Abstract
The use and popularity of maggot therapy (MT) – the treatment of wounds with live fly larvae – is increasing rapidly in many countries throughout the world. The advantages of MT, also called larval therapy, maggot debridement therapy (MDT), and biosurgery, include its profound efficacy in debriding necrotic tissue, its relative safety, and its simplicity. These factors, along with other advantages such as its efficiency, its low cost and its effectiveness even in the context of antibiotic-resistant infections, have been responsible for the recent revival in the use of MT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
The use and popularity of maggot therapy (MT) – the treatment of wounds with live fly larvae – is increasing rapidly in many countries throughout the world. The advantages of MT, also called larval therapy, maggot debridement therapy (MDT), and biosurgery, include its profound efficacy in debriding necrotic tissue, its relative safety, and its simplicity. These factors, along with other advantages such as its efficiency, its low cost and its effectiveness even in the context of antibiotic-resistant infections, have been responsible for the recent revival in the use of MT.
The end of the twentieth century witnessed the development of antibiotic resistance to some of the most potent antimicrobials yet created. Ironically (or perhaps as a consequence), the end of the century also witnessed the health care community, once again, embracing the maggot – a creature that thrives in the presence of bacteria, putrefaction and “filth.”
Maggot therapy is often used when conventional medical and surgical treatments fail to arrest the progressive tissue destruction and heal the wound. The poor blood supply to the deep wound and the consequent inability of immunological mediators and systemic antibiotics to reach the infected area prevent healing. For review see: Thomas et al. (1996), Church (1999), Sherman et al. (2000), Mumcuoglu (2001), Nigam et al. (2006a, b, 2010). Internet sites dealing with the subject of MDT are: BioTherapeutics Education and Research (BTER) Foundation; International Biotherapy Society; and World Wide Wounds.
2.2 History
For centuries, maggot-infested wounds have been associated with decreased infection, faster healing and increased survival. The beneficial effect of fly larvae for wounds was first observed by Ambroise Paré in the sixteenth century. While working with soldiers wounded and left on the battlefield for several days, Baron Larrey (physician-in-chief to Napoleon’s armies) and Dr. Joseph Jones (a medical officer during the American Civil war), both described their observations of maggots cleaning the soldiers’ wounds without destroying the viable tissue.
J.F. Zacharias, one of the Confederate Surgeons during the American Civil War, may even have facilitated the deposition of fly eggs on his wounded soldiers (Fleischmann et al. 2004). In non-Western civilizations, the intentional application of maggots for wound healing may date back even earlier (Pechter and Sherman 1983; Church 1996; Whitaker et al. 2007).
The earliest first-hand account of fly larvae intentionally applied for wound care was by William S. Baer, Chief of Orthopedic Surgery at Johns Hopkins Hospital in Baltimore (Baer 1929). As a military surgeon during World War I, he too witnessed the beneficial effects of maggot-infested wounds. He treated over 100 children, while observing the effects of maggots on chronic osteomyelitis (bone infection) and soft tissue wounds. He developed practical methods for keeping the larvae on wounds, and documented the mechanisms of action involved, i.e. debridement, disinfection and growth stimulation (Baer 1929, 1931). After witnessing serious wound infections, even in maggot-treated wounds, he concluded that medicinal maggots should be disinfected, and therefore developed methods for chemical disinfection (Baer 1931). Soon thereafter, thousands of surgeons were using Baer’s maggot treatment and over 90 % were pleased with their results (Robinson 1935a). The pharmaceutical company Lederle Laboratories (Pearl River, NY; taken over by Wyeth in 1994 and by Pfizer in 2009) commercially produced “Surgical Maggots” until the 1940s for those hospitals that did not have their own insectaries (Chernin 1986).
By the mid 1940s, this treatment modality was abandoned, most likely due to the availability of effective antibiotics. Not only were the antibiotics effective in controlling some of the infections that had previously been treated with maggot therapy, but most importantly, use of these new antibiotics pre-empted the bacteremia and local spread of infections that previously led to the soft-tissue complications that the maggots so effectively treated. During the next few decades, rare reports of intentional (Teich and Myers 1986) or accidental myiasis (Horn et al. 1976) illustrated the wound-healing benefits of blow fly larvae, when all else failed.
2.3 Current Status
The end of the twentieth century was the beginning of a new era for maggot therapy, and for that matter, for most of biotherapy: it marks the first controlled, comparative clinical trials of maggot therapy, which led to regulatory oversight and marketing clearance of medicinal maggots in 2004 by the U.S. Food and Drug Administration (FDA), as the first legally marketed “living therapeutic animal.”
In early 1990, a prospective clinical study was conducted to evaluate maggot therapy as a treatment for pressure ulcers (“bed sores”) in spinal cord injury patients at the Veterans’ Affairs Medical Center in Long Beach, CA (Sherman et al. 1995b). Over the next 5 years, the project was expanded to include other patient populations (Sherman 2002b, 2003). Together, these studies demonstrated unequivocally that maggot therapy was associated with faster and more thorough debridement than conventional surgical and non-surgical modalities. Maggot-treated wounds filled with healthy granulation tissue and became smaller more rapidly than those wounds treated with conventional modalities. Wounds scheduled for surgery or amputation but treated first with maggot therapy required fewer surgical interventions, and those that still required surgery had significantly fewer post-operative infections and wound-closure problems (Sherman et al. 2001; Sherman and Shimoda 2004).
In the two following decades, MT has again become accepted as a wound care treatment. Scores of reports, including clinical trials and basic research, were published in the medical and scientific literature since 1995. In 1995, medicinal maggots were produced in the U.S., Israel and the United Kingdom. By the year 2002, they were being produced by over a dozen labs; by 2011, an estimated 50,000 units were produced by at least 24 laboratories, and shipped to patients in over 30 countries. Today, 20 years after its reintroduction, we estimate that more than 80,000 patients were treated by MT (Mumcuoglu et al. 2012).
More recently, MT is taught in medical and surgical training programs, and medical device companies are investing in maggot laboratories, dressings and research. The growing acceptance of MT is due mainly to the growing need for effective, low cost wound care, combined with the growing recognition that maggots can provide exactly that: simple, safe, effective and low-cost wound care (Wayman et al. 2001; Thomas 2006).
2.4 The Fly
Medicinal blowfly maggots are selected for their ability to feed on dead tissue without disturbing viable tissue (Sherman 2002a). The most commonly employed larvae have been those of the green bottle fly, Lucilia sericata (or Phaenicia sericata, depending on authority) (Fig. 2.1).
The green bottle-fly, Lucilia sericata: (a) Female fly with visible ovipositor; (b) eggs; (c) apical part of a grown third instar larva with the two protruding mouth hooks; (d) dissected third instar larva with paired large salivary glands (arrows), the crop (Cr) rotated to the top left, esophagus (E), proventriculus (Pr), the light-brown midgut (Mg) and beginning of the darker hindgut (Hg). The large white masses on the upper and lower part of the picture are the larval fat bodies
Rarely, therapists have used related species, such as Lucilia cuprina and Calliphora vicina (Table 2.1); but these have not been studied as extensively (Paul et al. 2009; Tantawi et al. 2010; Kingu et al. 2012).
