Abstract
Natural products as disease remedies have a history of near 5,000 years (India, China and Greece), and even today, in this advanced technological age, a revival of interest is being witnessed in the use of natural or plant-based therapeutic agents for the treatment of several pathological conditions. Citrus fruits have been utilised as a traditional medicine in India, China, Korea and Japan, and many studies have highlighted the various biological properties of their phytophenolics which are suggested to be responsible for the prevention of degenerative diseases such as diabetes and cancer. With the background of comprehensive studies conducted on Mauritian citrus fruits, this chapter reviews some of the literature data on the phytophenolic contents, vitamin C composition and antioxidant functions of citrus extracts and emphasises on their potential applications in nutrition management programmes for diabetes and cancer chemoprevention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
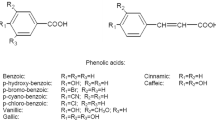
The role played by dietary factors on health status has long been recognised, but it has been only recently that epidemiological and clinical data have provided a deeper insight on some of the intricate mechanisms of the effect of bioactive foods on human health. Citrus genus is one of the most important fruit tree crop in the world with an annual fruit production of approximately 102 million tons. Tropical countries like Mauritius enjoy the right balance of sunshine and rainfall for the growth of a wide range of exotic fruits including citrus which is the second most consumed fruit after bananas (Central Statistics Office 2008). More than 30 different varieties of citrus fruits are grown in Mauritius, either in backyard orchards or on a commercial basis, and consumed as fresh fruits or are processed into a variety of products such as juices, jams, marmalades and fruit pastes. Citrus fruits, in addition to providing an ample supply of vitamins, minerals, dietary fibres and pectins, contain a host of active phytochemicals including phytophenolics (e.g. flavanones, flavones, flavonols, phenolic acids) that can protect health. In fact, literature abounds of examples of citrus fruits, citrus fruit extracts and citrus flavonoids, exhibiting a wide range of promising biological properties including anti-atherogenic, anti-inflammatory and antitumor activity, inhibition of blood clots and strong antioxidant activity (Middleton and Kandaswami 1994; Montanari et al. 1998; Samman et al. 1998). Citrus is consumed mostly as fresh produce and juice, and most often, the peel is discarded. This represents a huge waste as citrus peels are reported to possess highest amounts of flavonoids compared to other parts of the fruit (Manthey and Grohman 2001). Citrus peels are subdivided into the epicarp or flavedo and mesocarp or albedo. The flavedo is the coloured peripheral surface of the peel, whilst the albedo is the white soft middle layer of the peel (Fig. 3.1). The beneficial health effects of citrus flavonoids would be particularly relevant in the Mauritian context considering the high incidence of cardiovascular diseases, diabetes and cancer on the island. This chapter emphasises on the phytophenolic, vitamin C and antioxidant screening of flavedo, albedo and pulp extracts of citrus fruits commonly consumed in Mauritius (Table 3.1). With this background, the antidiabetic and cancer chemopreventive potential of these extracts are discussed.
3.1.1 Phytophenolics and Vitamin C in Citrus Extracts
Most citrus species accumulate substantial quantities of flavonoids during the development of their different organs (Castillo et al. 1992). Four types of flavonoids occur in citrus species, namely the flavanones, flavones, flavonols and anthocyanins with the latter group occurring only in blood oranges. More than 60 individual flavonoids have been identified (Horowitz and Gentili 1977). Studies on the quantitative distribution of these flavonoids have shown that the flavanones predominate in all species of the genus, and they occur as glycosides, in which the aglycones are linked to a sugar moiety (Fig. 3.2) (Lewinsohn et al. 1989). Although flavones and flavonols have been found in low concentrations in citrus fruit tissues, they have been shown to be powerful antioxidants and free-radical scavengers with the highly methoxylated flavones exhibiting the highest biological activity (Benavente-Garcia et al. 1997).
Flavanone skeleton with substitution pattern (a) Flavanone aglycone (b) Rutinoside (Diglycoside) (c) Neohesperidoside (Diglycoside) (Adapted from Merken and Beecher 2000)
Comprehensive studies conducted on 21 Mauritian citrus species demonstrated, with established correlations, that polyphenolic-rich extracts exhibited important antioxidant propensities in various test systems (Ramful et al. 2010a, b, 2011). Table 3.2 lists the citrus varieties having highest amounts of total phenolic, flavonoid and vitamin C in their flavedo, albedo and pulp extracts, respectively. The total phenolic content decreased in the following order: flavedo extracts > albedo extracts > pulp extracts. Gorinstein et al. (2001) also reported total polyphenols in the peels of lemons, oranges and grapefruits to be significantly higher than in the peeled fruits whilst quince peel extracts had threefold higher amounts than that of the pulp (Fattouch et al. 2007).
The levels of total phenolics in the Mauritian study are much higher than those measured in peels of similar varieties from Israel and New Zealand using the same methodology, indicating that the contents can be influenced by various factors such as genotypic differences, geographical and climatic conditions, cultural practices, harvest time, fruit maturity, environmental and growing conditions and extraction methods amongst others (Van der Sluis et al. 2001). Literature data on total phenolics of pulp extracts of citrus fruits are, however, comparable to the investigation on Mauritian citrus. Thus, Gorinstein et al. (2001) reported values for three varieties of citrus pulps in the range 1,350–1,640 μg/g FW, whilst the total phenolics content of pulp extracts in our study was between 406 and 1,694 μg/g FW.
The total flavonoid content of the extracts followed the same order as the total phenolics with highest levels in the flavedo extracts and lowest in the pulp extracts. Flavonoid derivatives, expressed in quercetin equivalents, in Mauritian citrus flavedos were generally high (>2,000 μg/g FW for the majority of samples analysed). Again, factors, including differences in variety and high sunlight conditions (a characteristic feature of tropical Mauritius), which can induce the accumulation of flavonoids (Li et al. 1993), are probably responsible for the relatively high yield. Using the same assay system but with catechin as standard, Gorinstein et al. (2004) reported that peeled Jaffa sweeties (a grapefruit hybrid) and white grapefruits contained 471 and 377 μg/g FW, whilst 925 and 744 μg/g FW were measured in their respective peels.
