taxa per region and descriptive statistics. As a first step, we summed up numbers of species or subspecies for the whole of Europe, and numbers of species and subspecies per region. The regions are of different sizes. At the moment it is impossible to reliably determine numbers of European endemic vascular plant taxa in relation to habitat for an artificially defined grid cell. This is the reason why certain statistical methods cannot be applied (see also the discussions of species-area and endemics-area relationships, e.g. in He and Hubbell 2011; Dengler 2009; Werner and Buszko 2005; Green and Ostling 2003). Preliminary investigations, for example, showed a negative correlation between area and endemism of vascular plants in Europe (log-log-space, all endemics) because many small regions in the South of Europe show high endemism, whereas very large regions in Scandinavia and Russia are poor in endemics. Further problems and restrictions related to the statistics are discussed in Bruchmann (2011).
If we compare numbers of regions of the same area then we can use these values as direct measurements for the density of endemics (Bruchmann 2011). Also in other cases we can compare density values directly (see Chap. 2). In our division of Europe this allows a direct comparison of density values of many pairs of regions.
3.2.2.2 Endemic Vascular Plants in Wetlands of Europe
The data base EvaplantE comprises at least 275 endemic plant taxa – species and subspecies – which occur more or less regularly in wetland communities (Table 5.6). All of these are restricted to the boundaries of Europe as defined in Fontaine et al. (2007). This number is a minimum value because c. 20 % of the listed taxa in EvaplantE have not yet, or have only inadequately, been characterized in relation to ecological conditions or habitat.
However, compared with endemics of other habitat types the number of 275 is low (Bruchmann 2011).
Only few of the taxa listed are hydrophytes living in standing or running waters. Many more taxa are not strongly associated with wet habitats and occur in both wet and other habitats (Hobohm and Bruchmann 2011, Table 5.6).
Figure 5.2 shows distribution patterns of endemic vascular plants related to wet habitats. The numbers in the South-west of Europe are higher than in the North-east. The numbers for a triangle-shaped region between Spain, former Yugoslavia and Germany are much higher than for the rest of Europe. The highest absolute numbers of taxa were found in France and Spain. Austria, Italy and Germany also have high numbers.
We assume that this fact reflects a combination of processes and conditions which favour endemism in general and endemism of wet habitats in particular (e.g. oceanic climate, high precipitation rate, humidity). Southern France is located within and between two high-mountain ranges with high environmental heterogeneity, the Pyrenees and the Alps. The country is also located between two marine environments which influence and stabilise the climate. Therefore, three major climate regimes occur in France: Mediterranean, temperate Atlantic and high-mountain climate. Mainland France is connected with other species-rich regions – e.g. the Iberian Peninsula, the Alps, Italy. Some of the Pleistocene refugia are located in the country or not far away. Thus, migration distances after glacial cycles might have been relatively short (Medail and Diadema 2009; Krebs et al. 2004).
The East of Europe shares many taxa with the West of Asia. Many landscapes, habitat types and ecological conditions are quite similar West and East of the border between Europe and Asia. The southern and south-eastern part of the continental border, in particular, is artificially defined (along the narrow Bosporus and along the Ural River). Thus, the marginal regions bordering Asia necessarily have fewer endemics than they would have if these regions were located in another part of Europe. This applies particularly to the European part of Turkey, which is rather small.
These factors together might explain the relatively high numbers of European endemic vascular plant taxa related to wet habitats in France and neighbouring countries (cf. Rull 2004; Rosenzweig 1995a, b) and relatively low numbers to the East. However, this cannot easily been verified at present.
Table 5.6 shows the result of all (250) pairwise comparisons of the density of endemism (E/A) that can be calculated directly. Some regions, such as the Madeira archipelago, Corsica, Switzerland, Austria, Italy, Germany, mainland Spain and France are only to be found in the left column. These countries show relatively high density values. Others occur only in the right column: Regions such as Svalbard and the eastern, northern and central divisions of the former Soviet Union have relatively low density values. All other regions occur in both columns.
The comparison shows that the density of endemics increases roughly from the continental regions in eastern Europe to the more oceanic regions towards the west. However, Switzerland has a higher density value than Ireland. Austria, the Czech Republic and Slovakia have higher values than Great Britain.
The highest density values on a N-S gradient are in general not represented by the Mediterranean regions, e.g. Austria has a higher density than Portugal, Greece or former Yugoslavia, and even the Faroe Islands towards the North-west have a higher density than the Canary Islands, Crete, or Cyprus, to the South. In general, temperate regions do not have lower values than Mediterranean regions. However, the boreal-arctic regions towards the north, such as Svalbard, Iceland, Finland and northern Russia, have extremely low values.
We obtained altitudinal range data for 156 taxa on the list. Wetland endemics occur at all altitudes between sea level and alpine zones. The average of the minima (median) is 300 m above sea level, the average of the maxima 1,800 m. This means that most endemics occur in the montane and subalpine zones. Gentiana bavarica occurs in damp places, e.g. moors with spring water, wet alpine meadows, and snow patches. This species represents the absolute maximum of 3,600 m above sea level in our list.
Forty-one endemics are basiphytes, 31 acidophytes. Many species are indifferent to pH or have not yet been characterized (Table 5.7).
3.3 Forest and Woodland
Many publications cited in Tables 5.1, 5.2, 5.3, 5.4, and 5.5 name forest or woodland as the only or as one of the main habitat types – often in the warm-temperate and tropical zones of the world. Data for the boreal-arctic and cold temperate zones is scarce and seldom provides quantified information on patterns of endemism.
In the coastal forest of the Eastern Africa Biodiversity Hotspot many endemics have been recorded from forest habitats. However, it is difficult to give quantified data because numbers for the areas studied diverge considerably between sources. While Burgess et al. (1998, 2003, 2004) state that of the 1,750 strictly endemic species 554 species are confined to the coastal forest habitat and 812 species inhabit the surrounding non-forested habitats, Mittermeier et al. (2005) claim that some 1,225 (about 70 % of all endemic species in the hotspot region) have been recorded from forest.
Approximately one half of the 1,605–1,957 endemic species within the Chilean-Winter-Rainfall-Valdivian-Forest Hotspot belong to shrub and forest ecosystems, which constitute 50 % of the whole area (Mittermeier et al. 2005).
In some biodiversity hotspots with high endemism, such as Wallacea or the Guinean forests of West Africa (Tables 5.3 or 5.5, respectively), tropical rain forest is also predominant (Mittermeier et al. 2005). In the wet tropics of Mesoamerica many endemic species inhabit rain forest, cloud forest and montane forest (e.g. Mittermeier et al. 2005) but it was not possible to find specific numbers at smaller scales for this region. However, according to Gentry (1986) in montane regions of Central America and especially the Andes, local endemism seems to result mostly from a veritable explosion of speciation in relatively few taxa, mostly of shrubs, herbs and epiphytes. These groups constitute almost half of the neotropical flora. Many of them are not associated with forest but can also be found there. And they account for most of the excess floristic diversity of the Neotropics compared with the Paleotropics. In lowland Amazonia endemism is prevalent to habitat islands. Most of the taxa involved are canopy trees and lianas, with derivative species in specialised habitats such as white sands or seasonally inundated swamp forests. In some cases, it is clear that local endemic habitat specialists are derived from wide-ranging ancestors of the terra firme forest. Habitat specialisation has obviously been the prevalent evolutionary pathway of giving rise to local endemics in Amazonia (Gentry 1986).
On New Caledonia (Table 5.5) endemism peaks at an extraordinarily high level, with the highest percentage numbers reached in humid evergreen and sclerophyllous forest (Lowry 1998; Morat 1993; Schneckenburger 1991).
Some authors claim that the wet tropics or the tropical rain forest harbour most (endemic) vascular plant species (e.g. Mittermeier et al. 2005; Parmentier et al. 2007). Very often, tropical rainforest is named as the centre of endemism, with extraordinarily high endemism; however, this assumption should be checked carefully, as results from several ecoregions show a contrary trend. For the Northern Territory of Australia, for example, Woinarski et al. (2006) showed that rain forest-associated species have less propensity for endemism (602 species with 41 endemics) than species associated with other habitats (3,611 species with 526 endemics). Knowledge of the occurrence, distribution patterns, and diversity of endemic vascular plants in forest ecosystems (all layers) is quite limited and sometimes fragmentary. The analyses of Kreft et al. (2004) indicate that knowledge of the structure and diversity of neotropical forests was for a long time based on incomplete floristic inventories which misjudged large groups of vascular plants.
