Abstract
p21Waf1/Cip1/Sdi1 is a potent inhibitor of cyclin-dependent kinases (CDKs) and one of the best characterized p53 direct target genes. Moreover, it is one of the principal inducers of senescence, a permanent arrest phenotype related to ageing and the prevention of cell transformation. It was initially thought that p21 had tumour suppressor properties, due to its ability to stop cell cycle at different stages, either transitorily or permanently. However, recent evidence points to a much more complex picture. It is now well established that p21 itself can trigger apoptosis in certain situations, even independently of p53. On the other hand, p21-mediated cell arrest can actually limit the sensitivity to apoptotic stimuli. To complicate matters even further, p21 has been shown to have other direct pro-survival functions and could even be contributing to tumourigenesis through the secretion of growth factors by arrested cells. At the core of these antagonistic functions of p21 is the generation of Reactive Oxygen Species (ROS), which have been shown to be important both in the induction of apoptosis and the establishment of senescence, and could also participate in the negative effects on tissue homeostasis of the senescent cell secretome. p21 has the ability to stop cancer cell growth and is not mutated in cancer. However, without a better understanding of its pleiotropic functions we will not be able to harness its clinical potential. It is therefore important to investigate the mechanisms by which p21 affects cell physiology and its use of ROS as messengers and effectors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The CDKN1a gene, localized on chromosome 6p21.2, encodes a 21 kDa protein that was discovered by three independent groups, which named it cyclin/cyclin-dependent kinases (CDKs)-interacting protein 1 (CIP1) (Harper et al. 1993), wild-type p53-activated fragment 1 (WAF1) (el-Deiry et al. 1994) and senescent cell-derived inhibitor 1 (SDI1) (Noda et al. 1994), according to the functions they observed it had. Thus, p21Waf1/Cip1/Sdi1 was known from the beginning to be a protein that played a role in the p53 pathway and the cell cycle arrest/senescence response. This placed p21 in a central position in the DNA damage and tumour suppressor mechanisms of the cell. However, in the following years it became clear that p21 functions were far more intricate than previously thought.
p21 is first and foremost a CDK inhibitor. It is a member of the CIP/KIP family, which also includes p27Kip1 and p57Kip2. They are all capable of inhibiting a broad spectrum of CDKs involved in cell cycle progression and therefore inducing an immediate arrest in different phases of the cell cycle (Sherr and Roberts 1999). In addition, p21 is one of the first direct target genes of tumour suppressor p53 that was characterized, to the point that p21 up-regulation is often used as a marker of activation of this pathway. After being induced by p53 in response to DNA damage and other stimuli, p21 mediates a transient cell cycle arrest. It also binds to proliferating cell nuclear antigen (PCNA), which affects DNA replication and repair processes. Moreover, it has been shown that, depending on the intensity and nature of the stress, this arrest can eventually become permanent as the cell enters a terminal differentiation state known as senescence. The role of p21 as regulator of this process was established when it was discovered that its expression increased in cultured human fibroblasts undergoing replicative senescence (Noda et al. 1994). In recent years, senescence has been identified as a major tumour suppressor mechanism and a determinant process in organismal ageing, and the central role of p21 in triggering it has been confirmed.
A part from its activity as a CDK inhibitor, p21 can also interact with transcription factors, proteins involved in the DNA synthesis and repair processes and apoptotic factors, among others. Because of this, p21 is involved in cell death, DNA repair, cellular differentiation and ageing. It is important to note that p21 can also be induced independently of p53 in response to stimuli such as TGFβ, Histone deacetylase (HDAC) inhibitors or oncogenes such as Ras. Recent studies have shown a completely different side of p21, in which it acts as a promoter of cellular proliferation by preventing apoptosis and inducing growth and pro-survival signals. This implies that p21 could actually be favouring cellular transformation instead of tumour suppression in certain situations. Considering all these aspects, the functions of p21 are still poorly understood. The mechanisms by which it induces senescence are not completely clear and the risk of triggering its potentially carcinogenic effects are a deterrent for exploiting these arresting capabilities in clinical settings.
The Complex Pleiotropy of p21
The Transcriptional Regulation of p21
The p21 promoter contains two highly conserved p53-responsive elements (p53-REs), to which p53 binds with high affinity. The two known p53 homologues, p63 and p73, can also transactivate p21 by binding to these p53-REs. External stress signals, including DNA damage and oxidative stress, activate p53, which in turn transcriptionally up-regulates p21. Similarly, intrinsic and oncogenic stresses activate p53 and subsequently p21 expression. Because of this downstream position of p21 in the p53 pathway, several proteins that play a role in the activation of p53 also contribute to p21 induction. For instance, BRCA1 functions as a p53-coactivator by recruiting p300/CBP, which in turn acetylates and stabilizes p53. In response to DNA damage, the propyl isomerase Pin1, the tumour suppressor LKB1and GADD34, a member of growth arrest and DNA damage-inducible proteins, facilitate p53 phosphorylation and therefore its transactivation capabilities. Cell division autoantigen 1 (CDA1) stabilizes p53 by inhibiting MDM2, an E3 ligase that ubiquitylates p53 and promotes its degradation. The monocytic leukemia zing finger (MOZ), a MYST-type histone acetyltransferase, induces p21 transcription by interacting with p53 and acetylating histones on the p21 promoter. NORE1A, a pro-apoptotic Ras effector, arrest cells via p21 by promoting the nuclear localization of p53. c-Myc activates p21 transcription through p19ARF which stabilizes p53 by inhibiting MDM2 activity. Interestingly, it has recently been shown that MDM2 not only degrades p53 through a ubiquitin-related proteasome pathway, but it is also able to target p21 to proteasome degradation independently of ubiquitin. All this shows that p21 expression can be modulated by many pathways that impinge on p53.