Vitamin C content was higher in the peel extracts than in the pulp juice. Gorinstein et al. (2001) also reported the ascorbic acid content of three varieties of citrus peels to be significantly higher than that of peeled fruits. In our study, pamplemousses varieties showed relatively high vitamin C content in peel and pulp extracts. Cano et al. (2008), on the other hand, reported that orange varieties had higher vitamin C concentrations than mandarin, clementine, satsume and hybrid varieties, supporting the argument of a wide variation of vitamin C content in literature. Vitamin C levels in fruits and vegetables, in fact, can also be influenced by various factors such as genotypic differences, climatic conditions and cultural practices (Lee and Kader 2000).
Citrus fruits contain a wide range of flavonoid constituents which are encompassed in the flavanones, flavones and flavonols subclasses (Nogata et al. 2006; Mata Bilbao et al. 2007). HPLC analyses of nine flavedo extracts showed that, consistent with literature data (Londoño-Londoño et al. 2010), the flavanone glycoside, hesperidin, was present at highest concentrations (83–234 mg/g FW) in all the extracts except for a variety of pamplemousse. The flavanone glycosides poncirin, didymin, narirutin and flavone glycosides diosmin and isorhoifolin were present in all flavedo extracts, whereas the flavanone glycoside naringin was present only in one variety of Mandarin (1A). The presence of naringin was observed in Mandarin 1A despite its reported absence from mandarin varieties (Tomás-Barberán and Clifford 2000). Similar to flavedo extracts, hesperidin was the most abundant flavonoid glycoside in the albedo extracts where it was detected at concentrations ranging from 132 to 540 mg/g FW. Didymin, hesperidin and narirutin were ubiquitously present in all the nine albedo extracts analysed, whereas naringin and diosmin were not present in any of them. Rhoifolin was measured only in Mandarin 2B and Mandarin 5. Rutin was detected in albedo extracts of Clementine A, Mandarin 1A and Tangor A only. In line with literature data (Cano et al. 2008), hesperidin was the most abundant flavanone glycoside in the citrus pulp extracts. The latter was found at concentrations ranging from 7 to 27 mg/g FW, followed by narirutin (0.3–21 mg/g FW). Hesperidin was present in all the varieties except for Pamplemousse 2A, whilst narirutin was absent only in Tangor A.
The amount of flavonoid glycosides in the pulp extracts was much lower than in the flavedo and albedo extracts of Mauritian citrus fruits; a trend that was consistent with the phenolic and vitamin C contents, as well as the antioxidant activities (Ramful et al. 2011). Berhow et al. (1998) reported that the concentration of flavanones was greater in the citrus albedo, whilst the levels of flavones and flavonols decreased in the following order: flavedo > albedo > juice sacs. The data reported for flavonoid composition of citrus pulp juices are fairly heterogeneous as a result of the different techniques employed and the different units of measurement used by the various authors (mg 100/mg juice, mg 100/mg lyophilised juice, mg 100/mL juice, mg 100/mg fresh product) (Peterson et al. 2006a, b). In a survey of phenolic compounds in 35 citrus species, the same authors reported that the dominant neohesperidosyl flavanones were naringin (found at high concentrations in grapefruit, kumquat and pummelo), neoeriocitrin (found in bergamot and sour orange tissues) and neohesperidin (in tangelo). The dominant rutinosyl flavanones were hesperidin (in lemon, lime, mandarin and sweet orange), eriocitrin and narirutin. The flavanone profile of sweet orange is relatively simple and varies little among cultivars. It is generally agreed that orange fruit and juice contain hesperidin (Anis and Aminuddin 1981), narirutin (Rousseff et al. 1987), and didymin (Matsubara et al. 1985). Berhow et al. (1998) also found some orange cultivars to contain eriocitrin. Mandarin contains predominantly hesperidin, occasionally narirutin, and trace levels of didymin (Horowitz and Gentili 1977). Pummelo (pamplemousses) is reportedly one of the three species (in addition to sour orange and Poncirus trifoliata) that accumulate neohesperidosyl glycosides (Albach and Redman 1969) with naringin being the major flavanone in most pummelo cultivars (Park et al. 1983; Berhow et al. 1998).
3.1.2 Antioxidant Propensity of Citrus Organs
Given that the mechanisms of action of naturally occurring antioxidants can be diverse in vivo, a comprehensive prediction of the antioxidant efficacy initially in vitro requires a multiplicity of assessing methods with various implications for molecular targets (Aruoma 1994, 2003; Pérez-Jiménez et al. 2008). It is noteworthy that synergism and concentration may also bring effects that are not observed when individual constituents are tested (Kaur and Kapoor 2001). There is therefore no universal method that can measure the antioxidant capacity of all samples accurately and consistently. Clearly, matching radical source and system characteristics to antioxidant reaction mechanisms is critical in the selection of appropriate antioxidant capacity assay assessing methods (Prior et al. 2005).The antioxidant characterisation of the flavedo, albedo and pulp extracts of 21 varieties of Mauritian citrus fruits was evaluated using independent methods: the TEAC, FRAP and HOCl assays. From the initial results, nine different flavedo, albedo and pulp extracts, which showed highest antioxidant activities in these three assays, were further assessed for their ability to protect DNA from damage and their iron-chelating activity (Ramful et al. 2010a, b).
Citrus flavedo extracts had wide antioxidant potential ranges, thereby supporting their classification as low, moderate and high. Table 3.3 classifies the citrus fruits according to the antioxidant activities of their flavedo and pulp extracts, as measured by the TEAC, FRAP and HOCl scavenging assays. Some clementine, mandarin, tangor, tangelo and pamplemousse varieties were all classified in the high-level range with TEAC values greater than 40 μmol/g FW. The free-radical scavenging activities of the pulp extracts were much lower compared to the flavedo and albedo extracts with values in the range 2.6–9.9 μmol/g FW. These data are consistent with those of Wang et al. (2011) who reported the antioxidant activity of peel extracts of Citrus sulcata to be twice that of the pulp extracts using the TEAC assay. Flavedo extracts of Orange 2B, Clementine A, Mandarin 1, 2 and 5, Tangor and Tangelo 1A and 2 had FRAP values greater than 50 μmol/g FW. FRAP values for albedo extracts ranged from 5.8 to 55 μmol/g FW, whilst values for pulp extracts ranged from 3.3 to 10.4 μmol/g FW, clearly depicting that the ferric reducing efficacy of the extracts decreased in the order flavedo extracts > albedo extracts > pulp extracts. Flavedo extracts of Orange 2, Clementine A, Mandarin 1, 2A and 5, Tangor A and Pamplemousse 2B were characterised by low IC50 values (3.70–4.41 mg FW/mL), indicating the high effectiveness of the extracts to scavenge hypochlorite. Pulp extracts were relatively poor scavengers of hypochlorite with IC50 values in the range 52.5–175 mg FW/mL. No literature data is available on the assessment of the HOCl scavenging activity of citrus fruits. However, the anti-inflammatory effects of Mangifera indica L. (Martynez et al. 2000), the brown algae Laminaria japonica (Zhao et al. 2004) and the medicinal plant Hypercum androsaerum (Valentão et al. 2002) have also been assessed using the HOCl assay. Among these, interesting radical scavenging capacities were observed in Mangifera indica L. (Vimang) with an IC50 value of 0.04%, thereby supporting its use in traditional medicine as an anti-inflammatory and cancer preventive agent (Martynez et al. 2000).