3.4 Scrub, Heath and Succulent Shrubland
Shrub-dominated communities may either be a mature vegetation type (e.g. in subalpine zones or some types of drylands) or may occur as temporary communities (successional stages) that develop following intense disturbances or degradations of woodland or forest. If disturbances such as fire, clearing, or heavy grazing regimes are regular phenomena then the adapted shrub vegetation may remain stable over a long period of time, even if forest could grow there.
Several sources state that Mediterranean climate and semi-desert ecosystems in Southern Africa, Southwest Australia, California, the Mediterranean Basin, and Mexico harbour a high number of endemic vascular plants. Many endemic-rich areas of the world have been found to be areas of shrubland vegetation.
In the Cape Floristic Region the highest numbers of endemic taxa are mostly found in the dominant fynbos vegetation (Mittermeier et al. 2005; Davis et al. 1994; Cowling 1992).
On New Caledonia the second largest group of endemics is associated with maquis vegetation (Lowry 1998; Morat 1993; Schneckenburger 1991).
We assume that the ecological conditions in many shrub-dominated landscapes promote endemism, especially under soil conditions and precipitation rates that are relatively stable over long evolutionary time periods (Desmet 2007; Ojeda et al. 2001; Wisheu et al. 2000; Cowling and Hilton-Taylor 1999; Cowling et al. 1992, 1994, 1996). Strong influences of the major ocean surface currents in the neighbourhood might favour high climatic constancy in these regions; thus relatively low precipitation totals (including mist) in some shrub- and dwarf-shrub dominated semi-desert and Mediterranean-climate areas are highly predictable (Hobbs et al. 1995) (Photos 5.8 and 5.9).
3.5 Grassland and Habitats Dominated by Forbs, Mosses or Lichens
As Mucina and Rutherford stated, “the term grassland is one of the most used, misused and abused terms of vegetation ecology” … (Mucina and Rutherford 2006: 350, see also Gibson 2009). In fact, the term grassland is often used as a generic term irrespective of the huge variety of environmental parameters, the different patterns of floristic or plant functional compositions, habitat extension and interface structures. Here we define grassland as a group of physiognomic habitats dominated by herbs and grasses which are not used as cropland. Sometimes shrubs or single trees occur but these cover less than 10 % of the area. This definition includes steppes, grassy savannas and paramo, and is consistent with other physiognomic classification systems (cf. EUNIS classification; Davies et al. 2004; White 1983).
Suttie et al. (2005) describe grassland ecosystems as one of the originally largest ecosystems in the world; estimates of the extent of this biome range from 31 to 40.5 % of the world’s terrestrial area, depending on the definition of the term ‘grassland’. Nowadays, large areas of grassland habitats are declining in quality and quantity, and some ecosystems are even dramatically threatened by extinction (Cremene et al. 2005; Mackay 2002; Dömpke and Succow 1998; Breymeyer 1990). Therefore, it is necessary that the great variety, and thus diversity, of grassland ecosystems should receive more attention than in the past (Photos 5.10, 5.11, 5.12, and 5.13).
The characteristic aspects of grassland-dominated ecosystems are widely influenced by their different developmental history. In the past, grassland was stabilised naturally either by specific climatic conditions such as low summer precipitation and winter drought, by periodical fire events, or by strong grazing regimes. Such habitats might be very old and thus characterised by long ecological continuity. On the other hand, there is the young semi-natural grassland that could potentially become forest if anthropogenic influences (cutting, burning, grazing, mowing) were less intensive (e.g. many grasslands at low- and mid altitudes in Europe).
The recent studies on occurrence of habitat specialists in grassland ecosystems show that, along with habitat persistence at the Holocene scale (Hájek et al. 2011), nutrient availability is also an important factor underlying specialist occurrence, while habitat-specialized grassland species tend to occur in nutrient-poor habitats (Fajmonová et al. 2012).
Mucina and Rutherford (2006) showed that some of the endemic-rich habitats of South Africa are linked to high altitude regions such as Drakensberg or Wolkensberg. The Drakensberg Alpine region, which is dominated by grassland, harbours about 334 endemic taxa (Mucina and Rutherford 2006) and for this reason it has been named a Centre of Plant Endemism (Van Wyk and Smith 2001). For many high altitude regions of the world, endemic-rich grassland such as alpine meadow, is mentioned in the literature, most often in connection with stony or rocky habitats (e.g. European Alps, Carpathians, Mountains of Central Asia and others). In the European flora, the group of grassland endemics is the second largest and comprises many more taxa than e.g. forest ecosystems; this is not an area-effect because the area size of forest is much larger than that of grassland (Hobohm 2008a; Hobohm and Bruchmann 2009).
Beard et al. (2000) listed 241 endemic species for the savanna landscape of the Northern Province of Western Australia.
In summary, grasslands around the world are inhabited by a huge amount of endemics. Because this type of habitat is globally decreasing in area and quality many of the endemics are threatened with extinction (cf. Smolenice Grassland Declaration; EDGG 2010, and Hohhot Declaration 2008; on the internet).
3.6 Rock and Scree Habitats
3.6.1 Overview
Rock and scree habitats are found all over the world. Rocky habitats are dominant in many high mountain zones, in landscapes with steep slopes, and in coastal and desert regions. In these habitats differences in ecological conditions (light, water, wind speed, etc.) are much higher within small distances – a few meters – than in other habitat categories, e.g. grassland or forest. This also means that the impact of climate change might be small compared to habitats which are less heterogeneous (cf. Leuzinger et al. 2011).
The environmental conditions of rock and scree vegetation seem to have been relatively constant through time. Erosion and sedimentation patches are narrowly meshed. To find adequate ecological conditions under the pressure of climate change (glaciation periods and global warming) adapted vegetation types and endemic species would simply have to move a few hundred metres up or down the mountains (vertical displacement, Rull 2004). Rock and scree habitats as stepping stones that support the survival probability of the (endemic) species can be found at almost all altitudes of mountain ranges.
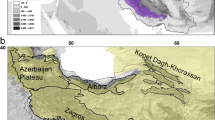
In the datasets analysed (Tables 5.1, 5.2, 5.3, 5.4, and 5.5) these habitats were not named very often as host areas for endemic species. On the other hand, in Europe, and possibly in many boreal-arctic and temperate regions worldwide, these are the habitats with very high or even the highest endemism. Studies on the endemic flora of Europe show that rocky habitats and screes represent the majority (>2,772 of c. 6,250–6,500 taxa) that are known to be endemic to Europe (Hobohm and Bruchmann 2009, data base EvaplantE in progress). In the Alps, 35–60 % of the 350–400 endemics species are found in habitats with rocks and screes, while in the Carpathians, which are not as high, only 10–15 % of 100–120 endemic species inhabit rocky habitats (Hobohm 2008a; Casazza et al. 2005, 2008; Dullinger et al. 2000; Pawlowski 1969). However, in Europe the highest endemism is confined to a habitat type which only represents a small percentage of the whole area (no exact data available yet). There is also data available on the endemic flora of the alpine regions of Iran (Noroozi et al. 2008) that reports a relationship between endemics and scree habitats. Talbot et al. (1999) list some endemic species of arctic regions that live in rock- and scree-dominated tundra vegetation.
Similarly high concentrations may exist in mountain areas of the tropics (e.g. the tropical Andes with about 15,000 endemic species) but no quantified data on endemics was found which could prove this assumption (Photo 5.14).
3.6.2 Comparison of High Endemism in Rocky Habitats with Low Endemism in Wetlands
In general, the number of endemics in wetlands is low compared to the numbers for rocky habitats and screes, grasslands, or scrub and heath landscapes (e.g. Bruchmann 2011; Bruchmann and Hobohm 2010 for Europe).
