Abstract
Coxiella burnetii is a highly infectious bacterial pathogen that replicates in a specialized vacuole inside eukaryotic cells. Due to a prolonged growth cycle, Coxiella continuously manipulates cellular processes to generate this parasitophorous vacuole (PV) and promote host cell viability. Here, we discuss recent findings that indicate Coxiella modulates several host signaling pathways to influence survival and ensure intracellular replication. The pathogen actively inhibits apoptotic cell death and activates the pro-survival kinases Akt and Erk1/2 to promote host viability. Coxiella’s anti-apoptotic activity also involves the interface between autophagy and apoptosis, which is regulated by the interaction of autophagy-related Beclin-1 and anti-apoptotic Bcl-2. Additionally, Coxiella requires host kinase activity for PV biogenesis and maintenance. Thus, signaling modulation by Coxiella is critical for multiple aspects of host cell parasitism. Collectively, recent signaling studies have enhanced our understanding of the unique Coxiella-host cell interaction. Identification of bacterial factors that regulate signaling events will further our ability to model this intriguing infectious process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Bacterial pathogens have evolved a battery of mechanisms to modulate the hostile host environment encountered during infection and cause disease. Intracellular bacterial parasites comprise a group of intriguing organisms that rely on the internal eukaryotic cell environment for nutrient acquisition and formation of a protected replication niche. Intracellular pathogens have adapted to their unique lifestyle by counteracting antibacterial responses such as cytokine production, immune detection, lysosomal degradation, and cell death. Manipulation of these processes intricately controls the infectious process and ultimately ensures an efficient infection. Conversely, combating these activities often results in clearance of the pathogen and resolution of disease. Thus, regulation of intracellular host processes has become a major focus of this growing field of infectious disease.
Coxiella burnetii is the intracellular bacterial agent of the zoonosis human Q fever, which can present as acute or chronic disease. Humans are typically exposed to Coxiella via inhalation of contaminated aerosols, resulting in deposition of infectious organisms in the alveolar spaces (Raoult et al. 2005). Hence, alveolar phagocytic cells are considered the initial reservoir wherein Coxiella promotes formation of a parasitophorous vacuole (PV) in which to replicate (Voth and Heinzen 2007). The early Coxiella-containing phagosome matures through interactions with host autophagosomes as a potential source of nutrients and membrane for the expanding vacuole (Romano et al. 2006). As the vacuole enlarges through continual fusion with lysosomes, autophagosomes, and fluid phase endosomes, Coxiella converts from a small cell variant morphological form into a replication-proficient large cell variant form that divides by binary fission throughout a prolonged growth cycle (Coleman et al. 2004). The mature PV is unique among intracellular pathogen compartments in possessing an acidic (pH ∼ 5.0), phagolysosome-like nature (Akporiaye et al. 1983). Indeed, the PV contains active acid hydrolases and retains degradative activity against other bacterial cells (Howe et al. 2010). Strikingly, Coxiella replicates to high numbers and thrives in this hostile environment designed to dispose of invading pathogens. Therefore, PV maintenance is of utmost importance to Coxiella and the bacterium actively promotes vacuole biogenesis and maintenance to ensure a successful infection.
Coxiella protein synthesis is required for PV biogenesis and antibiotic treatment results in accumulation of tight-fitting phagolysosomes containing individual organisms (Howe et al. 2003). These small vacuoles are unable to fuse with other PV or host vesicles and expansion and replication is stalled. To direct PV biogenesis, Coxiella likely employs its Dot/Icm type IV secretion system (T4SS), which delivers bacterial proteins, termed substrates or effectors, to the host cytosol where they interact with eukaryotic proteins to regulate infection events. Recent evidence indicates that Coxiella encodes numerous Dot/Icm substrates predicted to control a vast array of host processes. For example, Coxiella isolates collectively encode 11 Dot/Icm substrates with ankyrin repeat domains (Voth et al. 2009; Pan et al. 2008), which are eukaryotic motifs that mediate protein-protein interactions. These and other Dot/Icm-translocated proteins are predicted to be major virulence factors used by Coxiella to control infection events, including subversion of signaling pathways. In this chapter, recent discoveries will be discussed regarding intracellular host signaling pathways manipulated by Coxiella to control host viability and PV formation.