The metal complex copper 1,10-phenanthroline is known to promote hydroxyl radical formation from molecular oxygen by redox cycling and is therefore a suitable agent for the stimulation of oxidative DNA damage (Aruoma 1993, Halliwell 1997). Indeed DNA fragmentation detected in different cells treated with copper phenanthroline is considered to result from direct attack upon DNA by the hydroxyl radical (Tsang et al. 1996). DNA damage, such as single- and double-strand breakage, base modification, cross-linking of DNA with other biomolecules particularly proteins, is reported to be early events of cancer, cardiovascular diseases, diabetes, cataract and neurological disorders (Cadet et al. 1997), and phytochemicals have profound chemopreventive effects through modulation of molecular events that damage DNA (Bisht et al. 2008). The level of protection against copper-phenanthroline-mediated oxidative DNA damage was in the following order for the flavedo extracts: Tangelo 1A > Clementine A > Tangor A > Pamplemousse 2B > Mandarin 5 > Mandarin 1A ≈ Orange 2B > Mandarin 2A > Tangelo 2.
Among the transition metals, iron is known as the most important lipid pro-oxidant due to its high reactivity. Benherlal and Arumughan (2008) reported that phytochemicals/extracts with high antioxidant activity but without iron chelation capacity failed to protect DNA in Fenton’s system, suggesting that iron chelation was an essential requirement for extracts studied here to retard HO• generation by Fenton’s reaction. In the study conducted on Mauritian citrus, Clementine A, Tangor A and Mandarin 1A and 5 were the most potent Fe2+ ion chelator.
3.1.3 Antidiabetic Potential
Oxidative stress and alterations in glucose metabolism are important risk factors for diabetes and its related complications. Advanced glycated end products and their carbonyl derivatives are believed to contribute significantly to the pathogenesis of type 2 diabetes by their interaction with specific cell membrane receptors triggering, for instance, the nuclear factor-Kappa B (NF-κ B) signalling pathway to induce the expression of pro-inflammatory mediators and elicit oxidative stress which exacerbate diabetic complications (Stern et al. 2002). A great deal of effort has been focused on the identification of useful inhibitors of protein AGEs to delay or prevent glycation so as to alleviate the phenotype of these diseases (Pashikanti et al. 2010). Numerous AGEs inhibitors, including aminoguanidine, improved diabetic complications in animal models and clinical trials with, however, a number of adverse effects (Ho et al. 2010). It is suggested that AGEs inhibitors from natural foods/dietary biofactors may reasonably serve as valuable adjuvants. Using a diabetes-like oxidative stress model, the potential protective effect of antioxidant citrus fruit extracts on human adipocytes was evaluated (Ramful et al. 2010a, b). In spite of the determinant role of adipocytes in the aetiology of obesity-related disorders, there are very few reports on the effect of natural antioxidants on adipocyte-response to oxidative stress. The extracts were tested on SW872 liposarcoma cells subjected or not to H2O2 or AGEs. Cell viability, carbonyl accumulation, free-radical formation, tumour necrosis factor α (TNFα) and apolipoprotein E (apoE) secretions were assessed in treated cells (Ramful et al. 2010a, b). Data for two citrus species, namely Tangor and Tangelo showed pronounced abilities to delay free-radical-induced hemolysis in the red blood cell hemolysis test, thus providing complementary evidence of their antioxidative potency. This is the first report on the antioxidant propensity of nutritional compounds assessed by this red blood cell hemolysis test system patented in 1992 (Prost 1992). The low dose–response data therefore represent favourable applicable conditions to the in vivo environment, without affecting cellular viability and physiology.
Adipocyte cell viability was examined in the presence of different concentrations of tangelo and tangor flavedo, albedo and pulp extracts. Only the flavedo extract produced toxic effects at high concentrations (>0.75%). The phenolic richness of the extract could contribute to this observation. Analogous reports have previously been made, whereby phenolic-rich plant extracts exerted modulatory effects. These results indicated that phenolic constituents of complex plant mixtures possess the character of a ‘Janus’ (anti)genotoxicant, a term used to designate compounds behaving as genotoxin or antigenotoxin, depending upon the plant extract concentrations used (De Flora and Ramel 1988). The toxicity is suggested to be related to hydrogen peroxide formation arising from the auto-oxidation of phenolic molecules. In another work, Patil et al. (2009) showed that compounds purified from Mexican lime juice could induce apoptosis in human pancreatic cells (review in Roche et al. 2008) with the effects being shown to be proportionately linked to the flavonoid content. Our previous studies have shown that native albumin had strong antioxidant activities (Bourdon et al. 1999). Our data show an increase in the half time of AAPH-induced hemolysis in the presence of native albumin, whilst a significant reduction is observed with MGO-mediated glycation. Similar results were obtained on MGO-modified BSA by Faure et al. (2005). In the same vein, another work performed by our group showed oxidative damages in adipocytes subjected to oxidative stress induced by glycated albumin (Roche et al. 2009). The reduction of carbonyl formation at the adipocyte level is clearly reflective of the antioxidant power of tangor and tangelo flavedos (Fig. 3.3). This antioxidant propensity is reinforced with co-treatment with native albumin, whilst glycated albumin is devoid of antioxidant power. Consistently, similar data are observed in adipocytes submitted to an oxidative stress generated by H2O2 (Fig. 3.4). A significant decrease in carbonyl formation was observed when cells pretreated with tangelo flavedo extracts were incubated in the presence of H2O2. Along this line, it has been reported that polyphenolics, more particularly anthocyanins, have the ability to protect 3T3-L1 adipocytes against H2O2-induced insulin resistance (Guo et al. 2008).
