Abstract
Students’ achievement in learning chemistry through the design and construction approach to laboratory activity and the relation with their prior achievements and motivation to learn is discussed in this chapter by Vrtačnik, Sodja, and Juriševič. Authors claim that the design and construction approach to activities in chemistry lessons for middle school students is regarded as an authentic science activity, and that this approach to learn chemistry is quite rarely practised in science classes. In this approach, students were asked to design their own experiments and control variables. The results suggest that students’ success in the design and construction approach depended upon the complexity of a particular task. A significant drop off in achievements and motivation scores was found with tasks based on more abstract thinking, e.g., analyzing data and setting up hypotheses. In evaluating the design and construction approach, students expressed the highest appreciation for a positive classroom atmosphere and their active participation in the laboratory activity. The research findings revealed that students with higher achievement in chemistry are also highly extrinsically and intrinsically motivated for learning chemistry and have a higher academic self-concept.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Theoretical Framework
The majority of emerging active teaching strategies or student-centered strategies are rooted in constructivist learning theory. Their common feature is challenging open-ended investigations in a realistic, meaningful context which allows learners to explore and generate many possibilities, both affirmative and contradictory (Fosnot and Perry 2005). Thus the constructivist approach emphasizes teacher’s role in mediating learners to construct their own scientific models and to explore their domains of applicability (Matthews 2008). The relation of the constructivist learning model with Piaget’s theory of intellectual development and its implication for better understanding of some problems in teaching and learning chemistry is given by Bodner (1986). Research results that focused on laboratory activities which are inquiry-based and on an instructional technique (The Science Writing Heuristic) that combines inquiry, collaborative learning, and writing proved that these approaches have potential for improving the pedagogical value of laboratory work by changing the nature of the chemistry laboratory (Burke et al. 2006; Cacciatore et al. 2008; Furlan 2009; Rudd et al. 2001; Tarhan and Sezen 2010).
The hands-on/minds-on approach toward teaching and learning is therefore one of the active strategies which most science educators advocate, in spite of severe criticism toward constructivism derived from empirical studies on the effectiveness of this approach in comparison with the guided approach in teaching science (Kirschner et al. 2006; Kroesbergen et al. 2004; Mayer 2004). But according to current neurophysiological research findings, the doubt about and the criticism of the constructivist paradigm is not totally justified. Current neuroscience research has shown that information and knowledge are assimilated with different degrees of effectiveness, depending on the mood and tendency of emotions (Cohen and Magen 2004; Cozolino 2006; Erk et al. 2003). There are neurological reasons why learning contents should not be presented neutrally but in an emotional, interesting, and exciting manner (Thiel et al. 2002).
Design and construction of laboratory activity evokes a series of positive emotions in the students, since it is conducted in a relaxed atmosphere, without fear and pressure. Students design and plan their activities according to their own pace, knowing that they will support each other in achieving the mutual goal.
Unfortunately, many of the activities students perform in chemistry classrooms are usually related to listening to teacher’s explanations, following demonstrations, or conducting experiments in small groups according to a carefully prescribed procedure, and answering questions related to the experimental activity. Seldom are opportunities available to carry out more authentic science activities. However, when asked to design their own experiments and control variables, students are more likely to think like a scientist and apply science competencies to solving problems (Jones 1999). Construction and design activity therefore offers the possibility to develop some basic science competencies such as: define a problem, design experiments and/or observations, control variables, conduct experiments, take notes, analyze the results, set up hypotheses, check hypotheses, and report and communicate findings (Vrtačnik 2011).
It can be concluded that motivational initiatives which teachers apply while teaching have an important role for the neurophysiological processes in learning (Byrnes and Fox 1998; Jang 2008; Schunk 1998; Urdan and Schoenfelder 2006). They could be divided into two broader categories, didactic and psychological (Juriševič 2006). Didactic motivational initiatives represent the organizational side of the learning surroundings and learning process, type of instructional methods and resources used, while the psychological ones represent the mediation role of the teacher through the student’s learning process (i.e., coaching, scaffolding, and modeling, see Brophy 1999). Both of the initiatives have an important impact on the student’s motivation to learn and indirectly on the student’s performance and achievement. Especially when both of them are congruent at the relational and content level simultaneously (e.g., interest learning units and instructional methods, positive classroom climate, accepting and stimulating teacher, etc.), it is possible that students—beside responding to the external motivations—step by step develop also more intrinsic motivations for learning,—since in an acceptable and stimulating environment students feel psychologically safe, develop positive academic self-concept, interests, and curiosity (Eccles et al. 1998; Stipek 1998).
Learning motivation could be defined as a mediation variable of academic achievements, as it affects the academic performance through various quantitative and qualitative indicators of the learning process; it is also connected with some other personality traits of students as well as demographic characteristics, such as the nature of the temperament, anxiety, needs, abilities or nationality (Alexander and Murphy 1998; Jarvela and Niemivirta 2001; Juriševič 2006; Pintrich and Schunk 1996; Rheinberg et al. 2000; Rothbart and Hwang 2005). Research shows that motivation is connected with storing information into the long-term memory and with its recognition and retrieval (Schiefele and Rheinberg 1997). According to Corno (1994) and other contemporary authors motivation is attributed the key role in the decision-taking processes for certain learning behaviors.
In the opinion of Stipek (1998) learning motivation is mainly expressed in the attitudes of students toward learning and in their different approaches to learning. Jarvela and Niemivirta (2001) point out the fact that learning motivation encourages higher forms of learning, and consequently contributes to higher quality knowledge.
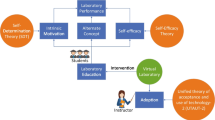
Rheinberg et al. (2000) provided for a detailed definition of the relationships among motivation, learning processes, and academic achievements. To their belief the influence of motivation upon learning is exercised on three different levels, namely: (1) in the duration and frequency of learning activities; (2) in the form of learning activities pursued; and (3) in functional disposition of the student during the learning activity. The first level of influence means active learning time (ALT)¸ in which the relation between motivation and academic achievement is a rather complex one. It is not necessarily positive in all cases, as it is interwoven in the network of other learning variables (e.g., abilities, learning strategies, previous knowledge). Motivation influences the form or nature of learning activities in a variety of ways: on the one hand it balances the effort invested by the pupil into learning (in proportion to the level of difficulty of the learning task), whereas on the other hand it influences the application of learning strategies, encouraging the student to learn and enabling the successful reaching of goals. The third level of motivational influence on the learning outcomes is related to the optimal psychological state of the student during learning (Fig. 11.1).