7.2 Coxiella Exhibits Potent Anti-apoptotic Activity
A costly detriment to an intracellular parasite is the demise of its preferred host cell. Many intracellular pathogens, including Coxiella (Coleman et al. 2004), display slow replication rates, necessitating a viable host cell throughout a prolonged growth cycle. Thus, these organisms have developed methods to promote host cell survival until replication is complete and cellular release can ensue. A common target of these organisms is apoptosis (Labbe and Saleh 2008), a form of ordered, non-inflammatory eukaryotic cell death broadly consisting of two pathways: extrinsic and intrinsic (Fig. 7.1). Extrinsic apoptosis is mediated by ligation of cell surface death receptors that activate intracellular proteolytic caspase cascades (Jin and El-Deiry 2005). Subsequent activation of downstream, or effector, caspases such as caspase-3 ultimately results in DNA fragmentation and cell death. Intrinsic apoptosis is regulated by intracellular events that trigger mitochondrial release of cytochrome c (Jin and El-Deiry 2005). Cytochrome c release is regulated by a panel of interacting anti- and pro-apoptotic mitochondrial surface proteins containing BH3 homology domains. In the cytosol, cytochrome c forms a complex with Apaf-1 and caspase-9 termed the apoptosome, which activates effector caspases, triggering DNA damage and death. It is increasingly clear that intracellular pathogens can completely halt cell death.
Overview of extrinsic and intrinsic apoptosis. Extrinsic apoptosis ensues following ligation of cell surface death receptors that trigger activation of proteolytic initiator caspase proteins, such as caspase-8. Intrinsic apoptosis is initiated by stress factors that allow mitochondrial release of cytochrome c through the interaction of pro-apoptotic BH3 domain proteins and anti-apoptotic proteins such as Bcl-2. Cytochrome c release results in formation of a multi-protein complex, termed the apoptosome, that mediates caspase-9 activation with the assistance of apoptotic protease activating factor-1 (Apaf-1). Following activation of initiator caspases, both apoptotic pathways converge on effector caspases, such as caspase-3 and -7, which trigger downstream DNA fragmentation and cell death
Anti-apoptotic activities have been described for multiple intracellular pathogens including Rickettsia, Salmonella, Mycobacterium, Legionella, and Chlamydia. Salmonella typhimurium secretes SopB, a type III effector protein that activates the host pro-survival kinase Akt during early stages of epithelial cell infection, resulting in decreased caspase activation (Knodler et al. 2005). Mycobacterium tuberculosis inhibits mitochondrial-dependent death by directly altering activity of the BH3 domain protein Bad (Maiti et al. 2001). Legionella pneumophila also directly engages mitochondrial proteins by secreting effector proteins that target pro-apoptotic BNIP3 and Bcl-rambo (Banga et al. 2007). Chlamydia spp. are perhaps the most prolific anti-apoptotic organisms described to date. Chlamydia spp. use a multi-faceted approach to inhibit apoptosis including activation of Akt (Verbeke et al. 2006), inhibition of cytochrome c release (Fan et al. 1998), and degradation of pro-apoptotic BH3 domain mitochondrial proteins (Fischer et al. 2004; Dong et al. 2005; Ying et al. 2005), an activity mediated by the chlamydial protease CPAF (Pirbhai et al. 2006). Finally, many intracellular pathogens promote a pro-survival host gene expression program that relies on the transcription factor NF-κB (Clifton et al. 1998; Losick and Isberg 2006; Abu-Zant et al. 2006; Wahl et al. 2003; Dhiman et al. 2007).
