Abstract
The serine/threonine kinase Mirk is an active kinase in pancreatic, ovarian and colon cancers, but is not activated by mutation. Mirk was upregulated or amplified in the majority of resected pancreatic or ovarian adenocarcinomas, and may be selected for by enabling cancer cells to enter a reversible quiescent state and thus survive suboptimal conditions. Mirk/dyrk1B levels and activity are highest when cells are quiescent. Some cancer cells can enter a reversible quiescent phase dependent on p130/Rb2 and Mirk/dyrk1B when deprived of growth factors, while others undergo autophagy or apoptosis. Mirk blocks cell cycle progression in G0 by complexing with GSK3ß and destabilizing cyclin D isoforms and by activating by phosphorylation Lin52, which is part of the DREAM complex including p130/Rb2 which sequesters E2F4 and other transcription factors necessary for cells to enter cycle. Mirk transcriptional co-activator activity allows Mirk to decrease ROS levels by increasing expression of a group of antioxidant genes. Since Mirk is activated by oncogenic K-ras/H-ras, its upregulation of antioxidant genes may compensate for the increase in ROS induced by ras oncoproteins. Mirk competes with the SAPK p38 for binding to their common activator MKK3. Thus Mirk is upregulated or amplified in certain pancreatic and ovarian cancers, is an active kinase in these cancers, and under suboptimal growth conditions, maintains these cancer cells in a viable, quiescent state.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Mirk/dyrk1B is a member of the Minibrain/dyrk family of kinases which mediate survival of differentiating cells in certain normal tissues: skeletal muscle (Mirk/dyrk1B) through blocking cell cycling and aiding the expression of MEF2, neuronal cells (Dyrk1A), erythropoietic cells (Dyrk3) and sperm (Dyrk4). Our group initially cloned Mirk (minibrain-related kinase)/dyrk1B from human colon cancer cells and then by stably overexpressing wild-type Mirk, demonstrated its capacity to mediate colon cancer cell survival under serum limited conditions, whereas kinase-dead mutant Mirk had no survival capacity (Lee et al. 2000). Mirk was an active kinase in colon cancer cells, but was not mutated. Mirk was most abundant and active as a kinase in G0 cells in pancreatic cancer, colon cancer, and ovarian cancer cultures (Friedman 2007).
Mirk Destabilization of Cyclin D
Mirk blocked cycling of tumor cells in a quiescent G0 state by destabilizing the G1 cyclins, cyclin D isoforms, by phosphorylation at a conserved ubiquitination site T288 (Zou et al. 2004). During in vivo phosphorylation experiments in both Mv1Lu and NIH3T3 cells, Mirk potently phosphorylated a construct mutated at the GSK3ß site, cyclin D1-T286A, but not T288A constructs (Zou et al. 2004). Both cyclin D1 residues, T286 and T288, are important for its ubiquitination, and the half-life of cyclin D1-T286A/T288A was three times that of wild-type (Germain et al. 2000). Expression of cyclin D1 mutant at the Mirk phosphorylation site, T288A, enabled serum-starved HD6 colon cancer cells to escape quiescence and move into cycle (Jin et al. 2009). Likewise, depletion of Mirk by RNA interference (Deng et al. 2009) or pharmacological inhibition of Mirk kinase activity (Ewton et al. 2011) allowed pancreatic cancer cells to escape the quiescent state by increasing their expression of cyclin D isoforms. The Mirk site of T288 is directly adjacent to the GSKß site of T286, and Mirk and GSK3ß can be co-immunoprecipitated, indicating that they form a complex. Thus, Mirk may act as a priming kinase for GSK3ß in phosphorylation and destabilization of cyclin D isoforms.
Mirk Elevated in Quiescent Cells
Mirk protein levels rise in cultures enriched in quiescent G0 cells, and are seven to tenfold higher than in cycling cultures by western analysis (Deng et al. 2004). Examination of sectioned pancreatic, colon or ovarian cancers by immunohistochemistry for markers of cycling cells such as Ki67 has revealed that the fraction of cycling cells is low. For example, in a series of 90 ovarian cancers the proliferative fraction had a median value of only 30% (Schindlbeck et al. 2007), while in a series of pancreatic cancers nuclear Ki67 was found in an average of only 28% of cells (Stanton et al. 2003). A subset of the nondividing cells may be in a quiescent state. Mirk reactivity and nuclear Ki67 were assayed on the same human ductal pancreatic adenocarcinomas in serial sections and were mutually exclusive, showing that Mirk was expressed in the nondividing fraction of tumor cells which would include any quiescent cells (Deng et al. 2009).
