Abstract
In the last decade, the availability of genetically modified animals has revealed interesting roles for phosphoinositide 3-kinases (PI3Ks) as signaling platforms orchestrating multiple cellular responses, both in health and pathology. By acting downstream distinct receptor types, PI3Ks nucleate complex signaling assemblies controlling several biological process, ranging from cell proliferation and survival to immunity, cancer, metabolism and cardiovascular control. While the involvement of these kinases in modulating immune reactions and neoplastic transformation has long been accepted, recent progress from our group and others has highlighted new and unforeseen roles of PI3Ks in controlling cardiovascular function. Hence, the view is emerging that pharmacological targeting of distinct PI3K isoforms could be successful in treating disorders such as myocardial infarction and heart failure, besides inflammatory diseases and cancer. Currently, PI3Ks represent attractive drug targets for companies interested in the development of novel and safe treatments for such diseases. Numerous hit and lead compounds are now becoming available and, for some of them, clinical trials can be envisaged in the near future. In the following sections, we will outline the impact of specific PI3K isoforms in regulating different cellular contexts, including immunity, metabolism, cancer and cardiovascular system, both in physiological and disease conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Class I PI3Ks in Cancer
The PI3K pathway participates in several processes of cancer biology including cell transformation, proliferation, survival, motility and angiogenesis. In cancer cells, hyperactivity of PI3K signaling results from (i) gain-of-function mutations of a class I PI3K, and/or (ii) abnormal activation upstream (e.g. tyrosine kinase receptors) or downstream PI3Ks (e.g. AKT and PTEN). In particular, the gene encoding for p110a (PIK3CA) is one of the most frequently mutated oncogenes in human tumors (Gymnopoulos et al. 2007), as somatic mutations of the PIK3CA gene have been reported in several cancer types including colon, ovary, breast, brain, liver, stomach, endometrial and lung cancer (Samuels et al. 2004). Three hot-spot mutations (E542K, E545K and H1047R) represent 80% of all PIK3CA mutations found in tumors and map two distinct domains of the p110a protein (Zhao and Vogt 2008). E542K and E545K mutations are situated within the helical domain, while H1047R lies in the kinase domain. Mutations in the helical domain seem to alter the binding of p110a to the regulatory subunit p85 and possibly interfere with the inhibitory action of p85 on p110a, thus mimicking an activation state by tyrosine kinase receptors. The H1047 is the most frequent mutation and occurs at the end of the activation loop of p110a, where it appears to directly influence the interaction between p110a and PIP2 (Chaussade et al. 2009).
PIK3CA mutations either participate in the initiation of tumorigenesis or sustain cell growth in advanced tumors. When expressed in chicken embryo fibroblasts, E542K, E545K and H1047R p110a mutants induce oncogenic transformation with high efficiency (Bader et al. 2006). Although the expression of p110a mutants in vitro results in constitutive activation of Akt even in the absence of growth factors, the impact of p110a mutation on Akt in vivo is variable (Morrow et al. 2005; Vasudevan et al. 2009). Importantly, gain-of-function mutations in PIK3CA genes often coexist with additional alterations in the PI3K pathway in several types of tumors. For instance, mutated p110a has been associated with mutations of PTEN and K-Ras (Silvestris et al. 2009; Velasco et al. 2006) or with ERBB2 over-expression (Bachman et al. 2004; Saal et al. 2005). Co-occurrence of p110a gain-of-function with other specific oncogenic alterations in the PI3K pathway suggests that the mutational status of p110a and p85a may have different consequences on tumor formation and progression depending on tissue specificity and cell type.