Other transcription factors induce p21 expression in a p53-independent manner. In the proximal p21 promoter there are several DNA-binding elements, including six Sp1/Sp3 binding sites. They are used to regulate p21 expression in response to various stimuli and stresses, including nerve growth factor (NGF), butyrate, phorbol myristate acetate (PMA) and TGFβ. The retinoblastoma protein, pRB, is also able to induce p21 through the Sp1/Sp3 sites and over-expression of integrin β1 subunit up-regulates p21 transcription by enhancing the recruitment of Sp1 to the p21 promoter. HDAC inhibitors are well-known p21 inducers, and they do so by enhancing histone acetylation around the p21 promoter and the Sp1 sites on the promoter itself, thus releasing the transcriptional repressor HDAC1 from its binding. FOXP3, an X-linked tumour suppressor, induces p21 expression by inhibiting the association of HDAC2 and HDAC4 to a site within the intron 1, and thus by increasing the local histone H3 acetylation. The Ski-interacting protein SKIP/SNW1(SKIP) is a transcription elongation factor critical for both basal and stress-induced p21 expression, confirmed by the fact that depletion of SKIP induces a rapid down-regulation of p21.
Moreover, retinoid X receptor (RXR) activates p21 expression through binding to two consensus RXR-responsive elements in the p21 promoter, and it has recently been shown that androgen receptor induces p21 independently of p53 in order to trigger a senescent response. Tumour suppressor IGFBP-rP1 (Insulin-like growth factor-binding protein related protein 1) is also capable of inducing senescence through p21 and bypassing p53. Intriguingly, caspase 2, which has still poorly characterized functions, is a translational regulator of p21 and participates in its induction after damage. p21 can be also transcriptionally repressed. For instance, c-Myc alleviates G1 cell cycle arrest via p21 repression by sequestering Sp1 from the p21 promoter. Also, CUT, a homeodomain transcription factor, blocks p21 expression via binding to a sequence that overlaps with the TATA box.
There are eight different p21 splice variants in humans, seven of which (p21V1, p21V2, p21C, p21 alt-a, p21 alt-a′, p21 alt-b, and p21 alt-c) encode for protein p21, while p21B encodes for an entirely different protein (also called p21B). The promoters for p21 and p21B contain common response elements for transcription factors, whereas some response elements are found exclusively in the p21 promoter. A part from the cyclin-cdk interacting region, p21 also has binding domains for procaspase-3 (in the N-terminal region) and PCNA (in the C-terminal region) binding domain, as well as a nuclear localization signal.
The Role of p21 in Cell Cycle Arrest
Upon exposure to growth stimuli, a series of CDKs, including CDK4, CDK6 and CDK2, are sequentially activated during the cell cycle, and this leads to cell proliferation. p21 was originally found in cyclin A, cyclin D1, cyclin E and CDK2 immunoprecipitates (Harper et al. 1993). It was proposed that p21 arrested cells by affecting the activity of cyclin D-, E- and A-dependent kinases, which regulate progression through the G1 phase of the cell cycle and initiation of DNA synthesis (Sherr and Roberts 1999). pRB is essential for the G1-S transition. pRB phosphorylation is initiated by cyclin D-CDK4/6 and phosphorylated pRB is then released from complexes with E2F transcription factors. These are then able to transactivate genes necessary for entry into S phase, including cyclin E. The resulting activation of the cyclin E-CDK2 complex further phosphorylates and completely releases pRB from its interaction with E2Fs. The association of p21 with cyclin D-CDK4/6 inhibits pRB phosphorylation and thus induces cell cycle arrest in G1. Recent studies suggest that Rb2/p130, a protein that belongs to the same family as Rb, is actually the one that mainly participants in the p53-p21 arrest response after damage (Helmbold et al. 2009).
p21 also associates with and directly inactivates E2F, thus mediating its degradation (Broude et al. 2007b). This is confirmed by the fact that p21 mutants that cannot block cyclin/CDK activity are still capable of inhibiting E2F transactivation. In a similar fashion, it has been proposed that p21 can also reduce p53 stability in some models (Broude et al. 2007a). This would provide a negative feedback loop to limit p53 induction and thus facilitate a recovery of the cell cycle once the transient arrest is resolved. p21 can also arrest cells in the S phase of the cell cycle. Since it forms quaternary complexes with a cyclin, a CDK and PCNA, this binding to PCNA interferes with DNA replication and triggers intra-S phase arrest. This is mainly due to the fact that the p21 binding site in PCNA overlaps with that of the DNA polymerase δ and the replication factor C. p21 may probably control both cyclin-CDK and PCNA activity within the quaternary complex providing a regulatory link between DNA replication and cyclin-CDKs complexes required for the S phase.
Finally, it has also been reported that p21 can arrest cells in G2 (Roninson 2002). This observation has been linked to the ability of p21 to inhibit both cyclin B-CDK1 and cyclin A-CDK1/2 complexes, which leads to a permanent cell cycle exit in G2. Furthermore, p21 cooperates with 14-3-3σ, which sequesters cyclin B-CDK1 complexes in the cytoplasm, and thus controls the G2-M transition. Of note, the ability of p21 to induce a G2 arrest is not shared by other CDK inhibitors, like p16.
When normal proliferating cells are subjected to DNA damage, the cell cycle temporarily pauses either at G1, S or G2 phase, due in great part to p53-dependent p21 up-regulation. Arrest at these checkpoints prevents DNA replication and mitosis in the presence of unrepaired DNA damage and presumably allows time for DNA repair to occur. The proportion of cells that arrest in G1, S or G2 after damage depends on cell type, growth conditions, type of damage and the checkpoints operative in the cells. Because of this, p21 is a necessary mediator of p53-induced cell cycle arrest (el-Deiry et al. 1994), as indicated by the fact that p53 cannot arrest cells after DNA damage in p21-null mice. p21−/− cells that fail to arrest in G1 even after up-regulation of p53 enter mitosis regardless of the presence of DNA damage or chromosome aberrations.