Effect of citrus flavedo extracts on carbonyl accumulation in AGEs-treated adicpocytes. Results expressed as % of control cells treated with only 1% (v/v) DMSO. Bars represent mean ± SEM of two independent experiments performed in triplicate. Significance using student’s t test for unpaired samples are: §P = 0.08, #P = 0.07 (vs. control); **P < 0.01
Effect of citrus flavedo extracts on carbonyl accumulation in H2O2-treated adicpocytes. Results expressed as % of control cells treated with only 1% (v/v) DMSO. Bars represent mean ± SEM of two independent experiments performed in triplicate. Significance was assessed using one-way ANOVA followed by Dunnett’s multiple comparison test; *P < 0.05
We further demonstrated that intracellular ROS formation is considerably lowered in cells pretreated with citrus flavedo extracts incubated in the presence or absence of H2O2 (unpublished data). Modulation of intracellular ROS production in the SW872 cells by citrus extracts was shown using the dichlorodihydrofluorescein diacetate (DCFH-DA) assay. This is a useful indicator of reactive oxygen species (ROS) and oxidative stress. The principle of the assay is summarised in Fig. 3.5. The nonpolar and nonionic DCFH-DA crosses cell membranes and is hydrolysed by intracellular esterases to non-fluorescent 2′,7′-dichlorofluorescin (DCFH). In the presence of ROS, such as hydrogen peroxide (H2O2), lipid hydroperoxides and peroxinitrite, DCFH is oxidised to fluorescent 2′,7′-dichlorofluorescein (DCF). In addition, DCFH can be oxidised by intracellular oxidases and oxidants formed during the reduction of H2O2. Altogether, these observations indicate that the oxidation of DCFH may be derived from several ROS intermediates (Wang and Joseph 1999). Therefore, DCFH is useful to indirectly measure the effect of intracellular antioxidant activities in scavenging ROS and in protecting DCFH from oxidation. Intracellular ROS formation was considerably lowered in cells pretreated with citrus flavedo extracts incubated in the presence or absence of H2O2 (Fig. 3.6). Hwang and Yen (2008) also reported that pretreatment of rat pheochromocytoma PC12 cells with citrus flavanones significantly eliminated the accumulation of intracellular ROS. Hesperetin and neohesperidin reduced the level of ROS by 16–24%, whilst hesperidin reduced the level of ROS by 32–48% in H2O2-indued PC12 cells (Hwang and Yen 2008). Other researchers have reported the use of the DCFH-DA assay in biological systems for the evaluation of natural antioxidants. Takamatsu et al. (2003) reported the screening of some flavonoids for their antioxidant properties on HL-60 cells using a DCFH-DA assay. Lu et al. (2004) used a DCFH-DA assay to assess the antioxidant activities of procyanidins from grape seeds whilst Eberhardt et al. (2005) evaluated the antioxidant activities of broccoli extracts. Recently, Girard-Lalancette et al. (2009) developed a sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of fruits and vegetable juices.
Principle of the DCFH-DA assay. Cells were pretreated with citrus extracts followed by the addition of the DCFH-DA. The antioxidants in the citrus extracts bound to the cell membrane and/or passed through the membrane to enter the cell. DCFH-DA diffused into the cell where cellular esterases cleaved the diacetate moiety to form the more polar DCFH, which was trapped within the cell. Cells were treated with H2O2 which diffused into the cells acting as ROS. These ROS oxidised the intracellular DCFH to the fluorescent DCF. Antioxidants present in the citrus extracts prevented the oxidation of DCFH (Adapted from Wolfe and Liu 2007)
Comparative antioxidant activities of citrus extracts in SW872 cells, as measured by the DCFH-DA assay, (a) in the absence of H2O2 and (b) in the presence of H2O2. Results are expressed as mean ± SEM of three independent experiments performed in triplicate using one-way ANOVA followed by Dunnett’s multiple comparison test; * P < 0.05, ** P <0.01 vs. control
ApoE, which is a component of lipoproteins, e.g. chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoproteins and high-density lipoprotein (HDL), is mainly produced and secreted by the liver (Beisiegel et al. 1988). ApoE is known to regulate both cellular and systemic cholesterol, as well as triglyceride metabolism (Mahley 1988; Tarnus et al. 2009), and has been extensively studied for its potential role in the aetiology of atherosclerosis, diabetes and obesity. ApoE, which was shown to exhibit anti-inflammatory, anti-atherogenic and antioxidant properties (Miyata and Smith 1996; Davignon 2005), has been found to be highly expressed by adipose tissue and adipocytes (Wassef et al. 2004; Zechner et al. 1991). However, if apoE expression by adipocytes has been known for many years, its importance in the adipose response to oxidative stress/antioxidants has never been thoroughly investigated. Significant reductions in apoE secretions were observed in albedo and pulp-extract-treated cells (Fig. 3.7). Recently, our group showed an increase in apoE secretion in SW872 cells subjected to oxidative stress induced by glucose or AAPH, a free-radical generator (Tarnus et al. 2009). We hypothesised that apoE may exert antioxidant effects at the adipocyte level, and its subsequent increase in expression may represent a defence response to oxidative stress (Tarnus et al. 2009). The decrease in apoE secretion in cells incubated with citrus extract seems to be an adaptive response to the presence of the exogenous citrus antioxidants.
Complementary to our data depicting the antidiabetic potential of citrus extracts, literature data on citrus phytophenolics suggest that naringenin is able to reduce glucose uptake and inhibit intestinal and renal Na+-glucose co-transporter (SGLT1) (Li et al. 2006) and that both naringin and hesperidin significantly increased the glucokinase mRNA level, whilst naringin reduced the mRNA expression of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in the liver (Jung et al. 2006). Recently, it was reported that a citrus extract of Dangyuja (citrus fruit from Korea), containing high levels of flavanone glycosides, could be used to control the blood glucose level of diabetic patients by inhibiting α amylase and α glucosidase in the intestinal tract (Gyo-Nam et al. 2009).