Somatic mutations have also been reported in the regulatory subunit p85a (PIK3R1 gene), even though they are less frequent compared to mutations of p110a. On the contrary, mutations in the genes encoding other PI3K regulatory subunits (p85b—PIK3R2, and p55g—PIK3R3), are rare events, which suggests an isoform-specific role for p85a in cancer. Most p85a mutations (i.e. D560Y, N564D, QYL579 deletion, DS459delN and DKRMNS560del) cluster in the two SH2 domains and in the inter-SH2 domain (Berenjeno and Vanhaesebroeck 2009; Jaiswal et al. 2009). Analysis of this region has revealed that these mutants, while retaining the ability to bind the p110 catalytic subunit, loose their inhibitory activity on p110. In addition, p85a mutations lead to unspecific activation of all class IA p110 isoforms. As p110b and p110d have been found over-expressed in certain human cancers, the co-expression of mutated p85a in these tumors may thus further enhance the tumorigenic activity of PI3Ks.
To date, no genetic alterations have been found in the genes encoding for p110b, g and d. Conversely, increased expression of p110b and p110d has been identified in glioblastomas (Knobbe and Reifenberger 2003), colon and bladder tumors (Benistant et al. 2000). Indeed, over-expression of wild-type p110b, d and g is sufficient to induce an oncogenic phenotype in cultured cells (Kang et al. 2006). Moreover, expression of myristoylated p110b induces the development of prostate intraepithelial tumors in mice (Lee et al. 2010) and expression of myristoylated p110g induces a constitutive activation of Akt in Rat1 fibroblasts (Link et al. 2005). In line with this finding, p110g has been found over-expressed in pancreatic cancer, where it is required for cell proliferation, as shown by reduced cell growth in the lack of p110g lipid kinase activity (Edling et al. 2010). Taken together, these findings suggest that contrary to p110a, p110b, p110g and 110d explicate their oncogenic potential as wild-type proteins.
p110b has emerged as an interesting target in tumors such as breast and prostate cancers (Carvalho et al. 2010; Hill et al. 2010). In a mouse model of breast cancer driven by hyperactivation of the HER-2 signaling pathway, the absence of p110b lipid kinase activity strongly delays the appearance of the first tumor and reduces tumor growth in vitro even in a context of PTEN down-regulation (Ciraolo et al. 2008). Similarly, ablation of p110b blocks PTEN loss-driven tumorigenesis in the prostate. In this model, PTEN-mediated transformation appears to strictly depend on p110b, since prostate-specific knockout of p110a fails to affect tumor formation (Jia et al. 2008). These observations demonstrate the existence of a link between PTEN loss and p110b signaling. As a matter of fact, in the absence of p110a mutations, cancer cells harboring PTEN-null alleles depend on p110b lipid kinase activity, as treatment with p110b-selective inhibitors can block cell growth (Wee et al. 2008).
The role of PI3Ks in cancer has been extensively described in the last decade. Since our group has mainly focused on the area of non-oncological diseases, for broader description of PI3Ks in cancer we refer to dedicated reviews of expert authors (Engelman et al. 2006; Wong et al. 2010).
6.2 PI3Ks in Immunity and Inflammation
Protection against pathogens is achieved through concerted and synergic actions of a variety of cell types, which constitute the innate and adaptive immune responses. Innate immunity has evolved to rapidly recognize and eliminate non-self molecules through specialized phagocytic/effector cells, neutrophils and macrophages, which cooperate by providing the first line of antimicrobial defense. Neutrophils are the first to infiltrate inflamed tissues, where they carry out their defensive strategy based on bulky production of reactive oxygen species. Macrophages participate in the later phases of the inflammatory response. These cells are characterized by a high phagocytic activity and represent the principal scavengers of the immune system. Moreover, they secrete pro-inflammatory cytokines, which in turn boost the host defense. Other cell types, including mast cells and eosinophils, participate in the response to parasites, by releasing important mediators. Adaptive immunity constitutes a more sophisticated mechanism of defense, whose key feature is represented by specific antigen recognition of the invaders by T and B lymphocytes, which clonally express a large repertoire of antigen receptors. Upon antigenic recognition, lymphocytes maintain a memory of this event, thus mounting a more rapid and efficient response upon subsequent exposures to the same agent.