Since p53 function is lost in most human cancers, p21 induction in response to stress is compromised. Because of this, neoplastic cells often have a defective G1 checkpoint response to DNA damaging agents. Although p21 also participates in the arrest at the G2 checkpoint, this can also be achieved by the activation of the Chk1 protein kinase, which maintains mitotic cyclin B/Cdc2 complexes in an inactive state. Thus, cancer cells are usually able to stop in G2 even in the absence of p53-p21 activity (Macip et al. 2006).
The ability of p21 to trigger arrest in different phases of the cell cycle allows p21 to participate in the maintenance of genomic stability. As we will comment below, the role of p21 in the induction of senescence following telomere shortening or certain kinds of stress further corroborates this view. Adding to this, p21 also plays a significant role in modulating DNA repair processes. By temporarily inhibiting cell cycle progression, p21 allows cell survival while DNA repair is completed. Also, p21 can compete for PCNA binding with several proteins that are directly involved in DNA repair processes and modulate PCNA-dependent DNA repair pathways. Finally, it has recently been shown that p21 regulates the Fanconi Anemia-BRCA1 repair pathway and thus plays an active role in repair processes following exposure to DNA crosslinking agents, which leads to accumulation of chromosomal aberrations in p21 null cells (Rego et al. 2012).
p21 Affects Gene Transcription
Even though p21 is not a transcription factor, some of p21 interactions have an effect on gene expression. In particular, p21 indirectly inhibits E2F1 factors, via pRB dephosphorylation after CDK inhibition, but also directly by binding to them. Moreover, p21 is also able to bind other transcription factors such as STAT3 and Myc, thereby inhibiting their transactivation properties. Thus, p21 may act as a transcriptional cofactor, being able to interact directly with DNA-binding proteins and modulate the activity of the co-activators and co-repressors that associate with them. p21 has also been shown to stimulate NFκB-mediated transcription. This effect is mediated by the activation of transcriptional cofactors p300 and CBP, which enhance not only NFκB but also many other inducible transcription factors (Roninson 2002). p21 can induce the expression of 55 genes, while it represses the expression of 77, including Polo-like kinase 1 (PLK1) and Topoisomerase IIα (TOPO IIα) (Chang et al. 2000b).
To Kill or Not to Kill?
Through its ability to promote cell cycle inhibition, especially after genotoxic insults, p21 is paradoxically preventing cells from undergoing apoptosis. Besides, it has been proposed that p21 could also block apoptosis directly. p21 is implicated in the Nrf2-mediated antioxidant pathway which is considered important for the pro-survival effects of p21 (Chen et al. 2009). Another indication of its anti-apoptotic potential is the observation that p21 is cleaved by caspase 3 at the onset of apoptosis. p21 also blocks the induction of pro-apoptotic genes by Myc and E2F1 through direct binding and inhibition of their transactivation functions. Thus, the ability of p21 to induce cell cycle arrest could be a double-edged sword that could actually act in detriment of the tumour suppressor mechanisms of the cell in certain situations.
Perhaps surprisingly, we and others have shown that p21 can also trigger cell death responses (Inoue et al. 2009; Masgras et al. 2012), although the mechanisms involved in these processes have not been fully elucidated. Tsao et al. (1999) showed that viruses expressing p21 could induce apoptosis as well as cell cycle arrest in cervical cancer cell lines. Consistent with this, p21 can sensitize cells to undergo apoptosis, as shown by the fact that p21 loss in colorectal and ovarian cancer cells or hepatocytes significantly reduced cell death in response to chemotherapy. Also, p21 enhances ceramide-induced apoptosis by increasing Bax expression and antagonizing Bcl2. It has been proposed that p21 can induce pro-apoptotic effectors such as Bax or members of the TNF family (Gartel 2005), as well as p53 (Inoue et al. 2009). However, we have shown that p21 can also induce apoptosis in sensitive cells independently of p53 through a ROS-dependent mechanism (Fig. 13.1). Consistent with this, up-regulation of p21 induces apoptosis in human cervical cell lines independently of p53 and pRB, and retinoic acid triggers senescence via RARβ2-mediated hypomethylation of the p21 promoter.
Increase in intracellular ROS correlates with p21 induction of apoptosis in HT1080p21-9. (a) PI-stained HT1080p21-9, uninduced or 5 days after p21 upregulation, in the presence or absence of 10 mM of the antioxidant NAC. Percentages indicate SubG1 events (dead cells). Right: the same cells stained with DCF (ROS measurement). (b) Immunoblot analysis of protein levels in HT1080p21-9 with p21 induced for 0 (c) to 3 days (From Masgras et al. 2012)
A part form apoptosis, p21-mediated depletion of proteins that control cell division can lead to abnormal mitosis and genetic destabilization when arrested cells attempt to re-enter cell cycle after p21 down-regulation, causing death by mitotic catastrophe independently of p53 or the apoptotic pathway (Chang et al. 2000a). At the same time, p21 could be inhibiting other pathways, like autophagy, to favour cell death. p21 is known to up-regulate Nrf2 by interfering with Keap1-mediated Nrf2 ubiquitination, and this triggers a series of cytoprotective effects. Moreover, p21-null mice are sensitive to hyperoxic lung injury and to lipopolysaccharide (LPS)-induced endotoxic shock, suggesting that p21 indeed participates in cell survival pathways.
