Abstract
It has been recently shown that some microRNAs (miRNAs) are involved in oxidative stress-induced cellular responses. Our studies elucidated a new role for miR-200 family miRNAs and of its target ZEB1 in endothelial cells (EC) respon se to hydrogen peroxide (H2O2). Specifically, ZEB1 expression down-modulation was observed upon oxidative stress in human umbilical vein endothelial cells (HUVEC). ZEB1 and miR-200 family miRNAs were previously shown to be involved in the molecular mechanisms underpinning apoptosis and senescence, both in non-transformed cells and in tumor cells. Our study confirmed a prominent role of ZEB1 down-modulation in the induction of growth arrest, apoptosis and senescence in EC, consequently to the oxidative stress increase of miR-200 family expression.
In vivo experiments in a mouse model of acute hindlimb ischemia, an insult that induces an increase in oxidative stress, enhanced miR-200 family expression in wild-type mice skeletal muscle. In contrast in p66ShcA–/– mice, which display lower levels of oxidative stress after ischemia, up-regulation of miR-200 family members was markedly inhibited. Further studies are needed in order to comprehend whether ZEB1 down-modulation following oxidative stress inducing stimuli is also elicited by other miRNAs and which molecular pathways are involved in ZEB1 ability to modulate cell proliferation, apoptosis and senescence.
In conclusion, miRNAs may represent novel targets to prevent the deleterious consequences of diseases associated with enhanced oxidative stress.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Reactive oxygen species (ROS) is a collective term that includes a number of reactive and partially reduced oxygen (O2) metabolites, with some of them being free radicals, such as superoxide anion (O2 −) and hydroxyl radicals (.OH), that are extremely reactive molecular species with an unpaired electron in their outer orbital. The third most relevant molecule included in ROS is hydrogen peroxide (H2O2), that is more properly a pro-oxidant and nonradical molecule. Formation of ROS is an unavoidable consequence of aerobic metabolism. ROS can be generated within living cells by the following major sources: mitochondria, plasma membrane NADPH oxidase (NOX) and different enzymes involved in redox reactions such as several oxidase, peroxidase, cytochromes, mono- and di-oxigenases (Finkel 2003).
Accumulation of ROS, can inflict damage to DNA, proteins, and fatty acids. It is generally believed that oxidative stress caused by ROS plays a causal role in numerous pathologies, including tissue ischemia and reperfusion, cancer, diabetic vasculopathy, pulmonary fibrosis as well as in aging (Giorgio et al. 2007). To cope with the burden of oxidative damage, a series of enzymatic and non-enzymatic antioxidant defences have evolved to neutralize ROS (Finkel 2003).
In addition, cells also utilize “adaptive responses” aimed at minimizing the damages caused by ROS. Mammalian cells respond to oxidative stress with an increase in expression of antioxidant enzymes and activation of protective genes. To defend themselves against DNA oxidation induced by ROS, proliferating cells arrest their cell cycle, preventing the mutated DNA from being replicated and transferred to daughter cells. This delay in cell growth enables the cells to repair the damaged DNA and to set up an adaptive response to counteract further oxidative damage. According to whether or not damaged DNA is repaired, cells either resume cell growth or enter a status of permanent proliferative arrest known as cellular senescence.
Cellular senescence can result from telomere shortening after multiple rounds of cell division (replicative senescence) or from various stresses such as oncogenes or oxidative stress and occurs independent of a change in telomere length (stress-induced premature senescence, SIPS) (Campisi 2011). While cellular senescence is considered a protective mechanism against cancer, it has also been hypothesized that the progressive accumulation of senescent cells in some tissues may contribute to several age-related diseases and organismal aging (Campisi 2011). Signaling that induces senescence has been extensively studied, and two major tumor suppressor cascades have been unravelled: one involves the retinoblastoma protein (pRb) pathway and the other involves p53; both pathways orchestrate the exit from cell cycle.
The retinoblastoma family of growth-inhibitory proteins is an integral part of the mechanisms that control cell growth under normal conditions and after exposure to genotoxic stimuli. This family includes three members: pRb and the related p107 and p130 (also known as p130Rb2) (Cobrinik 2005). Collectively, these proteins are called “pocket” proteins because they share a common domain, named the “pocket.” Pocket proteins control the G1 checkpoint of the cell cycle through their ability to bind and sequester members of the E2F family of transcription factors, which modulate the expression of genes involved in cell cycle progression. The ability of pocket proteins to bind their interactors is abolished through cell cycle-regulated phosphorylation by cyclin-dependent kinases (CDKs).