L. sericata is common all over the temperate and tropical regions of the world. It prefers warm and moist climates and accordingly is especially common in coastal regions. The female lays her eggs (up to 200 during her lifetime) on decaying organic material such as feces and animal corpses. Depending on external temperatures, the eggs hatch within 8–24 h, releasing larvae. The larvae will molt twice over the course of 4–7 days (again, depending on temperature). After the third stage (instar) of their larval period (Fig. 2.2), they will wander away from the host and enter their pupal stage. The pupal stage lasts approximately 10–20 days, at the end of which the organism will have transformed (metamorphosed) and emerged (eclosed) as an adult fly (Greenberg 1973) (Fig. 2.3).
At higher temperatures (37 °C) the newly hatched larvae enter their pre-pupal stage within 48 h.
2.5 Mechanisms of Action
The following mechanisms of action have been observed:
2.5.1 Debridement
The most obvious benefit of maggot therapy is the ability of medicinal maggots to effectively debride wounds by removing the sloughy, necrotic tissues. This process is accomplished both physically and chemically. Extracorporeal digestion by the maggots’ proteolytic enzymes is one mechanism by which wounds are cleaned. Secreted collagenases and trypsin-like and chymotrypsin-like enzymes have been described (Vistnes et al. 1981; Chambers et al. 2003; Horobin et al. 2003, 2005).
Each maggot is capable of removing 25 mg of necrotic material within just 24 h (Mumcuoglu 2001) (Fig. 2.4).
Maggots secrete their digestive juices directly into their environment, and these proteolytic enzymes are largely responsible for removing the infected, dead tissue from the wound by liquefying the tissue into a nutrient-rich fluid that can be imbibed by the maggots. These proteases may be the vehicle by which the maggots precipitate other wound healing effects, as well. Proteolysis is involved in tissue repair, haemostasis, thrombosis, inflammatory cell activation and tissue reconstruction. Proteinases (mainly matrix metalloproteinases, such as the serine proteases) are involved in collagen degradation, keratinocyte migration, and activation of endothelial cells, fibroblasts, keratinocytes and platelets, through proteinase-activated receptors (Chambers et al. 2003; Brown et al. 2012; Pritchard et al. 2012; Telford et al. 2012).
In addition to the serine proteases, two other classes of proteolytic enzymes are also present in L. sericata larval secretions (Chambers et al. 2003); but the predominant activity belongs to the trypsin-like and chymotrypsin-like serine proteases. All together, the larval proteinases are active across a wide pH range (pH 5.0 to pH 10.0).
In addition to enzymatic debridement, maggots also exert a physical debridement over the wound bed (Barnard 1977; Thomas et al. 2002). Blow fly larvae are covered by spines and two mouth-hooks, which aid in locomotion. As the maggots crawl about the wound, these rough structures loosen debris just like a surgeon’s “rasper.”
2.5.2 Disinfection
Since the natural habitats of blow flies are corpses, excrement, wounds, and similar decaying organic matter, it is obvious that they must be resistant to microbial attack. With the work of William Baer (1931), the antimicrobial activity of maggots began to be appreciated. Early theories were that the maggots killed bacteria through ingestion (Livingston and Prince 1932; Robinson and Norwood 1933, 1934). Greenberg (1968) demonstrated bacterial killing within the maggot gut, and provided evidence that the activity was due, at least in part, to metabolic products of Proteus mirabilis (a commensal gram-negative bacterium within the larval gut. Erdmann and Khalil (1986) went on to identify and isolate two antibacterial substances (phenylacetic acid and phenylacetaldehyde) produced by P. mirabilis that they isolated from the gut of screwworm larvae (Cochliomyia hominivorax).
Mumcuoglu et al. (2001) added to our understanding of alimentary disinfection, and demonstrated that P. mirabilis is not required for the process, by feeding green fluorescent protein-producing Escherichia coli to L. sericata, and using a laser scanning confocal microscope to detect the fluorescence as the bacteria traveled through the maggot’s gut (Fig. 2.5). The number of bacteria, very high in the crop (Fig. 2.6), decreased significantly in the mid- and hindgut, with essentially no bacteria reaching the end of the gut (anus) (Fig. 2.7).
Antimicrobial killing also occurs outside the maggot gut in the wound bed itself. Some early researchers pointed to the fact that the profuse exudates might be washing the bacteria out of the wounds. Changes in the alkalinity of the wound were also felt to be a mechanism of bacterial killing (Baer 1931) as well as the ammonium products themselves (Messer and McClellan 1935; Robinson and Baker 1939).
Simmons (1935) and Pavillard and Wright (1957) demonstrated that excretion of the antimicrobial gut contents was probably the major mechanism by which the maggots were killing bacteria even before ingesting them.
A preliminary study demonstrated that sterile L. sericata secretions exhibit marked antimicrobial activity against liquid cultures of the Gram positive Streptococcus sp., Staphylococcus aureus and a clinical strain of methicillin-resistant S. aureus (MRSA) (Thomas et al. 1999).
Bexfield et al. (2004) and Kerridge et al. (2005) isolated two antibacterial fractions from maggots of L. sericata; one with 0.5–3.5 kDa and the second <500 Da. It was shown that these antibacterials are active against a range of bacteria, including the Gram positive S. aureus, both methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA), Streptococcus pyogenes and, to a lesser extent, the Gram negative Pseudomonas aeruginosa.
Huberman et al. (2007b) isolated three molecules with a molecular weight (MW) of 194, 152 and 138 Da, having antibacterial activity. The antibacterial activity was observed in extracts of whole body, haemolymph and in the excretions of the maggots, and it was effective against a large number of pathogenic and non-pathogenic bacteria (Huberman et al. 2007a). By examining the influence of an infected environment and physical injury on maggots, it was found that the activity was higher in non-sterile reared maggots and even more so in injured maggots when compared with sterile ones, showing that maggots produce higher quantities of antibacterials in the presence of bacteria. Higher antibacterial levels were observed in L. sericata maggots removed from chronic wounds of patients. The low MW molecules found in this study have an antibacterial activity against Gram-positive bacteria including methicillin susceptible Staphylococcus aureus (MSSA) and MRSA and Gram-negative bacteria such as P. aeruginosa, Serratia marcescens, E. coli and Klebsiella pneumoniae, which are also found in chronic wounds. This is in agreement with the findings of other authors, who showed that maggot excretions/secretions are capable of neutralising bacteria such as E. coli, P. aeruginosa, MSSA and MRSA. The destruction of the bacteria by the low molecular fractions started after 2 min, and over 90 % of the bacteria were destroyed within 15 min. The quick influx of K+ and changes in the membrane’s potential showed that the low molecular fraction caused bacterial lysis, which was also demonstrated by scanning electron microscopy (SEM) (Huberman et al. 2007a, b).