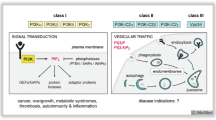
Several mechanisms have evolved to allow innate and adaptive immunity to recognize and eliminate foreign antigens, while maintaining tolerance to the self. Among these, the PI3K signaling pathway is a major example of a mechanism that requires fine tuning in order to ensure proper defense without developing excessive inflammation and autoimmunity. In this context, PI3Ks are activated by stimulation through a variety of receptor types, including antigen receptors, co-stimulatory receptors and certain cytokine receptors. Hence, PI3Ks profoundly impact on the pathophysiological regulation of innate and adaptive immunity. In the last decades, studies based on pharmacological and genetic inhibition of PI3Ks have highlighted the role of distinct PI3K isoforms in regulating specific aspects of immune responses (Fig. 6.1). Although immune cells express all class I PI3Ks, a prominent role is hold by PI3Kd and PI3Kg. Indeed, PI3Kd and PI3Kg mutant mice show relevant phenotypes in their immune responses (Clayton et al. 2002; Hirsch et al. 2000; Jou et al. 2002; Li et al. 2000; Patrucco et al. 2004; Sasaki et al. 2000).
PI3Ks control multiple aspects of innate and adaptive immunity. Class I PI3Kd and PI3Kg function as master regulators of distinct cell types, orchestrating innate and adaptive immune responses. In neutrophils and macrophages, both isoforms are required for correct directional movement, while ROS production is mainly controlled by PI3Kg. The functional interaction between PI3Kd and PI3Kg is also relevant for mast cell degranulation. On the other hand, only PI3Kg contributes to eosinophil migration. Within adaptive immunity, PI3Kd and PI3Kg cooperate in regulating distinct functions of T lymphocytes. While PI3Kg is essential for thymocyte maturation and T cell proliferation, PI3Kd mainly participates to T cell differentiation. Instead, B cell function is uniquely controlled by PI3Kd
In the following sections, we will summarize the role of PI3Ks in innate and adaptive immunity, with a main focus on PI3Kd and PI3Kg, both in physiological and disease conditions.
6.2.1 PI3Ks in Neutrophils and Macrophages
Neutrophils and macrophages provide the first defensive barrier against the invasion by pathogens and microbial agents. Recruitment of these cells to the site of infection is initiated by sensing of specific inflammatory signals (chemokines and cytokines) released by the inflamed tissue. This directional cell migration is known as chemotaxis. The importance of PI3Ks in chemotaxis has been firstly uncovered by means of pan-PI3K inhibition with wortmannin (Okada et al. 1994). In the last 10 years, the availability of isoform-selective compounds and genetic modified animals has allowed for further dissection of the specific contribution of distinct PI3K isoforms. Amongst class I PI3Ks, PI3Kd and PI3Kg have clearly emerged as the main determinants of leukocyte migration, both in vitro and in vivo.
Neutrophils and macrophages from mice lacking PI3Kg (PI3Kg−/−) show impaired in vitro migration in response to different G-protein coupled receptor (GPCR)-related stimuli such as fMLP, C5a, RANTES, IL-8 (Hirsch et al. 2000; Li et al. 2000; Patrucco et al. 2004; Sasaki et al. 2000). In agreement, PI3Kg−/− animals display reduced number of infiltrating cells in a peritonitis model (Hirsch et al. 2000; Li et al. 2000; Sasaki et al. 2000). This phenotype can be explained by the inability of PI3Kg−/− cells to correctly assemble and activate their molecular machinery controlling cell polarization, which represents an essential prerequisite for directional movement. Knock-in mice expressing a membrane-targeted PI3Kg enzyme (characterized by delocalized production of PIP3) recapitulate the phenotype of PI3Kg−/− mice, demonstrating the importance of controlled spatial and temporal production of PIP3 in leukocyte migration (Costa et al. 2007). Thus, PI3Kg catalytic function is crucial for proper PIP3 generation, and, in turn, for the regulation of Rac activity and cytoskeleton rearrangement at the leading edge (Barberis et al. 2009; Costa et al. 2007; Ferguson et al. 2007). In addition to PI3Kg, PI3Kd contributes to the fine modulation of directional movement, as the PI3Kd-specific inhibitor IC87114 significantly dampens migration of neutrophils in vitro (Puri et al. 2004; Sadhu et al. 2003), as well as recruitment of inflammatory cells in a in vivo model of pulmonary inflammation (Puri et al. 2004).