In view of these seemingly paradoxical results, it is logic to hypothesize that cell fate decisions after p21 induction, ranging from survival to death, largely vary depending on the cellular context. The magnitude of ROS induced by p21 and the involvement of a p21/ROS/p53 feedback signalling pathway will surely play a role in these decisions, as we will comment below (Inoue et al. 2009; Masgras et al. 2012). It has also been proposed that the number of molecules of p21 relative to the cyclin-CDK complex may dictate the ultimate response of a cell to p21.
Post-translational modifications of p21 and its localization may also affect its effects. p21 is post-translationally regulated by various serine and threonine kinases and this determines cell- and context-dependent dissociation of p21 from PCNA, translocation of p21 from nucleus to cytoplasm and stabilization or degradation of the protein. p21 phosphorylation at Thr145 by activated AKT1 or IKKβ signalling prevents the nuclear translocation of p21. Serine and threonine kinase Pim-1 stabilizes p21 protein and instead induces its shift from nucleus to cytoplasm. Whereas the growth-inhibitory functions of p21 are associated with its nuclear localization, the anti-apoptotic activities of p21 are frequently associated with its cytoplasmic accumulation. Cytoplasmic p21 binds to and inhibits the activity of proteins directly involved in the induction of apoptosis, including procaspase 3, caspases 8 and 10, stress-activated protein kinases (SAPKs) and apoptosis signal-regulating kinase 1 (ASK1), while at the same time it promotes cellular proliferation through both the alleviation of CDKs and PCNA inhibition.
The Dark Side of p21: Can It Act as an Oncogene?
As we have discussed, the main function p21 is to stop cell proliferation and, in some cases, even induce apoptosis. Because of this, it seems that its place in the complex network of tumour suppressor mechanisms of the cell is guaranteed. This is supported by the fact that, although p21 is never mutated in tumours, it can be down-regulated by several miRNA that are expressed in cancer cells (Borgdorff et al. 2010). However, while p53 and p16 are two of the most commonly inactivated genes in human cancer, it is usually difficult to correlate the status of p21 to tumour progression or to a prognostic value. In fact, p21 protein levels are often elevated in some cancers without signs of growth inhibition (Abbas and Dutta 2009) and a recent study showed that lack of p21 expression in colorectal tumours could be a good prognostic factor (Lin et al. 2012). Prolonged p21 induction has been related to events of abnormal mitosis and endoreduplication in recovering cells (Chang et al. 2000a). These events may conceivably lead to genetic destabilization and thus play a role in carcinogenesis and tumour progression of the cells that re-enter into the cell cycle after having had p21 induced.
Several genes induced after p21 up-regulation encode for secreted proteins with paracrine effects on cell growth and apoptosis (Chang et al. 2000b). In particular, it has been reported that conditioned media from p21-induced cells has anti-apoptotic and mitogenic activity and, consequently, the effect of p21 on the cell secretome may contribute to the onset of cancer. Thus, even in the situations where p21 is inducing cell cycle arrest, this may be accompanied by a paracrine growth-stimulatory effect on the surrounding cells. We will further discuss this phenomenon below in the context of senescence.
Because of all this, it has been proposed that p21 can actually favour transformation in certain contexts by inhibiting apoptosis and inducing growth and pro-survival signals, genomic destabilization and expression of secreted mitogenic factors. It is not well understood mechanistically how p21 could exert these radically different functions or even if they reside in separate domains of the protein. Due to the difficulty of selectively activating its tumour suppressor properties without also inducing the potentially oncogenic features, the design of antineoplastic therapies involving p21 regulation has so far been unsuccessful (Abbas and Dutta 2009).
Senescence and Tumourigenesis
The Importance of Senescence in Preventing Cancer
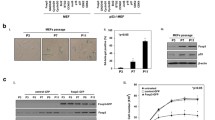
Senescence is a terminal differentiation state in which metabolically active cells are permanently arrested with distinctive morphological changes and phenotypic markers (Dimri et al. 1995). Senescent cells look distinctively flat and elongated under the microscope and are incapable of dividing despite the presence of nutrients and growth factors in the medium (Fig. 13.2a). Once a permanent cell-cycle arrest is established, cells remain viable for long periods of time but never regress to their original state. This phenomenon, named replicative senescence, was first observed after serial cell passaging as cells got older, and it was seen that it is a consequence of telomere shortening and/or dysfunction (Campisi et al. 2001).
p21-mediated ROS increases are necessary for maintaining the permanent growth arrest in senescent cells. (a) 501 T human fibroblasts infected with a retrovirus containing p21 and selected with puromycin for 1 week, showing morphology changes and growth inhibition compared to the vector-infected cells. (b) ROS levels of EJp21 cells (I) in the absence of exogenous p21 expression, (II) after 5 days of p21 induction and (III) after 5 days of p21 induction with the addition of 10 mM NAC. (c) Percentage of SA-β-gal positive cells in EJp21 induced to express p21 for 5 days in the presence or absence of 10 mM NAC. (d) Colony formation assay with cells maintained in the presence of induced p21 for 0–5 days before p21 expression was suppressed (From Macip et al. 2002)
Shortened and/or damaged chromosome telomeres provided the first molecular explanation for the senescence of cells in culture and it has been shown that they triggered senescence through the p53-p21 pathway. Telomere shortening can be reversed by telomerase, the ribonucleoprotein responsible for telomere lengthening during replication. Telomerase activation is actually one of the major mechanisms through which immortal tumour cells overcome the finite lifespan determined by the barrier of replicative senescence. In vivo, senescent cells increase with age in various tissues and in age-related diseases including atherosclerosis and diabetes.