Apparently, these molecules with low MW are a first-line defense mechanism against the intrusion of bacteria. Unlike antibacterial peptides, they exist already in sterile insects and can be recruited immediately upon invasion of any bacteria. The antibacterial peptides which take longer to be synthesized (Kerridge et al. 2005), strengthen the activity of the former.
Kawabata et al. (2010) applied sterile larvae of L. sericata to a test tube containing a bacterial suspension of S. aureus or P. aeruginosa. To collect the larval extracts, the incubated larvae were cut into multiple pieces with scissors, transferred to a test tube containing PBS and centrifuged. The supernatant was used to test for antibacterials activities. The results showed that infected larvae had better antibacterial capacities than sterile larvae. Antibacterial activities were induced by pretreatment with a single bacterial species, S. aureus or P. aeruginosa, within 24 and 12 h, respectively, and disappeared after 36 h. The activities were effective against S. aureus, but not against P. aeruginosa.
Kruglikova and Chernysh (2011) isolated two groups of antibacterial compounds from the excretion: polypeptides with molecular masses ranging from 6.5 to 9 kDa and small molecules with molecular masses ranging from 130 to 700 Da. The polypeptides characterized by the masses of 8.9 and 9 kDa and showing selective activity against Gram negative bacteria correspond well to diptericins, antimicrobial peptides previously found in the hemolymph of Calliphoridae maggots and known to be part of the immune response to bacterial pathogens.
Ceřovský et al. (2010) were the first to completely sequence a 40-residue defensin-like antimicrobial peptide from L. sericata, which they called “lucifensin.” Subsequently, the researchers managed to synthesize the molecule by several different methods (Ceřovský et al. 2011). Research continues to isolate other lucifensin-like molecules, since, in other animals where one defensin has been found, the discovery of other related molecules soon followed.
Other high-molecular compounds with masses 6.5, 6.6, 5.8 and 8.6 Da have no clear analogs among antimicrobial peptides present in the hemolymph. The nature of small molecules present in the excretion awaits further study. Thus, the diversity of antimicrobial compounds discovered in Lucilia excretion demonstrates a sophisticated strategy that helps the maggots to fight bacteria and other microorganisms settling their environment. The strategy combines secretion of a set of antibacterial peptides involved in insect immune response as well as molecules which function outside the host organism.
Barnes et al. (2010) studied the antibacterial activities of excretions/secretions (ES) from larvae of a carrion feeding beetle, Dermestes maculatus, a detritus feeding beetle, Tenebrio molitor, and those of Calliphora vicina and L. sericata. Viable counts were used to assess time-kill of ES against five bacterial species, S. aureus, E. coli, Bacillus cereus, P. aeruginosa and Proteus mirabilis. The two blowflies were more effective in controlling a wider range of both Gram-positive and Gram-negative bacteria.
Maggot secretions and excretions contain molecules which not only kill bacteria directly, but also molecules which dissolve biofilm and inhibit the growth of new biofilm (Cazander et al. 2009, 2010). At least two types of biofilm have been tested: S. aureus and P. aeruginosa. Anti-biofilm activity is a very important discovery because biofilm appears to be an important mechanism by which bacteria evade both the body’s immune system and also antibiotic activity.
Today, we have not only laboratory evidence for antimicrobial killing, but also clinical evidence that the maggots are disinfecting the wounds. In a study conducted by Bowling et al. (2007), it was demonstrated that MRSA was eliminated from all but one of 13 MRSA-colonized diabetic foot ulcers (92 %) after a mean of three maggot therapy applications (within an average of 19 days). Tantawi et al. (2007) and Contreras-Ruiz et al. (2005) demonstrated a significant decrease in the number of microbial organisms and species after maggot therapy. Armstrong et al. (2005) demonstrated the clinical relevance of this by establishing a case-controlled study of maggot therapy for lower extremity wounds in hospice patients: maggot therapy resulted in fewer clinical assessments of infection, less use of antibiotics, and fewer amputations.
2.5.3 Growth Stimulation
William Baer observed that wounds debrided with maggots heal normally thereafter. But if maggot therapy is continued beyond the point of complete debridement, then the wounds heal even faster than expected (Baer 1931). Maggot-induced wound healing has fascinated therapists and researchers ever since.
Many theories have arisen to explain the rapid growth of granulation tissue and the rapid wound closure associated with maggot therapy. Some researchers speculated that the maggots facilitated normal healing simply by removing the debris and infection that was impairing wound healing (Robinson and Norwood 1934). Others speculated that the simple action of maggots crawling over the wounds stimulated the healing process (Buchman and Blair 1932). This is a reasonable explanation, as we now know that such physical stimulation could very well cause the release of growth factors from the wound bed. Yet, for decades, researchers have believed there were other mechanisms at play, and the search to explain the rapid healing of maggot-treated wounds has continued through the present day.
Clinically, maggot therapy continues to be associated with increased rates of granulation tissue formation (Sherman 2002b, 2003) and faster wound closure (Markevich et al. 2000; Sherman 2002b, 2003; Armstrong et al. 2005) in all but one controlled study (Dumville et al. 2009). But in the latter study, maggot therapy was used only for debridement, and then withdrawn instead of continued; so perhaps it simply proved Baer’s observations about normal healing rates following maggot debridement.
Laboratory investigations to explain these observations of enhanced wound healing have also continued ever since Baer’s early work. In the 1930s, the alkalinity of maggot-treated wounds, along with the isolated allantoin and urea-containing compounds were believed to be responsible for wound-healing (Robinson 1935b). In fact, today, allantoin and urea are still components of many cosmetics.
More recently, maggot secretions have been shown to stimulate the proliferation of fibroblasts (Prete 1997) and endothelial tissue, in tissue culture. Biopsies of treated wounds reveal profound angiogenesis and luscious granulation tissue (Sherman 2002a). Wollina et al. (2002) demonstrated increased vascular perfusion and tissue oxygenation in patients treated with maggot therapy, using remittance spectroscopy to evaluate the patients before and after maggot therapy.
In 3-dimensional gel-based wound models, maggot secretions significantly enhanced fibroblast migration over the wound surface (Horobin et al. 2003, 2005), apparently as a result of proteolytic cleavage of fibronectin and other molecules which otherwise anchor the fibroblasts to each other and to the wound edges. These migrating fibroblasts are then free to replicate in all directions (not just from the margins of the wound towards the center). Since fragmented fibronectin may itself be bioactive in the wound-closure process, this, too, may be another mechanism by which the maggots stimulate wound healing.