In early G1, pRb is hypophosphorylated and becomes hyperphosphorylated in late G1 prior to entry into S phase of the cell cycle. pRb phosphorylation increases even further as cells progress through S and G2; p107 and p130 are regulated in a similar fashion (Cobrinik 2005). Inhibitors of CDKs, Cip/Kip and INK4 families, provide another level of regulation (Pei and Xiong 2005). Indeed, increased levels of CDK inhibitors in response to stress or differentiative cues inhibit pRb phosphorylation and cause growth arrest.
Two cell cycle inhibitors that are often expressed by senescent cells are the CDKIs p21 (also termed CDKN1a, p21Cip1, waf1 or SDI1) and p16 (also termed CDKN2a or p16INK4a) (Campisi 2011). These CDKIs are components of tumor suppressor pathways that are governed by p53 and pRb proteins, respectively. Both p53 and pRb are transcriptional regulators that are frequently deregulated in cancer; both pathways can establish and maintain growth arrest that is typical of senescence. The expression of p21 is induced directly by p53 but the mechanisms that induce p16 are not completely understood. Ultimately, p21 and p16 maintain pRb in a hypophosphorylated and growth arrest active state, but their activities are not equivalent. Cells that senesce solely due to p53–p21 activation can resume growth after inactivation of the p53 pathway. However, although cells that senesce due to oncogenic RAS (which induces p16 expression) can resume limited proliferation, cells that fully engage the p16–pRb pathway for several days usually cannot resume growth even after inactivation of p53, pRb or p16 (Campisi 2011). Certain genotoxic stimuli elicit the activation of p53, which, in turn, transactivates the transcription of the p21 gene. Alternatively, oxidative stress induces p21 protein levels, triggering the stabilization of p21 mRNA in a p53-independent manner (Gorospe et al. 1999).
Another mechanism of induction of growth arrest caused by oxidative stress requires phosphate removal from pRb by phosphatases. We have elucidated the molecular mechanisms underlying pRb dephosphorylation upon oxidative stress by the protein phosphatase 2A (PP2A) (Cicchillitti et al. 2003; Magenta et al. 2008). We demonstrated that pRb dephosphorylation by PP2A was very rapid and did not require p53 or p21 induction, nor CDKs down-modulation. Moreover, we showed that intracellular Ca2+ mobilization was necessary and sufficient to trigger pRb dephosphorylation and that PP2A activity was Ca2+-induced (Magenta et al. 2008). Thus, growth arrest and senescence are crucial component of the cellular responses to oxidative stress.
Moreover, ROS can also induce cell death, either by apoptosis or by necrosis, according to the level of oxidative stress experienced by the cell and its genotype (Ray et al. 2012). One mechanism of induction of apoptosis by oxidative stress involves p53 (Borras et al. 2011). A pivotal role in ROS induced apoptosis is also played by the p66 isoform of ShcA protein. The mammalian adaptor protein ShcA has three isoforms, p46, p52 and p66; all isoforms share a common structure, but p66ShcA has an additional domain at the N terminus. This domain contains a serine residue at position 36 (Ser-36) that is phosphorylated in response to several stimuli, including UV irradiation and H2O2 (Migliaccio et al. 1999). While p52/p46 are cytoplasmic signal transducers involved in mitogenic signaling from activated tyrosine kinase receptors to Ras, p66 isoform is not involved in Ras activation and regulates ROS metabolism and apoptosis (Giorgio et al. 2007). A fraction of p66ShcA is localized in the mitochondria and functions as a redox enzyme that generates mitochondrial ROS as signalling molecules for apoptosis (Giorgio et al. 2007). According to these data, both p66ShcA ko cells and mice display lower levels of intracellular ROS and are resistant to apoptosis induced by a variety of different stimuli (Giorgio et al. 2007). Likewise, our group demonstrated that p66ShcA ko mice are resistant to ischemia-induced apoptosis and show decreased vascular and muscle damage in response to hindlimb ischemia (Zaccagnini et al. 2004).
Since both ischemia and ischemia/reperfusion induce oxidative stress, we concluded that p66ShcA plays a crucial role in ROS induced cell death following acute ischemia and ischemia/reperfusion, indicating p66ShcA as a potential therapeutic target for prevention and treatment of ischemic tissue damage (Zaccagnini et al. 2004).
MicroRNAs
Oxidative Stress and MicroRNAs
MicroRNAs (miRNAs) are small non coding RNAs, usually 21–23 nucleotides long, which regulate the stability and/or the translational efficiency of target messenger RNAs (mRNAs) (Bartel 2009). A wide range of cell functions are under miRNAs control including regulation of proliferation, differentiation, senescence and death (Chang and Mendell 2007).