Coxiella has taken a page from the pathogens above and actively promotes host cell survival by altering multiple steps of the apoptotic pathway. The organism antagonizes caspase-3, caspase-9, and poly (ADP-ribose) polymerase (PARP) processing following either staurosporine (intrinsic apoptosis) or TNF-α (extrinsic apoptosis) treatment of THP-1 macrophage-like cells (Voth et al. 2007b). Coxiella also inhibits caspase-3 activation in primary primate alveolar macrophages, which represent a Coxiella target cell, implicating the in vivo importance of promoting cell survival. Coxiella also exhibits anti-apoptotic activity in HeLa (human epithelial) and CHO (Chinese hamster ovary) cells via inhibition of mitochondrial cytochrome c release (Luhrmann and Roy 2007). However, unlike Chlamydia spp., mitochondrial BH3 domain proteins are not degraded during Coxiella infection (Luhrmann and Roy 2007; Voth et al. 2007b). Importantly, virulent Coxiella also inhibits caspase-3 and PARP processing (Voth et al. 2007b), indicating anti-apoptotic activity is important during natural infection by disease-causing organisms. Furthermore, bacterial protein synthesis is required for the organism’s anti-apoptotic effects, indicating active regulation of host cell survival. It is reasonable to predict that one or more Dot/Icm substrates are responsible for this potent anti-apoptotic activity. Indeed, closely related Legionella pneumophila secretes the Dot/Icm substrates SidF (Banga et al. 2007), SdhA (Laguna et al. 2006), and LegK1 (Ge et al. 2009) to antagonize mitochondrial-mediated apoptosis and activate NF-κB. However, Coxiella does not encode homologs of these proteins, suggesting a pathogen-specific repertoire of anti-apoptotic effectors.
Coxiella also regulates host apoptosis at the transcriptional level by altering expression of 30 survival-related genes (Voth et al. 2007b). For example, infected THP-1 cells show increased expression of cIAP2, a1/bfl-1, and bag1, which promote survival, and decreased expression of pro-apoptotic bax, bim, bik, casp2, and casp6, contributing to an overall anti-apoptotic state. cIAP2 and A1/Bfl-1 protein production also dramatically increases during infection. Previous studies demonstrated that cIAP2 and a1/bfl-1 are regulated by NF-κB transcriptional activity (Zong et al. 1999; Chu et al. 1997), which is important for the anti-apoptotic potential of other intracellular pathogens (see above). Interestingly, NF-κB translocates to the host nucleus during early stages of Coxiella infection and substantial nuclear levels persist throughout intracellular growth (Voth et al. 2007a), suggesting Coxiella promotes sustained activation of this versatile transcription factor.
7.3 A Link Between Autophagy and Coxiella Anti-apoptotic Activity
Coxiella engages host autophagosomes throughout infection as a potential source of nutrients and membrane for the maturing PV. In fact, the PV decorates with the autophagy marker LC3 as early as 5 min post-infection (Gutierrez et al. 2005; Romano et al. 2006). Additionally, activation of autophagy by amino acid depravation or exogenous treatment with rapamycin stimulates infection and enhances PV formation. Recent evidence indicates a potential link between Coxiella interactions with autophagosomes and the pathogen’s ability to antagonize apoptosis. During intracellular growth, the PV decorates with the autophagy-related protein Beclin-1 and the anti-apoptotic mitochondrial protein Bcl-2 (Vazquez and Colombo 2010). The interaction between these two proteins is critical for both PV biogenesis and inhibition of apoptosis as evidenced by increased death of cells expressing low levels of Beclin-1 or a Beclin-1 mutant deficient for Bcl-2 binding. These intriguing new studies underscore the importance of crosstalk between multiple pathways for proper PV formation and sustenance of host viability.