The recruitment of inflammatory cells at the site of infection is a multistep process, including a first event of selectin-mediated “capture” of circulating leukocytes and subsequent “rolling” on the vascular endothelium, followed by integrin-mediated firm adhesion and extravasation. Both PI3Kg and PI3Kd are expressed in endothelial cells, where they regulate the complex interplay between leukocytes and the inflamed, sticky and leaky endothelium. Selective blockade of PI3Kg in endothelial cells has been shown to reduce selectin-mediated attachment of neutrophils and to increase their rolling velocity (Puri et al. 2005). Similarly, endothelial PI3Kd plays a central role in neutrophil adhesion and subsequent transendothelial migration in response to tumor necrosis factor a (TNF a) and leukotriene B4 (LTB4) (Puri et al. 2004). Consistent with an essential role for both isoforms in leukocyte migration, double knock-out PI3Kg−/−d−/− mice display a more dramatic phenotype than single mutants (Puri et al. 2005). Nonetheless, PI3Kg and PI3Kd do not play overlapping roles, as they regulate temporally distinct events. Neutrophil emigration toward CXCL2 or CXCL1 is severely impaired in PI3Kg−/− mice at an early time (first 90 min), but more prolonged responses are almost entirely PI3Kg-independent and largely dependent on PI3Kd (Liu et al. 2007).
After recruitment to the inflammation site, neutrophils and macrophages exert their antimicrobial function by producing and secreting reactive oxygen species (ROS), an event known as respiratory burst. In the absence of PI3Kg, ROS production evoked by cytokine-primed neutrophils in response to fMLP is significantly reduced (Hirsch et al. 2000; Li et al. 2000; Sasaki et al. 2000). Pharmacological inhibition of PI3Kg with selective inhibitors further demonstrates that in TNFa-primed human neutrophils PI3Kg is needed to initiate the first phase of a temporally biphasic pathway of ROS production induced by fMLP. Instead, PI3Kd and at least in part PI3Kα and PI3Kβ, are necessary for the subsequent amplification phase (Puri et al. 2004; Sadhu et al. 2003). Although the second phase of ROS production is mediated by PI3Kd, both phases actually depend entirely on the first phase of ROS production, which is regulated exclusively by PI3Kg (Condliffe et al. 2005).
6.2.2 PI3Ks in Mast Cells and Eosinophils
In their action against parasites and infections, neutrophils and macrophages are assisted by mast cells. On the other hand, aberrant activation of mast cells causes different allergic diseases. Mast cells are characterized by large intracytoplasmic granules, containing hystamin and heparin, which are rapidly released following cell activation, a process known as degranulation. In allergic reactions, mast cells remain inactive until an allergen binds to a special set of immunoglubulins of the IgE type, which are tightly associated to the IgE high affinity receptor (FCeRI) at the plasma membrane. At molecular level, allergen stimulation, through IgE binding, triggers the activation of the protein tyrosine kinase Lyn and recruitment of Syk, resulting in the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs). These phosphorylated motifs provide a docking sites for the SH2 domains of class IA PI3Ks adaptor subunits. The subsequent PIP3 production is then essential to activate Bruton’s tyrosine kinase (Btk) and subsequently phospholipase C-g (PLCg). These signaling pathways cause the opening of plasma membrane calcium channels and granules release (Rommel et al. 2007).