2.6 Clinical Use of Maggot Therapy
2.6.1 Indications
In the U.S., the production of medicinal maggots is regulated by the FDA. The FDA-cleared indications are for debriding non-healing necrotic skin and soft tissue wounds, including pressure ulcers, venous stasis ulcers, neuropathic foot ulcers, and non-healing traumatic or post surgical wounds (hence the acronym MDT). Maggot therapy has also been used to treat necrotic/sloughy wounds associated with a wide variety of pathology (including burns, arterial ulcers, Burger’s disease, cellulitis, lymphostasis, neuropathies, osteomyelitis, mastoiditis, thalassemia, polycythemia, and necrotic tumors), and to stimulate and close clean but non-healing wounds (Mumcuoglu et al. 1997, 1999; Angel et al. 2000; Namias et al. 2000; Wollina et al. 2002; Bowling et al. 2007; Sherman et al. 2007a; Gilead et al. 2012).
2.6.2 Contraindications and Adverse Events
Maggot therapy is relatively safe, but complications (“adverse events”) are possible. The most common complaint is pain, occurring in 6–40 % of reported patients. When first applied, medicinal maggots are usually too small to be felt, however as they grow larger (generally after the first 24 h of therapy), the movements of their rough exoskeleton and their two hook-like teeth, which are used for locomotion, can be appreciated by those with sensate wounds. Patients most likely to feel discomfort or pain with maggot therapy are easily identified, as they are the patients with wound pain prior to MDT. Once the larvae are satiated (at about 48–72 h), they will spend all of their time trying to escape, by prying the dressing off the skin or wound bed. Thus, MDT-associated pain is also associated with leaving the dressings on the wound for too long (beyond the point when the maggots have finished feeding (Dumville et al. 2009)). Pain is usually well controlled with analgesics; but if not, removal of the dressing and release of the maggots will immediately put an end to the discomfort.
In Israel, 30–35 % of the patients with superficial, painful wounds complain of increased pain during treatment with maggots, which can be treated successfully with analgesics (Mumcuoglu 2001; Mumcuoglu et al. 1998, 2012). Sherman et al. (2000) reported that during MDT, the most common patient complaint was the physical discomfort. Although treatment-associated pain has been reported in only 6 % of nursing home patients (n = 113), pain was reported in 38.1 % (n = 21) of ambulatory patients. Wollina et al. (2002) used MDT to debride the wounds of 30 patients with chronic leg ulcers of mixed origin. Twelve patients (40 %) reported temporary pain, but only two needed analgesic treatment. Wolff and Hansson (2003) treated 74 patients with necrotic or sloughy chronic ulcers of different etiologies and found that maggots effectively debrided 86 % of the necrotic ulcers, and a single application was clinically beneficial in two-thirds of the patients. One-quarter of the study group experienced less pain during treatment, while 41 % felt no difference in pain. Although 34 % noted an increase in pain, most of these patients wanted to continue the treatment because of subjective and objective improvement of the wound.
Steenvoorde et al. (2005a) determined pain levels in patients treated with MDT using a visual analogue scale. Paracetamol (acetaminophen; 1 g/3× daily) and fentanyl patch (25 μg/h every 3 days and 50 μg/h a day before the maggot challenge) were administered for pain relief in the outpatient clinic. Diabetic patients experienced the same amount of pain before and during MDT, while 8 out of 20 non-diabetic patients experienced more pain during MDT than before. The difference between diabetic and non-diabetic patients was statistically significant for all applications combined. In 78 % (n = 37) of patients, pain was adequately treated with analgesic therapy. The authors concluded that a standardized but individually tailored pain management protocol is mandatory.
MDT associated pain is probably the result of the maggots’ movements over the wound surface, especially because the maggots use their two mouth-hooks to pull their body forwards, and because the cuticular layer of their body is covered with thorn-like hairs.
In addition, the secretion/excretion products of the maggots, which include proteolytic enzymes, might have an influence on the exposed nerves and nerve endings of an open wound (Mumcuoglu et al. 2012).
Since patients prone to MDT-associated pain can readily be identified (they have pain before maggot therapy is even initiated), several interventions are generally undertaken to prevent or minimize additional discomfort during therapy: application of the maggots for shorter periods of time, e.g. 6–8 h, mainly during the daytime hours, use of maggot containment bags (which restrict the maggots’ access to the wound bed), and application of smaller maggots and/or fewer maggots.
The second most common problem associated with maggot therapy is late delivery of the maggots. Delivery delays and exposure to extreme temperatures decrease the survival of these young, starving larvae. The shipping container may restrict access to oxygen, especially when designed to be an air-tight thermal insulator. Even though medicinal maggots are generally shipped by overnight courier (and timed to be used within 24 h of arrival), post-marketing studies in the United States demonstrate that 1–2 % still arrive late or dead (unpublished data, RAS).
Occasionally the maggot dressings may come loose, especially if left in place for more than 48 h. Larvae which manage to escape often will pupate under furniture or between mattresses, and emerge from their hiding places 1–2 weeks later as adult flies. Although these flies are not yet mature enough to lay eggs, they can be a nuisance in the clinic or hospital. Moreover, “used maggots” and flies are essentially mobile fomites. There is no data on the frequency of this complication, but clearly it is related to the experience of the therapist in making secure dressings, and the length of time that those dressings are left in place (considering that once the maggots are satiated, they will instinctively attempt to break out of the dressing, leave the wound, and find a secluded place to pupate).
Patient anxiety (the “yuck factor”) is more frequently discussed than encountered. In studies of patients with chronic wounds, most patients were quite accepting of maggot therapy (Sherman and Shimoda 2004; Steenvoorde et al. 2005b).
Maggot therapy should not be performed on patients with allergies to the maggots, media or dressing materials used. Media and dressings may need to be specially adapted for those patients. No allergic reactions related to maggots have been described. Maggot therapy should not be performed in patients whose wounds are aggressively advancing, or need around-the-clock inspection (such as anaerobic or mixed aerobic-anaerobic soft tissue infections and fasciitis). The appropriate treatment for these life- and limb-threatening infections is immediate surgical drainage and/or resection. MDT can be a useful ancillary treatment, post-operatively; but should not be administered until all emergency surgical interventions have been delivered.
Maggot therapy is not recommended for use in cases where there is an opening to internal organs (open peritoneum or chest cavity), although successful use of maggot therapy in these situations has certainly been reported (Sherman et al. 2007a).
Therapists may reduce the anxiety of ambulatory patients by providing 24 h/day telephone access to medical assistance.
Escaping maggots could be unpleasant for patients, their relatives and medical staff. Care should be taken to restrict the maggots to the area of the wound, using appropriate dressings. In cases where escaping maggots could cause damage to sensitive structures (i.e. wounds near the eyes or mouth) or where confinement dressings are particularly difficult due to excessive perspiration or soiling (i.e. coccygeal ulcers), use of containment dressings could be helpful.
The digestive enzymes of the maggots can cause erythema or cellulitis. Therefore, the maggots should be restricted to the wound bed, and the periphery of the wound should be protected by the plaster or hydrocolloid dressing. Although maggots will always look for necrotic tissue and slough as a food source, they should not be left on a completely debrided tissue, since they could damage living tissue in their attempt to escape or scavenge for necrotic tissue.