7.4 Coxiella Activates Host Pro-survival Signaling Proteins
It is clear that intracellular pathogens control apoptosis at both transcriptional and post-translational levels to ensure a viable niche throughout their growth cycle. As discussed above, Coxiella inhibits mitochondrial cytochrome c release and caspase activation and induces a pro-survival transcriptional program (Luhrmann and Roy 2007; Voth et al. 2007b). A recent study also demonstrates the role of host kinase-directed signaling in the Coxiella anti-apoptotic response (Voth and Heinzen 2009). During infection of THP-1 cells, two kinase activation events are observed. First, c-Jun, Hsp27, JNK, and p38 MAPK are phosphorylated at 2 hours post-infection (hpi), then de-phosphorylated at 12–24 hpi, suggesting these proteins are activated as an initial host response to phagocytosis of Coxiella. Conversely, the pro-survival kinases Akt and Erk1/2 are phosphorylated at 6 hpi and remain phosphorylated throughout infection, indicating prolonged activation of both proteins and regulation of downstream targets by Coxiella. The pathogen actively promotes increased Akt and Erk1/2 activation as elevated phosphorylation levels are not observed in cells infected in the presence of bacterial protein synthesis inhibitors. Akt and Erk1/2 are heavily involved in maintaining a viable eukaryotic cell via phosphorylation. Akt regulates numerous pathways that promote survival including activation of the FOXO family of transcription factors and direct regulation of caspase-9 activity (Manning and Cantley 2007), while Erk1/2 activates survival-related transcription factors such as Elk-1 and cAMP response binding element, or CREB (McCubrey et al. 2006). Thus, Akt and Erk1/2 are prime targets for apoptosis intervention by Coxiella. Furthermore, treatment of infected cells with Akt and Erk1/2 pathway inhibitors negates Coxiella’s anti-apoptotic effect, suggesting these pathways are critical for the pathogen’s ability to promote survival. However, the upstream and downstream components of these cascades that are modulated during infection, and the bacterial proteins responsible, are currently unknown.
7.5 A Role for Host Signaling Cascades in PV Development
Mammalian cells also use phosphorylation-based signaling to control a diverse array of responses not directly related to apoptosis. Kinase-based cascades, such as Akt and Erk1/2, are efficient scaffolds that control sequential phosphorylation events to regulate downstream substrate activity (McCubrey et al. 2006). Eukaryotic kinases are also controlled by their own phosphorylation, providing for tightly regulated responses. Not surprisingly, bacterial pathogens have evolved mechanisms to subvert distinct signaling pathways and influence infection events for the benefit of the pathogen (Bhavsar et al. 2007). Aside from apoptosis, intracellular pathogens utilize eukaryotic signaling for cellular entry, modulation of cytokine production, and alteration of the host cell cycle (Knodler et al. 2001; Bhavsar et al. 2007). However, the role of mammalian kinase signaling in formation of bacterial replication vacuoles has not been elucidated.
Recent studies in our laboratory have uncovered a role for host phosphorylation signaling in PV biogenesis and maintenance (Hussain et al. 2010). Thirteen signaling proteins including protein kinase C (PKC), cAMP-dependent protein kinase, and calmodulin kinase II are involved in PV formation, and inhibition of these molecules adversely affects Coxiella growth. These results suggest the organism must engage host signaling that is not directly involved in survival to promote PV formation and maintenance. Additionally, several kinases are differentially phosphorylated throughout intracellular growth. Furthermore, virulent Coxiella isolates activate PKC during infection, suggesting PKC signaling is regulated during natural infection. However, the ultimate effects of pathogen-modulated kinase activity are unknown.