It was later discovered that senescence can also be prematurely triggered by oncogenes and DNA damage. This senescent phenotype slowly develops over the course of 5–10 days after the stimulus. It has been progressively evident that, apart from its impact on ageing, senescence acts as a tumour suppressor mechanism by controlling the emergence of immortal cells. This alternative type of senescence is mainly due to stress agents and has been called stress-induced premature senescence (SIPS). Also, excessive mitogenic signals, like those produced by oncogenes, also induce a premature senescence in normal cells, and this is known as oncogene-induced senescence (OIS). These forms of premature senescence have also been observed in vivo. For instance, senescent cells can be observed after chemotherapy and senescent cells have been found in benign nevi but not in the melanomas that arise from them. All this confirms that senescence is a mechanism to prevent malignant progression.
Like apoptosis, senescence is an extreme response to cellular stress and it is important to control damaged cells. It is still unclear what determines whether cells will undergo senescence or apoptosis after damage. Among the determinants that have been characterized there are the cell type and the nature, intensity and duration of the damage. The ability to undergo senescence has been primarily viewed as a property of normal cells, which is lost during neoplastic transformation. However, phenotypic and proliferative changes that resemble terminal stages of replicative senescence can apparently be induced in tumour cells not only through genetic modifications but also by treatment with different classes of chemotherapeutic agents. These findings suggest that certain tumour cells have retained at least some of the components of the senescence-like program of terminal proliferation arrest and that this can be exploited in chemotherapeutic treatments.
The Senescent Phenotype
The principal hallmark of cellular senescence is the inability of cells to progress through the cell cycle. Senescent cells arrest growth normally in G1 phase, and they fail to initiate DNA replication despite adequate growth conditions. However, they remain metabolically active and they may produce secreted proteins with important effects on neighbouring cells and tissues. In contrast to quiescence, the senescence growth arrest is essentially permanent and irreversible, since senescent cells cannot be stimulated to proliferate by known physiological stimuli. Another characteristic change in the phenotype of senescent cells is the development of resistance to apoptosis, although not all cell types display it. Senescence also causes an altered pattern of gene expression, including changes in cell-cycle inhibitors or activators. The two genes that are more often up-regulated in senescent cells are p21 and p16, and they have been used as markers.
Several other markers can identify senescent cells in culture and in vivo. For instance, lack of DNA replication, which can be detected by the incorporation of 5-bromodeoxyuridine or 3H-thymidine, or by immunostaining of proteins such as PCNA and Ki-67. Of note, these detect arrested cells but do not distinguish between senescent, quiescent and differentiated post-mitotic cells. The first marker to be used for the specific identification of senescent cells was the SA-β-gal (Dimri et al. 1995). SA-β-gal activity is thought to be due to the lysosomal β-galactosidase, encoded by the GLB1 gene, and reflects the increased lysosomal biogenesis that commonly occurs in senescent cells. The presence of senescence-associated DNA-damage foci (SDFs) and senescence-associated heterochromatin foci (SAHFs) are other possible markers.
The Side-Effects of Senescence
In spite of the obvious advantages that senescence has in tumour suppressor mechanisms, it has been proposed that it can also have negative effects on the homeostasis of the organism. First, cellular senescence has been linked to tissue and organismal ageing, and thus may play a role in the degenerative and cancerous pathologies related to it. It could also interfere with the effects of chemotherapy by blocking apoptosis.
An altered secretome is emerging as one of the most puzzling aspects of the senescence program. It has a potential wide-ranging impact on a tissue’s function, response to damage, and tissue degeneration. Initial studies correlated the senescent phenotype to a paracrine mitogenic and anti-apoptotic effect on the surrounding cells (Chang et al. 2000b). The senescence-associated secretory phenotype (SASP) is complex and includes factors that reinforce senescence-associated proliferation arrest, immune regulators, factors that remodel the extracellular matrix and probably reactive oxygen species that could cause oxidative stress in surrounding cells. Thus, senescent cells might seriously impair tissue function through their secretions. Moreover, although senescent cells cannot themselves form tumours, they may fuel the progression of nearby premalignant cells and facilitate the development of cancer in ageing organisms. A part from these potentially tumourigenic effects, it has been observed that senescent cells also secrete immune regulators that trigger the clearance of incipient cancer cells by the innate immune system. Thus, the real impact of the SASP on the growth of neighbouring cells may be dependent of the context and still needs to be fully elucidated.
How to Induce a Permanent Growth Arrest
Senescence is usually achieved by the activation of the p53-p21 and/or pRB-p16 pathways, which interact with each other but can also independently stop cell cycle progression. Different stimuli and cell-type specific factors may lead to the activation of one pathway rather than the other. For instance, the p53 mediated DNA damage response can induce a temporary arrest through p21 that, if the stimulus persists, can eventually turn into senescence. Similar stresses can also engage the pRB-p16 axis, but this is usually secondary to the activation of the p53. However, other factors, like the expression of oncogenic Ras, preferentially act through pRB-p16. The ability of p21 to trigger senescence was confirmed by the fact that p21 is capable of inducing a permanent arrest in normal and cancer cells in a p53 independent manner.
The importance of the pRB and p53 pathways in senescence is emphasized by the fact that inactivation of both usually abolishes the permanent proliferation arrest, regardless of the initial trigger. Despite this, there are some examples of senescence that appear to be independent of these pathways. The mechanisms involved in these situations have not been yet clearly identified. It has been proposed that genotoxic stresses can induce senescence in p53-null as well as wt p53-containing cancer cells (for review see Roninson 2003) and that this response plays a role in the suppression of tumour growth by chemo and radiotherapy. However, recent studies have indicated that cancer cells without functional p53 pathways do not undergo a proper senescence response after being treated with a variety of chemotherapeutic agents (te Poele et al. 2002; Macip et al. 2006). Thus, it is not clear whether what has been traditionally considered a G2-based senescence, observed in p53 null tumour cells after treatment with genotoxic drugs, is really a permanent arrest phenotype comparable to that observed in cells with intact p53-p21 activity.
p21 expression is progressively increased in human fibroblasts in culture, reaching a peak when these cells undergo senescence (Noda et al. 1994). However, this increase is only transient and its levels usually decrease after the establishment of the permanent growth arrest. As p21 level goes down, p16 becomes constitutively up-regulated, suggesting that while p21 acts as a trigger, p16 may be responsible for the maintenance of growth arrest in senescent cells. However, a fail-safe mechanism is also induced in the form of a stable increase in intracellular levels of ROS. We have shown that blocking p21-mediated ROS generation prevents the establishment of a permanent arrest and cells resume the cell cycle once p21 levels return to basal levels (Fig. 13.2). Thus, ROS seems to be an important part of the mechanism by which p21 is able to maintain senescence.