The first indication of the involvement of PI3Ks in mast cells came from the use of non-selective PI3K inhibitors. Indeed, pan-PI3K inhibitors like LY294002 and wortmannin impair mast cell degranulation (Tkaczyk et al. 2003). Class I PI3Kd and PI3Kg appear to be the main isoforms involved in this process. Treatment with the PI3Kd-selective inhibitor IC87114 or genetic inactivation of PI3Kd activity (PI3KdD910A) dampen mast cell activity (Ali et al. 2004, 2008). Accordingly, PI3KdD910A mice are protected from passive cutaneous anaphylaxis induced by IgE- and antigen-injection (Ali et al. 2004, 2008). Similarly, blockade of the GPCR-coupled PI3Kg activity reduces mast cell degranulation and PI3Kg−/− animals are resistant to passive systemic anaphylaxis (Laffargue et al. 2002). Recent findings suggest that only a restricted pool of PI3Kg is committed to the modulation of this process. Indeed, the mast cell phenotype of PI3Kg−/− animals is completed rescued by the co-expression of p110g catalytic subunit together with the regulatory subunit p84/87, but not with p101 (Bohnacker et al. 2009). The current view of the complex interplay between PI3Kg and PI3Kd in regulating mast cell function proposes a complex epistatic interaction, with PI3Kd acting earlier in response to IgE and PI3Kg functioning later to maximize degranulation (Hirsch et al. 2006).
Eosinophils are recruited and activated in response to mast cell degranulation, thus functioning as effector cells in the allergic disease. They are typical infiltrating cells at sites of allergen-IgE reactions, where they produce a wide array of mediators such as cytokines and ROS. PI3Ks have been shown to regulate eosinophil chemotaxis in response to different chemoattractants. IL-5-induced release of eosinophils from the bone marrow is severely impaired upon treatment with the pan-PI3K inhibitor wortmannin (Palframan et al. 1998). In addition, wortmannin decreases the number of eosinophils in the brochoalveolar lavage (BAL) of ovalbumin (OVA)-challenged animals (Tigani et al. 2001). Wortmannin and LY294002 have also been found to inhibit platelet-activating factor (PAF)-induced eosinophil chemotaxis and respiratory burst, but not eotaxin-induced migration (Mishra et al. 2005). Furthermore, intra-tracheal administration of PI3K inhibitors wortmannin or LY294002 could significantly attenuate inflammation symptoms and airway hyper-responsiveness, due to sensitization with OVA inhalation in a mouse model of asthma (Duan et al. 2005; Ezeamuzie et al. 2001).
The specific PI3K isoforms involved in regulating eosinophil chemotaxis are still unclear. OVA-sensitized PI3Kg−/− mice display reduced levels of allergen-induced eosinophilic airway inflammation and airway remodeling (Lim et al. 2009; Takeda et al. 2009), thus pointing to a crucial role of this class I isoform. However, other studies suggest that PI3Kg mainly regulates the maintenance of eosinophilic inflammation in vivo, rather than the recruitment process, which seems to be modulated by other PI3Ks (Pinho et al. 2005). Further complexity comes from the unexpected finding that double mutant mice PI3KgKO/dD910A display marked eosinophilic inflammation in multiple mucosal organs, as well as increased amount of serum IgE, IL-4 and IL-5 levels (Ji et al. 2007).
6.2.3 PI3Ks in T Lymphocytes
T and B lymphocytes orchestrate a sophisticated mechanism of protection, featured by recognition of specific antigens and pathogens. T lymphocytes are involved in cell-mediated immunity and contribute to the control of humoral immunity, by exerting a strict control on the activity of B lymphocytes. The first suggestion of a key role of PI3Ks in regulating T lymphocyte function has come from studies with pan-PI3K inhibitors. Wortmannin impairs antigen (Ag)-induced IL-2 production, as well as Ag-induced CD4+ T cells differentiation (Shi et al. 1997). In addition, wortmannin and LY294002 inhibit CD3-induced IL-2 synthesis and proliferation of CD8+ T cells (Phu et al. 2001).