The dynamics of motivation to learn (adapted from Rheinberg et al. 2000)
According to Ryan and Deci (2000), intrinsic motivation is an individual’s inherent inclination from which stems his/her tendency to learn about particular areas of life regardless of the presence of external enticements. In their Self-determination theory (SDT) the authors “assumed that all students, no matter how unskilled or how impoverished their backgrounds, possess inherent growth tendencies and innate psychological needs that provide a motivational foundation for their autonomous motivation and healthy psychological development” (Reeve et al. 2004, p. 33). The theory focuses on the degree to which human behaviors are self-determined (i.e., volitional) or controlled from external sources. The former means the degree to which people endorse their actions at the highest levels of reflection and engage in the action with a full sense of choice (i.e., they are intrinsically motivated and their learning is thus self-determined), while the latter means just the opposite side (i.e., learning is motivated by external events—rewards, praise, punishment, without internal regulation).
According to this theory, learning activities in the chemistry classroom should be designed in such a way that students would value and self-regulate these activities without or with a minimum of external pressure. This process is realized through internalization (the process of taking in a value or regulation) and integration (a process by which individuals transform the regulation into their own so that it will emanate from their sense of self) (Ryan and Deci 2000). Namely, research shows that learners with internalized, integrated, or pure internal motivation achieve better results in knowledge tests, get higher achievement scores, and have a highly positive learning self-concept. In comparison with their peers with more extrinsic motivation, they show also less academic anxiety, and are less dependent on external motivational stimuli (Green et al. 2007; Gottfried et al. 2001). Personal satisfaction experienced through learning is also linked to higher creativity (Amabile 1985, cited in Csikszentmihalyi and Nakamura 1989; Shachar and Fischer 2004). Highly intrinsically motivated students are more successful in learning new concepts and show better understanding of the learning matter (Stipek 1998). Rennie (1990), on the basis of the research study on science learning, also concluded that higher results in science are related to the learner’s active engagement in learning tasks, to his/her positive attitude toward the subject and to a highly positive self-concept in science, all of which imply the learner’s intrinsic motivation to learn.
Approaches to chemistry teaching and learning, based on theories of scientific literacy, motivation, and situated learning yielded positive results regarding students’ interest, achievements, and motivation for learning chemistry concepts (Bobich 2008; Nentwig et al. 2007; Chimeno et al. 2006).
Combining information technologies with the intent of improving the science-learning environment in terms of student motivation and learning efficiency, additionally increased students’ positive perception of their learning and confidence, Charlesworth and Vician (2003).
Providing direction for students to review topics from previous chemistry classes, designing courses for early introduction to current research topics, using applied chemistry examples for solving problems, and analogies to teach chemistry, are approaches reported in the literature, which aim to tackle motivation problems for learning chemistry (Rieck 1998; Holme 1994; Thiele and Treagust 1994; Woodburn 1977).
The model of expertise in chemistry problem solving based on Anderson’s Adaptive Control of Thought-Rational (ACT-R) theory, which was tested by Taasoobshirazi and Glynn (2009) showed how conceptualization, self-efficacy, and strategy interacted and contributed to the successful solution of quantitative, well-defined chemistry problems. The impact of self-concepts, self-efficiency, usefulness of science study, and interest in chemistry and physics, on students’ academic performance was also revealed in the study by Lavonen and Laaksonen (2009).
Research results of Glynn et al. (2009) provided evidence that the students conceptualized their motivation to learn science in terms of five dimensions: intrinsic motivation and personal relevance, self-efficacy and assessment anxiety, self-determination career motivation, and grade motivation, and especially the belief in the relevance of science to students’ careers was found by Glynn et al. (2007) as a strong predictor of students’ motivation for learning science. Palmer (2009) investigated situational interest as a short-term form of motivation which occurred during a inquiry-based science lesson. The results indicated that interest arousal was substantial but did fluctuate throughout the lesson, according to the types of activities in which students were involved. The main source of interest was novelty, although choice, physical activity, and social involvement were also implicated.
Reseach Problem and Research Questions
This study aimed to investigate the correlation between students’ academic achievements obtained through the design and construct approach to laboratory activities and their motivation to learn chemistry. The research questions were:
-
1.
Did the design and construct approach to laboratory activities in learning concepts related to foam in the 9th grade, enable the understanding of the concepts selected?
-
2.
How did the knowledge achieved through this approach correlate with students’ prior knowledge (chemistry achievements from the 8th grade, and science achievements from the 7th grade) and their motivation to learn chemistry (i.e., controlled motivation, regulated motivation, intrinsic motivation, and academic self-concept)?
-
3.
How did students evaluate the design and construct approach to laboratory activities in comparison with other teaching/learning strategies usually experienced in their chemistry classes.
We assume that this information will be useful to science (chemistry) teachers and science education researchers in applying more appropriate instructional methods, and thus fostering motivation for learning science (chemistry) with the objective of attaining a deeper understanding of chemical concepts and higher achievements.
Method
Participants
A total of 132 9th Grade students (58 males and 74 females) from four different Slovenian schools participated in the study. Their average age was 14.4 years. The sample represented an urban and rural population with mixed socioeconomic status and was randomly selected.