This fact should also be discussed in the context of zonal, extrazonal and azonal vegetation types. Walter (1954) defined zonobiomes as zonal vegetation types that are controlled by climate, orobiomes as different belts of mountain ranges, and pedobiomes as soil-dependent vegetation types that are azonal. For example, boreal spruce forest belongs to zonal vegetation, and aquatic vegetation, swamps, fens, bogs and riparian forest belong to azonal vegetation (cf. e.g. Bohn et al. 2000, 2003; Bailey 1998). However, the relationship between zonality and endemism is not clear. Some azonal vegetation types are very rich in endemics. Serpentine soils, for example, often give rise to sparse associations with many endemic plants (Chiarucci and Baker 2007; Alexander et al. 2007; Stevanović et al. 2003; Roberts and Proctor 1992). Rocky habitats which are strongly affected by both climate and substrate also harbour many endemic plants (Bruchmann 2011). In contrast, azonal vegetation types such as aquatic vegetation, reeds or bogs are normally poor in endemics (e.g. Parolly 2004; Meusel and Jäger 1992).
Wetlands cover a small part (a few percent) of the world’s surface (Hobohm and Bruchmann 2009, 2011; Revenga et al. 2000). This is also true for rocks and screes which are inhabited by far more endemic taxa than is the case for wetlands. Thus, the differences in endemism cannot be explained by the size of area because both habitat types represent vegetation types which cover only a very small proportion of the earth.
In contrast to rocky habitats, most freshwater habitats, mires and swamps are very young, often much younger than 10,000 years. These habitats are characterised by low ecological continuity during the late Pleistocene and Holocene. Many lakes, for example, originated as dead ice holes during the late Pleistocene and silted up or developed into fens, bogs or peaty substrates covered by forest during the Holocene (Pott 2010; Hobohm and Bruchmann 2009). Furthermore, sudden changes in the physico-chemical conditions can impact whole water bodies. Perhaps as an adaptation to this, many aquatic and wetland plants, such as Alisma plantago-aquatica, Eleocharis acicularis, Lemna minor, Phalaris arundinacea or Phragmites communis, have long-distance dispersal abilities (wind, migratory birds) and very large ranges of distribution (Meusel and Jäger 1992; Meusel et al. 1978, 1965; Hultén 1971). In Europe, even the endemics of wetlands have, on average, larger ranges than endemics of rocks and screes (Hobohm and Bruchmann 2009). The relationship between the distribution patterns of specialists and refugial history of mires in the West Carpathians and Bulgaria, for example, has been shown by Hájek et al. (2011) and Horsák et al. (2007).
Rocky habitats, in general, are composed of different micro-habitats with very different environmental conditions with respect to light, water, organic material, soil, dynamics, etc. Under changing conditions, inhabitants of rocky habitats can normally find suitable survival conditions very close to the place where they are located. The higher rates of endemism here might be due to the relatively fixed structural complexity of their environment, compared with the dynamics of the lotic and lentic aquatic environment (see Scherrer and Körner 2011). Therefore, we assume that rocky habitats and screes are less affected by changing physico-chemical conditions than standing and running waters, banks, mires and swamps.
3.7 Arable, Horticultural and Artificial Habitats
In Europe a few regional endemics (e.g. Anthemis lithuanica, Bromus secalinus ssp. multiflorus, Bromus interruptus, Carduus litigiosus, Centaurea polymorpha, Erucastrum gallicum, and Urtica atrovirens; see Tutin et al. 1996a, b, c, d, e) might be largely restricted to anthropogenic habitats, such as arable land or ruderal habitats, and so are not expected to occur in natural or seminatural habitats. Discussing the fact that certain plant species only occur in anthropogenic habitats, Gams (1938) and Pignatti (1978, 1979) explored the possibility that plant evolution is influenced by, or dependent on, human activities. Another explanation for the existence of endemic taxa in anthropogenic habitats could be that the original habitats of these taxa were destroyed, while associated endemics survived under similar environmental conditions. A combination of both explanations might also be correct.
413 European endemics occur in this group of relatively young but widespread habitats (Hobohm and Bruchmann 2009). However, we did not find much information about endemics in anthropogenic habitats outside of Europe.
Because of the duration of human influence we expect that this group of endemics might also exist in Africa and perhaps in Asia, but most probably neither in Australia nor in the Americas.
According to the IUCN Red List c. 63 vascular plant species on earth are extinct in the wild and still survive in horticulture. Rauer et al. (2000) have discussed the role of botanic gardens in protecting threatened vascular plant taxa (Photos 5.15 and 5.16).
References
Abbott I (1977) Species richness, turnover and equilibrium in insular floras near Perth, Western Australia. Aust J Bot 25:193–208
Adamson DA, Selkirk JM, Seppelt RD (1993) Serpentinite, harzburgite, and vegetation on subantarctic Macquarie Island. Arctic Alpine Res 25:216–219
Akpulat HA, Celik N (2005) Flora of gypsum areas in Sivas in the eastern part of Cappadocia in Central Anatolia, Turkey. J Arid Environ 61:27–46
Alexander EB, Coleman RG, Keeler-Wolf T, Harrison S (2007) Serpentine geoecology of western North America: geology, soils, and vegetation. Oxford University Press, New York
Allison A, Eldridge LG (2004) Polynesia-Micronesia. URL: http://multimedia.conservation.org/cabs/online_pubs/hotspots2. Downloaded 15 October 2012
Anádon-Irizarry V, Wege DC, Upgren A, Young R, Boom B, León YM, Arias Y, Koenig K, Morales AL, Burke W, Perez-Leroux A, Levy S, Koenig S, Gape L (2012) Sites for priority biodiversity conservation in the Caribbean Islands Biodiversity Hotspot. J Threatened Taxa 4(8):2806–2844
Ashworth AC, Vestal WD, Hokanson G, Joseph L, Martin M, McGlynn K, Newbrey MG, Schlecht N, Turnbull J, White A, Zimmerman T (2000) Tristan da Cunha Island Group and Gough Island. www.ndsu.edu/subantarctic. Last updated 2001
Ashworth AC, Vestal WD, Hokanson G, Joseph L, Martin M, McGlynn K, Newbrey MG, Schlecht N, Turnbull J, White A, Zimmerman T (2004) Biota Australis Terrestris. www.ndsu.edu/instruct/ashworth/subantarctic/index.htm. Last updated 01/2004.