Mirk Amplified in Cancers and Activated by Oncogenic Ras
Both pancreatic ductal adenocarcinoma and serous ovarian adenocarcinoma lack effective treatment strategies. Pancreatic cancer is the fourth deadliest cancer in the United States, even though it ranks 11th in incidence. Epithelial carcinoma of the ovary is one of the most common gynecologic malignancies and the fifth most frequent cause of cancer death in women (NIH database). The Mirk/dyrk1B kinase gene is part of the 19q13 amplicon found in pancreatic cancer (Karhu et al. 2006) and ovarian cancer (Thompson et al. 1996). Immunohistocytochemical analysis revealed that the Mirk protein is expressed in about 90% of pancreatic cancers (Deng et al. 2006) and about 75% of ovarian cancers (Hu et al. 2011). Furthermore, the Mirk gene has been localized to the 660 kb core region of the 19q13 amplicon which contains about 20 genes, only four of which might have some role in tumor growth (Mirk/dyrk1B, MED29, PAF1 and PAK4) (Kuuselo et al. 2007). The 660 kb core amplicon is found in 12.2% of all primary pancreatic cancers, but increases to 33.3% of the more advanced T3 and pT4 tumors, as well as in lymph node metastases and distant metastases (Kuuselo et al. 2010). Mirk is activated by signaling from activated Rac1 to MKK3 (Jin et al. 2005) or oncogenic K-ras or H-ras to Rac1 to MKK3 (Jin et al. 2007) and was an active kinase in pancreatic, ovarian or colon cancer cell cultures assayed by the immune complex kinase reaction. Mirk is an active kinase in pancreatic cancers in vivo where it restricts Hedgehog initiated Gli1 activity to the stromal compartment (Lauth et al. 2010). Significantly, Mirk maintains the viability of the most aggressive subset of pancreatic cancer cells that can undergo clonal growth and that should include the tumor stem cells (Jin et al. 2007).
Reactive Oxygen Species (ROS) in Quiescence
Factors that allow the prolonged survival of quiescent tumor cells in vivo are of clinical relevance. These include antioxidant proteins and factors which control their expression (Chen et al. 2008) such as Mirk, which decreases the level of toxic reactive oxygen species (ROS) in tumor cells, increasing their survival (Deng et al. 2009) and their clonogenic growth (Jin et al. 2007). ROS are constantly generated as cells grow, and increased growth rates and oncogenes like H-ras and Bcr-abl raise ROS levels in tumor cells (Trachootham et al. 2006). We hypothesize that tumor cells enter a quiescent state because of damage resulting from oxidative stress, with a few percent entering G0 at each cell cycle. After repair, cells can then re-enter the cell cycle if nutrients are available. Mirk maintains the viability of repaired cells in G0 by reducing ROS levels through activating expression of antioxidant genes, among them ferroxidase and SOD2 in some pancreatic cancers (Deng et al. 2009) and ovarian cancers (Hu and Friedman 2010).
Cycling Ovarian Cancer Cells Enter G0
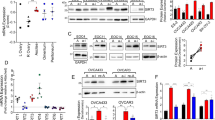
The presence of quiescent G0 tumor cells has been controversial. Quiescent cells degrade their ribosomes allowing G0 cells to be identified by their lower RNA content than G1 cells, but 2N DNA content. Cellular DNA was stained with Hoechst 33258, Pyronin Y then added to bind to RNA, and both fluorochromes measured by two parameter flow cytometry. The BJ strain of human normal diploid fibroblasts maintained in log phase growth had a low fraction (10%) of cells in G0 while serum-starvation placed the majority of cells (66%) in G0 as noted by others (Coller et al. 2006). Five ovarian cancer cell lines were assayed during log phase growth to determine whether any cells cycled into a G0 state even when the majority of cells were proliferating. Surprisingly, OVCAR3, SKOV3, TOV21G, OVCAR4 and OV90 cultures each contained a fraction of cells in G0. These averaged 17 ± 3% (SD) for SKOV3 cultures, 17 ± 4% for TOV21G cultures, 38 ± 2% for OVCAR3 cultures and 20 ± 2% for OVCAR4 and OV90 cultures (Fig. 10.1a and other data not shown). OVCAR3 cultures with the highest fraction of G0 cells and an amplified Mirk gene (Hu et al. 2011) proliferated the slowest. Reflecting their lower fraction of G0 cells, SKOV3 and TOV21G cultures grew over twice as fast as OVCAR3 cultures (Fig. 10.1b), as did OVCAR4 and OV90 cultures (data not shown). Thus the entry of cells into G0 lowers the fraction of cycling cells within the culture, increasing the average doubling time.