More recently, the development of isoform-selective inhibitors and genetically engineered animals has allowed to show that distinct PI3K isoforms control different processes of T lymphocyte function, including development, proliferation and migration. For instance, class I PI3Kg and PI3Kd are both required for proper T cell differentiation. Indeed, PI3Kg−/− mice show a reduced number of peripheral T lymphocyte due to impaired maturation of thymocytes. In particular, ablation of PI3Kg increases the ratio of double negative (CD4− CD8−) on double positive (CD4+ CD8+) cells in thymus, blocking thymocyte development (Sasaki et al. 2000). This phenotype is worsened by the double ablation of PI3Kg and PI3Kd, leading to a dramatic increase in the number of double negative thymocytes. On the contrary, PI3Kd inactivation (PI3KdD910A) alone does not affect thymocyte development (Ji et al. 2007; Webb et al. 2005).
Both PI3Kd and PI3Kg are essential for the subsequent phase of proliferation. In naïve T cells, PI3Ks are engaged by the cross-linking of T-cell receptor (TCR), with or without co-stimulation by CD28, or by activation of the IL-2 receptor or chemokine receptors (Alcazar et al. 2007; Fruman and Cantley 2002). T cells lacking PI3Kg show abnormal TCR-mediated signaling and reduced immunological synapse organization, as well as reduced proliferation (Sasaki et al. 2000). Similarly, knock-in mice expressing a kinase-inactive PI3Kd display impaired antigen-specific T-cell responses and a reduction in T-cell activation and proliferation upon in vitro stimulation (Okkenhaug et al. 2002).
On the other hand, PI3Kd is central for maturation of CD4+ T cell and differentiation in distinct T cell subsets (Th1, Th2, Th17, Treg). PI3KdD910A mice display reduction of both Th1 and Th2, in vitro and in vivo (Okkenhaug et al. 2006). Furthermore, PI3Kd cooperates with SHIP to maintain the correct ratio of Th17 and Treg cells. SHIP1−/− mice show preferential differentiation in Treg compared to Th17 (Locke et al. 2009). On the other hand, in PI3KdD910A mice peripheral Treg maturation is impaired (Ji et al. 2007; Liu et al. 2009; Oak et al. 2006; Patton et al. 2006). Reduction of Treg function, associated with increased B cell-mediated IgE production, renders PI3KdD910A mice prone to autoimmunity (Ji et al. 2007; Oak et al. 2006). By contrast, the impaired Treg immunosuppressive function of PI3KdD910A mice appears beneficial in the case of infection by the parasite Leishmania major. Indeed, the reduced Treg expansion of PI3KdD910A mice seems to be responsible for a weakened Th1 response, thus preventing disease development (Liu et al. 2009).
The role of PI3Ks in the regulation of T cell migration is more controverse. In some circumstances, PI3Kg signaling appears important for T cell chemotaxis in the mouse (Camps et al. 2005; Reif et al. 2004; Sasaki et al. 2000; Webb et al. 2005). In addition, treatment with the PI3Kg specific inhibitor AS605240 has indicated that PI3Kg plays a dominant role in the migratory response to CXCL12 (Smith et al. 2007) in primary human T lymphocytes. In contrast, migratory responses to a range of chemokines, including CXCL12, of T cells derived from mice expressing a catalytically-inactive form of PI3Kd are largely unaffected (Reif et al. 2004), indicating that PI3Kg is the predominant isoform involved in T cell migration. However, recent works have suggested that T cell migration principally depends on pathways involving the Rac guanine nucleotide exchange factor (GEF) DOCK2, rather than on PI3Kd- and PI3Kg-dependent signaling (Nombela-Arrieta et al. 2004). Instead, PI3Kd is crucial for T lymphocyte trafficking, by regulating shedding and transcriptional shut off of the lymph node-homing receptor CD62L (L-selectin) (Sinclair et al. 2008).
Overall, these findings suggest that class I PI3Kd and PI3Kg cooperate in regulating T cell signaling, with PI3Kd impacting more profoundly than PI3Kg on regulation of cell-based immunity.
