Abstract
Alkaline slag, rice chaff, and peanut straw were chosen to investigate the ameliorating effects of these industrial and agricultural by-products on soil microbial and enzyme properties in a field experiment on an acidic Ultisol. The alkaline slag was superior to rice chaff and peanut straw in correcting soil acidity and induced a larger increase in catalase activity, acid phosphatase activity, and the microbial quotient and thus depressed the metabolic quotient more, since soil acidity was an important stress factor for microbial and enzyme activities in the Ultisol. Incorporation of rice chaff and peanut straw markedly increased the soil microbial C, urease activity, and basal respiration and improved soil fertility to a greater extent, by more greatly enriching soil with organic C and the nutrients nitrogen, phosphorus, and potassium, which were also significant factors affecting microorganism properties. Consequently, the application of alkaline slag and rice chaff together was the most effective in enhancing the geometric mean of enzyme activities (an important index of soil quality), suggesting that the combined industrial and agricultural by-products together as an amendment was the better choice to improve soil quality in the Ultisol.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Soil quality
- Industrial by-product
- Agricultural by-product
- Enzyme activity
- Microbial activity
- Acid Ultisol
Introduction
The influence of soil microorganisms on soil quality has been emphasized more recently since soil microorganisms provide the primary driving force for many chemical and biochemical processes and thus affect nutrient cycling and soil fertility (Mele and Crowley 2008). The growth and activity of microorganisms are functions of soil properties. The chemical properties of industrial and agricultural by-products are different; thus, they result in different changes in soil chemical and microorganism properties when applied to acidic soils as ameliorants. Thus, to evaluate the effects of industrial and agricultural by-products on soil quality, alkaline slag (AS), rice chaff (RC), and peanut straw (PS) were chosen as amendments to compare the effect on enzyme activity and microbial properties under field conditions. The geometric mean of enzyme activities (GMea) index was used to estimate soil quality by considering that it is sensitive to the changes in soil management (Paz-Ferreiro et al. 2012).
Materials and Methods
A field experiment was established in September 2007 in cropland of Langxi County (119°8′E, 31°6′N), Anhui Province, in the middle of China. The soil was a strongly acidic Ultisol with pH 4.1. The crop rotation in the experiment was winter canola (Brassica chinensis L.) followed by summer peanut (Arachis hypogaea) every year. N, P, and K were applied based on the local recommended dose and methods and were applied the same in all treatments. AS, as an industrial by-product, and RC and PS, as agricultural by-products, were chosen for this field experiment. AS is the by-product obtained from ammonia-alkali production of sodium carbonate, with the main chemical composition of calcium carbonate, calcium sulfate, and calcium chloride. RC is the residue from production of rice, and PS is the aboveground part of the plants collected after peanut harvest. The experiment included five different treatments: application of RC, PS, AS, and AS together with RC and control with no ameliorant. The total amounts applied were 15 t ha−1 for RC and PS and 0.56 t ha−1 for AS. Treatments were arranged in a randomized complete block design with three replicates. The size of each field plot was 2 m × 10 m. Soil surface samples (0–15 cm) were taken immediately after peanut harvest in September 2011 by pooling 15 soil cores sampled with a 3.0-cm-diameter stainless steel auger. The samples were sieved (mesh size <2 mm) and were then either air-dried for chemical analysis or stored at 4°C before the analysis of enzyme and microbial properties.
Soil pH, exchangeable acidity (EA), and available N, P, and K were measured as common methods. Soil organic C was determined by dichromate oxidation method. Catalase activity was measured following the method of Guan (1986). The activities of urease, invertase, and acid phosphatase were analyzed using the constant temperature incubation method. The GMea (a general index to integrate information from variables that possess different units and range of variation) of the assayed enzyme activities was calculated for each sample as
where Cat, Ure, Inv, and Pho are catalase, urease, invertase, and acid phosphatase activities, respectively. Soil microbial biomass C was analyzed by the fumigation–extraction method. Soil basal respiration was determined using the sealed incubation–alkali absorption method. The metabolic quotient was calculated as the ratio of basal respiration to microbial biomass C.