Different stimuli that produce ROS are known to induce modulation of miRNAs expression such as UV, H2O2, ionizing radiation, and anticancer drugs such as etoposide and anthracyclines. A number of studies have underlined the role of different miRNAs in ROS induced cell responses. For example, UV radiation induces a significant increase of miR-22 expression which may promote cell survival via the repression of tumor suppressor gene phosphatase and tensin homolog (PTEN) expression (Tan et al. 2012). Different reports showed miR-21 up-regulation upon oxidative stress (Lin et al. 2009; Simone et al. 2009). The increase of miR-21 expression following 6 h (hrs) of 200 μM H2O2 exposure in rat vascular smooth muscle cells (VSMC) was shown to have an anti-apoptotic function, since programmed cell death protein 4 (PDCD4) was demonstrated to be a miR-21 target (Lin et al. 2009). Moreover, screening of miRNAs in human fibroblasts exposed to radiation, H2O2 or etoposide had been performed in order to find common signature in response to genotoxic oxidative stress (Simone et al. 2009).
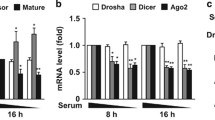
Our group recently demonstrated that miR-200 family was induced upon oxidative stress (Magenta et al. 2011). This miRNA family consists of five members: miR-200c and miR-141 clustered on chromosome 12, and miR-200a, miR-200b, and miR-429 clustered on chromosome 1. We demonstrated that miR-200c and miR-141 were the most up-regulated miRNAs in human umbilical vein endothelial cells (HUVEC) exposed to 200 μM H2O2 for different period of times whereas the other three clustered members were up-regulated to a lower level (Fig. 14.1). Since miR-200c is well expressed in HUVEC, whereas the other family miRNAs are barely detectable, miR-200c is likely the main effector of oxidative stress-induced biological responses in endothelial cells (EC). The induction of miR-200c upon H2O2 was also confirmed in other cell lines, such as human fibroblasts and C2C12 myoblasts and myotubes (Magenta et al. 2011). In order to demonstrate that miR-200c up-regulation was oxidative stress-dependent we used other interventions that cause red/ox imbalance, i.e. the alkylating agent 1,3-bis(2 chloroethyl)-1-nitrosourea (BCNU) that is a glutathione reductase inhibitor that blocks the conversion of oxidized to reduced glutathione, ultimately leading to an intracellular increase of oxidative stress. Incubation of HUVEC with BCNU enhanced miR-200c and this phenomenon was inhibited by a free-radical scavenger N-acetyl-L-cysteine (NAC). Therefore we concluded that miR-200c induction was oxidative stress dependent.
Our data are also supported by another study showing that miR-200c and miR-141 were up-regulated in a cell model of oxidative stress by treatment of House Ear Institute-Organ of Corti 1 (HEI-OC1) cells with different concentrations of tertbutyl hydroperoxide (t-BHP) (Wang et al. 2010).
MicroRNAs in Apoptosis and Senescence
It is well known the crucial role played by miRNAs in different cellular responses including apoptosis and senescence. Specifically, numerous miRNAs, referred to as “apoptomirs”, have targets involved in apoptotic signaling and both pro-apoptotic and anti-apoptotic miRNAs have been described (Vecchione and Croce 2010). For example, miR-1 has a pro-apoptotic function targeting HSP60 and HSP70, which are primarily anti-apoptotic proteins since they inhibit the mitochondrial death pathway at different points. On the other end miR-133 targets caspase-9 and consequently it has an anti-apoptotic effect (Vecchione and Croce 2010). Similarly, let 7a, one of the let-7 miRNA members, targets caspase-3 and therefore displays an anti-apoptotic function as well (Vecchione and Croce 2010).
As previously described, miR-21 expression is induced by ROS and has an anti-apoptotic function, since its targets molecules are involved in the induction of apoptosis, i.e. phosphatase and tensin homolog (PTEN) and PDCD4 (Vecchione and Croce 2010).
The pro-survival protein Bcl-2 is a crucial molecule that contributes to cell death inhibition, nowadays different miRNAs are known to target Bcl-2, including miR-15a and miR-16-1, miR-1 and miR-34a (Vecchione and Croce 2010).
The miR-34 family plays a crucial role both in cell cycle progression and in apoptosis and senescence. The double positive feedback loop between miR-34 and p53, determines a miR-34 family role in the cellular responses activated by DNA damage such as oxidative stress, radiation and chemotherapeutic drugs (Yamakuchi et al. 2008). This family of miRNA includes three members: miR-34a, miR-34b and miR-34c.
Different targets have been attributed to miR-34 family; specifically, Cyclin E (CCNE2), cyclin dependent kinase 4 (CDK4) and 6 (CDK4), E2F3, E2F4, hepatocyte growth factor receptor (MET) are miR-34a targets and contribute to the arrest of proliferation (Yamakuchi and Lowenstein 2009). Over-expression of miR-34a, in fact, promoted cell cycle arrest, pRb dephosphorylation; apoptosis was also induced, possibly due to Bcl2 targeting (Yamakuchi and Lowenstein 2009).