3.1.4 Cancer Chemoprevention Potential
The concept of chemoprevention by dietary means is gaining momentum in a number of chronic degenerative diseases primarily due to the dramatic rise of cancer and type 2 diabetes mellitus and the increasing incidence of cardiovascular diseases as major and interlinked healthcare problems. As it stands today, cancer is the second leading death cause in the world. In 2005, out of 58 million deaths worldwide, 7.6 million people died of cancer. Based on projections, cancer deaths will continue to rise with an estimated 9 million people dying from cancer in 2015, and 11.4 million dying in 2030. Cancer is a multifactorial and multistage process consisting of three distinct phases: initiation, promotion and progression phases. Whilst current clinical therapies including radiation, chemotherapy, immunosuppression and surgery are limited as indicated by the high morbidity and mortality rate from cancer, there is an imperative need for new treatment modalities. Chemoprevention which involves the use of pharmacological, dietary biofactors, phytochemicals and even whole plant extracts to prevent, arrest or reverse the cellular and molecular processes of carcinogenesis has been proposed due to its multiple intervention strategies.
The preventive mechanisms of tumour promotion by natural phytochemicals range from the inhibition of genotoxic effects, increased antioxidant and anti-inflammatory activity, inhibition of proteases and cell proliferation, protection of intercellular communications to modulation of apoptosis and signal transduction pathways (Chen and Kong 2005; De Flora and Ferguson 2005; Holmes-McNary and Baldwin 2000; Aruoma et al. 2005; Soobrattee et al. 2008). Dietary polyphenols can induce the phase I and II detoxifying enzymes involved in the biotransformation and elimination of potential carcinogens. The natural chemopreventive compounds serve as transcriptional activators for the expression of glutathione S-transferase, NAD(P)H: quinine oxidoreductase (NQO), heme oxygenase 1 (HO 1), γ-glutamylcysteine synthetase (γGCS) and antioxidant enzymes via the antioxidant/electrophile response element (ARE/EpRE) (Neergheen and Bahorun 2009). The induction effects of phase II detoxifying agents by natural phytochemicals is mediated in part through the activation of Nrf 2 signalling pathways by upstream kinases (Fig. 3.8).
Schematic representation of the intracellular signal transduction cascades activated by reactive oxygen species and converging on downstream transcription factors and the site where dietary phenolics possibly intervene. Activation of Nrf2 signalling and induction of phase II detoxifying and antioxidant genes by chemopreventive polyphenols represented by (). () indicates the sites where phenolic compounds have been reported to modulate and suppress the cell signal transduction cascades (Neergheen and Bahorun 2009)
Citrus flavonoids have been reported to protect DNA by their ability to absorb ultraviolet light (Stapleton and Walbot 1994). The role of Naringin as an important modulator of superoxide dismutase and catalase activities and upregulator of gene expressions of superoxide dismutase, catalase and glutathione peroxidase in cholesterol-rich diet-fed rabbits has been highlighted (Jeon et al. 2001). It has been further suggested naringin might affect H2O2-induced expression of an apoptosis-associated gene or proteins as one of its pharmacological actions (Kanno et al. 2003). Orange juice containing hesperetin and naringenin delayed tumour development, suggesting their effectiveness to inhibit human breast cancer cell proliferation in vitro (So et al. 1996).
Naringenin was also found to inhibit proliferation of HT-29 colon cancer cell lines at concentrations of 0.71–2.85 mmol. These amounts are found to be effective in plasma and can be provided by drinking between 2 and 3 l of grapefruit juice daily. Taking into account its low bioavailability, higher volumes of grapefruit juice may however be required, thereby suggesting that naringenin in capsular form may be more practical and efficient. (Tripoli et al. 2007). Another study on naringenin showed that its administration to gastric carcinoma–induced rats largely up-regulated the redox status to decrease the risk of cancer. The authors concluded that up-regulation of antioxidants by naringenin treatment might be responsible for the anticancer effect in gastric carcinoma (Ekambaram et al. 2008).
Numerous reports indicate that citrus flavonoids affect cellular metabolism in various ways, thereby influencing cancer proliferation, e.g. inhibition of glycolysis (the most active metabolic pathway in tumoural cells (Manach et al. 1996)), depress production of lactate in leukaemia cell lines or in Ehrlich tumour cells (Suolinna et al. 1975), inhibition of the Na/K ATPase pump that could negatively influence the energetic metabolism, the synthesis of the proteins and DNA replication, by pH reduction of the cells (Hirano et al. 1989). Furthermore citrus flavonoids have been shown to potentiate the drug therapies effects against cancer. For example, quercetin prominently enhances the effect of Adriamycin in a multidrug-resistant MCF-7 human breast cancer cell line (Scambia et al. 1994) and cells of colon MCT-15 (Critchfield et al. 1994). The anti-metastatic and anti-invasive activities of citrus flavonoids, based on cell mobility inhibition (Bracke et al. 1991), have been observed in several human neoplastic cellular line proliferations: lymphoid and myeloid leukaemias (Larocca et al. 1990), gastric carcinoma (Yoshida et al. 1990), ovarian carcinoma (Scambia et al. 1990), prostate carcinoma (Peterson and Barnes 1993) and squamous cellular carcinoma (Kandaswami et al. 1991).
Nobiletin, which is a predominant methoxylated flavone in mandarins, has shown direct cytotoxicity on TMK-1, MKN-45, MKN-74 and KATO-III with a dose–response relationship. Loss of cell viability at low doses was found to be a consequence of apoptosis (Yoshimizu et al. 2004). A screening of 78 citrus species showed inhibitory effects of the Epstein-Barr virus antigen (EBV-EA) activation, indicating that citrus phytochemicals may inhibit susceptibility factors involved in the events leading to the development of cancer (Iwase et al. 1999). Hirata and his group, in a study conducted on citrus peels, isolated polymethoxyflavones and coumarin derivatives with anti-corpulence activities and the ability to inhibit the proliferation of human colon cancer HT-29 cells (Hirata et al. 2009).
More recently, the modulatory effects of hesperidin on attenuating the lipid peroxidation and down-regulation of key membrane bound marker enzyme activities and up-regulation of protein content affording an assurance for its potential use for the treatment of breast cancer have been reported (Nandakumar et al. 2011).