7.6 Conclusions and Future Perspectives
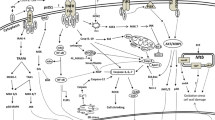
Intracellular pathogens are adept at directing host pathways during intracellular growth to ensure a viable, sustainable niche in which to replicate. As discussed in this chapter, Coxiella actively regulates host apoptosis, autophagy, and phosphorylation cascades to provide a proper replication environment (Fig. 7.2). However, the bacterial proteins that regulate signaling events have not been identified and represent a substantial void in our understanding of Coxiella-host cell interactions. As mentioned above, Coxiella produces a specialized Dot/Icm T4SS during infection that delivers bacterial proteins directly to the host cytoplasm. It is intriguing to predict that a subset of effector proteins controls the pathogen’s anti-apoptotic activity through direct interactions with host proteins. Indeed, exciting new evidence demonstrates Coxiella Dot/Icm-translocated AnkG binds to host p32, inhibiting the pro-apoptotic properties of this protein and promoting cell survival (Luhrmann et al. 2010). In addition to identifying novel Dot/Icm substrates using heterologous models such as closely-related L. pneumophila, recent advances in Coxiella host cell-free growth (Omsland et al. 2009) and genetic manipulation (Beare et al. 2009) will enhance our ability to identify and functionally characterize pathogen virulence factors. Furthermore, recent success using transposon mutagenesis (Beare et al. 2009) will allow screening of Coxiella mutant libraries to identify strains defective for anti-apoptotic activity and kinase activation. These anticipated studies will shed important insight into Coxiella virulence factors and pathogenic mechanisms.
Manipulation of intracellular host signaling by Coxiella . In susceptible host cells, Coxiella generates a membrane bound vacuolar compartment with features of phagolysosomes, including acidic pH and the presence of acid hydrolases and lysosomal membrane markers (e.g., LAMP-1, -2, -3). PV formation is aided by interactions with autophagosomes (denoted by labeling with LC3) that provide membrane for the expanding vacuole and may provide nutrients to replicating organisms. PV biogenesis relies on functional host kinase activity as evidenced by phosphorylation (p) of downstream substrates. During a lengthy infectious cycle, Coxiella actively antagonizes apoptotic cell death through inhibition of cytochrome c release and caspase processing, up-regulation of an anti-apoptotic transcriptional program, and activation of pro-survival kinases. Additionally, the autophagy-related protein Beclin-1 interacts with anti-apoptotic Bcl-2 to promote cell survival. These processes are likely controlled by the activity of numerous Coxiella proteins, such as anti-apoptotic AnkG, that are delivered to the host cytosol by the Dot/Icm T4SS
References
Abu-Zant A, Jones S, Asare R, Suttles J, Price C, Graham J, Kwaik YA (2006) Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol 9:246–264
Akporiaye ET, Rowatt JD, Aragon AA, Baca OG (1983) Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect Immun 40:1155–1162
Banga S, Gao P, Shen X, Fiscus V, Zong WX, Chen L, Luo ZQ (2007) Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci U S A 104:5121–5126
Beare PA, Howe D, Cockrell DC, Omsland A, Hansen B, Heinzen RA (2009) Characterization of a Coxiella burnetii ftsZ mutant generated by Himar1 transposon mutagenesis. J Bacteriol 191:1369–1381
Bhavsar AP, Guttman JA, Finlay BB (2007) Manipulation of host-cell pathways by bacterial pathogens. Nature 449:827–834
Chu ZL, Mckinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW (1997) Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A 94:10057–10062
Clifton DR, Goss RA, Sahni SK, Van Antwerp D, Baggs RB, Marder VJ, Silverman DJ, Sporn LA (1998) NF-kappa B-dependent inhibition of apoptosis is essential for host cellsurvival during Rickettsia rickettsii infection. Proc Natl Acad Sci U S A 95:4646–4651
Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA (2004) Temporal analysis of Coxiella burnetii morphological differentiation. J Bacteriol 186:7344–7352
Dhiman R, Raje M, Majumdar S (2007) Differential expression of NF-kappaB in mycobacteria infected THP-1 affects apoptosis. Biochim Biophys Acta 1770:649–658
Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, Zhong G (2005) Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun 73:1861–1864
Fan T, Lu H, Hu H, Shi L, Mcclarty GA, Nance DM, Greenberg AH, Zhong G (1998) Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496
Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, Ying S, Hacker G (2004) Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med 200:905–916
Ge J, Xu H, Li T, Zhou Y, Zhang Z, Li S, Liu L, Shao F (2009) A Legionella type IV effector activates the NF-kappaB pathway by phosphorylating the IkappaB family of inhibitors. Proc Natl Acad Sci U S A 106:13725–13730
Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI (2005) Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol 7:981–993
Howe D, Melnicakova J, Barak I, Heinzen RA (2003) Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell Microbiol 5:469–480
Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA (2010) Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect Immun 78:3465–3474
Hussain SK, Broederdorf LJ, Sharma UM, Voth DE (2010) Host kinase activity is required for Coxiella burnetii parasitophorous vacuole formation. Front Microbiol 1:137. doi:10.3389/fmicb.2010.00137
Jin Z, El-Deiry WS (2005) Overview of cell death signaling pathways. Cancer Biol Ther 4:139–163
Knodler LA, Celli J, Finlay BB (2001) Pathogenic trickery: deception of host cell processes. Nat Rev Mol Cell Biol 2:578–588
Knodler LA, Finlay BB, Steele-Mortimer O (2005) The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem 280:9058–9064
Labbe K, Saleh M (2008) Cell death in the host response to infection. Cell Death Differ 15:1339–1349
Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR (2006) A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A 103:18745–18750
Losick VP, Isberg RR (2006) NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med 203:2177–2189
Luhrmann A, Roy CR (2007) Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect Immun 75:5282–5289
Luhrmann A, Nogueira CV, Carey KL, Roy CR (2010) Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci USA 107:18997–19001
Maiti D, Bhattacharyya A, Basu J (2001) Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating bad through a phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem 276:329–333
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Mccubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M (2006) Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 46:249–279
Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, Porcella SF, Heinzen RA (2009) Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc Natl Acad Sci U S A 106:4430–4434
Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR (2008) Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651–1654
Pirbhai M, Dong F, Zhong Y, Pan KZ, Zhong G (2006) The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J Biol Chem 281:31495–31501
Raoult D, Marrie T, Mege J (2005) Natural history and pathophysiology of Q fever. Lancet Infect Dis 5:219–226
Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI (2006) The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol 9:891–909
Vazquez CL, Colombo MI (2010) Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell Death Differ 17:421–438
Verbeke P, Welter-Stahl L, Ying S, Hansen J, Hacker G, Darville T, Ojcius DM (2006) Recruitment of BAD by the Chlamydia trachomatis vacuole correlates with host-cell survival. PLoS Pathog 2:e45
Voth DE, Heinzen RA (2007) Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell Microbiol 9:829–840
Voth DE, Heinzen RA (2009) Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity. Infect Immun 77:205–213
Voth DE, Howe D, Heinzen RA (2007a) Coxiella burnetii inhibits apoptosis and modulates cell survival signaling in macrophages FASEB Microbial Pathogenesis Meeting
Voth DE, Howe D, Heinzen RA (2007b) Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun 75:4263–4271
Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, Heinzen RA (2009) The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol 191:4232–4242
Wahl C, Maier S, Marre R, Essig A (2003) Chlamydia pneumoniae induces the expression of inhibitor of apoptosis 2 (c-IAP2) in a human monocytic cell line by an NF-kappaB-dependent pathway. Int J Med Microbiol 293:377–381
Ying S, Seiffert BM, Hacker G, Fischer SF (2005) Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun 73:1399–1403
Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C (1999) The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev 13:382–387
Acknowledgements
Research in the Voth laboratory is supported by funding to D. E. V. from NIH/NIAID (K22AI081753 and R01AI087669), the American Heart Association (BGIA3080001), and the Arkansas Biosciences Institute.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Hussain, S.K., Voth, D.E. (2012). Coxiella Subversion of Intracellular Host Signaling. In: Toman, R., Heinzen, R., Samuel, J., Mege, JL. (eds) Coxiella burnetii: Recent Advances and New Perspectives in Research of the Q Fever Bacterium. Advances in Experimental Medicine and Biology, vol 984. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4315-1_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-4315-1_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4314-4
Online ISBN: 978-94-007-4315-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)