Results and Discussion
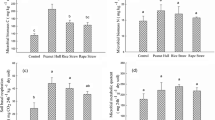
Results in Fig. 1 indicated that AS was more effective to increase soil pH, decrease exchangeable acidity, and thus correct soil acidity but reduced the soil in available P and K. Incorporation of RC and PS increased the organic C and available P and K of the soil and also depressed soil acidity. The different soil properties interacted with each other, which influenced both soil microbial and enzyme properties. Among soil basic physicochemical properties, the properties of soil acidity were very important factors affecting soil microorganisms. More favorable environmental conditions in amended acid soils (e.g., reduction of Al toxicity and increased concentration of exchangeable cations such as Mg2+ and Ca2+) could increase soil microbial biomass and therefore produce significant changes in enzyme activities (Bending et al. 2002). Thus, decreases in soil acidity resulted in increased activities of catalase and acid phosphatase and microbial quotient and decreased the metabolic quotient in this Ultisol (Fig. 2). As a consequence, reducing soil acidity could significantly decrease the H2O2 toxicity and acid stress and enhance P availability and quality of organic matter and thus the soil environment. Since the AS was superior to RC and PS in correcting soil acidity, the former was also more effective in increasing the catalase and acid phosphatase activities and the microbial quotient and decreasing the metabolic quotient.
Increasing organic C was beneficial for the increase of urease activity, microbial C, and GMea (Fig. 2). The activities of most soil enzymes, such as urease, increase as native soil organic matter content increases, reflecting larger microbial communities and stabilization of enzymes on humic materials (Bending et al. 2002). Soil microbial C is the most labile C pool in soils. The turnover time for C immobilized in the microbial biomass is about ten times faster than that derived from plant materials. Hence, the change of the soil microbial C could lead to a change in the rate of nutrient cycling and the size of the nutrient pool. Therefore, RC and PS were more effective than AS in increasing soil organic C, and thus improving N availability as well as other aspects of soil fertility to a greater degree. The application of AS with RC was the most effective in increasing GMea, followed in order by PS, RC, and AS, which suggested that increasing organic C was more effective than correcting soil acidity to improve soil quality, and both aspects were key factors for soil quality in Ultisol.
Additionally, microorganisms under balanced fertilizer treatments had higher efficiencies of C utilization, and the decrease in efficient metabolism under nutrient-deficient treatments was chiefly due to the unavailability of P, followed by N and K (Zheng et al. 2009). In this study, basal respiration was positively correlated with the available P and K, which indicated that the AS markedly decreased basal respiration mainly through the reduction in soil available P and K. Basal respiration is a valuable tool for understanding changes in soil microbial activities. RC and PS were superior to AS in increasing available P and K and thus more effective in increasing soil microbial activities as expressed by their basal respiration.
References
Bending, G.D., M.K. Turner, and J.E. Jones. 2002. Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biology and Biochemistry 34: 1073–1082.
Guan, S.Y. 1986. Soil enzymes and research methods. Beijing: China Agricultural Science Press (in Chinese).
Mele, P.M., and D.E. Crowley. 2008. Application of self-organizing maps for assessing soil biological quality. Agriculture, Ecosystems and Environment 126: 139–152.
Paz-Ferreiro, J., G. Gascó, B. Gutiérrez, and A. Méndez. 2012. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biology and Fertility of Soils. doi:10.1007/s00374-011-0644-3.
Zheng, S., J. Hu, K. Chen, J. Yao, Z. Yu, and X. Lin. 2009. Soil microbial activity measured by micro-calorimetry in response to long-term fertilization regimes and available phosphorous on heat evolution. Soil Biology and Biochemistry 41: 2094–2099.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (40901110 and 40971135).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Zhejiang University Press and Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Li, J., Liu, Z., Zhao, A., Xu, R. (2013). Microbial and Enzyme Properties in Response to Amelioration of an Acidic Ultisol by Industrial and Agricultural By-Products. In: Xu, J., Wu, J., He, Y. (eds) Functions of Natural Organic Matter in Changing Environment. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5634-2_151
Download citation
DOI: https://doi.org/10.1007/978-94-007-5634-2_151
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5633-5
Online ISBN: 978-94-007-5634-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)