Ovarian cancer cell cultures contain a fraction in G0. a Log phase SKOV3, TOV21G, and OVCAR4 ovarian cancer cells, then grown serum-free medium 3 days before cell cycle analysis by two parameter flow cytometry, with G0 cells indicated by arrows. b Log phase cells replated at similar cell densities, and cultured in growth medium, with relative cell numbers determined by MTT assay +/− SD (n = 6). c Cells depleted of p130, made quiescent, and the fraction of cells in G0 then determined as above, mean +/−SE. d Western blot of immunoprecipitated p130/Rb2, E2F4 bound to immunoprecipitated p130/Rb2, and total p130/Rb2 and antibody heavy chain in the respective lysates from cells log phase or serum-starved 3 days
Stress Conditions Increase G0 Cells
TOV21G, SKOV3, OVCAR3, OVCAR4 cells, and OV90 cells were cultured for 3 days either in serum-free DMEM or maintained as log phase cultures in DMEM plus 7% FBS. Culture under serum-free conditions increased the fraction of G0 cells an average of 3.2 (±0.2) fold for SKOV3 cultures, 6.5 (±0.4) fold for TOV21G cultures, and 1.5 (±0.1) for OVCAR3 cultures but did not increase the fraction of G0 cells in OVCAR4 or OV90 cultures (Fig. 10.1a and other data not shown). Similar results were found when cells were cultured to high density.
Were the cells in G0 in the ovarian cancer cell lines terminally arrested, undergoing apoptosis or were they in a transient quiescent state like dormant tumor cells in vivo? SKOV3 cells were accumulated in G0 by serum-free culture for 2 days, then replated at lower density in fresh serum-free growth medium containing the mitotic inhibitor nocodazole. Within 2 days, about 90% of the cells had exited the quiescent G0 state, traversed G1 and S and had been arrested in G2+M by nodocazole, without the appearance of a sub-G1 peak of apoptotic cells (Hu et al. 2011). Thus quiescent SKOV3 cells can eventually re-enter the cell cycle in the presence of fresh nutrients showing that G0 arrest is transient.
Quiescence Markers in G0 Cancer Cells
Possibly a molecular defect in OVCAR4 and OV90 cells prevented their accumulation in G0 under suboptimal culture conditions. The retinoblastoma protein family member p130/Rb2 sequesters the E2F4 transcription factor, preventing progression of mammalian cells from G0 into G1. Consistent with this function, ectopic expression of the p130/Rb2 gene mediated growth arrest of human ovarian cancer cells (D’Andrilli et al. 2004). The protein level of p130/Rb2 is elevated in quiescent cells where p130/Rb2 functions, but is lower in proliferating cells due to turnover (Smith et al. 1998), so an increase in p130/Rb2 levels should occur to facilitate the entry of ovarian cancer cells into G0. A time-course analysis showed that when TOV21G and SKOV3 cells were cultured in serum-free medium for 3 days and cells entered G0, the protein level of p130/Rb2 increased several-fold, while neither increase in p130/Rb2 nor accumulation in G0 occurred in OVCAR4 cells. This difference led to levels of p130/Rb2 sixfold higher in TOV21G cells and fourfold higher in SKOV3 cells than in OVCAR4 cells (Hu et al. 2011). Levels of p130/Rb2 can be controlled through the Foxo3a transcription factor (Kops et al. 2002), or post-translationally through protein stabilization. One or both of these regulations may be aberrant in OVCAR4 cells. Immunohistochemical analysis by others revealed loss or decrease in expression of p130/Rb2 in about 40% of 45 resected human ovarian cancers assayed (D’Andrilli et al. 2004), so the OVCAR4 line may reflect this large subgroup of ovarian cancers with low p130/Rb2 expression.
