Abstract
Ionizing radiation inflicts damage to cells in large part through the generation of chemical intermediates that damage DNA and generate DNA strand breaks. Radiation-induced late normal tissue toxicity is the outcome of changes in tissue following exposure to radiation that precede overt toxicity. These events include mitotic cell death especially in bone marrow and mucosal tissue, and the activation of inflammatory responses that can lead to blood vessel damage, tissue necrosis resulting from lack of oxygen and excessive extracellular matrix deposition (fibrosis). While antioxidants can prevent damage by moderating the chemistry of DNA strand breaks, other agents known as radiation mitigators can be used soon after exposure to protect essential compartments such as the bone marrow from collapse. These include agents that reinforce the rapid development of mature bone marrow and mucosa. Medical management of acute radiation syndrome following accidental exposures to ionizing radiation (IR) involves attempts to reduce the risks of infection and hemorrhage resulting from bone marrow aplasia. This involves stimulating the proliferation and differentiation of residual non-impacted or radioresistant hematopoietic stem and progenitor cells (HSPC) with hematopoietic growth factors. Soon after irradiation radiosensitive HSPC have been shown to undergo apoptosis. It has therefore been proposed that antiapoptotic cytokines including stem cell factor, Flt-3 ligand, thrombopoietin, and interleukin-3 could be employed acutely to prevent this cell death. Moreover, acute exposure to high doses of IR induces sequential, deleterious effects responsible for a delayed multiple organ dysfunction syndrome. Of course, the caveat of preventing the death of cells with damaged DNA is carcinogenesis resulting from DNA mutations in critical genes. NF-κB constitutes a family of transcription factors best associated with mediating the inflammatory response. In cancer cells the activation of the inhibitor of-κB kinase (IKK) and consequently the NF-κB pathway increases resistance to ionizing radiation (IR) by facilitating cell survival, despite the presence of DNA damage and mutations. A number of small molecule inhibitors of NF-κB have been described. I discuss the potential benefit of targeting NF-κB for the prevention of radiation-induced cancers.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Therapeutics of Exposure to Ionizing Radiation
Ionizing radiation inflicts damage to cells in large part through the generation of chemical intermediates that damage DNA and generate DNA strand breaks. Radiation-induced late normal tissue toxicity is the outcome of changes in tissue following exposure to radiation that precede overt toxicity. These events include mitotic cell death especially in bone marrow and mucosal tissue, and the activation of inflammatory responses that can lead to blood vessel damage, tissue necrosis resulting from lack of oxygen and excessive extracellular matrix deposition (fibrosis). While antioxidants can prevent damage by moderating the chemistry of DNA strand breaks, other agents known as radiation mitigators can be used soon after exposure to protect essential compartments such as the bone marrow from collapse. These include agents that reinforce the rapid development of mature bone marrow and mucosa. Medical management of acute radiation syndrome following accidental exposures to ionizing radiation (IR) involves attempts to reduce the risks of infection and hemorrhage resulting from bone marrow aplasia. This involves stimulating the proliferation and differentiation of residual non-impacted or radioresistant hematopoietic stem and progenitor cells (HSPC) with hematopoietic growth factors including granulocyte-macrophage colony stimulating factor (GM-CSF). GM-CSF treatment promotes rapid development of mature bone marrow and mucosa from the stem cell compartment. Colony-stimulating factor remains the therapeutic standard after exposure to less than the lethal dose 50 % (Haematopoietic [H] score 3-H3) while higher scores are treated with stem cell transplantation. Soon after irradiation radiosensitive HSPC have also been shown to undergo apoptosis [1, 2]. It has therefore been proposed that antiapoptotic cytokines including stem cell factor, Flt-3 ligand, thrombopoietin, and interleukin-3 could be rapidly employed to prevent acute cell death [1].
2 The NF-κB Pathway and Its Regulation by Receptors and Ionizing Radiation
Of course, the caveat of preventing the death of cells with damaged DNA or promoting their proliferation is carcinogenesis resulting from DNA mutations in critical genes. Tissue damage arising from IR results in activation of the inflammatory process. Activated macrophages secrete cytokines including TNF-α and IL-1, both of which interact with receptors on T lymphocytes. There are two TNF receptors, I and II, which both result in activation of NF-κB and have been implicated in cancer (reviewed in [3]). NF-κB is a transcription factor that is composed of members of the Rel family including p65RelA and p105/p50 (NF-κB1) and p100/p52 (NF-κB2), RelB and c-Rel. These proteins function as heterodimers or in the case of p52 and p50, can also form homodimers to regulate target genes. The inhibitor of κB (IκBα) kinase (IKK) complex consists of IKK-α,-β and -γ (NEMO) which negatively regulates IκBα activity. The canonical NF-κB pathway is activated by numerous cellular stresses and inflammatory cytokines including TNF-α. In this pathway IκBα binds and retains p65 in the cytoplasm until stimulated IKK-β-mediated phosphorylation results in ubiquitin-mediated degradation of IκBα allowing p65/p50 heterodimerization and nuclear translocation (reviewed in [4, 5]). A second pathway, called the alternative NF-κB pathway (NF-κB AP), is usually induced by activation of specific members of the TNF receptor family including the lymphotoxin (LT)α/β, B-cell activating factor (BAFF) receptors, CD40, and receptor activator of NF-κB (RANK) and is involved in lymphoid organ development and adaptive immunity [6]. Here, activation of the NF-κB inducing kinase, NIK, activates IKK-α homodimers which phosphorylates p100 to signal partial proteolytic processing to mature p52. p52 then translocates to the nucleus to participate in transcriptional activation [4–6]. RelB associates with unprocessed p100 in the cytoplasm and its nuclear translocation occurs coordinately with p52 [7]. Homodimers of p52 are transcriptionally inactive but can also combine with Bcl-3 to mediate transactivation [8, 9].