Atamuradov H, Fet GN, Fet V, Valdez R, Feldman W (1999) Biodiversity, genetic diversity, and projected areas in Turkmenistan. J Sustain For 9:73–88
Bailey RG (1998) Ecoregions: the ecosystem geography of the oceans and continents. Springer, New York
Barthlott W, Mutke J, Rafiqpoor D, Kier G, Kreft H (2005) Global centers of vascular plant diversity. Nova Acta Leopoldina NF 92(342):61–83
Beard JS, Chapman AR, Gioia P (2000) Species richness and endemism in the Western Australian flora. J Biogeogr 27:1257–1268
Bhandari NN (1979) Phytogeography of the tropical flora of the Indian Desert. In: Larsen K, Holm-Nielsen LB (eds) Tropical botany. Academic, London, pp 143–152
Blom A (2001) Mount Cameroon and Bioko montane forests (AT0121). In: Word Wildlife Fund (ed) Wild world. URL: http://www.worldwildlife.org/wildworld/profiles/terrestrial/at/at0121_full.html
Bohn U, Neuhäusel R, Gollub G, Hettwer C, Neuhäuslová Z, Schlüter H, Weber H (2000/2003) Karte der natürlichen Vegetation Europas/Map of the natural vegetation of Europe. Maßstab/scale 1:2500000. Teil 1: Erläuterungstext mit CD-ROM. -Landwirtschaftsverlag, Bonn
Bolay E (1997) The Dominicain Republic. A country between rain forest and desert. Contributions to the ecology of a Caribbean Island. Markgraf, Weikersheim
Borges PAV, Abreu C, Aguiar AMF, Carvalho P, Jardim R, Melo I, Oliveira P, Sergio C, Serrano ARM, Vieira P (eds) (2008) A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. Direccao Regional do Ambiente da Madeira and Universidade dos Acores, Funchal und Angro do Heroismo
Breckle S-W (2000) Biodiversität von Wüsten und Halbwüsten. Berichte der RTG 12:207–222
Breymeyer AI (ed) (1990) Managed grasslands (Ecosystems of the world 17A). Elsevier, Amsterdam
Brochmann C, Rustan OH, Lobin W, Kilian N (1997) The endemic vascular plants of the Cape Verde Islands, W. Africa. Sommerfeltia 24. Botanical Garden and Museum, University of Oslo, Oslo, 356pp
Bruchmann I (2011) Plant endemism in Europe: spatial distribution and habitat affinities of endemic vascular plants. Dissertation, University of Flensburg, Flensburg. www.zhb-flensburg.de/dissert/bruchmann/bruchmann_endemism.pdf
Bruchmann I, Hobohm C (2010) Halting the loss of biodiversity: endemic vascular plants in grassland of Europe. Grassland Sci Eur 15:776–778
Buckley RC (1985) Distinguishing the effects of area and habitat type on island species richness by separating floristic elements and substrate types and controlling for island isolation. J Biogeogr 12:527–535
Burga CA, Zanola S (2007) Madagaskar – Hot Spot der Biodiversität. Exkursionsbericht und Landeskunde. Schriftenreihe Physische Geographie Bodenkunde und Biogeographie 55:1–201
Burga CA, Klötzli F, Grabherr G (eds) (2004) Gebirge der Erde. Ulmer, Stuttgart, p 504 S
Burgess ND, Clarke GP, Rodgers WA (1998) Coastal forests of eastern Africa: status, endemism patterns and their potential causes. Biol J Linn Soc 64:337–367
Burgess N, Doggart N, Doody K, Negussie G, Sumbi P, Perkin A (2003) New information on the lowland coastal forests of Eastern Africa. Oryx 37:280–281
Burgess N, D’Amico Hales J, Underwood E, Dinerstein E, Olson D, Itoua I, Schipper J, Ricketts T, Newmann K (2004) Terrestrial ecoregions of Africa and Madagascar. Island Press, Washington, DC
Cable S, Cheek M (eds) (1998) Plants of mount Cameroon: a conservation checklist. Kew Publishing, Cumbria
Callmander MW, Phillipson PB, Schatz GE, Andriambololonera S, Rabarimanarivo M, Rakotonirina N, Raharimampionona J, Chatelain C, Gautier L, Lowry II, Porter P (2011) The endemic and non-endemic vascular flora of Madagascar updated. Plant Ecol Evol 144:121–125
Carbonao E, Lozano-Contreras G (1997) Endemismos y otras singularidades de la Sierra Nevada de Santa Marta, Colombia. Posibles causas de origen y necesidad de conservarlos. Revista de la Academia Colombiana de ciencias exactas, fisicas y naturales 21:409–419
Carbutt C, Edwards TJ (2006) The endemic and near-endemic angiosperms of the Drakensberg Alpine Centre. S Afr J Bot 72:105–132
Casazza C, Barberis G, Minuto L (2005) Ecological characteristics and rarity of endemic plants of the Italian Maritime Alps. Biol Conserv 123:361–371
Casazza G, Zappa E, Mariotti MG, Medail F, Minuto L (2008) Ecological and historical factors affecting distribution pattern and richness of endemic plant species: the case of the maritime and Ligurian Alps hotspot. Divers Distrib 14:47–58
Caujape-Castells J, Tye A, Crawford DJ, Santos-Guerra A, Sakai A, Beaver K, Lobin W, Vincent Florens FB, Moura M, Jardim R, Gomes I, Kueffer C (2010) Conservation of oceanic island floras: present and future global challenges. Perspect Plant Ecol Evol Syst 12:107–129
Chiarucci A, Baker AJM (2007) Advances in the ecology of serpentine soils. Plant Soil 293:1–2
Chown SL, Gaston KJ (2000) Reply from S.L. Chown and K.J. Gaston. In: Cleveland CJ (ed) Encyclopedia of earth. Trends Ecol Evol 15(12):514. URL: http://www.eoearth.org
Clark VR, Barker NP, Mucina L (2008) The Sneeuberg: a new centre of floristic endemism on the Great Escarpment, South Africa. S Afr J Bot 75:196–238
Clough LD (2008) Peninsula Valdés, Argentina. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Pennsula Valds, Argentina
Connor HE (2002) Regional endemism in New Zealand grasses. New Zeal J Bot 40(1):189–200
Conservation International, Duffy JE (eds) (2008) Biological diversity in Japan. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www-eoaerth.org/article/Biological_diversity_in_Japan. Downloaded 20.10.2012
Cooper J, Ryan PG (1994) Management plan for the Gough Island Wildlife Reserve. Government of Tristan da Cunha, Edinburgh
Cowling RM (ed) (1992) The ecology of Fynbos. Nutrients, fire and diversity. Oxford University Press, Cape Town
Cowling RM, Hilton-Taylor CI (1999) Plant geography, endemism and diversity. In: Richard W, Dean J, Milton SJ (eds) The Karoo: ecological patterns and processes. Cambridge University Press, Cambridge, pp 42–56
Cowling RM, Lombard AT (2002) Heterogeneity, speciation/extinction history and climate: explaining regional plant diversity patterns in the Cape Floristic Region. Divers Distrib 8:163–179
Cowling RM, Holmes PM, Rebelo AM (1992) Plant diversity and endemism. In: Cowling RM (ed) The ecology of Fynbos. Nutrients, fire and diversity. Oxford University Press, Oxford, pp 62–112
Cowling RM, Witkowski ETF, Milewski AV, Newbey KR (1994) Taxonomic, edaphic and biological aspects of narrow plant endemism on matched sites in Mediterranean South-Africa and Australia. J Biogeogr 21:651–664
Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianoutsou M (1996) Plant diversity in Mediterranean-climate regions. Trends Ecol Evol 11:362–366
Cremene C, Groza G, Rakosy L, Schileyko A, Baur A, Erhardt A, Baur B (2005) Alterations of steppe-like grasslands in Eastern Europe: a threat to regional biodiversity hotspots. Conserv Biol 19:1606–1618
Cribb P, Hermans J (2009) Field guide to the orchids of Madagascar. Kew Publishing, Kew
Crisp MD, Laffan S, Linder HP, Monro A (2001) Endemism in the Australian flora. J Biogeogr 28:183–198
Daniels FJA, Elvebakk A, Talbot SS, Walker DA (eds) (2005) Classification and mapping of arctic vegetation. Phytocoenologia 35(4):715–1079
Davies CE, Moss D, Hill MO (2004) EUNIS habitat classification, revised 2004. Report to European Environment Agency. European Topic Centre on Nature Protection and Biodiversity. Paris
Davis SD, Heywood VH, Hamilton AC (eds) (1994) Centres of plant diversity, vol 1, Europe, Africa, South West Asia and the Middle East. IUCN Publications, Unit, Cambridge