A special device should be built for ambulatory patients with plantar wounds in order to prevent them from squashing the maggots. It is possible that ammonium salts produced by maggots (Robinson and Baker 1939), if not adequately absorbed by the maggot dressing, could precipitate an increase in the patient’s body temperature. In rare cases, bleeding of the wound has been observed during maggot therapy, especially if surgical debridement was performed shortly before. If non-sterile maggots are used, there is a danger of bacteremias and/or sepsis (Nuesch et al. 2002).
Fetor has been noted by a few patients and therapists, and probably results from the liquefaction of necrotic tissue, followed by the vaporization of volatile products of putrefaction. Ammonium salts excreted by maggots and the odor of bacteria such as Pseudomonas might contribute to fetor, which can be minimized or eliminated by frequently changing the absorbent gauze covering the maggot dressing (Mumcuoglu 2001).
Although the standard of care for bone infection is surgical resection, when surgery is not feasible, maggot debridement may be a reasonable option.
Maggot therapy will dissolve the necrotic soft tissue, even when it surrounds or involves the artery wall. If this occurs, blood vessels – especially those under pressure, such as arteries – may leak blood or perforate. Coaggulopathy (natural or induced) increases the risk of bleeding even further (Steenvoorde and Oskam 2005). Often, the preferred treatment of a necrotic wound involving major vessels is surgery, because it allows direct visualization of the vessels during debridement. However, when this is not feasible, maggot therapy can be performed in the context of potential rupture of a major vessel as long as the patient remains under close observation, i.e., in the hospital or intensive care unit, if necessary.
Without adequate blood flow, even the best-cleaned (debrided) wound will not heal. In fact, it is often argued that a wound that no longer is “protected” by its shell of hard, leathery dead tissue (eschar) is more susceptible to microbial invasion and spread. However, the precise definition or assessment of that critical level of blood flow remains elusive. In published studies of wounds with arterial insufficiency or for any other reason scheduled for amputation, when maggot debridement was performed as a last resort, 40–60 % of wounds healed, and the patients avoided amputation (Mumcuoglu et al. 1998; Sherman et al. 2001; Jukema et al. 2002; Sherman and Shimoda 2004; Armstrong et al. 2005). Therefore, it is well justified to offer a trial of maggot therapy, even in patients with severe arterial insufficiency, as long as they can be appropriately monitored and treated if maggot therapy is not successful. Such patients may require peri-debridement antibiotic therapy to avoid bacteremia or cellulitis or even surgical intervention.
Maggot therapy should not be left to unproven or inadequately disinfected larvae. Strain differences may affect efficacy and safety, and even experienced laboratories have witnessed infectious complications in their patients as a result of inadequate processing and disinfection (Nuesch et al. 2002).
The administration of systemic antibiotics is not a contraindication for maggot therapy (Sherman et al. 1995a; Peck and Kirkup 2012). Antibiotics may be needed to prevent serious infections from spreading to the neighboring skin (cellulitis) or bloodstream (bacteremia). Topical antibiotic ointments should not be used, however, as these may coat and plug the maggots’ spiracles, through which they obtain oxygen.
Therapists should always read and follow the packaging information accompanying the disinfected maggots. Like any other treatment, the risks associated with maggot therapy must be weighed against its potential benefits and against the risks of alternative strategies.
2.6.3 How to Apply Medicinal Maggots
Maggot dressings are intended to maintain the medicinal maggots on the wound in a manner that will be acceptable to the patient and therapist. Accordingly, the dressing should keep the maggots from wandering off, prevent the wound drainage from pooling on the skin (which could lead to maceration, inflammation or dermatitis), and prevent the maggots from drowning, suffocating, or otherwise becoming injured during treatment. The dressing must allow oxygen to reach the maggots, must allow the egress of fluid from the wound, should be relatively inexpensive to produce and simple to maintain, and should prevent the premature escape of maggots (Sherman 1997).
There are two general types of maggot dressings:
-
Confinement Dressings . Confinement dressings restrict the maggots to the wound area, but allow them complete and free access to the wound tissue. They are sometimes referred to as “cage dressings,” or “free range” maggot dressings. The dressings usually are comprised of a foundation or “fence” on the skin that surrounds the wound, topped by a porous polyester net. The maggots are placed inside (at a dose of 5–10 larvae/cm2) along with some light wet gauze, before sealing the netted roof of this dressing cage (Fig. 2.8). An absorbent pad (i.e. gauze pad) is then held in place just over the porous net, to collect the drainage. This absorbent pad should be changed at least once daily, but more frequently if it becomes soiled (always follow manufacturer guidelines). The cage dressing itself, however, should be left in place until the maggots are satiated, at about 28–72 h. At that time, the maggots and their cage dressings are all removed. Confinement dressings can be constructed at the bedside, from readily available materials (Sherman et al. 1996; Sherman 1997; AMCICHAC 2010) or they can be purchased ready-made, specifically manufactured for maggot therapy (LeFlap and LeSoc, by Monarch Labs [Irvine, CA]; AgilPad by Agiltera [Dormagen, Germany]).
-
Containment Dressings . Containment dressings completely contain or envelop the maggots (Grassberger and Fleischmann 2002). These bags may be constructed from simple polyester net (e.g. Biobag by BioMonde [Bridgend, Wales]) or they can be constructed from polyvinyl alcohol foam (Vitapad, by BioMonde). The sealed maggot-bags are then placed on the ulcer bed, covered with a light gauze wrap, and left in place for 3–5 days.
Each dressing style has its advantages and disadvantages (Table 2.2). Limited clinical (Steenvoorde et al. 2005c) and laboratory (Thomas et al. 2002; Blake et al. 2007) comparisons have been made, but only once have confinement and containment dressings been compared in a prospective clinical trial (Dumville et al. 2009). The results of that study demonstrated no significant differences other than faster debridement with the confinement dressings (average of 14 days) compared to bagged maggots (debridement achieved in an average of 28 days).
2.6.4 Case Reports
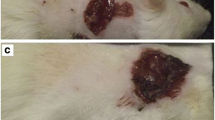
Patient #1. A 68-years old female with venous stasis had a painful chronic wound on her right leg for 24 months, during which she was treated with several conventional debridement methods (Fig. 2.9a). Complete debridement of the wound was achieved within 2 weeks, with six treatment cycles (each for 24 h) of 50–300 confined maggots (Fig. 2.9b).
Patient #2. A 66-years-old male with venous stasis presented to the Department of Dermatology of the Hadassah Hospital in Jerusalem with a chronic wound, which first appeared 6 months earlier (Fig. 2.10a). The wound was completely debrided after four maggot cycles within a week (Fig. 2.10b), each lasting 24–48 h.