Instruments
Student’s Handouts
A handout composed of five segments was designed for collecting feed-back on student achievements in designing and carrying out experiments of the teaching unit “Foam, foam” (Vrtačnik 2009). The structure of the handout followed hierarchically ordered steps by the process complexity of the design and construction approach. At the beginning of each segment there was a short explanation of the purpose of the segment. The first segment was dedicated to designing the experiments; students had to write down all possible pairs of salt solutions they could form from four different salt solutions (con. 0.5 mol/L) they were given on the tray. The second segment involved to carrying out wet experiments and taking notes of observations. For the purpose of the evaluation of students’ results, a sketch of an empty table was attached as a hint for colleting data and a legend for uniform marking of the amount of foam produced in mixing pairs of salt solutions. The third segment was dedicated to setting up reasons for abundant and stable foam formation. A table was included for marking the pH of salt solutions, and a short guideline for setting up the hypothesis was also added. The fourth segment was intended to find out the role of detergent in foam formation and the nature of the gas trapped in the foam. The fifth segment was intended to link the macroscopic findings in foam formation with submicroscopic presentation of the role of detergent and water molecules in foam stabilization. This part was also connected with a short animation, which shows how detergent molecules are oriented around the bubble of gas and how water molecules surround the polar heads of detergent molecules. Scoring of the handouts was done for each task of the experimental procedure separately; for the first task (each combination of reagents 1 point), for the second task (see Fig. 11.3 for the combinations of salt solutions Al/Zn, Al/Na, Zn/Na, and Na/NaHC assigned as no foam or very little foam 0.5 points, for Al/NaHC and Zn/NaHC very abundant or abundant 1.5 points), for the third task (each correct determination of pH of salt solution 0.5 points, and for correct statement of the hypothesis 3 points, for partially correct 1.5 points), for the fourth task 6 points (1 point for each correct observation and 1 point for each correct explanation), and for the fifth task 5 points (for correct orientations of detergent molecules and water molecules around gas bubbles, and partially correct, meaning that the majority of the presented molecules were oriented correctly, 2.5 points). The total score achieved by each student (score maximum 31 points) was defined as student achievement. In order to draw a distinction between correct and partial correct answers, 20 % of students’ handouts were collected and their answers analyzed. A list of accepted correct and partially correct answers was prepared and used in the further scoring procedure.
Students’ Motivation for Learning Chemistry
A 37-item questionnaire for assessment of students’ motivation was constructed on the basis of two questionnaires used in previous research (Black and Deci 2000; Juriševič et al. 2008) with the theoretical background from educational psychology research on motivation and self-concept (Ryan and Deci 2000; Marsh 1990). Specifically, the instrument was designed to assess (1) different components of students’ motivation for learning chemistry (i.e., controlled motivation based on extrinsic motivational stimuli, regulated motivation based on internalized and integrated motivational stimuli, intrinsic motivation, and academic self-concept), (2) students’ reasons for preference regarding the instructional method used in the study, and (3) students’ preferences for different learning methods usually applied in chemistry classrooms.
Administration of the instrument took approximately 15 min in the classroom; students were asked to respond to a simple declarative sentence on a 5-point Likert scale ranging from 1—not at all true to 5—very true for me.
Reseach Design
The teacher brought some foam products into the classroom and initiated discussion on foams and their usage. Afterward he demonstrated an experiment in which very abundant and stable foam was formed. The teacher poured into 200 mL beaker 50 mL of 0.5 mol/L aqueous solution of NaHCO3, added 2–3 drops of liquid detergent and 50 mL of 0.5 mol/L aqueous solution of Al2(SO4)3, but the students were not told which salt solutions were mixed. This experiment was the starting point of the experimental design and construction approach. From the teacher’s demonstration, the students had to observe that equal volumes of two salt solutions were mixed and that only a few drops of liquid detergent were added to one of the salt solutions. These observations were crucial for their own experimental design, which followed the demonstration. Students worked in pairs, each pair of students was given reagents on a plastic tray: 0.5 mol/L solutions of the following salts: NaHCO3, Al2(SO4)3, ZnSO4, Na2SO4, beakers, measuring cylinders, pH papers, liquid detergent, and other materials. On the handouts they were presented with the following problems: (1) to find out the combination of two salt solutions which would upon mixing form the most stable and abundant foam, (2) to find out which gas was trapped in the foam, and (3) to find out the role of detergent in the experiments. The teaching unit lasted 40 min; during the lesson students were filling in the handouts. After one week, during regular chemistry class, students’ motivation to learn chemistry was assessed.
Data were statistically analyzed with the SPPS package, version 17.0 on descriptive and bivariate levels of analysis. On the descriptive level, the basic statistics of variables were calculated. Correlations between variables were calculated based on Pearson correlation coefficients.
Results with Discussion
Students’ Achievement in the Design and Construction Approach Used in Teaching the Unit » Foam, Foam «
Task: Combinations of Pairs of Salt Solutions
Students had to predict pairs of all possible combinations of salt solutions which they were given on plastic trays in 250 mL reagent flasks. As an example of how to write the combinations, Al2(SO4)3 + ZnSO4 was already written on the handouts. The results are presented in Fig. 11.2.
The majority of students (90.4 %) found and correctly wrote the formulae of all five combinations of salt solutions. Mistakes were due to presenting the same combination of salt solutions several times (6.3 %) or writing the incorrect formula of the salt (4.3 %).
Task: Carrying Out Experiments—Collecting Data
Planning experiments was followed by carrying out wet-experiments according to the plan. As a hint for collecting data an empty layout of the table, and a legend for assigning the amount of foam were included in the handout. The students had to calculate in advance the amount of salt solutions they should use for one experiment in order to be able to complete the task successfully. Their achievements are presented in Fig. 11.3.
Over 90 % (92 %) of students correctly observed that the maximum amount of stable foam was formed upon mixing the water solution of aluminum sulfate and sodium hydrogen carbonate. Further, 84 % of students found out correctly that medium rich foam was formed upon mixing the solution of zinc sulfate with sodium hydrogen carbonate. During carrying out the experiments, careful observation and precision were necessary. If students were careless and did not pay attention to the amount of foam formed, or forgot which salt solutions they were mixing, their results were incorrect. These observations are supported with the findings that 16 % of students additionally found that the medium amount of foam was formed also in cases where no foam could be expected, and 8 % of students found for the same combination of salts that very rich foam was formed. Some students (4 %), in spite of the guidelines, used nearly all solutions of salts for only a few experiments, and 4 % of students mixed the same salt solutions twice.
Task: Setting up the Hypothesis on the Correlation Between the pH of the Salt Solution and the Amount of Foam
Students had to measure the pH of salt solutions and find out the relation between pH of salts and the amount of foam formation. Teacher helped them by focusing their attention on pH papers. This teacher intervention acted as a support for directing students’ thinking. The results are presented in Fig. 11.4a.