Davis SD, Heywood VH, Hamilton AC (eds) (1995) Centres of plant diversity, vol 2, Asia, Australasia and the Pacific. IUCN Publications, Unit, Cambridge
Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J, Hamilton AC (eds) (1997) Centres of plant diversity, vol 3, The Americas. IUCN Publications, Unit, Cambridge
De Lange PJ, Sawyer JWD, Jensen CA (2006) New Zealand indigenous vascular plant checklist. New Zealand Plant Conservation Network, Wellington, 94pp
De Lange PJ, Norton DA, Courtney SP, Heenan PB, Barkla JW, Cameron EK, Hitchmough RA, Townsend AJ (2009) Threatened and uncommon plants of New Zealand. New Zeal J Bot 47(1):61–96
Dengler J (2009) Which function describes the species-area relationship best? A review and empirical evaluation. J Biogeogr 36:728–744
Desmet PG (2007) Namaqualand – a brief overview of the physical and floristic environment. J Arid Environ 70:570–587
Dhar U (2002) Conservation implications of plant endemism in high-altitude Himalaya. Curr Sci 82:141–148
Dömpke S, Succow M (1998) Cultural landscapes and nature conservation in Northern Eurasia. NABU, Bonn
Duarte MC, Rego F, Romeiras MM, Moreira I (2008) Plant species richness in the Cape Verde islands – eco-geographical determinants. Biodivers Conserv 17:453–466
Dullinger S, Dirnböck T, Grabherr G (2000) Reconsidering endemism in the North-eastern Limestone Alps. Acta Botanica Croatica 59:55–82
EDGG (ed) (2010) Smolenice grassland declaration. Bull Eur Dry Grassland Group 7:7
Euro+Med (2006–2011) Euro+Med PlantBase – the information resource for Euro-Mediterranean plant diversity. URL: www.bgbm.org/EuroPlusMed/. Downloaded 2/2012
Ewald J (2003) The calcareous riddle: why are there so many calciphilous species in the central European flora? Folia Geobot 38:357–366
Fajmonová Z, Zelený D, Syrovátka V, Vončina G, Hájek M (2012) Distribution of habitat specialists in semi-natural grasslands. J Veg Sci. doi:10.1111/jvs.12005
Ferry L, Robinson L, Ranarijaona H, Gasse F (1999) Les Lacs de Madagascar: présentation et typologie. Rapport Laboratoire Hydrologie, Montpellier
Fleischmann K (1997) Invasion of alien woody plants on the islands of Mahe and Silhouette, Seychelles. J Veg Sci 8:5–12
Fleischmann K, Héritier P, Meuwly C, Küffer C, Edwards PJ (2003) Virtual gallery of the vegetation and flora of the Seychelles. Bull Geobot Inst ETH 69:57–64
Florence J, Lorence DH (1997) Introduction to the flora and vegetation of the Marquesas Islands. Allertonia 7:226–237
Fontaine B, Bouchet P, Van Achterberg K, Alonso-Zarazaga MA, Araujo R, Asche M, Aspock U, Audisio P, Aukema B, Bailly N, Balsamo M, Bank RA, Barnard P, Belfiore C, Bogdanowicz W, Bongers T, Boxshall G, Burckhardt D, Camicas JL, Chylarecki P, Crucitti P, Davarveng L, Dubois A, Enghoff H, Faubel A, Fochetti R, Gargominy O, Gibson D, Gibson R, Gomez Lopez MS, Goujet D, Harvey MS, Heller K-G, Van Helsdingen P, Hoch H, De Jong H, De Jong Y, Karsholt O, Los W, Lundqvist L, Magowski W, Manconi R, Martens J, Massard JA, Massard-Geimer G, Mcinnes SJ, Mendes LF, Mey E, Michelsen V, Minelli A, Nielsen C, Nieto Nafria JM, Van Nieukerken EJ, Noyes J, Papa T, Ohl H, De Prins W, Ramos M, Ricci C, Roselaar C, Rota E, Schmidt-Rhaesa A, Segers H, Zur Strassen R, Szeptycki A, Thibaud J-M, Thomas A, Timm T, Van Tol J, Vervoort W, Willmann R (2007) The European Union’s 2010 target: putting rare species in focus. Biol Conserv 139:167–185
Foster MN, Brooks TM, Cuttelod A, De Silva N, Fishpool LDC, Radford EA, Woodley S (2012) The identification of sites of biodiversity conservation significance: progress with the application of a global standard. J Threatened Taxa 4(8):2733–2744
Francisco-Ortega J, Wang Z-S, Wang F-G, Xing F-W, Liu H, Xu H, Xu W-X, Luo Y-B, Song X-Q, Gale S, Boufford DE, Maunder M, An S-Q (2010) Seed plant endemism on Hainan Island: a framework for conservation actions. Bot Rev 76(3):346–376
Fuwu X, Telin W, Zexian L, Huagu Y, Binghui C (1995) Endemic plants of Hainan Island. J Trop Subtrop Bot 3:1–12
Gamisans J, Marzocchi J-F (1996) La flore endémique de la Corse. Edisud, Aix-en-Provence
Gams H (1938) Die nacheiszeitliche Geschichte der Alpenflora. Jahrbuch der Vereinigung zum Schutze der Alpenpflanzen und -tiere 10:9–34
Gaston KJ (ed) (1996) Biodiversity: a biology of numbers and difference. Blackwell Science, Oxford
Gautier L, Goodman SM (2003) Introduction to the flora of Madagascar. In: Goodman SM, Benstedad JP (eds) The natural history of Madagascar. The University of Chicago Press, Chicago/London, pp 229–250
Gentry AH (1986) Endemism in tropical versus temperate plant communities. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer Associates, Inc.-Publisher, Sunderland, pp 153–182
Ghazanfar SA (2004) Biology of the central desert of Oman. Turk J Bot 28:65–71
Gibson DJ (2009) Grasses and grassland ecology. Oxford University Press, Oxford
Gillespie RG, Clague DA (eds) (2009) Encyclopedia of islands. University Press of California, Berkeley
Goodman SM, Benstead JP (eds) (2003) The natural history of Madagascar. The University of Chicago Press, Chicago/London
Grainger J (2003) ‘People are living in the park’. Linking biodiversity conservation to community development in the middle east region: a case study from the Saint Katherine Protectorate, Southern Sinai. J Arid Environ 54:29–38
Green JW (1985) Census of vascular plants of western Australia, vol 2. W.A. Herbarium, Department of Agriculture, Perth
Green JL, Ostling A (2003) Endemics-area relationships: the influence of species dominance and spatial aggregation. Ecology 84(11):3090–3097
Greuter W, von Raab-Straube E (2012) Euro+Med Notulae, 6. Willdenowia 42:283–285
Groombridge B, Jenkins MD (2002) World atlas of biodiversity: earth’s living resources in the 21st century. University of California Press, Berkeley
Hájek M, Horsák M, Tichý L, Hájková P, Dítě D, Jamrichová E (2011) Testing a relict distributional pattern of fen plant and terrestrial snail species at the Holocene scale: a null model approach. J Biogeogr 38:742–755
He F, Hubbell S (2011) Species-area relationships always overestimate extinction rates from habitat loss. Nature 473:368–371
Hendrych R (1982) Material and notes about the geography of the highly stenochoric to monotopic endemic species of the European flora. Acta Universitatis Carolinae-Biologica 335–372
Hobbs RJ, Richardson DM, Davis GW (1995) Mediterranean-type ecosystems: Opportunities and constraints for studying the function of biodiversity. In: Davis, GW, Richardson DM (eds) Mediterranean-type ecosystems. Ecol Stud 109:1–42. Springer, Berlin/Heidelberg/New York
Hobohm C (2000a) Biodiversität. Quelle & Meyer, Wiebelsheim
Hobohm C (2000b) Plant species diversity and endemism on islands and archipelagos, with special reference to the Macaronesian Islands. Flora 195:9–24
Hobohm C (2004) Ökologische Aspekte der Vielfalt endemischer Pflanzenarten in Europa unter besonderer Berücksichtigung großflächiger Beweidungsmaßnahmen als Instrumentarium für den Arten- und Biotopschutz. Schriftenreihe für Landschaftspflege und Naturschutz 78:281–292
Hobohm C (2008a) Ökologie und Verbreitung endemischer Gefäßpflanzen in Europa. Tuexenia 28:7–22
Hobohm C (2008b) Gibt es endemische Gefäßpflanzen in Mooren Europas? Mitt AG Geobot in Schl-Holst und Hambg 65:143–150
Hobohm C, Bruchmann I (2009) Endemische Gefäßpflanzen und ihre Habitate in Europa - Plädoyer für den Schutz der Grasland-Ökosysteme. Berichte der Reinh-Tüxen-Gesellschaft 21:142–161