2.7 Maggot Therapy in the Veterinary Medicine
Many small animals succumb to complications of serious wounds. Sometimes infection and sepsis overwhelm the animal, while sometimes the costs of intensive care overwhelm the owner. In a study conducted by Sherman et al. (2007b), all eight US veterinarians who had been provided with medicinal maggots were surveyed to determine if this treatment was being used for small animals, and for what indications. At least two dogs, four cats and one rabbit were treated with maggot therapy between 1997 and 2003. The most common indications for using maggot therapy were to effect debridement and control infection, especially if the wound failed to respond to conventional medical and/or surgical therapy. Between 1997 and 2003, 13 horses were treated by 8 veterinarians who used MDT to control infection or debride wounds, which could not easily be reached surgically or were not responding to conventional therapy. Seven animals were lame, and six were expected to require euthanasia. Following maggot therapy, all infections were eradicated or controlled, and only one horse had to be euthanized. No adverse events were attributed to maggot therapy for any of these cases, other than presumed discomfort during therapy (Sherman et al. 2007c). The data collected suggested that maggot therapy was useful for treating some serious equine hoof and leg wounds. Practitioners reported the treatment to be safe and often beneficial. Amputation and euthanasia may have been avoided. It was concluded that maggot therapy may have utility for small animals, and should be evaluated further.
2.8 Future of Maggot Therapy
Over the past 75 years, maggot therapy has saved countless lives and limbs. For just as long, researchers have attempted to isolate the therapeutic molecules in hopes of being able to provide “maggot therapy” without the maggots. These efforts are valuable because they will yield a better understanding of the mechanisms of maggot therapy, as well as a better understanding of wound healing itself. Still, the efficacy of maggot therapy does not lie with a single molecule, nor even a whole family of molecules, but rather a family of molecules in the ideal relative concentrations, combined with the maggot’s anatomy and ambulatory behavior that bring these molecules into the recesses of the wound while the maggots physically debride the wound. Perhaps, someday, maggot therapy will be replaced by maggot-derived pharmaceuticals. However, in the short run, it is more likely that we will continue to improve the delivery of maggot therapy by extending their shelf-life, shortening their delivery time, preventing or mitigating the occasional discomfort, and simplifying dressing application. Additional comparative, clinical studies are necessary to further define the advantages of maggot therapy in general, and to investigate the medical utility of species other than L. sericata.
In the near future, maggot therapy will continue to spread around the world, as more and more countries recognize the benefits (Contreras-Ruiz et al. 2005). For now, thousands of patients each year are already singing praises and gratitude for the lowly maggot.
References
Angel K, Grassberger M, Huemer F, Stackl W (2000) Maggot therapy in Fournier’s gangrene – first results with a new form of treatment. Aktuelle Urologie 31:440–443
Armstrong DG, Salas P, Short B, Martin BR, Kimbriel HR, Nixon BP, Boulton AJ (2005) Maggot therapy in “lower-extremity hospice” wound care: fewer amputations and more antibiotic-free days. J Am Podiatr Med Assoc 95:254–257
Asociación Mexicana para el Cuidado Integral y Cicatrización de Heridas A.C. [AMCICHAC – Mexican Association for Wound Care and Healing] (2010) Clinical practice guidelines for the treatment of acute and chronic wounds with maggot debridement therapy. http://aawconline.org/wp-content/uploads/2011/09/GPC_larvatherapy.pdf. Last accessed 26 Aug 2012
Baer WS (1929) Sarco-iliac joint-arthritis deformans-viable antiseptic in chronic osteomyelitis. Proc Int Assemb Inter-state Postgrad Med Assoc North Am 371:365–372
Baer WS (1931) The treatment of osteomyelitis with the maggot (larva of the blowfly). J Bone Joint Surg 13:438–475
Barnard DR (1977) Skeletal-muscular mechanisms of the larva of Lucilia sericata (Meigen) in relation to feeding habit. Pan-Pac Entomol 53:223–229
Barnes KM, Gennard DE, Dixon RA (2010) An assessment of the antibacterial activity in larval excretion/secretion of four species of insects recorded in association with corpses, using Lucilia sericata Meigen as the marker species. Bull Entomol Res 100:635–640
Bexfield A, Nigam Y, Thomas S, Ratcliffe NA (2004) Detection and partial characterization of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microbes Infect 6:1297–1304
Blake FA, Abromeit N, Bubenheim M, Li L, Schmelzle R (2007) The biosurgical wound debridement: experimental investigation of efficiency and practicability. Wound Repair Regen 15:756–761
Bowling FL, Salgami EV, Boulton AJ (2007) Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabetes Care 30:370–371
Brown A, Horobin A, Blount DG, Hill PJ, English J, Rich A, Williams PM, Pritchard DI (2012) Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med Vet Entomol 26(4):432–439
Buchman J, Blair JE (1932) Maggots and their use in the treatment of chronic osteomyelitis. Surg Gynecol Obstet 55:177–190
Cazander G, van Veen KE, Bouwman LH, Bernards AT, Jukema GN (2009) The influence of maggot excretions on PAO1 biofilm formation on different biomaterials. Clin Orthop Relat Res 467:536–545
Cazander G, van de Veerdonk MC, Vandenbroucke-Grauls CM, Schreurs MW, Jukema GN (2010) Maggot excretions inhibit biofilm formation on biomaterials. Clin Orthop Relat Res 468:2789–2796
Ceřovský V, Zdárek J, Fucík V, Monincová L, Voburka Z, Bém R (2010) Lucifensin, the long-sought antimicrobial factor of medicinal maggots of the blowfly Lucilia sericata. Cell Mol Life Sci 67:455–466
Ceřovský V, Slaninová J, Fučík V, Monincová L, Bednárová L, Maloň P, Stokrová J (2011) Lucifensin, a novel insect defensin of medicinal maggots: synthesis and structural study. Chembiochem 12:1352–1361
Chambers L, Woodrow S, Brown AP, Harris PD, Phillipes D, Hall M, Church JCT, Pritchard DI (2003) Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol 148:14–23
Chernin E (1986) Surgical maggots. South Med J 79:1143–1145
Church JCT (1996) The traditional use of maggots in wound healing, and the use of larva therapy (biosurgery) in modern medicine. J Altern Complem Med 2:525–527