Another important target of miR-34a is the class III histone deacetylase silent information regulator 1 (SIRT-1), a gene that regulates cellular senescence and limits longevity. SIRT1 plays an important protective role against oxidative stress and DNA damage, deacetylates different proteins such as pRb, FOXO, ku70 NFkB, pGC1 alpha and p53. Deacetylation of p53 diminished its transcriptional activity, inhibiting the expression of p21 and PUMA. The latter is a proapoptotic Bcl-2 homology 3 (BH3)-only Bcl-2 family member, that is considered as one of the main downstream effectors of p53 in apoptosis induction, since it directly binds and antagonizes all known antiapoptotic Bcl-2 family members, inducing mitochondrial dysfunction and caspase activation. Therefore p53 deacetylation mediates cell survival during periods of severe stress inhibiting apoptosis. Yamakuchi et al. (2008) demonstrated that miR-34a over-expression suppresses SIRT1 leading to an increase of deacetylated p53 and of its targets p21 and PUMA expression, ultimately inducing apoptosis (Yamakuchi et al. 2008).
The effects of miR-34a on senescence have been largely demonstrated; miR-34a is known to regulate senescence in several cancer cell lines and elevated level of miR-34a were found in senescent HUVEC as well as in heart and spleen of older mice (Ito et al. 2010). Moreover, miR-34a over-expression induced EC senescence and introduction of miR-34a and b/c in human diploid fibroblasts caused senescence (Ito et al. 2010; Vecchione and Croce 2010). Ito et al. (2010) demonstrated that SIRT1 mediates miR-34a regulation of senescence in HUVEC, given SIRT1 role in cellular senescence, and forced expression of SIRT1 blocked the ability of miR-34a to induce senescence.
Another important miRNA that targets SIRT1 is miR-217. Over-expression of miR-217 induces EC senescence whereas miR-217 inhibition delays senescence. Further, high levels of mir-217 had been found in human atherosclerotic lesion and its expression was associated with reduced level of SIRT1 (Vecchione and Croce 2010).
Somehow, miR-200 family resembles miR-34 gene family in many aspects. First, our study and other reports demonstrated that this miRNA family is under p53 control (Chang et al. 2011; Kim et al. 2011; Magenta et al. 2011) and, secondly, our group demonstrated that miR-200c over-expression in EC is able to induce growth arrest, apoptosis and senescence (Magenta et al. 2011). We found that over-expression of miR-200c significantly inhibited EC proliferation inducing pRb dephosphorylation and moreover, induced an increase in apoptosis, measured by apoptotic DNA fragmentation and by the percentage of cells displaying subdiploid DNA content. Finally, miR-200c over-expression induced EC senescence, testified by an increase of senescence-associated β-galactosidase (SA-β-gal) activity, characteristic feature of senescence-related growth arrest, and by expression of p21 protein, which increases in response to oxidative stress and, as previously described, plays a major role in permanent growth arrest/senescence. Since all these effects are also induced by oxidative stress we next evaluated whether miR-200c inhibition prevented oxidative stress-induced effects.
Our results demonstrated that H2O2 induction of growth arrest, apoptosis and senescence, in EC were all diminished when miR-200c increase was prevented by the means of an anti-miR-200c oligos. Different studies underlined miR-200 family role in apoptosis and senescence also in tumor cells. For example, a pro-apoptotic role of miR-200c was discovered showing that this miRNA targets the apoptosis inhibitor FAP1, thereby sensitizing tumor cells to apoptosis (Brabletz and Brabletz 2010). In keeping with a miR-200c role in senescence, it has been shown that miR-200c is up-regulated by chronic oxidative stress-induced senescence in human fibroblasts and in human trabecular meshwork cells (Li et al. 2009). Notably, SIRT1 was also demonstrated a direct gene target of miR-200a. Eades et al. (2011) showed that loss of miR-200a expression is associated with breast cancer transformation and is responsible, at least in part, for SIRT1 over-expression.
Zinc Finger E-Box Binding Homeobox 1 (ZEB1)
ZEB1 in Normal and Tumor Cells
Zinc finger E-box binding homeobox 1 (ZEB1; also known as Zfhx1A, δEF1, Tcf8 and Zfhep) is a transcriptional repressors that binds a set of E-box-like promoter elements that overlap with those bound by ZEB2 (also known as Sip1) and the Snail family of transcription factors (Brabletz and Brabletz 2010). Each of these E-box-binding proteins can act as a transcriptional repressor through recruitment of the co-repressor, C-terminal binding protein (CtBP; Ctbp1) HDACs and BRGG1. In contrast to Snail proteins, ZEB factors (ZEB1 and ZEB2) are capable of interacting with the transcriptional co-activators p300 and pCAF and can subsequently switch to a transcriptional activator under still poorly defined conditions.