Mirk protein levels increased four to sevenfold in SKOV3 and TOV21G cells, but not in OVCAR4 cells, cultured under these suboptimal growth conditions. In time-course studies Mirk levels increased within 24 h of the switch to serum-free culture and then continued to increase. Thus an increase in level of Mirk protein was found only when ovarian cancer cells accumulated in G0. The CDK inhibitor p27kip1 helps to maintain the G0 state by binding to CDK/cyclin complexes. Levels of p27 were increased 20-fold when SU86.86 pancreatic cancer cells were made quiescent in G0 (Deng et al. 2009). Levels of p27 differed dramatically between the OVCAR4, SKOV3 and TOV21G cell lines, but were increased several fold in each line by serum-free culture (Hu et al. 2011). The inability of OVCAR4 cells to arrest in G0 could not be ascribed to alterations in abundance or regulation of p27.
Some Ovarian Cancers Defective in G0 Arrest
SKOV3, TOV21G and OVCAR4 cells were cultured in serum-free conditions for up to 6 days. Measurement of relative cell number by MTT assay showed that both SKOV3 and TOV21G cultures grew to a higher cell density than the OVCAR4 cultures that exhibited declining cell numbers after 3 days of serum-free culture (Fig. 10.2a). Analysis of parallel cultures revealed that OVCAR4 cells underwent more apoptosis than TOV21G or SKOV3 cells (Fig. 10.2b). Critical steps in apoptosis are cleavages of poly (ADP-ribose) polymerase (PARP) and caspase 3, which were prominent only in OVCAR4 cells after 4–6 days of serum-free culture. Measurement of cell viability by dye exclusion showed that about 60% of the OVCAR4 cells were nonviable and unable to exclude dye after 4–6 days of serum-free culture, compared with about 30% of SKOV3 and TOV21G cells (Fig. 10.2c). These nonviable OVCAR4 cells then underwent apoptosis (Fig. 10.2b), reducing total cell numbers (Fig. 10.2a). Other suboptimal culture conditions also led TOV21G and SKOV3 cells to accumulate in G0. Cells were cultured for 3 days either in serum-free DMEM, in normal growth medium to high cell density or in low glucose DMEM without FBS. In each case the fraction of G0 cells increased to 60–80% of the culture, while no such increase was seen with OVCAR4 cultures. Thus ovarian cancer cells that could enter a reversible quiescent arrest in G0 were more protected from suboptimal growth conditions than tumor cells that lacked this capacity. In vivo, such quiescent cells could re-enter the cell cycle under favorable clues from the microenvironment.
Cultures of OVCAR4 cells with low fractions in G0 under serum-free culture conditions undergo more cell death than cells that can arrest in G0. a Cells were plated, and allowed to enter cycle by culture in growth medium for 24 h, then switched to serum-free medium. MTT assay, mean +/−SD shown, n = 2. b (upper panel) Parallel cultures from panel a analyzed by western blotting for markers of apoptosis, cleaved PARP and cleaved caspase 3. (lower panel) Cells depleted of Mirk using two different RNAi duplexes independently (siD or siC) or GC-matched controls, then cultured serum-free for 4 days before western blotting for Mirk, actin, cleaved PARP and cleaved caspase 3. Only data with siD shown. c The cultures from panel a were sampled for the fraction of dead cells incapable of exclusion of trypan blue dye. Mean +/− SD shown, with n = 4 for each point. An average of 488+/−40 cells were assayed per point. d TOV21G, OVCAR4 and BJ normal human diploid fibroblasts were depleted of Mirk using two different RNAi duplexes independently (siD or siC) or GC-matched controls as in panel b, then cultured serum-free for four and for 6 days before assay of the percentage of dead cells by trypan blue dye exclusion. Mean +/−SD shown (n = 4, with two measurements per RNAi duplex). Student’s two-tailed t test used to analyze cultures of TOV21G gave p < 0.001. (lower panel) Western blot shows depletion of Mirk in parallel cultures after 4 days