Church JCT (1999) Larva therapy in modern wound care: a review. Prim Intent 7:63–68
Contreras-Ruiz J, Fuentes-Suarez A, Karam-Orantes M, MdL E-M, Dominguez-Cherit J (2005) Larval debridement therapy in Mexico. Wound Care Can 3:42–45
Dumville JC, Worthy G, Jm B, Cullum N, Dowson C, Iglesias C, Mitchell JL, Nelson EA, Soares MO, Torgerson DJ, on behalf of the VenUS II team (2009) Larval therapy for leg ulcers (VenUS II): randomised controlled trial. Br Med J 338:1047–1050
Erdmann GR, Khalil SKW (1986) Isolation and identification of two antibacterial agents produced by a strain of Proteus mirabilis isolated from larvae of the screwworm (Cochliomyia hominivorax) (Diptera: Calliphoridae). J Med Entomol 23:208–211
Fine A, Alexander H (1934) Maggot therapy— technique and clinical application. J Bone Joint Surg 16:572–582
Fleischmann W, Grassberger M, Sherman R (2004) Maggot therapy: a handbook of maggot-assisted wound healing, 1st edn. Thieme, Stuttgart
Gilead L, Mumcuoglu K, Ingber A (2012) The use of maggot debridement therapy in the treatment of chronic wounds in hospitalised and ambulatory patients. J Wound Care 21:78–85
Grantham-Hill C (1933) Preliminary note on the treatment of infected wounds with the larva of Wohlfahrtia nuba. Trans R Soc Trop Med Hyg 27:93–98
Grassberger M, Fleischmann W (2002) The biobag – a new device for the application of medicinal maggots. Dermatology 204:306
Greenberg B (1968) Model for destruction of bacteria in the midgut of blow fly maggots. J Med Entomol 5:31–38
Greenberg B (1973) Flies and disease, vol. 2. Biology and disease transmission. Princeton University Press, Princeton
Horn KL, Cobb AH Jr, Gates GA (1976) Maggot therapy for subacute mastoiditis. Arch Otolaryngol 102:377–379
Horobin AJ, Shakesheff KM, Woodrow S, Robinson C, Pritchard DI (2003) Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon interactions between human dermal fibroblasts and extracellular matrix components. Br J Dermatol 148:923–933
Horobin AJ, Shakesheff KM, Pritchard DI (2005) Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon the migration of human dermal fibroblasts over a fibronectin-coated surface. Wound Repair Regen 13:422–433
Huberman L, Gollop N, Mumcuoglu KY, Block C, Galun R (2007a) Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. J Wound Care 16:123–127
Huberman L, Gollop N, Mumcuoglu KY, Breuer E, Bhusare SR, Shai Y, Galun R (2007b) Antibacterial substances of low molecular weight isolated from the blowfly, Lucilia sericata. Med Vet Entomol 21:127–131
Jukema GN, Menon AG, Bernards AT, Steenvoorde P, Rastegar AT, van Dissel JT (2002) Amputation-sparing treatment by nature: “surgical” maggots revisited. Clin Infect Dis 35:1566–1571
Kawabata T, Mitsui H, Yokota K, Shino KI, Guma KO, Sano S (2010) Induction of antibacterial activity in larvae of the blowfly Lucilia sericata by an infected environment. Med Vet Entomol 24:375–381
Kerridge A, Lappin-Scott H, Stevens JR (2005) Antibacterial properties of larval secretions of the blowfly, Lucilia sericata. Med Vet Entomol 19:333–337
Kingu HJ, Kuria SK, Villet MH, Mkhize JN, Dhaffala A, Iisa JM (2012) Cutaneous myiasis: is Lucilia cuprina safe and acceptable for maggot debridement therapy? J Cosmet Dermatol Sci Appl 2:79–82
Kruglikova AA, Chernysh SI (2011) Antimicrobial compounds from the excretions of surgical maggots, Lucilia sericata (Meigen) (Diptera, Calliphoridae). Entomol Rev 91:813–819
Leclercq M (1990) Utilisation de larves de Dipteres – maggot therapy – en medicine: historique et actualite. Bull Annls Soc belge Entomol 126:41–50
Livingston SK, Prince LH (1932) The treatment of chronic osteomyelitis with special reference to the use of the maggot active principle. J Am Med Assoc 98:1143–1149
Markevich YO, McLeod-Roberts J, Mousley M, Melloy E (2000) Maggot therapy for diabetic neuropathic foot wounds: a randomized study. In: Abstract of the 59th European association for the study of diabetes, Jerusalem, 17–21 September 2000
McClellan NW (1932) The maggot treatment of osteomyelitis. Can Med Assoc J 27:256–260
Messer FC, McClellan RH (1935) Surgical maggots. A study of their functions in wound healing. J Lab Clin Med 20:1219–1226
Mumcuoglu KY (2001) Clinical applications for maggots in wound care. Am J Clin Dermatol 2:219–227
Mumcuoglu KY, Lipo M, Ioffe-Uspensky I, Miller J, Galun R (1997) Maggot therapy for gangrene and osteomyelitis (in Hebrew). Harefuah 132:323–325, 382
Mumcuoglu KY, Ingber A, Gilead L, Stessman J, Friedman R, Schulman H, Bichucher H, Ioffe-Uspensky I, Miller J, Galun R, Raz I (1998) Maggot therapy for the treatment of diabetic foot ulcers. Diabetes Care 21:2030–2031
Mumcuoglu KY, Ingber A, Gilead L, Stessman J, Friedmann R, Schulman H, Bichucher H, Ioffe-Uspensky I, Miller J, Galun R, Raz I (1999) Maggot therapy for the treatment of intractable wounds. Int J Dermatol 8:623–627
Mumcuoglu KY, Miller J, Mumcuoglu M, Friger M, Tarshis M (2001) Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae). J Med Entomol 38:161–166
Mumcuoglu KY, Davidson E, Avidan A, Gilead L (2012) Pain related to maggot debridement therapy. J Wound Care 21:400, 402, 404–405
Namias NN, Varela E, Varas RP, Quintana O, Ward CG (2000) Biodebridement: a case report of maggot therapy for limb salvage after fourth-degree burns. J Burn Care Rehabil 21:254–257
Nigam Y, Bexfield A, Thomas S, Ratcliffe NA (2006a) Maggot therapy: the science and implication for CAM. Part I—history and bacterial resistance. eCAM 3:223–227
Nigam Y, Bexfield A, Thomas S, Ratcliffe NA (2006b) Maggot therapy: the science and implication for CAM. Part II—maggots combat infection. eCAM 3:303–308
Nigam Y, Dudley E, Bexfield A, Bond AE, Evans J, James J (2010) The physiology of wound healing by the medicinal maggot, Lucilia sericata. Adv Insect Physiol 39:39–81
Nuesch R, Rahm G, Rudin W, Steffen I, Frei R, Rufli T, Zimmerli W (2002) Clustering of bloodstream infections during maggot debridement therapy using contaminated larvae of Protophormia terraenovae. Infection 30:306–309
Paul AG, Ahmad NW, Lee HL, Ariff AM, Saranum M, Naicker AS, Osman Z (2009) Maggot debridement therapy with Lucilia cuprina: a comparison with conventional debridement in diabetic foot ulcers. Int Wound J 6:39–46
Pavillard ER, Wright EA (1957) An antibiotic from maggots. Nature 180(4592):916–917
Pechter EA, Sherman RA (1983) Maggot therapy: the surgical metamorphosis. Plast Reconstr Surg 72:567–570