ZEB factors are strong inductors of epithelial-to-mesenchimal transition (EMT) by suppressing the expression of many epithelial genes, including E-cadherin, that is a key event in tumor invasion. Therefore ZEB proteins are increasingly recognized as important contributors to tumor progression and metastatic spread. A crucial activator of ZEB factors is the TGF-β signaling pathway, indicating that they are pivotal players in TGF-β-induced EMT. Forced expression of ZEB factors in epithelial cells results in a rapid EMT characterized by cell polarity impairment, loss of cellular adhesion and induction of cell motility. On the other end, ZEB factors knockdown in undifferentiated cancer cells induces a mesenchymal-to-epithelial transition (MET).
In a number of EMT model systems it has been shown that autocrine TGF-β signaling contributes to the stability of the mesenchymal state, moreover a TGF-β family members, TGF-β2 was shown to be targeted by miR-141/200a in cancer cells (Burk et al. 2008), leading to the hypothesis that repression of miR-200a during EMT may facilitate induction of autocrine TGF-β signaling.
In normal conditions ZEB1 is expressed in proliferating cells in the developing embryo, and in cell culture (Liu et al. 2007). Its expression is under the Rb/E2F cell cycle pathway. Mutation of the Rb or E2F1 genes lead to induction of ZEB1 mRNA, implying that the Rb–E2F1 repressor complex is important for repression of ZEB1. Vice versa, ZEB2 is also regulated by E2Fs, but in contrast with ZEB1 this regulation is Rb-family-independent. In keeping with these notions, ZEB1 mRNA was induced in mouse embryonic fibroblasts (MEF) from mice where all three Rb family member genes had been mutated; in contrast, expression of ZEB2 mRNA was not induced (Liu et al. 2007).
Moreover, ZEB1 is also a negative regulator of muscle differentiation; ZEB1, in fact, binds to a subset of E boxes in muscle genes and actively represses transcription (Postigo and Dean 1997). The relative affinity of ZEB1 for myogenic basic helix–loop–helix (bHLH) family proteins (i.e. myoD, myf-5, myogenin and MRF-4) varies for E boxes in different genes; as myogenic bHLH proteins accumulate during myogenesis, ZEB1 would be displaced from different genes at distinct times, thus providing a mechanism to modulate the timing of gene expression (Postigo and Dean 1997).
Data from several research groups pointed to the involvement of miR-200 family in EMT (Brabletz and Brabletz 2010); the entire miR-200 family is downmodulated in EMT and the most prominent target factors identified in all studies are ZEB1 and ZEB2. The miR-200 family can be divided in two subgroups according to their seed sequences (a conserved heptametrical miRNA sequence responsible for targets’ mRNAs recognition). Subgroup I: miR-141 and miR-200a and subgroup II: miR-200b, -200c and -429. The ZEB1 3′ UTR contains eight miR-200 binding sites (five for subgroup II members and three for subgroup I members), and the ZEB2 3′ UTR contains nine binding sites (six for subgroup II members and three for subgroup I members). Moreover, a double-negative feedback loop between miR-200 family and ZEB factors has been elucidated, ZEB factors, in fact, are transcriptional inhibitors of all miR-200 family members; as a result ZEB factors and miR-200 family miRNAs not only have opposite functions, but also reciprocally control the expression of each other (Brabletz and Brabletz 2010). Since ZEB factors are strong EMT inducers, the consequence of miR-200 over-expression is the reduced expression of ZEB factors and subsequent epithelial differentiation. Different studies demonstrated that in EMT miR-200 family down-modulation enhances cancer aggressiveness and metastases, whereas reintroduction of miR-200 family miRNAs reverted this phenomenon (Brabletz and Brabletz 2010).
ZEB1 in Apoptosis and Senescence
It has been well documented a role of ZEB1 both in apoptosis induction and in the establishment of senescence. In cortical neurons ZEB1 protein is induced by ischemia and activates a prosurvival response involving p73 proteins. ZEB1, in fact, is a transcriptional repressor of the pro-apoptotic isoform of p73, therefore ZEB1 induction is part of neuronal protective response to ischemia (Bui et al. 2009). Moreover, ZEB1 expression has also been shown to protect tumor cells from apoptosis. It is known that the induction of EMT is generally associated with reduced apoptosis and increased cell survival, as a consequence, cells that have undergone EMT are more resistant to toxic stress, including chemotherapy and radiotherapy (Brabletz and Brabletz 2010). In agreement, it has been shown that long-term exposure of breast cancer cells to TGF-β, a highly potent inducer of ZEB1 expression, induces EMT and inhibits apoptosis (Brabletz and Brabletz 2010). Further, knockdown of ZEB1 in pancreatic and colorectal cancer cell lines not only affects their stem-cell properties, but also increases the sensitivity of the cells to chemotherapeutic agents.