Hobohm C, Bruchmann I (2011) Are there endemic vascular plants in wet habitats of Europe? Transylvanian Rev Syst Ecol Res 12:1–14
Holland RF, Jain SK (1988) Vernal pools. In: Barbour MJ, Major J (eds) Terrestrial vegetation of California, Special Publication 9. California Native Plant Society, Sacramento, pp 515–531
Holmgren M, Poorter L (2007) Does a ruderal strategy dominate the endemic flora of the West African forests? J Biogeogr 34:1100–1111
Honeycutt RL, McGinley M (2007) Rapa Nui and Sala-y-Gomez subtropical broadleaf forests. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Rapa_Nui_and_Sala-y-Gomez_subtropical_broadleaf_forests
Honeycutt RL, McGinley M (2008) Samoan tropical moist forests. In: Encyclopedia of earth. URL: http://www.eoearth.org/article/Samoan_tropical_moist_forests
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650
Horsák M, Hájek M, Dítě D, Tichý L (2007) Modern distribution patterns of snails and plants in the Western Carpathian spring fens: is it a result of historical development? J Molluscan Stud 73:53–60
Hsieh CF (2002) Composition, endemisms and phytogeographical affinities of the Taiwan flora. Taiwania 47(4):298–310
Huang T-C (ed) (1993–2003) Flora of Taiwan, vols 1–6. National Taiwan University, Department of Botany, Taipai
Huang J-H, Chen B, Ying J-S, Ma K (2011a) Features and distribution patterns of Chinese endemic seed plant species. J Syst Evol 49(2):81–94
Huang J-H, Chen B, Liu C, Lai J, Zhang J, Ma K (2011b) Identifying hotspots of endemic woody seed plant diversity in China. Divers Distrib:1–16
Huber O (1995) Vegetation. In: Berry PE, Holst BK, Yatskievych K (eds) Flora of the Venzuelan Guayana. Missouri Botanical Garden and Timber Press, St. Louis, pp 97–160
Hultén E (1971) Atlas över växternas utbredning i Norden. Generalstabens Litografiska Anstalts Förlag, Stockholm
Huston MA (1994) Biological diversity. Cambridge University Press, Cambridge
Ito M (1998) Origin and evolution of endemic plants of the Bonin (Ogasawara) Islands. Res Popul Ecol 40:205–212
Izquierdo I, Martin JL, Zurita N & Arechavaleta M (eds) (2004) Lista de especies silvestres de Canarias (hongos, plantas y animales terrestres) 2004. Consejeria de Medio Ambiente y Ordenacion Territorial, Gobierno de Canarias
Jansson R (2003) Global patterns in endemism explained by past climatic change. Proc R Soc Lond 270:583–590
Jonsell B, Karlsson T (2004) Endemic vascular plants in Norden. In: Jonsell B (ed) Flora Nordica. General volume. Bergius Foundation, Stockholm, pp 139–159
Juste JB, Fa JE (1994) Biodiversity conservation in the Gulf of Guinea islands: taking stock and preparing action. Biodivers Conserv 3:759–771
Kalla J (2003) Submission for nomination of Tropical Rainforsést Heritage of Sumatra. URL: http://whc.unesco.org/uploads/nominations/1167.pdf
Keeler-Wolf T, Elam DR, Lewis K, Flint SA (1998) California vernal pool assessment: preliminary report, 161pp
Khan TI (1997) Biodiversity conservation in the Thar Desert, with emphasis on endemic and medicinal plants. Environmentalist 17:283–287
Kier G, Mutke J, Dinerstein E, Ricketts TH, Kuper W, Kreft H, Barthlott W (2005) Global patterns of plant diversity and floristic knowledge. J Biogeogr 32:1107–1116
Kim KOK, Hong SH, Lee YH, Na CS, Kang BH, Son Y (2009) Taxonomic status of endemic plants in Korea. J Ecol Field Biol 32(4):277–293
Kliment J (1999) Komentovaný prehlád vyšších rastlín flóry Slovenska, uvádzaných v literatúre ako endemické taxóny. Bull Slov Bot Spoločn Bratislava 21(suppl 4):1–434
Körner C (2000) Why are there global gradients in species richness? Mountains might hold the answer. Trends Ecol Evol 15(12):513
Krebs P, Conedera M, Pradella M, Torriani D, Felber M, Tinner W (2004) Quaternary refugia of the sweet chestnut (Castanea sativa Mill.): an extended palynological approach. Veg Hist Archaeobot 13:145–160
Kreft H, Köster N, Küper W, Nieder J, Barthlott W (2004) Diversity and biogeography of vascular epiphytes in Western Amazonia, Yasuni, Ecuador. J Biogeogr 31:1463–1476
Kruckeberg AR (1992) Plant life of western North America ultramafics. In: Roberts BA, Proctor J (eds) The ecology of areas with serpentinized rocks: a world view. Kluwer Academic Publishers, Dordrecht, pp 31–74
Lawesson JE, Hamann O, Rogers G, Reck G, Ochoa H (eds) (1990) Botanical research and management in Galapagos, vol 32, Monographs in systematic botany from the Missouri Botanical Garden. Missouri Botanical Garden, St Louis, 301pp
Lazar KA (2004) Characterization of rare plant species in the vernal pools of California. Master thesis, University of California, 344pp. www.vernalpools.org/documents/LazarThesisFinal.pdf
Lee WG (1992) New Zealand ultramafics. In: Roberts BA, Proctor J (eds) The ecology of areas with serpentinized rocks: a world view. Kluwer Academic Publishers, Dordrecht, pp 375–417
Lepping O, Daniels FJA (2006) Phytosociology of beach and saltmarsh vegetation in northern West Greenland. Polarforschung 76(3):95–108
Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C (2011) Do global change experiments overestimate impacts on terrestrial ecosystems? TREE 26:236–241
Lowry Il PP (1998) Diversity, endemism, and extinction in the flora of New Caledonia: a review. In: Peng CI, Lowry Il PP (eds) Rare, threatened, and endangered floras of Asia and the Pacific Rim, Academia Sinica Monograph Series No. 16. pp 181–206
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princetown University Press, Princetown
Mackay R (2002) The atlas of endangered species: threatened plants and animals of the world. Earthscan, London
Major J (1988) Endemism: a botanical perspective. In: Myers AA, Giller PS (eds) Analytical biogeography. Chapman & Hall, London, pp 117–146
McGinley M (2007a) Bermuda subtropical conifer forests. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Bermuda_subtropical_conifer_forests
McGinley M (2007b) Caatinga. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Caatinga
McGinley M (2007c) Florida sand pine scrub. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Florida_sand_pine_scrub
McGinley M (2007d) Galapagos Islands xeric scrub. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Galpagos Islands xeric scrub
McGinley M (2007e) Mediterranean conifer and mixed forests. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Mediterranean_conifer_and_mixed_forests
McGinley M (2007f) San Félix-San Ambrosio Islands temperate forests. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/San Flix-San Ambrosio Islands temperate forests
McGinley M (2007g) Sierra Madre del Sur pine-oak forests In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Sierra_Madre_del_Sur_pine-oak_forests
McGinley M (2008a) Badkhiz-Karabil semi-desert. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Badkhiz-Karabil_semi-desert
McGinley M (2008b) Garamba National Park, Democratic Republic of Congo. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Garamba_National_Park,_Democratic_Republic_of_Congo
McGinley M (2008c) Gough Island Wildlife Reserve, United Kingdom. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Gough_Island_Wildlife_Reserve,_United_Kingdom
McGinley M (2008d) Granitic Seychelles forests. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Granitic_Seychelles_forests
McGinley M (2008e) Mount Emei and Leshan Giant Buddha, China. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Mount_Emei_and_Leshan_Giant_Buddha,_China
McGinley M (2008f) Nile Delta flooded savanna. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Nile_Delta_flooded_savanna
McGinley M (2008g) Tepuis. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/tepuis