Peck GW, Kirkup BC (2012) Biocompatibility of antimicrobials to maggot debridement therapy: medical maggots Lucilia sericata (Diptera: Calliphoridae) exhibit tolerance to clinical maximum doses of antimicrobials. J Med Entomol 49:1137–1143
Prete PE (1997) Growth effects of Phaenicia sericata larval extracts on fibroblasts: mechanism for wound healing by maggot therapy. Life Sci 60:505–510
Pritchard DI, Telford G, Diab M, Low W (2012) Expression of a cGMP compatible Lucilia sericata insect serine proteinase debridement enzyme. Biotechnol Prog 28:567–572
Reames MK, Christensen C, Luce EA (1988) The use of maggots in wound debridement. Ann Plast Surg 21:388–391
Robinson W (1933) The use of blowfly larvae in the treatment of infected wounds. Ann Entomol Soc Am 26:270–276
Robinson W (1935a) Progress of maggot therapy in the United States and Canada in the treatment of suppurative diseases. Am J Surg 29:67–71
Robinson W (1935b) Stimulation of healing in non-healing wounds: by allantoin occurring in maggot secretions and of wide biological distribution. J Bone Joint Surg Am 17:267–271
Robinson W, Baker FL (1939) The enzyme urease and occurrence of ammonia in maggot infected wounds. J Parasitol 25:149–155
Robinson W, Norwood VH (1933) The role of surgical maggots in the disinfection of osteomyelitis and other infected wounds. J Bone Joint Surg 15:409–412
Robinson W, Norwood VH (1934) Destruction of pyogenic bacteria in the alimentary tract of surgical maggots implanted in infected wounds. J Lab Clin Med 19:581–586
Sherman RA (1997) A new dressing design for use with maggot therapy. Plast Reconstr Surg 100:451–456
Sherman RA (2002a) Maggot therapy for foot and leg wounds. Int J Low Extrem Wounds 1:135–142
Sherman RA (2002b) Maggot versus conservative debridement therapy for the treatment of pressure ulcers. Wound Repair Regen 10:208–214
Sherman RA (2003) Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care 26:446–451
Sherman RA, Shimoda KJ (2004) Presurgical maggot debridement of soft tissue wounds is associated with decreased rates of postoperative infection. Clin Infect Dis 39:1067–1070
Sherman RA, Wyle FA, Thrupp L (1995a) Effects of seven antibiotics on the growth and development of Phaenicia sericata (Diptera: Calliphoridae) larvae. J Med Entomol 32:646–649
Sherman RA, Wyle F, Vulpe M (1995b) Maggot therapy for treating pressure ulcers in spinal cord injury patients. J Spinal Cord Med 18:71–74
Sherman RA, Tran JM-T, Sullivan R (1996) Maggot therapy for venous stasis ulcers. Arch Dermatol 132:254–256
Sherman RA, Hall MJ, Thomas S (2000) Medicinal maggots: an ancient remedy for some contemporary afflictions. Annu Rev Entomol 45:55–81
Sherman RA, Sherman JM-T, Gilead L, Lipo M, Mumcuoglu KY (2001) Maggot debridement therapy in outpatients. Arch Phys Med Rehabil 82:1226–1229
Sherman RA, Morrison S, Ng D (2007a) Maggot debridement therapy for serious horse wounds – a survey of practitioners. Vet J 174:86–91
Sherman RA, Shapiro CE, Yang RM (2007b) Maggot therapy for problematic wounds: uncommon and off-label applications. Adv Skin Wound Care 20:602–610
Sherman RA, Stevens H, Ng D, Iversen E (2007c) Treating wounds in small animals with maggot debridement therapy: a survey of practitioners. Vet J 173:138–143
Simmons SW (1935) A bactericidal principle in excretions of surgical maggots which destroys important etiological agents of pyogenic infections. J Bacteriol 30:253–267
Steenvoorde P, Oskam J (2005) Bleeding complications in patients treated with maggot debridement therapy. Int J Low Extrem Wounds 4:57–58
Steenvoorde P, Budding T, Oskam J (2005a) Determining pain levels in patients treated with maggot debridement therapy. J Wound Care 14:485–488. Erratum in J Wound Care 2006 15:71
Steenvoorde P, Budding TJ, van Engeland A, Oskam J (2005b) Maggot therapy and the “Yuk” factor: an issue for the patient? Wound Repair Regen 13:350–352
Steenvoorde P, Jacobi CE, Oskam J (2005c) Maggot debridement therapy: free-range or contained? An in-vivo study. Adv Skin Wound Care 18:430–435
Tantawi TI, Gohar YM, Kotb MM, Beshara FM, El-Naggar MM (2007) Clinical and microbiological efficacy of MDT in the treatment of diabetic foot ulcers. J Wound Care 16:379–383
Tantawi TI, Williams KA, Villet MH (2010) An accidental but safe and effective use of Lucilia cuprina (Diptera: Calliphoridae) in maggot debridement therapy in Alexandria, Egypt. J Med Entomol 47:491–494
Teich S, Myers RAM (1986) Maggot therapy for severe skin infections. South Med J 79:1153–1155
Telford G, Brown AP, Rich A, English JS, Pritchard DI (2012) Wound debridement potential of glycosidases of the wound-healing maggot, Lucilia sericata. Med Vet Entomol 26:291–299
Thomas S (2006) Costs of managing chronic wounds in the UK, with particular emphasis on maggot debridement therapy. J Wound Care 15:465–469
Thomas S, Jones M, Shutler S, Jones S (1996) Using larvae in modern wound management. J Wound Care 5:60–69
Thomas S, Andrews AM, Hay NP, Bourgoise S (1999) The anti-microbial activity of maggot secretions: results of a preliminary study. J Tissue Viability 9:127–132
Thomas S, Wynn K, Fowler T, Jones M (2002) The effect of containment on the properties of sterile maggots. Br J Nurs 11:S21–S28
Vistnes LM, Lee R, Ksander GA (1981) Proteolytic activity of blowfly larvae secretions in experimental burns. Surgery 90:835–841
Wayman J, Nirojogi V, Walker A, Sowinski A, Walker MA (2001) The cost effectiveness of larval therapy in venous ulcers. J Tissue Viability 10:91–94
Whitaker IS, Twine C, Whitaker MJ, Welck M, Brown CS, Shandall A (2007) Larval therapy from antiquity to the present day: mechanisms of action, clinical applications and future potential. Postgrad Med J 83:409–413
Wolff H, Hansson C (2003) Larval therapy– an effective method of ulcer debridement. Clin Exp Dermatol 28:134–137
Wollina U, Liebold K, Schmidt WD, Hartmann M, Fassler D (2002) Biosurgery supports granulation and debridement in chronic wounds – clinical data and remittance spectroscopy measurement. Int J Dermatol 41:635–639
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Sherman, R.A., Mumcuoglu, K.Y., Grassberger, M., Tantawi, T.I. (2013). Maggot Therapy. In: Grassberger, M., Sherman, R., Gileva, O., Kim, C., Mumcuoglu, K. (eds) Biotherapy - History, Principles and Practice. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6585-6_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-6585-6_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6584-9
Online ISBN: 978-94-007-6585-6
eBook Packages: MedicineMedicine (R0)