The Importance of Relative Oxygen Species (ROS) in Cell Fate Decisions
Reactive Oxygen Species
ROS are generated by normal oxidative processes related to cell metabolism and buffered by antioxidant mechanisms. They are produced initially by the reduction of singlet O2 to superoxide anion and then H2O2 that, if not eliminated, generates the highly reactive hydroxyl free radical that causes DNA damage. Metabolic processes, primarily oxidative metabolism in the mitochondria, and pathological processes, such as inflammation and ischemic reperfusion, are the major endogenous sources of ROS (Bai and Cederbaum 2003). They can also be generated by specific plasma membrane oxidases in response to growth factors and cytokines and serve as secondary messengers in signalling pathways. Both Ras and p53 cause ROS accumulation, which play a role in subsequent cell fate responses (Lee et al. 1999; Macip et al. 2003).
Depending on the level of oxidative stress and the extent of the induced DNA damage, cell fate can vary from temporary arrest to death (Barzilai and Yamamoto 2004). For instance, exposure to H2O2 has been shown to induce apoptosis or necrosis depending on concentrations and cellular context (Hampton and Orrenius 1998), whereas low concentrations of oxidants can force normal human fibroblasts to permanently arrest in a senescent-like state (von Zglinicki et al. 1995). The impact of ROS on cellular structures such as DNA and mitochondria depends not only on the magnitude of ROS but also on other physiological factors, for instance the active anti-oxidant mechanisms of the cell. Thus, the threshold of cellular oxidation may vary between different cell types and between normal and cancerous cells.
It is also known that senescent cells have higher levels of ROS than normal cells. Moreover, overexpression of antioxidant genes like Superoxide Dismutase or Catalase causes extension of lifespan in Drosophila. This effect can also be observed in cell cultures maintained in low oxygen environments. All of these findings point to a strong relationship between oxidative damage, senescence and ageing. Of note, ROS present at normal physiological levels play a role in regulating signalling pathways and gene expression, and therefore, their production is of vital importance for cellular homeostasis.
Relative Oxygen Species Modulate Cell Fate Decisions
ROS have been implicated as potential modulators of apoptosis that act downstream of p53. Rather than being just a consequence of the cellular changes associated with apoptosis, p53-generated ROS have been shown to constitute a signal for cell death pathways, presumably mediated by the transcriptional influence of p53 on pro-oxidant genes. One of the classic models for p53-induced apoptosis proposes that p53 triggers cell death through a multistep process that requires transcriptional induction of redox-related genes, formation of reactive oxygen species and oxidative degradation of mitochondrial components.
We have shown that ROS are actually important in determining cell fate after p53 up-regulation, with the redox balance of the cell even being able to turn an initial arrest response into apoptosis (Macip et al. 2003). Moreover, we have reported that p21 can increase ROS levels independently of p53 and that this is required for the permanent arrest observed in senescence. Recent evidence suggests the existence of a threshold of cellular oxidation above which the apoptotic program is initiated (Inoue et al. 2009; Macip et al. 2003). In view of this, a model of p53-p21 activity could be argued, in which cell senescence is triggered via a persistent lower magnitude of ROS accumulation while apoptotic cell death is induced in response to a higher, and sometimes faster, increase in ROS. The intrinsic resistance of the cell to oxidative stress, and particularly that of its mitochondria, may also play a decisive role.
Mitochondria and Relative Oxygen Species in Senescence
Mitochondria are highly dynamic organelles prone to fusion and fission processes. Mitochondrial DNA and proteins can be continuously exchanged and distributed throughout the whole mitochondria population, suggesting that fusion and fission processes may function as a rescue mechanism for damaged mitochondria. The number of mitochondria in dividing cells is thought to be maintained constant through de novo biogenesis and degradation of damaged mitochondria. Some studies have reported an increase in the mitochondrial mass and mitochondrial DNA copy number related to the senescent phenotype. Moreover, mitochondria in senescent cells are elongated due to a decreased expression of Drp1 and Fis1, and it is thought that this protects them against oxidative stress. Increase in mitochondrial mass has also been observed prior to release of cytochrome c from the mitochondria and apoptosis, which suggests that these different mechanisms of tumour suppression could have some common mechanisms of initiation.
These changes in the mitochondrial biogenesis process have been correlated to a mechanism termed retrograde response, a nuclear response to mitochondrial dysfunction that has been well characterized in Saccharomyces cerevisiae but which is not fully understood in mammalian cells. The retrograde response includes deregulation of Ca2+-dependent signalling, up-regulation of mitochondrial biogenesis and major metabolic and anti-apoptotic adjustments. Calcium appears to play an important role in this process as there is an increase in the cytoplasmic Ca2+ levels because of the low Ca2+ storage capacity of dysfunctional mitochondria with low membrane potential. Such changes in mitochondrial metabolism are most probably an attempt at adaptation of cells to senescence-associated high levels of ROS.