McGinley M (2008h) Venezuelan Andes montane forests. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Venezuelan_Andes_montane_forests
McGinley M (2008i) Moja desert. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Mojave_Desert
McGinley M (2009) Dong Phayayan Khao-Yai Forest Complex, Thailand. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Dong_Phayayan_Khao-Yai_Forest_Complex,_Thailand
McGlone MS, Duncan RP, Heenan PB (2001) Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. J Biogeogr 28:199–216
Médail F, Diadema K (2009) Glacial refugia influence plant diversity patterns in the Mediterranean basin. J Biogeogr 36:1333–1345
Médail F, Verlaque R (1997) Ecological characteristics and rarity of endemic vascular plants from Southeastern France and Corsica: implications for biodiversity conservation. Biol Conserv 80:269–281
Meikle RD (1977/1985) Flora of Cyprus. The Bentham-Moxon Trust Royal Botanic Gardens, Kew
Meusel H, Jäger EJ (1992) Vergleichende Chorologie der zentraleuropäischen Flora. Text u. Karten. Bd. 3. Gustav Fischer Verlag, Stuttgart/New York
Meusel H, Jäger EJ, Weinert E (1965) Vergleichende Chorologie der zentraleuropäischen Flora. Text u. Karten. Bd. 1. VEB Fischer, Jena
Meusel H, Jäger EJ, Rauschert SW, Weinert E (1978) Vergleichende Chorologie der zentraleuropäischen Flora. Text u. Karten. Bd. 2. VEB Fischer, Jena
Miller AG, Morris M (2004) Ethnoflora of Soqotra Archipelago. Royal Botanic Garden, Edinburgh
Ministry for Natural Resources and the Environment (ed) (2007) National report on the status of biodiversity in S. Tomé and Principe. URL: www.cbd.int/doc/world/st/st-nr-03-en.pdf, 109pp
Mittermeier RA, Gil PR, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, da Fonseconda GAB (2005) Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions. Cemex, Mexico City
Moat J, Smith P (eds) (2007) Atlas of the vegetation of Madagascar. Kew Publishing, Kew
Morat P (1993) Our knowledge of the flora of New Caledonia: endemism and diversity in relation to vegetation types and substrates. Biodivers Lett 1:72–81
Moustafa A, Zaghloul MS (1996) Environment and vegetation in the montane Saint Catherine area, south Sinai, Egypt. J Arid Environ 34:331–349
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria
Mueller-Dombois D, Fosberg FR (1998) Vegetation of the tropical Pacific Islands. Springer, New York
Myers N (1988) Threatened biotas: hotspots in tropical forests. Environmentalist 8:1–20
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Natori Y, Kohri M, Hayama S, De Silva N (2012) Key biodiversity areas identification in Japan Hotspot. J Threatened Taxa 4(8):2797–2805
Nau C (2003) Das Insel-Lexikon. Heel Verlag, Barcelona, 360S
Noroozi J, Akhani H, Breckle SW (2008) Biodiversity and phytogeography of alpine flora of Iran. Biodivers Conserv 17:493–521
Nowak A, Nobis M (2010) Tentative list of endemic vascular plants of the Zeravshan Mts in Tajikistan: distribution, habitat preferences and conservation status of species. Biodivers Res Conserv 19:65–80
Ojeda F, Simmons M, Arroyo J, Marañón T, Cowling RM (2001) Biodiversity in South African fynbos and Mediterranean heathland. J Veg Sci 12:867–874
Owiunji I, Nkuutu D, Kujirakwinja D, Liengola I, Plumptre AJ, Nsanzurwimo A, Fawcett K, Gray M, McNeilage A (2005) The biodiversity of the Virunga Volcanoes. URL: http://programs.wcs.org/portals/49/media/file/Volcanoes_Biodiv_survey.pdf. Downloaded 3/1/2012
Parks and Wildlife Service (2006) Macquarie island nature reserve and world heritage area management plan. Parks and Wildlife Service, Hobart
Parmentier I, Malhi Y, Senterre B, Whittaker RJ, Alonso A, Balinga MPB, Bakayoko A, Bongers FJJM, Chatelein C, Comiskey J et al (2007) The odd man out? Might climate explain the lower tree α-diversity of African rain forests relative to Amazonian rain forests? J Ecol 95:1058–1071
Parolly G (2004) The high mountain vegetation of Turkey – a state of the art report, including a first annotated conspectus of the major syntaxa. Turk J Bot 28:39–63
Pawar SS, Birand AC, Ahmed MF, Sengupta S, Shankar Raman TR (2007) Conservation biogeography in North-East India: hierarchical analysis of cross-taxon distributional congruence. Divers Distrib 13(1):53–65
Pawlowski B (1969) Der Endemismus in der Flora der Alpen, der Karpaten und der Balkanischen Gebirge im Verhältnis zu den Pflanzengesellschaften. Mitteilungen der ostalpin-dinarischen pflanzensoziologischen Arbeitsgemeinschaft 9:167–178
Pennington RT, Lewis GP, Ratter JA (eds) (2006) Neotropical savannas and seasonally dry forests. Plant diversity, biogeography, and conservation. CRC Press, Boca Raton
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100:33–46
Pignatti S (1978) Evolutionary trends in Mediterranean flora and vegetation. Vegetatio 37:175–185
Pignatti S (1979) Plant geographical and morphological evidences in the evolution of the Mediterranean flora (with particular reference to the Italian representatives). Webbia 34:243–255
Plumptre AJ, Davenport TRB, Behangana M, Kityo R, Eilu G, Ssegawa P, Ewango C, Meirte D, Kahindo C, Herremans M et al (2007) The biodiversity of the Albertine Rift. Biol Conserv 134:178–194
Pott R (2010) Klimawandel im System Erde. Berichte der RTG 22:7–33
Pott R, Hüppe J, Wildpret de la Torre W (2003) Die Kanarischen Inseln. Ulmer, Stuttgart
Press JR, Short MJ (eds) (1994) Flora of Madeira. HMSO, London
Pryce M, Mitchell S, Burke A, McKenzie C, Stirling S, Ryan J, Simpson W, McGlashan D (2008) Jamaica: country report to the FAO international technical conference on plant genetic resources for food and agriculture. URL: www.moa.gov.jm/jam/jamaica2.pdf. Kingston, 59pp
Pyak AI, Shaw SC, Ebel AL, Zverev AA, Hodgson JG, Wheeler BD, Gaston KJ, Morenke MO, Revushkin AS, Kotukhov YA, Oyunchimeg D (2008) Endemic plants of the Altai mountain country. Wild Guides, Hampshire
Rabitsch W, Essl F (eds) (2009) Endemiten – Kostbarkeiten in Österreichs Pflanzen- und Tierwelt. Umweltbundesamt, Klagenfurt, Wien
Rapoport EH (1982) Areography: geographical strategies of species. Bergamon Press, New York
Rauer G, Ibisch PL, von den Driesch M, Lobin W, Barthlott W (2000) The convention on biodiversity and botanic gardens. In: Bundesamt für Naturschutz (ed) Botanic gardens and biodiversity. Landwirtschaftsverlag, Münster, pp 25–64
Raven PH, Axelrod DI (1978) Origin and relationships of the California flora. University of California Press, Berkeley
Republica de Cuba (ed) (2009) IV informe nacional al convenio sobre la diversidad biologica. URL: www.cbd.int/doc/world/cu/cu-nr-04-es.pdf, 162pp
Revenga C, Brunner J, Henninger N, Kassem K, Payne R (eds) (2000) Pilot analysis of global ecosystems: freshwater systems. World Resources Institute, Washington, DC
Ricklefs RE, Lovette IJ (1999) The roles of island area per se and habitat diversity in the species-area relationships of four Lesser Antiellean faunal groups. J Anim Ecol 68:1142–1160
Riemann H, Ezcurra E (2005) Plant endemism and natural protected areas in the Peninsula of Baja California, Mexico. Biol Conserv 122:141–150
Riemann H, Ezcurra E (2007) Endemic regions of the vascular flora of the peninsula of Baja California, Mexico. J Veg Sci 18:327–336
Roberts BA, Proctor J (eds) (1992) The ecology of areas with serpentinized rocks: a world view. Kluwer Academic Publishers, Dordrecht
Robertson SA (1989) Flowering plants of the Seychelles. Royal Botanic Gardens, Kew
Rohde K (1992) Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65:514–527