Moreover, ZEB1 had been shown to play a role in cellular senescence. Zeb1 mutant MEFs, in fact, revealed proliferation defects and undergo premature replicative senescence through a mechanism involving the up-regulation of p21 and p15INK4b expression, and not the INK4a locus (Liu et al. 2008). In keeping with these results, Liu et al. (2008) demonstrated that ZEB1 binds to p21 and p15INK4b promoters, inhibiting their expression. Notably, both CDK inhibitors take part in TGF-β signaling.
In line with the above described studies we found that ZEB1 knockdown in HUVEC had effects similar to those caused by miR-200c over-expression and by oxidative stress, i.e. induced cell growth arrest apoptosis and senescence (Magenta et al. 2011). Indeed, ZEB1 knockdown resembles the effects described in Zeb1 mutant MEFs, it induced cellular senescence, testified by SA-β-gal-positive cells and by the increase of p21 protein, in contrast we did not find a p16INK4a increase. We also demonstrated a direct implication of ZEB1 down-modulation in the establishment of miR-200c-mediated effects. In fact, the expression of a ZEB1 allele devoid of most-3′UTR sequence, and that therefore cannot be targeted by miR-200 family miRNAs, reverted miR-200c-induced phenotype, enhancing cell growth and inhibiting apoptosis and senescence (Fig. 14.2).
HUVEC were co-infected with lentiviruses encoding miR-200c and a ZEB1 lacking the 3′UTR (ZEB1Δ). Single infection either of miR-200c or ZEB1Δ was performed together with ZEB1Δ backbone vector (vec) or miR-scramble viruses, respectively. Control cells were co-infected with miR-scramble and vec viruses. (a) Representative western blot demonstrating the expression level of ZEB1 in cells co-infected with miR-200c and ZEB1Δ. miR-200c forced expression decreased ZEB1, this effect was inhibited in cells expressing ZEB1Δ. Tub, α-tubulin (n = 3) (b) HUVEC co-infected with miR-200c and ZEB1Δ showed a higher proliferation rate compared with miR-200c over-expressing cells (n = 5 at each time point;*P < 0.001; #P < 0.05). (c) The proapoptotic effect of miR-200c was abolished by the expression of ZEB1Δ (n = 3; *P < 0.05). (d) Cells co-infected with miR-200c and ZEB1Δ showed a decrease of the % of SA-b-gal-positive cells compared with miR-200c-infected cells (n = 3; *P < 0.001) (Magenta et al. 2011)
Oxidative Stress and ZEB1 Expression
We also investigated H2O2 effects on ZEB factors expression in HUVEC, given the role of miR-200 family in oxidative stress-induced phenotype. We found that upon H2O2 exposure both ZEB1 mRNA and protein expression were down-modulated and interestingly, ZEB1 mRNA was inversely related to miR-200c and miR-141 induction. Indeed, the lowest expression level of ZEB1 mRNA (73.7% reduction) was achieved after 16 h exposure to H2O2, that was the time point were miR-200c and miR-141 reached their maximum expression levels compared to control (29.3 and 23.3 fold induction respectively). ZEB1 protein down-modulation was also observed in HUVEC exposed to BCNU treatment and it was prevented by pre-treatment with the ROS scavenger NAC.
The H2O2 effect on ZEB2 protein could not be established because its protein level was undetectable in HUVEC. In contrast, ZEB2 mRNA was expressed, but the H2O2 effect was relatively minor, and no statistically significant difference from control was achieved.
In agreement with these results, miR-200c-overexpressing HUVEC exhibited a 53% decrease in ZEB1 mRNA, whereas ZEB2 mRNA decrease was minor and did not reach a statistical significance (Magenta et al. 2011).
The oxidative stress effects on miR-200 family expression were reproduced in vivo, in a mouse model of hindlimb ischemia. We found that miR-200c, miR-200b and miR-141 increased in ischemic skeletal muscles and the increase in miR-200c and miR-200b was markedly attenuated in p66ShcA−/− mice, which display lower levels of oxidative stress after ischemia, confirming the causal role of oxidative stress in the modulation of these miRNAs also in vivo (Magenta et al. 2011).
Discussion
Our work on oxidative stress and miRNAs underlined the role of miR-200 family and in particular of ZEB1 protein in ROS-induced cellular responses. We could establish a direct link between miR-200c induction by oxidative stress, and the establishment of cell growth arrest, apoptosis and senescence through a mechanism involving ZEB1 down-modulation. Indeed, we clarified the complex mechanism of miR-200 family induction. First of all we could establish that miR-200c induction was transcriptionally induced (Magenta et al. 2011). This was achieved demonstrating that the precursor of mature miR-141 and miR-200c, that are cotranscribed in a common precursor, pri-miR-200c-141 was up-regulated upon H2O2 exposure in HUVEC. Further, miR-200c and miR-141 common promoter was induced by H2O2, supporting the transcriptional regulation of these miRNAs; moreover the promoter mutated in ZEB1 binding sites was not significantly induced upon H2O2 exposure, suggesting that H2O2 induction of the promoter was largely mediated by ZEB1 down-regulation.