6.2.4 PI3Ks in B Lymphocytes
B lymphocytes represent the other major cellular component of the adaptive immune response. In contrast to what found in T cells, PI3Kg does not play a significant role in B cells. Conversely, PI3Kd has been shown to control different aspects of B lymphocyte function, including their maturation process and proliferation.
B cell development occurs through several stages. Immature B cells are produced in bone marrow, through Ig chain rearrangement of B cell progenitors (at pro-B and pre-B stages), followed by repertoire selection. These immature B cells then migrate to the spleen where, upon a further selection process, they differentiate into mature B lymphocytes by forming follicular (FO) and marginal zone (MZ) niches. Lack of PI3Kd has been shown to affect the early steps of B cell maturation, as suggested by the increased pro B/pre B ratio. In agreement, the few immature B cells of PI3KdD910A mice are unable to sustain FO and MZ pools (Clayton et al. 2002; Jou et al. 2002; Okkenhaug et al. 2002). Interestingly, B cell development is not further impaired when both PI3Kd and PI3Kg are eliminated and PI3Kg−/− mice do not show any clear defect in B cell maturation, thus demonstrating that PI3Kg does not contribute to regulation of B cell development (Webb et al. 2005).
Also B cell proliferation is strictly dependent on PI3Kd signaling. B-cell proliferation in response to IgM stimulation and BAFF is decreased in cells expressing a catalytically-inactive form of PI3Kd, whereas proliferation induced by IL-4, CD40 or LPS is only partially affected (Henley et al. 2008; Okkenhaug et al. 2002). In addition, PI3Kd activity is indispensable for B-cell-receptor-induced DNA synthesis and proliferation, as well as IL-4-induced survival (Bilancio et al. 2006; Sujobert et al. 2005). PI3Kd−/− and PI3KdD910A B lymphocytes also display reduced antibody production upon T cell-dependent and independent stimulation (Clayton et al. 2002; Jou et al. 2002; Okkenhaug et al. 2002). More specifically, PI3KdD910A mice show reduced IgM and IgG antibody responses (Okkenhaug et al. 2002). By contrast, IgE production is paradoxically increased by genetic or pharmacological inactivation of PI3Kd, despite reduced Th2 responses (Zhang et al. 2008) due to the ability of PI3Kd to modulate IgE switch (Omori et al. 2006; Zhang et al. 2008).
Similar to the case of T cells, migration of B cells is not regulated by PI3Ks. Rather, this process is mediated by DOCK2 and PI3K-independent Btk signaling (de Gorter et al. 2007; Nombela-Arrieta et al. 2004). Nonetheless, the complete response to CXCL13 is reduced in PI3KdD910A, but not in PI3Kg−/− B lymphocytes, revealing a surprising and not well clarified role of PI3Kd downstream G-protein coupled chemokine receptors (Nombela-Arrieta et al. 2004; Reif et al. 2004).
6.2.5 PI3Ks in Inflammatory and Autoimmune Diseases
Given the central role of class I PI3Kd and PI3Kg in the homeostasis of both innate and adaptive responses, these enzymes can also participate to the onset and/or progression of diseases characterized by deregulated activation of innate and/or adaptive immunity. Indeed, genetic inactivation of PI3Kg or PI3Kd modulates the susceptibility to specific diseases.