On average 90.7 % of students found correctly the pH of three salt solutions, the only exception being zinc sulfate, for which only 74 % of answers were correct. Observation of students’ work showed that mistakes were due to exchanging the names of solutions or using of the same pH paper several times.
In the second part of this task, students had to set up the hypothesis about the correlation between the pH of salt solutions and the amount of foam formed upon mixing two salt solutions. For the students this part was much more difficult than the previous one, Fig. 11.4b.
Less than half of the students (43 %) stated the hypothesis correctly: » The greater the difference between pH values of two salts in combination, the greater is the amount of foam formed «. Less than one third of students (30 %) gave a partially correct answer, meaning that the hypothesis was not correctly formulated, e.g., » Because there is the greatest difference in pH. «, or » The greater the difference in water solutions, the more foam is formed «. 13 % of answers were totally incorrect and 14 % of students did not state the hypothesis. These results proved our assumption that in Slovenian schools chemistry is mostly taught in the traditional way, and that teachers are not paying enough attention to science process skills and hands-on activities. Consequently, students are not used to formulating their own opinions during the school experimental work. In addition, the level of student chemistry literacy is rather low, therefore the majority of students were not able to formulate meaningful sentences from their observations, because they are lacking opportunities to discuss and express openly their own opinions about the concept taught.
Task: The Role of Detergent and the Nature of Gas
According to the instructions, students had to repeat the experiment (mixing solutions of aluminum sulfate and sodium hydrogen carbonate with detergent and without detergent) in order to find out the role of detergent and the nature of the gas trapped in the foam they obtained by using the burning splint. Their task was to describe in a coherent way the results of both experiments. The results are presented in Fig. 11.5.
Expected observations and explanations:
-
1.
Foam is formed also when detergent is not added, but is not very stable or rich.
-
2.
Gas which evolves upon mixing, extinguishes the flame of the burning splint when the splint reaches the rim of the beaker filled with gas. The gas is carbon dioxide.
-
3.
Detergent traps the gas bubbles, thus preventing them from escaping from the beaker.
One fifth (21 %) of student observations and explanations were in line with our expectations, while two thirds (62 %) of students were able to describe correctly only one observation, 7 % of students did not describe correctly any of the observations and 10 % of students did not give answers. The majority (62 %) focused their attention only on one experimental observation e.g., results of the reaction without detergent, or the experiment with a burning splint, or describing the role of detergent. Only one fifth of them linked correctly all observations into a coherent set of explanations.
Explaining the Role of Detergent at Submicroscopic Level
Students had to follow a short animation which showed at the molecular level how detergent and water molecules stabilize gas bubbles. Then they had to use models of particles (Table 11.1) and draw their own presentation of the stabilization process.
Nearly two thirds of students (60 %) were able to draw correctly the orientation of detergent and water molecules around the bubble of gas, 40 % of students oriented the models incorrectly or else they did not draw the scheme, because they probably did not understand the meaning of the animation.
Synthesis of Results: Steps of the Design and Construction Procedure and Students’ Achievements
Figure 11.6 shows how the percent of correct answers differs according to different steps of the design and construction procedure. Students’ achievements on the learning method used depend on the complexity of the thinking process required for finding correct answers. 90.4 % of students were able to predict all possible combinations of salt solutions, 92 % of students carried out experiments precisely enough that without difficulty they found the combination of salts which upon mixing gave the most stable and rich foam. 86.5 % of students determined correctly, within experimental error, the pH of salt solutions. Students were able to design and conduct simple experiments, they also proved to be good observers, however when confronted with more difficult tasks, where chemical literacy and analytical thinking were required, a great drop off in the number of correct answers was observed. Less than half of the students (40 %) were able to set up the hypothesis on the impact of pH of salts on the amount and stability of foam, and only 20 % explained correctly the role of detergent in foam formation. Surprisingly, 60 % of students drew the correct scheme showing at molecular level the stabilization of foam with detergent and water molecules. The results might confirm that students proved to be good observers.
Relation Between Students’ Achievement in the Design and Construction Activity and their Prior Achievement
Table 11.2 summarizes the results related to student achievements in the design and construction approach with their prior achievements: science in 7th grade and chemistry in 8th grade. The values of the Pearson correlation coefficients are 0.49 (p < .001) for science grade and 0.55 (p < 0.001) for chemistry grade.
The results indicate the importance of pre-knowledge in learning chemistry concepts. New concepts could not be understood if pre-knowledge of the concepts in which they are rooted did not exist. According to the expectations, students with better pre-knowledge of chemistry and science were better in planning, controlling, and executing experiments as well as in analyzing data and correlating results with theory. These results are in line with the findings of Doppelt et al. (2008), Zangyuan (2003), who reported that students’ prior knowledge and free exploration in teaching scientific concepts may have the advantage of engaging more students in the learning process and advancing their achievements. The relevance of prior knowledge on students’ performance in science and more specifically chemistry, and its effect on the instructional design has also been proved in a series of studies e.g., Seery (2009), Hailikari et al. (2008), Chambers and Andre (1997), Hewson and Hewson (1983).
Students’ Achievement with Four Motivational Components
The next set of analyses encompassed assessing relations between students’ chemistry achievements and four motivational components. Target correlations are displayed in Table 11.3.
Correlations between students’ achievement in handouts on the topic of foam, based on the design and construction approach to laboratory activities in Table 11.3 are bolded. All target correlations are of medium–high level and are significant, ranging between 0.54 and 0.65. Correlations between students’ achievement in chemistry and science from grade 7 and 8 follow the same path, but they are slightly lower, ranging between 0.32 and 0.64. The strongest correlations are between students’ current achievement and motivational measures, especially with academic self-concept in chemistry, the one highly correlated also with the other two measures of students’ achievement in chemistry and science in grade 7 and grade 8. However, it is possible that the finding is in line with the benefits of the instructional method used in the study, under the assumption that the design and construction approach is more appropriate for students with a higher academic self-concept, or that it has a positive impact on its enhancement. Kaya and Rice (2010) investigated the effects of individual student factors, among them self-confidence, and classroom factors on elementary science achievement within and across five countries. At the student level, higher levels of home resources and self-confidence yielded higher science scores on the TIMSS 2003. Statistically significant correlation between students’ science achievement and their self-confidence and interest in science as well as instructional design, was also revealed in the studies of Chang and Cheng (2008), Feltham and Downs (2002), Romance and Vitale (1992, 2001), and Tarhan and Sesen (2010). The results of the study by Nieswandt (2007) also revealed the importance of a strong and positive self-concept, the feeling of doing well in the chemistry class, for developing a meaningful understanding of scientific concepts.