Extracts from lemon seed were also shown to have potent antioxidant activity and to induce apoptosis in MCF-7 cells, leading to the inhibition of proliferation, suggesting that aglycones and glucosides of the limonoids and flavonoid present may potentially serve as a chemopreventive agent for breast cancer (Kim et al. 2011). Recent advanced molecular studies focused to understand the structure-function relationship of citrus flavonoids in terms of their ability to alter the gene expression in the colon adenocarcinoma cells. Structurally related flavonoids (apigenin and quercetagetin) found in citrus were shown to have pronounced ability to inhibit colon cancer (SW480) cells as well as change the expression of apoptosis-related genes/proteins (Chidambara Murthy et al. 2011).
3.2 Conclusion
Never before has the focus on the health benefits of commonly available foods been so strong. The philosophy that food can be health promoting beyond its nutritional value is gaining acceptance within the public arena and among the scientific community as mounting research links diet/food components to disease prevention and treatment. The efficacy of citrus extracts is supported by conclusive evidence from animal models which have provided the concepts for underlying mechanisms of action (Fig. 3.9). However, research must go far beyond the simplistic claims of positive properties in vitro. It must be heavily supplemented by well-designed observational epidemiological studies, bioavailability investigations and intervention trials. The World Health Organisation (WHO) has declared that diabetes and cancer epidemics are underway. Although there are various antidiabetic and anticancer drug therapies available on the market, tolerance and side effects are still an issue with many of these. There is thus a clear opportunity to improve on the current standard of care. In that regard, citrus fruit extracts represent an excellent candidate to be developed into nutraceuticals and functional foods geared towards the management of diabetes and cancer.
3.3 Future Research
Further studies are needed to achieve a better understanding of the cellular and molecular mechanisms of nutritional antioxidants as well as their clinical effects. The outcome of the various above-mentioned ongoing studies in our group under the Centre for Biomedical and Biomaterials Research (CBBR) (an African Network for Diagnostics and Innovation (ANDI) centre of excellence) in partnership with established collaborations (University of Reunion, American University of Health Sciences (USA), Chhatrapati Shahu Ji Maharaj University, (Kanpur, India), Seoul National University, etc.) would thus constitute the basis for the selection of citrus fruit varieties with high polyphenolic content and antioxidant activities for the development of functional foods that would contain the right mix and amounts of antioxidant prophylactics to be used as supplements in a balanced diet within existing nutrition programmes.
References
Albach RF, Redman GH (1969) Composition and inheritance of flavanones in citrus fruit. Phytochemistry 8:127–143
Anis M, Aminuddin M (1981) Flavonoid patterns of leaves of some citrus species and their hybrids. Plant Biochem J 8(1):56–60
Aruoma OI (1993) Use of DNA damage as a measure of pro-oxidant actions of antioxidant food additives and nutritive components. In: Halliwel B, Arouma OL (eds) DNA and free radicals. London, Eliis Horwood, pp 315–317
Aruoma OI (1994) Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol 32:671–683
Aruoma OI (2003) Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res 523–524:9–20
Aruoma OI, Bahorun T, Clement Y et al (2005) Inflammation, cellular and redox signalling mechanisms in cancer and degenerative diseases. Mutat Res 579:1–5
Beisiegel U, Weber W, Havinga JR et al (1988) Apolipoprotein E-binding proteins isolated from dog and human liver. Arteriosclerosis 8:288–297
Benavente-Garcia O, Castillo J, Marin FR et al (1997) Use and properties of citrus flavonoids. J Agric Food Chem 45:4506–4515
Benherlal PS, Arumughan C (2008) Studies on modulation of DNA integrity in Fenton’s system by phytochemicals. Mutat Res 648:1–8
Berhow M, Tisserat B, Kanes K et al (1998) Survey of phenolic compounds produced in citrus. Technical Bulletin No 1856. United States Department of Agriculture, Agricultural Research Service, Washington, DC. http://www.ars.usda.gov/IS/np/phenolics.htm
Bisht K, Wagner KH, Bulmer AC (2008) Curcumin, resveratrol and flavanoids as anti-inflammatory, cyto-and DNA protective dietary compounds. Toxicology 278:88–100
Bourdon E, Loreau N, Blache D (1999) Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J 13:233–244
Bracke ME, Vyncke B, Opdenakker JM et al (1991) Effect of catechins and citrus flavonoids on invasion in vitro. Clin Exp Metastasis 9:13–25
Cadet J, Berger M, Douki T et al (1997) Oxidative damage to DNA: formation, measurement and biological significance. Rev Physiol Biochem Pharmacol 131:1–87
Cano A, Medina A, Bermejo A (2008) Bioactive compounds in different citrus varieties, discrimination among cultivars. J Food Compos Anal 21:377–381
Castillo J, Benavente-Garcia O, Delrio JA (1992) Naringin and neohesperidin levels during development of leaves, flower, buds and fruits of Citrus aurantium. Plant Physiol 99:67–73
Central Statistics Office (CSO) (2008) Digest of agricultural statistics. CSO, Port Louis. http://statsmauritius.gov.mu
Chen C, Kong ANT (2005) Dietary cancer-chemopreventive compounds: from signalling and gene expression to pharmacological effects. Trends Pharmacol Sci 26:318–326
Chidambara Murthy KN, Kim J et al (2011) Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. doi:10.1016/j.foodchem.2011.10.014
Critchfield JW, Welsh CJ, Phang JM et al (1994) Modulation of adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells: activation of P-glycoprotein as a putative mechanism. Biochem Pharmacol 48:1437–1445
Davignon J (2005) Apolipoprotein E and atherosclerosis: beyond lipid effect. Arterioscler Thromb Vasc Biol 25:267–269
De Flora S, Ferguson LR (2005) Overview of mechanisms of cancer chemo preventive agents. Mutat Res 591:8–15
De Flora S, Ramel C (1988) Mechanisms of inhibitors of mutagenesis and carcinogenesis: classification and overview. Mutat Res 202:285–306