Rosenzweig ML (1995a) Species diversity gradients: we know more and less than we thought. J Mammol 73:715–730
Rosenzweig ML (1995b) Species diversity in space and time. Cambridge University Press, Cambridge
Rull V (2004) Biogeography of the ‘Lost World’: a palaeoecological perspective. Earth Sci Rev 67:125–137
Schaefer H, Carine MA, Rumsey FJ (2011) From European priority species to invasive weed: Marsilea azorica (Marsileaceae) is a misidentified alien. Syst Bot 36(4):845–853
Schäfer H (2005a) Endemic vascular plants of the Azores: an updated list. Hoppea 66:275–283
Schäfer H (2005b) Flora of the Azores. Markgraf Publishers, Weikersheim
Schatz GE (2001) Generic tree flora of Madagascar. Royal Botanic Gardens/Missouri Botanical Garden, Kew/St. Louis
Scherrer D, Körner C (2011) Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J Biogeogr 38:406–416
Schneckenburger S (1991) Neukaledonien. Palmengarten, Sonderheft 16: 78pp
Schroeder F-G (1998) Lehrbuch der Pflanzengeographie. Quelle & Meyer, Wiesbaden
Şekercioğlu ÇH, Anderson S, Akçay E, Bilgin R, Can ÖE, Semiz G, Tavşanoğlu Ç, Yokeş MB, Soyumert A, İpekdal K, Sağlam İK, Yücel M, Nüzhet Dalfes H (2011) Turkey’s globally important biodiversity in crisis. Biol Conserv 144:2752–2769
Shou-qing Z (1988) The vulnerable and endangered plants of Xishuangbanna Prefecture, Yunnan Province, China. Arnoldiana 48(2):2–7
Song Y-C, Xu G-S (2003) A scheme of vegetation classification of Taiwan, China. Acta Botanica Sinica 45(8):883–895
Stebbins GL, Major J (1965) Endemism and speciation in the California flora. Ecol Monogr 35:2–35
Sterrer W (1998) How many species are there in Bermuda? Bull Mar Sci 62:809–840
Stevanović V, Tan K, Iatrou G (2003) Distribution of the endemic Balkan flora on serpentine I: obligate serpentine endemics. Plant Syst Evol 242(1):149–170
Stevens GC (1992) The elevation gradient in altitudinal range: an extension of Rapoport’s latitudinal rule to altitude. Am Nat 140:893–911
Steyermark JA (1986) Speciation and endemism in the flora of the Venezuelan Tepuis. In: Vuilleumier F, Monasterio M (eds) High altitude tropical biogeography. Oxford University Press, New York, pp 317–373
Storch D (2000) Rapoport effect and speciation/extinction rates in the tropics. Trends Ecol Evol 15(12):514
Suttie JM, Reynolds SG, Batello C (2005) Grasslands of the world. Food and Agriculture Organisation of the United Nations, Rome
Talbot SS, Yurtsev BA, Murray DF, Argus GW, Bay C, Elvebakk A (1999) Atlas of rare endemic vascular plants of the arctic. In: Conservation of Arctic flora and fauna (CAFF). U.S. Fish and Wildlife Service, p. 73
Tokumaru H (2003) Nature conservation on Yakushima Island: Kagishima Prefecture’s efforts. Global Environ Res 7(1):103–111
Tordorff AW, Baltzer MC, Fellowes JR, Pilgrim JD, Langhammer PF (2012) Key biodiversity areas in the Indo-Burma Hotspot: process, progress and future directions. J Threatened Taxa 4(8):2779–2787
Trinder-Smith TH, Cowling RM, Linder HP (1996) Profiling a besieged flora: endemic and threatened plants of the Cape Peninsula, South Africa. Biodivers Conserv 5:575–589
Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA (1993 [1996a]). Flora Europaea, 2nd edn, vol 1, Psilotaceae-Platanaceae. Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (1968 [1996b]) Flora Europaea, vol 2, Rosaceae-Umbelliferae. Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (1968 [1996c]) Flora Europaea, vol 3, Diapensiaceae-Myoporaceae. Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (1976 [1996d]) Flora Europaea, vol 4, Plantaginaceae-Compositae (and Rubiaceae). Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (1980[1996e]) Flora Europaea, vol 5, Alismataceae-Orchidaceae. Cambridge University Press, Cambridge
United Nations Environment Programme, Clough LD (eds) (2008) Yakushima (Yaku Island), Japan. In: Cleveland CJ (ed) Encyclopedia of earth. URL: http://www.eoearth.org/article/Yakushima. Downloaded 20.10.2012
Valencia R, Pitman N, León-Yánez S, Jørgensen PM (eds) (2000) Libro Rojo de las Plantas Endémicas del Ecuador 2000. Publicaciones del Herbario QCA, Ponticicia Universidad Católica del Ecuador, Quito
Van der Maarel E, Van der Maarel-Versluys M (1996) Distribution and conservation status of littoral vascular plant species along the European coasts. J Coast Conserv 2:73–92
Van der Werff H, Consiglio T (2004) Distribution and conservation significance of endemic species of flowering plants in Peru. Biodivers Conserv 13:1699–1713
Van Wyk AE, Smith GF (2001) Regions of floristic endemism in southern Africa: a review with emphasis on succulents. Umdaus Press, Pretoria
Vetaas OR, Grytnes J-A (2002) Distribution of vascular species richness and endemic richness along the Himalayan elevation gradient in Nepal. Glob Ecol Biogeogr 11:291–301
Villarreal-Quintanilla JA, Encina-Dominguez JA (2005) Endemic vascular plants of Coahuila and adjacent areas, Mexico. Acta Botanica Mexicana 70:1–46
Vogiatsakis I (ed) (2012) Mediterranean mountain environments. Wiley-Blackwell, Oxford
Wagner WL, Lorence DH (1997) Studies of Marquesan vascular plants: introduction. Allertonia 7:221–225
Wagner WL, Herbst DR, Lorence DH (2005) Flora of the Hawaiian Islands – online. Smithsonia National Museum of History: http://ravenel.si.edu/botany/pacificislandbiodiversity/hawaiianflora/index.htm
Walter H (1954) Klimax und zonale Vegetation. Angewandte Pflanzensoziologie, Festschrift Aichinger 1:144–150
Werner U, Buszko J (2005) Detecting biodiversity hotspots using species-area and endemics-area relationships: the case of butterflies. Biodivers Conserv 14:1977–1988
Whistler A (2011) Rare plants of Samoa. Conservation International, Apia
White F (1983) The vegetation of Africa: a descriptive memoir to accompany the UNSECO/AEFTAT/UNSO vegetation map of Africa. United Nations Educational, Scientific and Cultural Organization, Paris
Wikramanayake E, Dinerstein E, Loucks CJ, Olson DM, Morrison J, Lamoreux J, McKnight M, Hedao P (2002) Terrestrial ecoregions of the Indo-Pacific. A conservation assessment. Island Press, Washington, DC
Wisheu IC, Rosenzweig ML, Olsvig-Whittaker L, Shmida A (2000) What makes nutrient-poor mediterranean heathlands so rich in plant diversity? Evol Ecol Res 2:935–955
Woinarski JCZ, Hempel C, Cowie I, Brennan K, Kerrigan R, Leach G, Russell-Smith J (2006) Distributional pattern of plant species endemic to the Northern Territory, Australia. Aust J Bot 54:627–640
World Wildlife Fund (ed) (2011) Tongan tropical moist forests. The Encyclopedia of Earth. URL: http://www.eoearth.org/article/Tongan_tropical_moist_forests. Downloaded 15 July 2012
WRI (World Resources Institute) (2003) Earth Trends. Country Profiles. URL: http://www.eoearth.org (downloaded for many regions; May-October 2012)
Yena AV (2007) Floristic endemism in the Crimea. Fritschiana 55:1–8
Zedler PH (1990) Life histories of vernal pool vascular plants. In: Ikeda DH, Schlising RA (eds) Vernal pool plants: their habitat and biology, Studies from the herbarium no. 8. California State University, Chico, pp 123–146
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Hobohm, C., Janišová, M., Jansen, J., Bruchmann, I., Deppe, U. (2014). Biogeography of Endemic Vascular Plants – Overview. In: Hobohm, C. (eds) Endemism in Vascular Plants. Plant and Vegetation, vol 9. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6913-7_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-6913-7_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6912-0
Online ISBN: 978-94-007-6913-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)