Research shows that didactic and psychological motivations based on active learning methods encourage students to learn more confidently and autonomously, making the learning context personal, interesting, and meaningful (Schunk and Pajers 2009; Reeve et al. 2004; Urdan and Schoenfelder 2006). On the other hand, it is important to take into consideration also two principles in motivation development—differentiation and multidimensionality. The first principle claims that the more the students develop different motivational components for the chemistry topics, the better is the result on their achievements (DeBacker and Nelson 2000). The second principle, multidimensionality, states that different motivational components are integrated in the motivational patterns that students develop through their schooling (Juriševič 2006) and “…allows us to understand the extent to which domain specificity might vary as a function of the construct under focus” (Green et al. 2007, p. 271). From this point of view it is important to note also the relation among motivational constructs as shown in Table 11.4. All correlations are significant and of medium–high level, indicating that different motivational constructs are correlated but still different enough to confirm the multidimensional framework.
On the basis of these results, it can be concluded that students’ chemistry achievements and their motivation to learn chemistry are correlated; students with high achievements in chemistry have a higher academic self-concept and are also highly motivated—they have a strongly expressed extrinsic as well as intrinsic motivation to learn chemistry.
Students’ Appreciation of the Design and Construction Approach to Laboratory Activities and Correlation with their Prior and Current Achievements
In evaluating the design and construction approach to laboratory activities, students had to estimate their opinion by specifying their levels of agreement with the five-level Likert item statements (1—meaning “I totally disagree.” and 5—meaning “I fully agree.”) for the following attributes of the teaching units: (a) correlation of concepts with their prior experience with foam, (b) teacher guidance, (c) communication with peers, (b) help between and within groups, (d) relaxing and working atmosphere, (e) understanding new concepts, and (f) learning by doing experiments. The results of the descriptive statistics are presented in Table 11.5.
The results show that students most appreciated the relaxed, yet working atmosphere which prevailed during the lesson, (mean = 3.58, mode = 4, frequency = 36), and learning through doing experiments (mean = 3.42, mode = 5, frequency = 41). Students’ high appreciation of the relaxing atmosphere and learning through doing experiments, as experienced throughout the teaching unit, is in accordance with the findings of neurophysiologic research on the impact of emotions on motivation for learning. In such an atmosphere, positive emotions could be easily evoked, thus supporting a more positive attitude for fulfilling different tasks of the construction and design approach (Pecrun 2009). They expressed a neutral opinion about help between and among groups (mean = 3.16, mode = 3, frequency = 35). They did not have high opinions about the relation between the concepts presented in the teaching unit and their life experiences with foams, (mean = 2.04, mode = 1, frequency 53) and about the role of teacher who was, in this case, more a guide through different steps of the approach than a presenter of knowledge (mean = 2.38, mode = 1, frequency = 40). They also disagreed with the statement that the teaching unit contributes to a better understanding of the concept of foam (mean = 2.54, mode = 1, frequency = 36).
We were also interested in correlations between attributes of the teaching unit and students’ achievement in the teaching unit, and their pre-knowledge. Results are summarized in Table 11.6.
A statistically significant medium–strong correlation at the level less then 0.001 was found only for one attribute of the teaching unit—relaxing and working atmosphere—with prior knowledge of science 7th grade and chemistry 8th grade (r = 0.319 and r = 0.321, respectively). Students with better grades in chemistry felt more self-confident and they appreciated more the relaxing and working atmosphere than did students with poorer prior achievements. Better students were also more successful at filling in handouts correctly and they appreciated more the relaxing atmosphere during the lesson, which evoked positive emotions. These results are in line with the study by Randler (2009), which also showed a positive association of emotion with achievement.
Students’ Appreciation of Different Teaching/Learning Strategies
With the last set of the five-level Likert item statements, students had to estimate their level of agreement with selected teaching/learning strategies used in chemistry classes: teacher’s lecturing, learning in pairs, independent learning with computer, independent learning with textbook, learning by doing experiments, Table 11.7.
The results reveal that in chemistry classrooms students most prefer learning by doing experiments (mean = 3.88, mode = 5, frequency = 55). This result is in accordance with students’ estimation of the attribute (learning by doing experiments) of the design and construction approach. Regarding other learning strategies, students expressed rather neutral opinions about teacher’s explanations, learning in pairs and independent learning with the computer (means = 2.69, 3.37, 2.97, modes = 3.00, frequencies = 40, 40, and 28), however the majority of students did not like to learn independently with the textbook (mean = 2.01, mode = 1, frequency = 31). The question is: why is the independent learning with the textbook so unpopular?
One possible answer is that teachers are not giving enough encouragement to their students to use more regularly the textbook as an important source of data and knowledge in chemistry classes. According to Harder (1989), science teachers, should be aware of the students’ frustration when confronted with reading technical material in science textbooks. Acknowledging this problem by recommending possible solutions (e.g., model of a variety of comprehension strategies, guided discussions, small group discussions) can produce a positive change in attitude.
We were further interested in how student preferences toward different learning strategies correlate with students’ pre-knowledge and their achievements in the design and construction approach, Table 11.8.
Statistically significant medium–high correlations at the 0.01 level were found only for learning based on doing experiments and pre-knowledge (r = 0.389, science—7th grade and r = 0.405, chemistry 8th grade). Statistically significant weak correlations at the 0.05 level were also found for learning in pairs and science grade (r = 0.207), teachers’ explanations and student achievements in the design and construction approach (r = 0.223), and independent learning with the textbook and knowledge gained (r = 0.200).