Mirk and p130/RB2 Required for G0 Viability
The effect of Mirk on viability of ovarian cancer cells under suboptimal growth conditions was determined by depletion of Mirk by two RNAi duplexes, each added independently to a parallel culture of either TOV21G or OVCAR4 ovarian cancer cells, or the BJ strain of normal human diploid fibroblasts. The Mirk depleted and control-depleted cells were then maintained in serum-free media for 4 or 6 days. About 60% of the OVCAR4 cells, 30% of the TOV21G cells, but only 10% of the normal fibroblasts died under these conditions (average of Ctsi treated cultures, Fig. 10.2d). However, these three cell types differed in their sensitivity to depletion of Mirk. There was no detectable effect on the viability of normal BJ cells on either day, while Mirk depletion did not further decrease OVCAR4 viability after 4 days of serum-starvation with a marginal effect after 6 days (Fig. 10.2d). In contrast, Mirk depletion led to a mean 50% increase in TOV21G cell death after either 4 or 6 days of serum starvation. In similar studies, Mirk depletion led to increased cleavages of poly(ADP-ribose) polymerase (PARP) and caspase 3 in SKOV3 and in TOV21G cells, not in OVCAR4 cells, indicating that the loss of Mirk led to cell death by apoptosis (Fig. 10.2b, lower panel). Thus, Mirk helps to maintain TOV21G ovarian cells in a viable quiescent state by blocking apoptosis, while having little protective effect for OVCAR4 cells which are not accumulated in G0 by suboptimal growth conditions. In addition, Mirk protein was expressed at much lower levels in normal BJ fibroblasts compared to either SKOV3 or TOV21G cancer cells, and depletion of Mirk in BJ cells led to no detectable increase in cell death (Fig. 10.2d). This data is consistent with earlier studies on Mirk knockout in mice (Leder et al. 2003) and colony formation assays (Jin et al. 2007)that showed a selective sensitivity of cancer cells to Mirk depletion compared with normal diploid cells.
The mechanism for G0 arrest includes the Mirk-mediated reduction of cyclin D1 to prevent escape into G1. Mirk has been shown to slow the exit of SU86.86 pancreatic cancer cells (Deng et al. 2009), and HD6 colon cancer cells (Jin et al. 2009) from G0 quiescence by phosphorylating their cyclin D isoforms at a conserved ubiquitination site that initiates rapid turnover. Mirk was shown to also alter ovarian cancer cell cycling through cyclin D1. Efficient depletion of Mirk in SKOV3, OVCAR3, and TOV21G cells by either of two synthetic RNAi duplexes led to an increase in cyclin D1. Thus Mirk reduced cyclin D1 levels in these cells, restricting their entry into G1 and the cell cycle.
The role of p130/Rb2 in G0 arrest in ovarian cancer cells was examined by depleting p130 in SKOV3, TOV21G and OVCAR4 cells, then placing cells in serum-free medium for 3–5 days in an attempt to induce quiescence. Although the p130 depletion was only partial, fewer p130-depleted SKOV3 and TOV21G cells were found in G0 (Fig. 10.1c). In contrast, depletion of p130 had almost no effect on the G0 fraction of OVCAR4 cells. These data suggest that p130/Rb2 enables TOV21G cells to remain in G0 as part of a stress response, and loss of this capacity for G0 arrest decreases their viability.
When normal diploid cells enter G1 from G0 by addition of mitogens, p130/Rb2 is phosphorylated by G1 cyclin/CDK complexes and other kinases at up to 22 sites, thus freeing the transcription factor E2F4. E2F4 was expressed at similar levels in the three cell lines under different culture conditions (data not shown). However, about fivefold more E2F4 was bound to p130/Rb2 immunoprecipitated from SKOV3 and TOV21G cells arrested in G0 compared with p130/Rb2 from OVCAR4 cells grown under similar conditions (Fig. 10.1d). Thus fewer serum-starved OVCAR4 cells were found in G0 because they did not express enough p130/Rb2 capable of sequestering E2F4 to block entry into G1.