We also demonstrated that pRb and p53 were both involved in H2O2-induced miR-200c up-regulation. We showed that miR-200c increase upon H2O2 exposure was lower in MEF Rb−/− compared to MEF RbLoxP/LoxP, indicating a role of pRb in this induction. These data are in agreement with Liu et al. (2007) results that showed an increase of ZEB1 mRNA in MEF mutated in all three Rb family genes. It is tempting to speculate that ZEB1 expression could be elicited by the lack of pRb, inhibiting through the negative feedback loop miR-200c induction upon oxidative stress. We also demonstrated that miR-200c forced expression in HUVEC induced pRb dephosphorylation, and p21 expression, in line with the arrest of cell proliferation and establishment of senescence. Moreover, as further confirmation of these data, H2O2-induced dephosphorylation of pRb was partially prevented by miR-200c inhibition.
The results of our own studies and of previous reports indicate that in EC, H2O2 causes pRb dephosphorylation by different mechanisms: PP2A activity, p53 and p21 increase, and also by a miR-200c-dependent mechanism involving a ZEB1-mediated up-regulation of p21. As a consequence, H2O2-dependent pRb dephosphorylation inhibits E2F activity causing the down-modulation of ZEB1 mRNA, contributing to ZEB1/miR-200 double feedback loop reinforcement (Fig. 14.3). Further, we demonstrated a role of p53 in miR-200c induction by H2O2, since p53 knockdown markedly inhibited H2O2-dependent miR-200c up-regulation, and p53 over-expression induced miR-200c expression in EC, in keeping with other papers indicating a role of p53 in EMT, as p53 it is involved in miR-200c transcription (Chang et al. 2011; Kim et al. 2011). Therefore, H2O2-mediated p53 increase and pRb de-phosphorylation are both involved in miR-200c up-regulation, reinforcing ZEB1/miR-200-feedback loop (Fig. 14.3).
H2O2 causes pRb dephosphorylation by a PP2A dependent pathway and by the increase of p53 and p21, which inhibits the cyclin-dependent kinases (CDKs). pRb dephosphorylation, in turn, inhibits E2F activity down-modulating ZEB1 mRNA and consequently ZEB1 protein, allowing p21 transcription. Moreover, ZEB1 down-modulation induces miR-200c up-regulation because of the ZEB1/mir-200 inhibitory feed-back loop. miR-200c up-regulation is also caused by p53. Finally, miR-200c increase caused growth arrest and senescence, by pRb dephosphorylation and ZEB1 down-modulation, and apoptosis by ZEB1 decrease (Magenta et al. 2011)
We cannot rule out that other targets of miR-200 family are involved in the establishment of oxidative stress induced phenotype. Moreover it is also possible that other miRNAs that target ZEB1 could reinforce this mechanism. Indeed, another miRNA that is induced by p53, miR-192 (Georges et al. 2008) that has ZEB1 protein as a target, is slightly induced by H2O2 in our profiling in HUVEC.
An interesting link between miR-200 family and oxidative stress is also described in a recent article that showed a crosstalk between oxidative stress and miR-200 family (Mateescu et al. 2011). The authors confirmed miR-200 family induction following H2O2 exposure in different cell lines i.e., human and mouse immortalized fibroblasts, colon carcinoma (CT26), mammary gland epithelial cells (NMuMG) and human cell lines, melanoma cells (MDA-MB-435S), kidney cells (293T), breast adenocarcinoma (MDA-MB-436 and BT-549) and ovarian adenocarcinoma (SKOV3). Notably, in all these cell lines all miR-200s were upregulated (Mateescu et al. 2011). The authors found similar kinetics in fibroblasts and epithelial cells from mouse and human and, interestingly, miR-200c and miR-141 were the most up-regulated family members, as we found in HUVEC.
Mateescu et al. (2011) found that miR-141 and miR-200a, which display the same seed sequence, targeted p38 α mitogen-activated protein (MAP) kinase, which is a broadly expressed signaling molecule that participates in the regulation of cellular responses to stress, as well as in the control of proliferation and survival of many cell types. Indeed, p38α acts as a sensor of oxidative stress, and its redox-sensing function is essential in the control of tumor development (Mateescu et al. 2011). Enhanced expression of miR-200 family miRNAs mimics p38α deficiency and increases tumor growth in mouse models, but it also improves the response to chemotherapeutic agents. High-grade human ovarian adenocarcinomas, in fact, are associated with an oxidative stress signature and display high levels of miR-200a and low concentrations of p38α (Mateescu et al. 2011). Since p38α inactivation, pharmacologically or genetically, is known to induce ROS accumulation and activation of antioxidant defences, the authors unravel a new interaction between miR-200s and oxidative stress response that affects human ovarian carcinogenesis and prognosis.