Conclusions
The main problem of the present study was to evaluate the 9th graders’ chemistry knowledge constructed through experimental work—design and construction approach to the topic “Foam, foam.” It was presumed that an active instructional approach would enhance students’ learning and thus the level of their chemistry knowledge. The results show that the majority of students (>90 %) did not have problems in resolving easier tasks within the learning activity, e.g., in predicting combinations of salt solutions, carrying out wet experiments, estimating the amount of reagents, and finding out the combination of two salts which upon mixing gave the most stable and rich foam (Fig. 11.6). However it is necessary to stress that only simple experimental skills (e.g., using measuring cylinders and beakers, pH papers) were expected for successful fulfillment of the mentioned tasks. But on those tasks of the approach where abstract thinking and higher order thinking skills were needed (e.g., analyzing data and setting up the hypothesis, determining the role of detergent, evaluating the results) less than half of the students were successful in completing the tasks (40 and 20 %, Fig. 11.6). Actually, students showed weakness in science literacy by having serious problems with formulation and verbalization of the hypothesis (Laugksch 2000). This is an important finding of the study itself, and at the same time also a contribution of the instructional method used for the purposes of this study. Namely, it is probably hard to discover and consequently also to deal with such kind of problems, although they are crucial for improving understanding of chemistry, if the prevailing teaching method is the classic (i.e., frontal method) without any possibility of detecting these obstacles while students are learning in school.
Another conclusion of the study is also valuable, as it is based on analyses of correlations between students’ achievement in the design and construction approach to laboratory activity and pre-knowledge of science in 7th grade and chemistry in 8th grade, where modest correlations were found at the α level of 0.1 % (Table 11.2). This means that students with a better background did better also on the present learning tasks, so it can be concluded that the students’ chemistry knowledge is upgrading or deepening throughout schooling, from middle to high school, and that in this case the grades have a relatively strong predictive value for students’ enhancement and achievements.
Further statistical analyses revealed also that students’ achievement through the design and construction approach is correlated with four motivational components: controlled and regulated motivation, and especially with intrinsic motivation and academic self-concept (Table 11.3). Only students with prior higher achievements and higher motivation for learning science/chemistry successfully accomplished the more demanding and complex tasks of the design and construction approach. This finding suggests that the pedagogical work in the chemistry classroom should focus more fully on internal motivation constructs (e.g., interest, self-concept) in order to empower students to use deep learning strategies, which leads to higher levels of knowledge (Green et al. 2007; Reeve et al. 2004; Zimmerman and Clearly 2009).
These findings should not discourage teachers from applying the design and construction approach to laboratory activity in their chemistry classes, since in evaluating different attributes of the approach, students reported that they most appreciate the relaxing and working atmosphere and the opportunity to learn chemistry by doing (Table 11.5). In other words, it means that more authentic learning tasks, together with the mediating role of the teacher within the supportive learning environment, create the best conditions for learning (Brophy 1999; DeBacker and Nelson 2000; Urdan and Schhoenfelder 2006). This is especially valuable when also the rationale for learning is mediated through teaching, so that students can understand immediately the usefulness of learning in everyday life; as Jang (2008) reports, the rationale enhances students’ autonomously motivated learning behavior, which is needed to engage them actively and constructively in learning, regardless of its difficulty.
References
Alexander, P. A., & Murphy, P. K. (1998). The research base for APA’s learner-centred psychological principles. In N. M. Lambert & B. L. McCombs (Eds.), How students learn: Reforming schools through learner-centred education (pp. 25–60). Washington, DC: APA.
Bobich, J. A. (2008). Active learning of biochemistry made easy (for the teacher). Journal of Chemical Education, 85(4), 234–236.
Bodner, G. M. (1986). Constructivism – A theory of knowledge. Journal of Chemical Education, 63(10), 873–878.
Black, A. E., & Deci, E. L. (2000). The effects of instructors’ autonomy support and students’ autonomous motivation on learning organic chemistry: A self-determination theory perspective. Science Education, 84(6), 740–756.
Brophy, J. (1999). Toward a model of the value aspects of motivation in education: Developing appreciation for particular learning domains and activities. Educational Psychologist, 34(2), 75–85.
Burke, K. A., Greenbowe, T. J., & Hand, B. M. (2006). Implementing the science writing heuristic in the chemistry laboratory. Journal of Chemical Education, 83(7), 1032–1038.
Byrnes, J. P., & Fox, N. A. (1998). The educational relevance of research in cognitive neuroscience. Educational Psychology Review, 10(3), 297–342.
Cacciatore, K. L., Amado, J., Evans, J. J., & Sevian, H. (2008). Connecting solubility, equilibrium, and periodicity in a green, inquiry experiment for the general chemistry laboratory. Journal of Chemical Education, 85(2), 251–253.
Chambers, S. K., & Andre, T. (1997). Gender, prior knowledge, interest, and experience in electricity and conceptual change text manipulations in learning about direct current. Journal of Research in Science Teaching, 34(2), 107–123.
Chang, C. Y., & Cheng, W. Y. (2008). Science achievement and students’ self-confidence and interest in science: A Taiwanese representative sample study. International Journal of Science Education, 30(9), 1183–1200.
Charlesworth, P., & Vician, C. (2003). Leveraging technology for chemical sciences education: An early assessment of WebCT usage in first-year chemistry courses. Journal of Chemical Education, 80(11), 1333–1337.
Chimeno, J. S., Wulfsberg, G. P., Sanger, M. J., & Melton, T. J. (2006). The rainbow wheel and rainbow matrix: Two effective tools for learning ionic nomenclature. Journal of Chemical Education, 83(4), 651–654.
Cohen, A., & Magen, H. (2004). Hierarchical systems of attention and action. In G. W. Humphreys & M. J. Riddoch (Eds.), Attention in action: Advances from cognitive neuroscience (pp. 27–68). Howe: Taylor & Francis.
Corno, L. (1994). Student volition and education: outcomes, influences, and practices. In D. H. Schunk & B. J. Zimmerman (Eds.), Self-regulation of learning and performance: Issues and educational applications (pp. 229–251). Hillsdale, New Jersey: Erlbaum.
Cozolino, L. (2006). The neuroscience of human relationships: Attachment and the developing social brain. New York: Norton.
Csikszentmihalyi, M., & Nakamura, J. (1989). The dynamics of intrinsic motivation: A study of adolescents. In R. Ames & C. Ames (Eds.), Research on motivation in education: Goals and cognitions (Vol. 3, pp. 45–72). San Diego, CA: Academic Press.
DeBacker, T. K., & Nelson, R. M. (2000). Motivation to learn science: Differences related to gender, class type, and ability level. Journal of Educational Research, 93(4), 245–254.