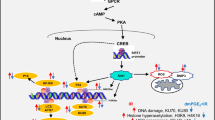
Importantly, NF-κB can also be activated in an atypical manner by DNA damage (Fig. 1.1). NEMO becomes SUMOylated by PIASy following DNA damage, and then undergoes nuclear translocation where it is phosphorylated at serine 85 by the ATM DNA damage-induced kinase which stimulates cIAP1 E3 ligase-mediated mono-ubiquitination of NEMO resulting in nuclear export and subsequent activation of IKK-α and IKK-β [10].
The NF-κB alternative pathway is also activated by DNA damage as evidenced by induced p100 processing and nuclear localization of the p52 subunit in an IKK-α, ATM and NEMO-dependent manner following DNA damage [11]. While the canonical pathway has been associated with cell survival following DNA damage, the alternative pathway is reported to promote cell death.
3 NF-κB Induction by TNFα and Cancer Progression
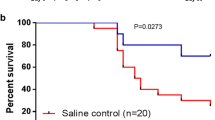
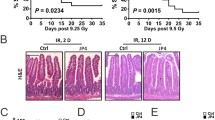
Interestingly, ATM has been shown to primarily mediate cell survival since downregulation sensitizes cells to IR [12]. In cancer cells the activation of the inhibitor of κB kinase (IKK) and consequently the NF-κB pathway increases resistance to ionizing radiation (IR) by facilitating cell survival, despite the presence of DNA damage/mutations. Importantly, TNFα and IL-1 can stimulate the production of NF-κB-responsive cytokines which further induce growth factors to promote survival and stem cell expansion. The therapeutic value of promoting bone marrow maturation from stem cells is clear promoting expansion and maturation of cells that have either escaped IR damage or those that have successfully repaired their DNA as shown in Fig. 1.2.
However, survival signalling combined with the establishment of a growth factor: cytokine feedforward pathway may allow the progression of hematopoetic stem cells harbouring cancer initiating mutations through TNF mediated signalling to NF-κB as shown in Fig. 1.3.
Clearly the risk to benefit ratio associated with replenishing the bone marrow compartment and the risk of enhancing cancer progression needs to be carefully considered. Should, for example, both high dose and very high dose radiation exposure be treated similar to multiple myeloma avoid potential expansion of an initiated population? Treatment of multiple myeloma involves induction therapy with high-dose chemotherapy to ablate the haematopoetic stem cell compartment which is then followed by autologous stem cell transplant.
Maintenance and/or induction can include bortezomib and steroids [13] both of which block NF-κB to prevent further signalling through this pathway. Of course the drawbacks include that applying this approach would be difficult on a large scale and would require further induction treatment to ablate residual cells. Another option could be a more limited exposure to GM-CSF with a set minimum recovery of the haematopoetic index followed by intermittent bortemozib or steroids to block initiated cell survival and proliferation. Promotion of initiated cells might also be better prevented by measures to block inflammation arising from tissue damage which would increase the production of TNFα and IL-1 from activated macrophages. To this end, therapeutics could include factors such as keratinocyte growth factor and erythropoietin as suggested by Herodin and Drouet [1] to ensure tissue damage repair and mitigate the inflammatory processes.
4 Conclusion
It is difficult to establish standard therapies for radiation exposure given the inability to run randomized clinical trials. However, our knowledge of mechanisms of long term cell survival and cancer promotion should guide in the rational treatment of high level exposure to ionizing radiation and efforts to spare damaged cells balanced with the potential for mediating cancer progression.
References
Hérodin F, Drouet M (2005) Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol 33:1071–1080
Dainiak N, Gent RN, Carr Z et al (2011) First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep 5:202–212
Walczak H (2011) TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev 244:9–28
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-κB transcription factors in the immune system. Ann Rev Immunol 27:693–733
Hayden MS, Ghosh S (2008) Shared principles in NF-κB signalling. Cell 132:344–362
Dejardin E (2006) The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol 72:1161–1179
Solan N, Miyoshi H, Carmona E et al (2002) RelB cellular regulation and transcriptional activity are regulated by p100. J Biol Chem 277:1405–1418
Nolan GP, Fujita T, Bhatia K et al (1993) The Bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that preferentially interacts with NF-kappa B p50 and p52 in a phosphorylation dependent manner. Mol Cell Biol 13:3557–3566
Wantanabe N, Iwamura T, Shinoda T et al (1997) Regulation of NF-kB1 proteins by the candidate oncoprotein BCL-3: a generation of NF-kB homodimers form the cytoplasmic pool of p50-p105 and nuclear translocation. EMBO J 16:3609–3620
Miyamoto S (2011) Nuclear initiated NF-κB signaling: NEMO and ATM take center stage. Cell Res 21:116–130
Barré B, Coqueret O, Perkins N (2010) Regulation of activity and function of the p52 NF-κB subunit following DNA damage. Cell Cycle 10:4795–4804
Gueven N, Keating KE, Chen P et al (2001) Epidermal growth factor sensitizes cells to ionizing radiation by down-regulating protein mutated in ataxia-telangiectasia. J Biol Chem 276:8884–8891
Giralt S (2012) Stem cell transplantation for multiple myeloma: current and future status. Hematology 17(Suppl 1):117–120
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Pratt, M.A.C. (2013). Targeting NF-κB to Prevent Radiation-Induced Carcinogenesis. In: Pierce, G., Mizin, V., Omelchenko, A. (eds) Advanced Bioactive Compounds Countering the Effects of Radiological, Chemical and Biological Agents. NATO Science for Peace and Security Series A: Chemistry and Biology. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6513-9_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-6513-9_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6512-2
Online ISBN: 978-94-007-6513-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)