Quiescence is maintained by the DREAM complex (p130/Rb2, E2F4, DP1 and a stable core including the LIN52 protein), which disassembles when cells leave quiescence and enter cycle. In quiescent cells Mirk/dyrk1B and the closely related Dyrk1A phosphorylate the core protein LIN52 at a site necessary for its quiescence function (Litovchick et al. 2011). Mirk was capable of phosphorylating LIN52 in SKOV3 and TOV21G ovarian cancer cells, but its function in OVCAR4 was not determined.
The Stress-Activated Kinase p38 and Mirk Compete
The SAPK p38 regulates a transcription factor network required for tumor cell quiescence (Adam et al. 2009). High levels of p38 coupled with low levels of Erks induce a G0/G1 arrest of HEp3 cells into a dormant state (Sosa et al. 2011). Interestingly, p38 can suppress Mirk function when p38 levels are higher than Mirk levels by competing for their common activator MKK3. The MAPK kinase MKK3 activates Mirk/dyrk1B as a protein kinase (Lim et al. 2002a), implicating Mirk/dyrk1B in the biological response to certain stress agents.
Mirk and p38 directly interact in vivo as they co-immunoprecipitate. Flag-epitope-tagged wild-type p38 or dominant negative p38AF were co-transfected into 293T cells with either wild-type Mirk or Mirk plus its activator, constitutively active mutant MKK3E (Fig. 10.3a). Mirk, p38 wild-type and kinase-dead p38AF were synthesized at comparable levels in each experimental mixture, as shown by western blotting of the cell lysates (Fig. 10.3a, lower two lanes) (Lim et al. 2002b). In vivo interaction between p38 and Mirk was demonstrated by their co-immunoprecipitation (Fig. 10.3a). Kinase-inactive p38AF bound Mirk about twice as avidly as wild-type p38. Each of the four isoforms of p38, α,β, γ, δ, were expressed in vivo alone or in the presence of Mirk, and only α and ß were capable of complexing and co-immunoprecipitating with Mirk (Fig. 10.3b).
Physical interaction between Mirk and p38 SAPK. a 293T cells were co-transfected with either MKK3E, wild-type Mirk, and either flag-p38 wild-type or kinase-inactive flag-p38AF. Mirk and its associated proteins in immunoprecipitates were examined by western blotting for the flag-epitope on the expressed p38 and p38AF proteins. Similar amounts of p38 wild type and p38AF were synthesized in the lysates, and equal amounts of Mirk were found in the appropriate lysates. b Physical interaction between Mirk and only the α or ß isoforms of p38 MAP kinase. Equal amounts of p38 SAPK isoforms, α,β2,γ δ, were synthesized in 293T cells following transfection in the presence of co-expressed Mirk or vector DNA (western blots of lysates shown in lower two panels). Immunoprecipitates western blotted for the flag-epitope on the expressed p38 isoforms. c Kinase mixtures in vitro contained either recombinant purified Mirk or increasing concentrations of recombinant purified p38, as indicated. Kinases were added to MBP and in vitro kinase assays were performed with [32]P-ATP and analyzed by autoradiography after SDS-PAGE. d 293T cells were co-transfected with either MKK3E, wild-type Mirk, and kinase-inactive flag-p38AF. Western blots of total lysates shown in upper two panels. Mirk immunoprecipitates were western blotted for the flag-epitope for complexed p38AF proteins and for complexed MKK3E by antibody to MKK3. e 293T cells were co-transfected as above. Western blot of total lysates is shown in upper three panels. The MKK3 immunoprecipitates were western blotted for the flag-epitope for complexed p38AF proteins and for complexed Mirk
The complexing of p38 to Mirk blocked Mirk kinase activity. Increasing amounts of purified recombinant non-activated p38 (0.1–3 ug) were mixed together with 1 ug of purified GST-Mirk, and an in vitro kinase assay was performed on myelin basic protein (MBP). Without activation p38 had little kinase activity on MBP, while Mirk exhibited MBP kinase activity (Fig. 10.3c). Mirk kinase activity was inhibited by roughly equimolar concentrations of inactive p38, consistent with the model that p38 sequesters Mirk. Since p38AF is a potent inhibitor of Mirk activation of HNF1 (Lim et al. 2002b), it is likely that p38 α and ß sequester Mirk, and prevent its activation by MKK3E.