Doppelt, Y., Mehalik, M. M., Schunn, C. D., Silk, E., & Krysinski, D. (2008). Engagement and achievements: A case study of design-based learning in a science context. Journal of Technology Education, 19(2), 22–39.
Eccles, J. S., Wigfield, A., & Schiefele, U. (1998). Motivation to succeed. In W. Damon & N. Eisenberg (Eds.), Handbook of child psychology (Vol. 3, pp. 1017–1095). New York, NY: Wiley.
Erk, S., Kiefer, M., Grothe, J., Wunderlich, A. P., Spitzer, M., & Walter, H. (2003). Emotional context modulates subsequent memory effect. Neuroimage, 18(2), 439–447.
Feltham, N. F., & Downs, C. T. (2002). Three forms of assessment of prior knowledge, and improved performance following an enrichment programme, of English second language biology students within the context of a marine theme. International Journal of Science Education, 24(2), 157–184.
Fosnot, C. T., & Perry, R. S. (2005). Constructivism: A psychological theory of learning. In C. T. Fosnot (Ed.), Constructivism: Theory, perspectives and practice. New York: Teachers College Press, Columbia University.
Furlan, P. Y. (2009). Engaging students in early exploration of nanoscience topics using hands-on activities and scanning tunneling microscopy. Journal of Chemical Education, 86(6), 705–711.
Glynn, S. M., Taasoobshirazi, G., & Brickman, P. (2009). Science motivation questionnaire: Construct validation with nonscience majors. Journal of Research in Science Teaching, 46(2), 127–146.
Glynn, S. M., Taasoobshirazi, G., & Brickman, P. (2007). Nonscience majors learning science: A theoretical model of motivation. Journal of Research in Science Teaching, 44(8), 1088–1107.
Gottfried, A. E., Fleming, J. S., & Gottfried, A. W. (2001). Continuity of academic intrinsic motivation from childhood through late adolescence: A longitudinal study. Journal of Educational Psychology, 93, 3–13.
Green, J., Martin, J. A., & Marsh, H. W. (2007). Motivation and engagement in english, mathematics and science high school subjects: Towards an understanding of multidimensional domain specificity. Learning and Individual Differences, 17(3), 269–279.
Hailikari, T., Katajavuori, N., & Lindblom-Ylanne, S. (2008). The relevance of prior knowledge in learning and instructional design. American Journal of Pharmaceutical Education, 72(5), 113.
Harder, A. K. (1989). Attitudes toward reading science textbooks. The American Biology Teacher, 51(4), 208–212.
Hewson, M. G., & Hewson, P. W. (1983). Effect of instruction using students’ prior knowledge and conceptual change strategies on science learning. Journal of Research in Science Teaching, 20(8), 731–743.
Holme, T. A. (1994). Providing motivation for the general-chemistry course through early introduction of current research topics. Journal of Chemical Education, 71(11), 919–921.
Jang, H. (2008). Supporting students’ motivation, engagement, and learning during an uninteresting activity. Journal of Educational Psychology, 100(4), 798–811.
Jarvela, S., & Niemivirta, M. (2001). Motivation in context: Challenges and possibilities in studying the role of motivation in new pedagogical cultures. In S. Volet & S. Jarvela (Eds.), Motivation in learning context: Theoretical advances and methodological implications (pp. 105–127). Amsterdam: Pergamon.
Jones, L. L. (1999). Learning chemistry through design and construction. UniServe Science News, 14, 3–7.
Juriševič, M. (2006). Učna motivacija in razlike med učenci [Motivation to learn and individual differences]. Ljubljana: Univerza v Ljubljani, Pedagoška fakulteta.
Juriševič, M., Razdevšek, Pučko C., Devetak, I., & Glažar, S. A. (2008). Intrinsic motivation of pre-service primary school teachers for learning chemistry in relation to their academic achievement. International Journal of Science Education, 30(1), 87–107.
Kaya, S., & Rice, D. C. (2010). Multilevel effects of student and classroom factors on elementary science achievement in five countries. International Journal of Science Education, 32(10), 1337–1363.
Kirschner, P. A., Sweller, J., & Clark, R. E. (2006). Why minimal guidance during instruction does not work: An analysis of the failure of constructivist, discovery, problem-based, experiential, and inquiry-based. Educational Psychologist, 41(2), 75–86.
Kroesbergen, E. H., Van Luit, J. E. H., & Maas, C. J. M. (2004). Effectiveness of explicit and constructivist mathematics instruction for low-achieving students in the Netherlands. Elementary School Journal, 104(3), 233–251.
Laugksch, R. C. (2000). Scientific literacy: A conceptual overview. Science Education, 84(1), 71–94.
Lavonen, J., & Laaksonen, S. (2009). Context of teaching and learning school science in Finland: Reflections on PISA 2006 results. Journal of Research in Science Teaching, 46(8), 922–944.
Marsh, H. W. (1990). The structure of academic self-concept: The Marsh/Shavelson model. Journal of Educational Psychology, 82(4), 623–636.
Mayer, R. E. (2004). Should there be a three-strikes rule against pure discovery learning? The case for guided methods of instruction. American Psychologist, 59(1), 14–19.
Matthews, M. R. (2008). Philosophical and pedagogical problems with constructivism. Sydney: University of New South Wales. Retrieved from http://per.physics.helsinki.fi/Tutkijakoulun_kesaseminaari_2008/Michael_R_Matthews_helsinki_2008.pdf.
Nentwig, P. M., Demuth, R., Parchmann, I., Grasel, C., & Ralle, B. (2007). Chemie im kontext: Situating learning in relevant contexts while systematically developing basic chemical concepts. Journal of Chemical Education, 84(9), 1439–1444.
Nieswandt, M. (2007). Student affect and conceptual understanding in learning chemistry. Journal of Research in Science Teaching, 44(7), 908–937.
Palmer, D. H. (2009). Student interest generated during an inquiry skills lesson. Journal of Research in Science Teaching, 46(2), 147–165.
Pecrun, R. (2009). Emotions in school. In K. R.Wentzel & A. Wigfield (Eds.), Handbook of motivation at school (pp. 575–604). New York, NY: Routledge.