In summary, miR-200 family and its targets play an important role in oxidative stress-induced cellular responses and also in the regulation of ROS production.
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Borras C, Gomez-Cabrera MC, Vina J (2011) The dual role of p53: DNA protection and antioxidant. Free Radic Res 45:643–652
Brabletz S, Brabletz T (2010) The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 11:670–677
Bui T, Sequeira J, Wen TC, Sola A, Higashi Y, Kondoh H, Genettea T (2009) ZEB1 links p63 and p73 in a novel neuronal survival pathway rapidly induced in response to cortical ischemia. PLoS One 4:e4373
Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9:582–589
Campisi J (2011) Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 21:107–112
Chang TC, Mendell JT (2007) MicroRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet 8:215–239
Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, Liu M, Chen CT, Yu D, Hung MC et al (2011) p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13:317–323
Cicchillitti L, Fasanaro P, Biglioli P, Capogrossi MC, Martelli F (2003) Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107, and p130. J Biol Chem 278:19509–19517
Cobrinik D (2005) Pocket proteins and cell cycle control. Oncogene 24:2796–2809
Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q (2011) miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem 286:25992–26002
Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15:247–254
Georges SA, Biery MC, Kim SY, Schelter JM, Guo J, Chang AN, Jackson AL, Carleton MO, Linsley PS, Cleary MA, Chau BN (2008) Coordinated regulation of cell cycle transcripts by p53-inducible microRNAs, miR-192 and miR-215. Cancer Res 68:10105–10112
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8:722–728
Gorospe M, Wang X, Holbrook NJ (1999) Functional role of p21 during the cellular response to stress. Gene Express 7:377–385
Ito T, Yagi S, Yamakuchi M (2010) MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun 398:735–740
Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM (2011) p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 208:875–883
Li G, Luna C, Qiu J, Epstein DL, Gonzalez P (2009) Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev 130:731–741
Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C (2009) Involvement of microRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem 284:7903–7913
Liu Y, Costantino ME, Montoya-Durango D, Higashi Y, Darling DS, Dean DC (2007) The zinc finger transcription factor ZFHX1A is linked to cell proliferation by Rb-E2F1. Biochem J 408:79–85
Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC (2008) Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 135:579–588
Magenta A, Fasanaro P, Romani S, Di Stefano V, Capogrossi MC, Martelli F (2008) Protein phosphatase 2A subunit PR70 interacts with pRb and mediates its dephosphorylation. Mol Cell Biol 28:873–882
Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC (2011) miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ 18:1628–1639
Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P, Sastre-Garau X, Mechta-Grigoriou F (2011) miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med 17:1627–1635
Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG (1999) The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313
Pei XH, Xiong Y (2005) Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene 24:2787–2795
Postigo AA, Dean DC (1997) ZEB, a vertebrate homolog of drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J 16:3935–3943
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W, Cook J, Harris CC, Gius D, Mitchell JB (2009) Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One 4:e6377
Tan G, Shi Y, Wu ZH (2012) MicroRNA-22 promotes cell survival upon UV radiation by repressing PTEN. Biochem Biophys Res Commun 417:546–551
Vecchione A, Croce CM (2010) Apoptomirs: small molecules have gained the license to kill. Endocr Relat Cancer 17:F37–F50
Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang W, Zou F (2010) Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res 1346:14–25
Yamakuchi M, Lowenstein CJ (2009) MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle 8:712–715
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105:13421–13426
Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, Biglioli P, Giorgio M, Martin-Padura I, Pelicci PG, Capogrossi MC (2004) p66ShcA modulates tissue response to hindlimb ischemia. Circulation 109:2917–2923
Acknowledgements
This work has been partly supported by Ministero della Salute (R.C.07-1.13, RF07-56.1, RC4.01, RF07 85.1, RF07 onc 26.1, RFS07).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Magenta, A., Capogrossi, M.C. (2013). Role of MicroRNAs and ZEB1 Downmodulation in Oxidative Stress-Induced Apoptosis and Senescence. In: Hayat, M. (eds) Tumor Dormancy, Quiescence, and Senescence, Volume 1. Tumor Dormancy and Cellular Quiescence and Senescence, vol 1. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5958-9_14
Download citation
DOI: https://doi.org/10.1007/978-94-007-5958-9_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5957-2
Online ISBN: 978-94-007-5958-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)