As cells age, mitochondria increase their superoxide production and it is thought that this contributes to replicative senescence. Also, dysfunctional mitochondria that accumulate around the nucleus are thought to participate in oncogene-induced senescence after mutant Ras expression, with the increase in mitochondria mass and ROS generation being dependent on intact p53 and pRB pathways. Furthermore, the interconnected mitochondria phenotype and the down-regulation of the fission mechanism are accompanied by major resistance to apoptosis of senescent cells. This further demonstrates that mitochondria of senescent cells have evolved mechanisms of protection against the increased levels of ROS.
The pathways leading to the establishment of senescence by p21 and ROS are complex and not fully understood. However, an elegant study by Passos et al. (2010) has defined the implication of a dynamic feedback loop triggered by a DNA damage response, which locks the cell into an actively maintained state of “deep” cellular senescence. According to this, high levels of ROS may damage mitochondria, leading to the opening of mitochondrial pores and allowing the influx of protons and ions. Thus, increased mitochondrial membrane permeability leads to loss of the proton ion gradient across the mitochondrial membrane and to the consequent decrease in potential which, in turn, may increase ROS production. Since damaged mitochondria generate more ROS, this suggests a self-amplifying feedback cycle. The mechanisms involved in mitochondrial dysfunction are likely to be common to all types of cellular senescence regardless of the trigger, since these changes have been detected in both telomere-dependent replicative senescence and premature senescence due to oncogenic stress. Their results also suggest that during the early establishment phase of senescence, the presence of DNA damage foci, replenished by the increase in ROS, is necessary to maintain growth arrest long enough for the process to become irreversible. All these data together supports the hypothesis that senescence could be a prolonged arrest phenotype sustained by the self-maintained DNA damage caused by a permanent elevation in intracellular ROS. Genes such as p53 or p21, capable of definitely shifting the oxidative balance of the cell towards increased ROS levels, possibly through engagement of mitochondria, could therefore hold the key to the induction and preservation of the phenotype.
Discussion
It is no doubt surprising that 20 years after its discovery, we are still not able to fully describe the basic functions of p21. What initially seemed like a straightforward CDK inhibitor with links to tumour suppressor pathways has become the picture of a complex network of interactions and cell fates, some of them radically opposed. It is even difficult sometimes to place p21 in one of the sides of the pro- and anti-neoplastic balance in the cell. There is obviously a strong dependence on the cellular context when it comes to decide cell fates after p21 induction and we will need more research to understand exactly what p21 is doing in a given model and why. It seems that ROS are important mediators of more than one of p21 functions, and their intracellular balance may hold the key to solve this riddle.
p21 can increase ROS in both normal and tumour cells proportionally to its protein levels, which shows a direct link between these two events (Macip et al. 2002). However, the mechanism implicated is still not clear. It is not just a consequence of cell cycle arrest, since p16 is not able to induce ROS. Prolonged expression of p16 induces a senescence-like arrest in cancer cells, but following p16 down-regulation this was found to be reversible (Macip et al. 2002). These and other findings strengthen the hypothesis that ROS accumulation is necessary for the permanent growth arrest phenotype induced by p21.
p21 blocks cell cycle progression in both G1 and G2 through inhibition of several CDKs, as well as PCNA. These functions, which reside in different domains of the protein, are each sufficient to cause growth arrest. In contrast, p16 specifically inhibits CDK 4 and 6 and has no effect on PCNA. Therefore, it was hypothesized that this could be the critical difference that allows p21 but not p16 to induce ROS. However, a p21 mutant that lacks PCNA binding ability retains the ability to induce growth arrest specifically in G1, implying that PCNA binding is required for maintenance of the G2 arrest function of p21. This mutant p21 can also induce ROS accumulation and senescence, therefore binding to PCNA and its G2 arrest function are not required for these responses.
Fibroblasts undergoing replicative senescence exhibit increased ROS, p16 and p21 levels, although p21 up-regulation is only transient. The use of ROS increases as mediator of the permanent growth arrest, which then becomes independent of the presence of p21 or p16, provides a possible explanation for these observations. p21 can also induce a p53-independent cell type-specific and, at least in part, ROS-dependent apoptotic response (Masgras et al. 2012). This confirms that the elevation of intracellular ROS levels is an important part of the mechanism by which p21 exerts its functions.
The source for p21-mediated ROS is still not clear. Some results point at a mitochondrial origin, maybe through induction of mitochondria-related genes. Since p21 can act as a modulator of transcription, this could explain its effect on the redox balance of the cell. Consistent with this, we observed that p21 induced up-regulation of PIG3, a pro-oxidant gene (Macip et al. 2002). We have also observed that p21 induction of cell death is not immediate and required prolonged expression of p21, which could reflect the necessity to accumulate sufficient intracellular ROS to trigger a certain amount of mitochondrial damage.
According to this model, short-term expression of p21 would induce cell cycle arrest, while apoptosis would only be achieved at a later time point if the stimulus is maintained and a threshold of oxidative stress is surpassed. The difference between inducing senescence or apoptosis could be then determined by the intensity of the stimulus, the levels of p21 induced and, importantly, cell-specific factors. p21 levels could indeed have a dose-dependent effect in cell fate decisions (Inoue et al. 2009). However, protein levels are not necessary determinant, since similar p21 induction can cause different effects depending on cell context (Masgras et al. 2012).
The sensitivity of mitochondria to oxidative stress is likely to be one of these factors. Recent data showed that cancer cells with primed mitochondria respond better to cell death stimuli (Ni Chonghaile et al. 2011). The apoptotic functions of p21 are likely to be preferentially observed in those cancer cells that have accumulated higher mitochondrial damage or defects in the intracellular/mitochondrial ROS buffers. Otherwise, cells would choose to undergo a less drastic response in the form of senescence. Cell fate decisions after p21 up-regulation would no doubt be better understood if we could determine cell sensitivity to oxidative stress and, specifically, which factors make mitochondria resistant to the elevation in intracellular ROS.