Pintrich, P. R., & Schunk, D. H. (1996). Motivation in education: Theory, research, and applications. Englewood Cliffs, New Jersey: Prentice Hall.
Randler, C. (2009). Association between emotional variables and school achievement. International Journal of Instruction, 2(2), 3–10.
Reeve, J., Deci, E. L., & Ryan, R. M. (2004). Self-Determination theory: A dialectical framework for understanding sociocultural influences on student motivation. In D. M. McInerney & S. V. Etten (Eds.), Research on sociocultural influences on motivation and learning (Vol. 4, pp. 31–60). Greenwich: Information Age.
Rennie, L. J. (1990). Student participation and motivational orientation: What do students do in science? In K. Tobin, J. Butler, & B. J. Fraser (Eds.), Windows into science classrooms: Problems associated with higher-level cognitive learning (pp. 164–198). London: The Falmer Press.
Rheinberg, F., Vollmeyer, R., & Rollett, W. (2000). Motivation and action in self-regulated learning. In M. Boekaerts, P. R. Pintrich, & M. Zeidner (Eds.), Handbook of self-regulation (pp. 503–531). San Diego: Academic Press.
Rieck, D. F. (1998). Providing direction and motivation for students to review topics from previous chemistry classes. Journal of Chemical Education, 75(7), 850.
Romance, N. R., & Vitale, M. R. (1992). A Curriculum strategy that expands time for in-depth elementary science instruction by using science-based reading strategies—Effects of a year-long study in grade 4. Journal of Research in Science Teaching, 29(6), 545–554.
Romance, N. R., & Vitale, M. R. (2001). Implementing an in-depth expanded science model in elementary schools: Multi-year findings, research issues, and policy implications. International Journal of Science Education, 23(4), 373–404.
Rothbart, M. K., & Hwang, J. (2005). Temperament and the development of competence and motivation. In A. J. Elliot & C. S. Dweck (Eds.), Handbook of competence and motivation (pp. 167–184). New York, NY: The Guilford Press.
Rudd, J. A., Greenbowe, T. J., Hand, B. M., & Legg, M. J. (2001). Using the science writing heuristic to move toward an inquiry-based laboratory curriculum: An example from physical equilibrium. Journal of Chemical Education, 78(12), 1680–1686.
Ryan, R. M., & Deci, E. L. (2000). Intrinsic and extrinsic motivation: Classic definitions and new directions. Contemporary Educational Psychology, 25(1), 54–67.
Schiefele, U., & Rheinberg, F. (1997). Motivation and knowledge acquisition: Searching for mediating processes. Advances in Motivation and Achievement, 10, 251–301.
Seery, M. K. (2009). The role of prior knowledge and student aptitude in undergraduate performance in chemistry: A correlation-prediction study. Chemistry Education Research and Practice, 10(3), 227–232.
Schunk, D. H. (1998). An educational psychologist’s perspective on cognitive neuroscience. Educational Psychology Review, 10(4), 297–342.
Schunk, D. H., & Pajers, F. (2009). Self-efficacy theory. In K. R.Wentzel & A. Wigfield (Eds.), Handbook of motivation at school (pp. 35–54). New York, NY: Routledge.
Shachar, H., & Fischer, S. (2004). Cooperative learning and the achievement of motivation and perceptions of students in 11th grade chemistry classes. Learning and Instructions, 14(1), 69–87.
Stipek, D. (1998). Motivation to learn: From theory to practice. Boston, MA: Allyn and Bacon.
Urdan, T., & Schoenfelder, E. (2006). Classroom effects on student motivation: Goal structures, social relationships, and competence beliefs. Journal of School Psychology, 44(5), 331–349.
Taasoobshirazi, G., & Glynn, S. M. (2009). College students solving chemistry problems: A theoretical model of expertise. Journal of Research in Science Education, 46(10), 1070–1089.
Tarhan, L., & Sesen, B. A. (2010). Investigation of the effectiveness of laboratory works related to “acids and basis” on learning achievements and attitudes toward laboratory. Procedia-Social and Behavioral Sciences, 2(2), 2631–2636.
Thiel, A., Eurich, C. W., & Schwegler, H. (2002). Stabilized dynamics in physiological and neural systems despite strongly delayed feedback. In J. R. Dorronsoro (Ed.), Artificial Neural Networks (Proceedings International Conference on Artificial Neural Networks, ICANN 2002) (pp. 15–20). Berlin; Heidelberg: Springer.
Thiele, R. B., & Treagust, D. F. (1994). An interpretive examination of high school chemistry teachers’ analogical explanations. Journal of Research in Science Teaching, 31(3), 227–242.
Vrtačnik, M. (2011). Development of the basic science competencies through experimental work. The Teachers’ Guidelines for Implementing New Chemistry Curricula for High School, General and Inorganic Chemistry, (pp. 19–23). Slovene Board of Education.
Vrtačnik, M. (2009). » Foam, foam « Teaching unit based on the design and construction laboratory approach, Project European Social Fund and Slovenian Ministry for Education and Sports, » Development of Science Competencies « .
Woodburn, J. H. (1977). Classroom mechanics—using applied chemistry to tackle motivation problems. Journal of Chemical Education, 54(12), 763.
Zangyuan, O. (2003). The application of adaptive learning environment on oxidation-reduction reactions. International Journal of Instructional Media, 30(1), 223–235.
Zimmerman, B. J., & Cleary, T. J. (2009). Motives to regulate Learning: A social cognitive account. In K. R.Wentzel & A. Wigfield (Eds.), Handbook of motivation at school (pp. 247–264). New York, NY: Routledge.
Acknowledgments
The authors wish to thank the financers of this project and especially all participating students and teachers, without whose dedication and willingness to cooperate the realization of the project would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Vrtačnik, M., Sodja, K., Juriševič, M. (2014). Students’ Achievement in Learning Chemistry Through the Design and Construction Approach to Laboratory Activity and the Relation with their Prior Achievements and Motivation to Learn. In: Devetak, I., Glažar, S. (eds) Learning with Understanding in the Chemistry Classroom. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4366-3_11
Download citation
DOI: https://doi.org/10.1007/978-94-007-4366-3_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4365-6
Online ISBN: 978-94-007-4366-3
eBook Packages: Humanities, Social Sciences and LawEducation (R0)