The ability of p38 to sequester Mirk was tested by co-expressing Mirk with MKK3E in the presence of increasing levels of flag-p38AF, and then analyzing the molecules bound to either immunoprecipitated Mirk (Fig. 10.3d) or immunoprecipitated MKK3E by western blotting (Fig. 10.3e). In the absence of p38AF, Mirk bound well to MKK3E (Fig. 10.3d). When the concentration of p38AF increased tenfold, more flag-p38AF and less MKK3E was found associated with Mirk in a dose-dependent manner (Fig. 10.3d, lower panels). Similarly when MKK3E immunocomplexes were analyzed, increasing amounts of p38AF led to the displacement of Mirk (Fig. 10.3e, lower panels). These experiments demonstrated that p38 blocked the association of MKK3E and Mirk in a kinase-independent manner. Synchronization experiments demonstrated that Mirk/dyrk1B levels fluctuate about tenfold within the cell cycle, while p38 levels do not, leading to the speculation that endogenous p38 could only block Mirk function when Mirk levels were low in S phase and not when Mirk levels were elevated in G0/G1. These data suggest a novel cell cycle dependent function for p38, suppression of Mirk functions only when cells are proliferating, and thus limiting Mirk functions to growth arrested cells.
References
Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, Kourtidis A, Conklin DS, Aguirre-Ghiso JA (2009) Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res 69:5664–5672
Chen C, Liu Y, Liu R, Ikenoue T, Guan K-L, Liu Y, Zheng P (2008) TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 205:2397–2408
Coller H, Sang L, Roberts JM (2006) A new description of cellular quiescence. PLoS Biol 4:329–349
D’Andrilli G, Masciullo V, Bagella L, Tonini T, Minimo C, Zannoni GF, Giuntoli RL II, Carlson JA Jr, Soprano DR, Soprano KJ, Scambia G, Giordano A (2004) Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin Cancer Res 10:3098–3103
Deng X, Mercer SE, Shah S, Ewton DZ, Friedman E (2004) The cyclin-dependent kinase inhibitor p27kip1 is stabilized in G0 by Mirk/dyrk1b kinase. J Biol Chem 279:22498–22504
Deng X, Ewton DZ, Li S, Naqvi A, Mercer SE, Landas S, Friedman E (2006) The kinase Mirk/Dyrk1B mediates cell survival in pancreatic ductal adenocarcinoma. Cancer Res 66:4149–4158
Deng X, Ewton DZ, Friedman E (2009) Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by decreasing ROS levels. Cancer Res 69:3317–3324
Ewton D, Hu J, Vilenchik M, Deng X, Luk K-C, Polonskaia A, Hoffman A, Zipf K, Heimbrook D, Boylan J, Friedman E (2011) Inactivation of Mirk/dyrk1b kinase targets quiescent pancreatic cancer cells. Mol Cancer Ther 10:2104–2114
Friedman E (2007) Mirk/dyrk1B in cancer. J Cell Biochem 102:274–279
Germain D, Russell A, Thompson A, Hendley J (2000) Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J Biol Chem 275:12074–12079
Hu J, Friedman E (2010) Depleting Mirk kinase increases cisplatin toxicity in ovarian cancer cells. Genes Cancer 1:803–811
Hu J, Nakhla H, Friedman E (2011) Mirk/dyrk1B and p130/Rb2 mediate quiescence in ovarian cancer cells. Int J Cancer 129:307–318
Jin K, Lim S, Mercer SE, Friedman E (2005) The survival kinase Mirk/dyrk1B is activated through Rac1-MKK3 signaling. J Biol Chem 280:42097–42105
Jin K, Park S-J, Ewton D, Friedman E (2007) The survival kinase Mirk/dyrk1B is a downstream effector of oncogenic K-ras. Cancer Res 67:7247–7255
Jin K, Ewton D, Park S, Hu J, Friedman E (2009) Mirk regulates the exit of colon cancer cells from quiescence. J Biol Chem 284:22916–22925
Karhu R, Mahlamaki E, Kallioniemi A (2006) Pancreatic adenocarcinoma- genetic portrait from chromosomes to microarrays. Genes Chromosomes Cancer 45:721–730