Since normal cells usually have intact antioxidant and DNA repair mechanisms, therapies that up-regulate p21 could have the potential to be more toxic for sensitive cancer cells. For instance, compounds that induce p21 independently of p53, like MLN4924, could trigger cell death in p53-null cancer types. The mitochondrial response to ROS could be a predictive marker of cancer cell sensitivity to p21. However, we have to keep in mind that p21 may have several pro-survival effects that could interfere with its tumour suppressor activity. Because of this, there are no antineoplastic therapies specifically directed at up-regulating p21 expression in cancer cells, despite its promising potential. The antitumoural functions of p21 could be enhanced if its abilities to cause cell death were favoured over induction of arrest and senescence, and thus the negative effects of the senescent cell secretome could be averted.
The study of p21 is likely to provide more surprises. We are just beginning to understand the complexity of its functions and further discoveries will determine why and how p21 promotes or suppresses tumourigenesis depending of the context. The ability to induce ROS and the cellular sensitivity to oxidative stress will no doubt take a prominent role in this and could even allow us to modulate p21 activity to selectively induce senescence or apoptosis when needed.
References
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9:400–414
Bai J, Cederbaum AI (2003) Catalase protects HepG2 cells from apoptosis induced by DNA-damaging agents by accelerating the degradation of p53. J Biol Chem 278:4660–4667
Barzilai A, Yamamoto K (2004) DNA damage responses to oxidative stress. DNA Repair 3:1109–1115
Borgdorff V, Lleonart ME, Bishop CL, Fessart D, Bergin AH, Overhoff MG, Beach DH (2010) Multiple microRNAs rescue from Ras-induced senescence by inhibiting p21(Waf1/Cip1). Oncogene 29:2262–2271
Broude EV, Demidenko ZN, Vivo C, Swift ME, Davis BM, Blagosklonny MV, Roninson IB (2007a) p21 (CDKN1A) is a negative regulator of p53 stability. Cell Cycle 6:1468–1471
Broude EV, Swift ME, Vivo C, Chang BD, Davis BM, Kalurupalle S, Blagosklonny MV, Roninson IB (2007b) p21(Waf1/Cip1/Sdi1) mediates retinoblastoma protein degradation. Oncogene 26:6954–6958
Campisi J, Kim S, Lim CS, Rubio M (2001) Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol 36:1619–1637
Chang BD, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC, Roninson IB (2000a) p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene 19:2165–2170
Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB (2000b) Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA 97:4291–4296
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD (2009) Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 34:663–673
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363–9367
el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54:1169–1174
Gartel AL (2005) The conflicting roles of the cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk Res 29:1237–1238
Hampton MB, Orrenius S (1998) Redox regulation of apoptotic cell death in the immune system. Toxicol Lett 102–103:355–358
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin- dependent kinases. Cell 75:805–816
Helmbold H, Komm N, Deppert W, Bohn W (2009) Rb2/p130 is the dominating pocket protein in the p53-p21 DNA damage response pathway leading to senescence. Oncogene 28:3456–3467
Inoue T, Kato K, Kato H, Asanoma K, Kuboyama A, Ueoka Y, Yamaguchi SI, Ohgami T, Wake N (2009) Level of reactive oxygen species induced by p21(WAF(1)CIP(1)) is critical for the determination of cell fate. Cancer Sci 100(7):1275–1283
Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, Yu ZX, Ferrans VJ, Howard BH, Finkel T (1999) Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem 274:7936–7940
Lin JH, Morikawa T, Chan AT, Kuchiba A, Shima K, Nosho K, Kirkner G, Zhang SM, Manson JE, Giovannucci E, Fuchs CS, Ogino S (2012) Postmenopausal hormone therapy is associated with a reduced risk of colorectal cancer lacking CDKN1A expression. Cancer Res 72:3020–3028
Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, Lee SW, Aaronson SA (2002) Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J 21:2180–2188
Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA (2003) Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol 23:8576–8585
Macip S, Kosoy A, Lee SW, O’Connell MJ, Aaronson SA (2006) Oxidative stress induces a prolonged but reversible arrest in p53-null cancer cells, involving a Chk1-dependent G2 checkpoint. Oncogene 25:6037–6047
Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, Tulchinksy E, Jones GD, Roninson IB, Macip S (2012) Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem 23 287(13):9845–9854
Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, del Moore VG, Deng J, Anderson KC, Richardson P, Tai YT, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, Deangelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A (2011) Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334:1129–1133
Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 211:90–98
Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6:347
Rego MA, Harney JA, Mauro M, Shen M, Howlett NG (2012) Regulation of the activation of the fanconi anemia pathway by the p21 cyclin-dependent kinase inhibitor. Oncogene 31:366–375
Roninson IB (2002) Oncogenic functions of tumour suppressor p21(Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett 179:1–14
Roninson IB (2003) Tumor cell senescence in cancer treatment. Cancer Res 63:2705–2715
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501–1512
te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62:1876–1883
Tsao YP, Huang SJ, Chang JL, Hsieh JT, Pong RC, Chen SL (1999) Adenovirus-mediated p21((WAF1/SDII/CIP1)) gene transfer induces apoptosis of human cervical cancer cell lines. J Virol 73:4983–4990
von Zglinicki T, Saretzki G, Docke W, Lotze C (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 220:186–193
Acknowledgements
This work was supported by the MRC and the University of Leicester. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Masgras, I., Macip, S. (2013). p21 Mediates Senescence by a Mechanism Involving Accumulation of Reactive Oxygen Species. In: Hayat, M. (eds) Tumor Dormancy, Quiescence, and Senescence, Volume 1. Tumor Dormancy and Cellular Quiescence and Senescence, vol 1. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5958-9_13
Download citation
DOI: https://doi.org/10.1007/978-94-007-5958-9_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5957-2
Online ISBN: 978-94-007-5958-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)