Eberhardt MV, Kobira Keck AS, Juvik JA et al (2005) Correlation analyses of phytochemical composition, chemical, and cellular measures of antioxidant activity of broccoli (Brassica oleracea L. Var. italica). J Agric Food Chem 53:7421–7431
Ekambaram G, Rajendran P, Magesh V et al (2008) Naringenin reduces tumor size and weight lost in N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Nutr Res 28:106–112
Fattouch S, Caboni P, Coroneo V et al (2007) Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem 55:963–969
Faure P, Troncy L, Lecomte M et al (2005) Albumin antioxidant capacity is modified by methylglyoxal. Diabetes Metab 31:169–177
Girard-Lalancette K, Pichette A, Legault J (2009) Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: analysis of fruit and vegetable juices. Food Chem 115:720–726
Gorinstein S, Martin-Belloso O, Park YS et al (2001) Comparison of some biochemical characteristics of different citrus fruits. Food Chem 74:309–315
Gorinstein S, Cvikrova M, Machackova I et al (2004) Characterization of antioxidant compounds in Jaffa sweeties and white grapefruits. Food Chem 84:503–510
Guo H, Ling W, Wang Q et al (2008) Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-R induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation. Biochem Pharmacol 75:1393–1401
Gyo-Nam K, Jung-Geun S, Hae-Dong J (2009) Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem 117:35–41
Halliwell B (1997) Antioxidants: the basics – what they are and how to evaluate them. Adv Pharmacol 38:3–20
Hirano T, Oka K, Akiba M (1989) Effects of synthetic and naturally occurring flavonoids on Na+, K+−ATPase: aspects of structure–activity relationship and action mechanisms. Life Sci 45:1111–1117
Hirata T, Fujii M, Akita K et al (2009) Identification and physiological evaluation of the components from citrus fruits as potential drugs for anti-corpulence and anticancer. Bioorg Med Chem 17:25–28
Ho S, Wu S, Lin S, Tang Y (2010) Comparison of anti-glycation capacities of several herbal infusions with that of green tea. Food Chem 122:768–774
Holmes-McNary M, Baldwin ABS Jr (2000) Chemopreventive properties of transresveratrol are associated with inhibition of activation of the IкB kinase. Cancer Res 60:3477–3483
Horowitz R, Gentili B (1977) Flavonoids constituents of citrus. In: Nagy S, Shaw PE, Veldhuis MK (eds) Citrus science and technology, vol 1. AVI Publishing, Westport, pp 397–426
Hwang SL, Yen GC (2008) Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J Agric Food Chem 56:859–864
Iwase Y, Takemura Y, Ju-ichi M et al (1999) Inhibitory effect of Epstein-Barr virus activation by citrus fruits, a cancer chemopreventor. Cancer Lett 139:227–236
Jeon SM, Bok SH, Jang MK et al (2001) Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci 69:2855–2866
Jung UJ, Lee MK, Park YB et al (2006) Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 38:1134–1145
Kandaswami C, Perkins E, Soloniuk DS et al (1991) Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro. Cancer Lett 56:147–152
Kanno SI, Shouji A, Asou K et al (2003) Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci Jpn Pharmacol Soc 92:166–170
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables – the millennium’s health. Int J Food Sci Technol 36(7):703–725
Kim J, Guddadarangavvanahally K, Uckoo RM, Patil BS (2011) Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Food Chem Toxicol. doi:10.1016/j.fct.2011.10.057
Larocca LM, Piantelli M, Leone G et al (1990) Type II oestrogen binding sites in acute lymphoid and myeloid leukaemias: growth inhibitory effect of oestrogen and flavonoids. Br J Haematol 75:489–495
Lee SK, Kader AA (2000) Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol 20:207–220
Lewinsohn E, Berman E, Mazur Y et al (1989) (7) Glucosilation and (1–6) rhamnosylation of exogeneous flavanones by undifferentiated citrus cell cultures. Plant Sci 61:23–28
Li Y, Ou-Lee TM, Raba R et al (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5:171–175
Li JM, Che CT, Lau CB et al (2006) Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. Int J Biochem Cell Biol 38:985–995
Londoño-Londoño J, Delima VR, Lara O et al (2010) Clean recovery of antioxidant flavonoids from citrus peel: optimizingan aqueous ultrasound-assisted extraction method. Food Chem 119:81–87
Lu Y, Zhao WZ, Chang Z et al (2004) Procyanidins from grape seeds protect against phorbol ester-induced oxidative cellular and genotoxic damage. Acta Pharmacol Sin 25:1083–1089
Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622–630
Manach C, Regerat F, Texier O et al (1996) Bioavailability, metabolism and physiological impact of 4- oxo-flavonoids. Nutr Res 16:517–544
Manthey JA, Grohman K (2001) Phenols in citrus peel by products: concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agric Food Chem 49:3268–3273
Martynez G, Delgado R, Perez G et al (2000) Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother Res 14:424–427
Mata Bilbao ML, Andres-Lacueva C, Uregui O et al (2007) Determination of flavonoids in a citrus fruit extract by LC-DAD and LC-MS. Food Chem 101:1742–1747
Matsubara Y, Kumamoto H, Iizuka Y et al (1985) Structure and hypotensive effect of flavonoid glycosides in citrus unshiu peelings. Agric Biol Chem 49:909–914
Merken HM, Beecher GR (2000) Measurement of food flavonoids by high-performance liquid chromatography: a review. J Agric Food Chem 48:577–599
Middleton E, Kandaswami C (1994) Potential health-promoting properties of citrus flavonoids. Food Technol 48:115–119
Miyata M, Smith JD (1996) Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat Genet 14:55–61
Montanari A, Chen J, Widmer W (1998) Citrus flavonoids: a review of past biological activity against disease. In: Manthey JA, Buslig BS (eds) Flavonoids in the living system. Plenum Press, New York, pp 103–113
Nandakumar N, Jayaprakash R, Rengarajan T et al (2011) Hesperidin, a natural citrus flavonoglycoside, normalizes lipid peroxidation and membrane bound marker enzymes in 7, 12-Dimethylbenz (a) anthracene induced experimental breast cancer rats. Biomed Prev Nutr. doi:10.1016/j.bionut 2011.06.004
Neergheen VS, Bahorun T (2009) Therapeutic relevance of dietary polyphenols as natural antioxidants and modulators of cell signal transduction pathways in cancer and other degenerative diseases. In: Farombi EO (ed) Nutritional antioxidants in cancer and degenerative diseases. Transworld Research Network, Trivandrum, pp 131–152