Kops G, Dansen TB, Polderman PE, Saarloos I, Wirtz KWA, Coffer PJ, Huang T-T, Bos JL, Medema RH, Burgering BMT (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316
Kuuselo R, Savinainen K, Azorsa DO, Basu GD, Karhu R, Tuzmen S, Mousses S, Kallioniemi A (2007) Intersex-like (IXL) is a cell survival regulator in pancreatic cancer with 19q13 amplification. Cancer Res 67:1943–1949
Kuuselo R, Simon R, Karhu R, Tennstedt P, Marx AH, Izbicki JR, Yekebas E, Sauter G, Kallioniemi A (2010) 19q13 amplification is associated with high grade and stage in pancreatic cancer. Genes Chromosomes Cancer 49:569–575
Lauth M, Bergstrom A, Shimokawa T, Tostar U, Jin Q, Fendrich V, Guerra C, Barbacid M, Toftgard R (2010) DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol 17:718–725
Leder S, Czajkowska H, Maenz B, de Graaf K, Barthel A, Joost H-G, Becker W (2003) Alternative splicing variants of the protein kinase DYRK1B exhibit distinct patterns of expression and functional properties. Biochem J 372:881–888
Lee K, Deng X, Friedman E (2000) Mirk protein kinase is a mitogen-activated protein kinase substrate that mediates survival of colon cancer cells. Cancer Res 60:3631–3637
Lim S, Jin K, Friedman E (2002a) Mirk protein kinase is activated by MKK3 and functions as a transcriptional activator of HNF1alpha. J Biol Chem 277:25040–25046
Lim S, Zou Y, Friedman E (2002b) The transcriptional activator Mirk/Dyrk1B is sequestered by p38alpha/beta MAP Kinase. J Biol Chem 277:49438–49445
Litovchick L, Florens LA, Swanson SK, Washburn MP, DeCaprio JA (2011) DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev 25:801–813
Schindlbeck C, Hantschmann P, Zerzer M, Jahns B, Rjosk D, Janni W, Rack B, Sommer H, Friese K (2007) Prognostic impact of KI67, p53, human epithelial growth factor receptor 2, topoisomerase IIalpha, epidermal growth factor receptor, and nm23 expression of ovarian carcinomas and disseminated tumor cells in the bone marrow. Int J Gynecol Cancer 17(5):1047–1055
Smith E, Leone G, Nevins J (1998) Distinct mechanisms control the accumulation of the Rb-related p107 and p130 proteins during cell growth. Cell Growth Differ 9:297–303
Sosa MS, Avivar-Valderas A, Bragado P, Wen H-C, Aguirre-Ghiso JA (2011) ERK1/2 and p38 signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res 17:5850–5857
Stanton KJ, Sidner RA, Miller GA, Cummings OW, Schmidt CM, Howard TJ, Wiebke EA (2003) Analysis of Ki-67 antigen expression, DNA proliferative fraction, and survival in resected cancer of the pancreas. Am J Surg 186:486–492
Thompson FH, Nelson MA, Trent JM, Guan X-Y, Liu Y, Yang J-M, Emerson J, Adair L, Wymer J, Balfour C, Massey K, Weinstein R, Alberts DS, Taetle R (1996) Amplification of 19q13.1-q13.2 sequences in ovarian cancer: G-band, FISH, and molecular studies. Cancer Genet Cytogenet 87:55
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao P, Achanta G, Arlinghaus R, Liu J, Huang P (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10:241–252
Zou Y, Ewton D, Deng D, Mercer S, Friedman E (2004) Mirk/dyrk1B kinase destabilizes cyclin D1 by phosphorylation at threonine 288. J Biol Chem 279:27790–27798
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Friedman, E.A. (2013). The Kinase MIRK/DYRK1B Mediates a Reversible Quiescent State in a Subset of Ovarian, Pancreatic and Colon Cancers. In: Hayat, M. (eds) Tumor Dormancy, Quiescence, and Senescence, Volume 1. Tumor Dormancy and Cellular Quiescence and Senescence, vol 1. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5958-9_10
Download citation
DOI: https://doi.org/10.1007/978-94-007-5958-9_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5957-2
Online ISBN: 978-94-007-5958-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)