Nogata Y, Sakamoto K, Shiratsuchi H et al (2006) Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem 70:178–192
Park GL, Avery SM, Byers JL et al (1983) Identification of bioflavonoids from citrus. Food Technol 37:98–105
Pashikanti S, de Alba DR, Boissonneault GA et al (2010) Rutin metabolites: novel inhibitors of nonoxidative advanced glycation end products. Free Radic Biol Med 48:656–663
Patil JR, Chidambara Murthy KN, Jayaprakasha GK et al (2009) Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J Agric Food Chem 57:10933–10942
Pérez-Jiménez J, Arranz S, Tabernero M et al (2008) Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int 41:274–285
Peterson G, Barnes S (1993) Genistein and biochain A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 22:335–345
Peterson JJ, Beecher GR, Bhagwat SA et al (2006a) Flavanones in grapefruit, lemons, and limes: a compilation and review of the data from the analytical literature. J Food Compos Anal 19:74–80
Peterson JJ, Dwyer JT, Beecher GR et al (2006b) Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J Food Compos Anal 19:66–73
Prior RI, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Prost M (1992) Process for the determination by means of free radicals of the antioxidant properties of a living organism or a potentially agressive agents. US Patent 5,135,850, 4 Aug 1992
Ramful D, Tarnus E, Philippe R et al (2010a) Citrus fruit extracts reduces AGEs- and H2O2-induced oxidative stress in human adipocytes. J Agric Food Chem 58:11119–11129
Ramful D, Bahorun T, Bourdon E et al (2010b) Bioactive, phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: potential prophylactic ingredients for functional foods application. Toxicology 278:75–87
Ramful D, Tarnus E, Aruoma OI et al (2011) Polyphenolics, Vitamin C composition and antioxidant propensities of Mauritian citrus fruit pulps. Food Res Int 44:2088–2099
Roche M, Rondeau P, Singh NR et al (2008) The antioxidant properties of serum albumin. FEBS Lett 582:1783–1787
Roche M, Tarnus E, Rondeau P et al (2009) Effects of nutritional antioxidants on AAPH- or AGEs-induced oxidative stress in human SW872 liposarcoma cells. Cell Biol Toxicol 25:635–664
Rousseff RL, Martin SF, Youtsef CO (1987) Quantitative survey of narirutin, naringin, hesperidin and neohesperidin in citrus. J Agric Food Chem 35:1027–1030
Samman S, Lyons Wall PM, Cook NC (1998) Flavonoids and coronary heart disease: dietary perspectives. In: Rice-Evans CA, Packer L (eds) Flavonoids in health and disease. Marcel Dekker, New York, pp 469–482
Scambia G, Ranelletti FO, Benedetti-Panici P et al (1990) Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumors and cultured cells. Br J Cancer 62:942–946
So FV, Guthrie N, Chambers AF et al (1996) Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer 26:167–181
Soobrattee MA, Bahorun T, Neergheen VS et al (2008) Phenolics content and antioxidant actions of the Rubiaceae, Ebenaceae, Celastraceae, Erythroxylaceae and Sterculaceae families of Mauritian endemic plants. Toxicol In Vitro 22:45–56
Stapleton AE, Walbot V (1994) Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiol 105:881–889
Stern DM, Yan SD, Yan SF et al (2002) Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 1:1–15
Suolinna EM, Buchsbaum RN, Racker E (1975) The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res 35:1865–1872
Takamatsu S, Galal AM, Ross SA et al (2003) Antioxidant effect of flavonoids on DCF production in HL-60 cells. Phytother Res 17:963–966
Tarnus E, Wassef H, Carmel JF et al (2009) Apolipoprotein E limits oxidative stress-induced cell dysfunctions in human adipocytes. FEBS Lett 583:2042–2048
Tomás-Barberán FA, Clifford MN (2000) Flavanones, chalcones and dihydrochalcones – nature, occurrence and dietary burden. J Sci Food Agric 80:1073–1080
Tripoli E, La Guardia M, Giammanco S et al (2007) Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem 104:466–479
Tsang SY, Tam SC, Bremner L et al (1996) Copper-1,10-phenanthrolin induces internucleosomal DNA fragmentation in HepG2 cells, resulting from direct oxidation by the hydroxyl radical. Biochem J 317:13–16
Valentão P, Fernandes E, Carvalho F et al (2002) Antioxidant activity of Hypericum androsaemum infusion: scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull 25(10):1320–1327
Van der Sluis AA, Dekker M, De Jager A et al (2001) Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. J Agric Food Chem 49:3606–3613
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Wang AY, Zhou MY, Lin WC (2011) Antioxidative and anti-inflammatory properties of citrus sulcata extracts. Food Chem 124:958–963
Wassef H, Bernier L, Davignon J et al (2004) Synthesis and secretion of apoC-I and apoE during maturation of human SW872 liposarcoma cells. J Nutr 134:2935–2941
Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55:8896–8907
Yoshida M, Sakai T, Hosokawa N et al (1990) The effect of quercetin on cell-cycle progression and growth of human gastric cancer cells. FEBS Lett 260:10–13
Yoshimizu N, Otani Y, Saikawa Y et al (2004) Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment Pharmacol Ther 20:95–101
Zechner R, Moser R, Newman TC et al (1991) Apolipoprotein E gene expression in mouse 3T3-L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J Biol Chem 266:10583–10588
Zhao X, Xue CH, Li Z-J et al (2004) Antioxidant and hepatoprotective activities of low molecular weight sulphated polysaccharide from Laminaria japonica. J Appl Phycol 16:111–115
Acknowledgments
The authors are thankful to the University of Mauritius, University of Réunion (France) and Chhatrapati Shahu Ji Maharaj University Kanpur (India) for their full support. One of the authors (TB) is particularly thankful to Mr Gaurav Gaur, the Federation of Indian Chambers of Commerce & Industry (FICCI) and the Government of India for the award of the CV Raman International Senior Fellowship for African Researchers. Part of this work has been carried out in the context of this fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Bahorun, T. et al. (2012). Phytophenolic Nutrients in Citrus: Biochemical and Molecular Evidence. In: Srivastava, A. (eds) Advances in Citrus Nutrition. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4171-3_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-4171-3_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4170-6
Online ISBN: 978-94-007-4171-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)