6.2.5.1 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disorder that affects the joints and is characterized by the progressive destruction of articular structures (Harris 1990). The pathogenesis of RA is not completely understood. Chemokines and other chemoattractants have been detected in the inflamed joints and are responsible for the local recruitment of leukocytes. Amongst these, neutrophils constitute the most abundant population and actively induce inflammatory response and tissue damage (Brennan and Feldmann 1996; Edwards and Hallett 1997; Szekanecz et al. 2003). As PI3Kg is key in neutrophil chemotaxis, PI3Kg deficiency is protective in different mouse models of RA. Camps et al. first showed that PI3Kg−/− animals are largely resistant to aCII-induced arthritis, where type II collagen-specific monoclonal antibodies are injected to initiate RA. Moreover, blockade of PI3Kg by oral delivery of the isoform-selective compound AS605240 reproduces the protective effect of PI3Kg−/− mice in a model of collagen-induced arthritis, where typical features of the disease are triggered by intra-dermally injection of collagen II (Camps et al. 2005). In both cases, the protection correlates with defective neutrophil migration and thus to reduced accumulation of neutrophils in the joints. PI3Kg inactivation is associated to a milder inflammatory arthritis also in an alternative mouse model of RA based on the transgenic overexpression of the human TNFa (Hayer et al. 2009). Interestingly, the genetic disruption of PI3Kg reduces the severity of arthritis through both reduced invasion of leukocytes and reduced proliferation of synovial mesenchymal-derived fibroblasts. These findings challenge the concept of a leukocyte-restricted role of PI3Kg in the pathogenesis of RA and suggest that the therapeutic potential of specific PI3Kg inhibitors might be expanded to a broader spectrum of cell targets, thus yielding superior results in the potential treatment of RA.
In line with a cooperative role of PI3Kg and PI3Kd in regulating neutrophil function, also PI3Kd−/− mice are protected from RA. Randis et al. (2008) have shown that administration of arthritogenic serum to PI3Kd−/− mice results in a significant reduction of paw edema, similar to what observed in PI3Kg−/− animals. A more pronounced protection is also observed in double PI3Kd−/−g−/− mice, indicating the existence of a functional interaction between PI3Kg and PI3Kd in inflammatory arthritis. Accordingly, combined inhibition of PI3Kd and PI3Kg might represent an intriguing innovative treatment for RA.
6.2.5.2 Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by deregulation of T-cell mediated B-cell activation, resulting in generalized B-cell expansion and hypergammaglobulinemia (Liu and Wakeland 2001). The involvement of PI3Ks in the pathogenesis of SLE was first uncovered by two works showing that increased PI3K activity, due to either PTEN ablation (PTEN+/−) or overexpression of an activating form of the p85 regulatory subunit (p65PI3K) in T lymphocytes, leads to a SLE-like phenotype (Borlado et al. 2000; Di Cristofano et al. 1999). Interestingly, the severity of the disease is attenuated in p65PI3KTg/PI3Kg−/− animals, thus suggesting that PI3Kg plays a crucial role in SLE (Barber et al. 2006). Blockade of PI3Kg by the selective inhibitor AS605240 has also found effective in another mouse model of SLE, the MRL-lpr SLE-prone model (Barber et al. 2006). Taken together, these data encourage further study of selective PI3K inhibitors in the treatment of SLE.
6.2.5.3 Asthma
Asthma is a pulmonary disease characterized by bronchial hypersensitivity and involving a Th2 immune response mounted by CD4+ T cells. Other inflammatory cells such as mast cells and eosinophils also play important roles. In particular, neutrophils contribute to the chronic evolution of the disease (Baraldo et al. 2007). The availability of pan-PI3K inhibitors first allowed to uncover the correlation between PI3K hyperactivity and the development of allergic conditions. Indeed, intratracheal administration of wortmannin or LY294002 significantly attenuates inflammation symptoms and airway hyperresponsiveness in a mouse model of asthma (Duan et al. 2005; Ezeamuzie et al. 2001).
PI3Kd and PI3Kg act as master regulators of mast cells and eosinophils, which constitute the principal mediators of allergy. Accordingly, PI3Kg−/− animals are completely protected against systemic anaphylaxis (Laffargue et al. 2002). Similarly, PI3Kd knock-in mice are partially resistant to passive cutaneous anaphylaxis induced by IgE- and antigen-injection (Ali et al. 2004, 2008). Furthermore, intratracheal administration of the PI3Kd-selective inhibitor IC87114 significantly attenuates allergic airway inflammation and suppresses OVA-induced airway hyper-responsiveness to inhaled methacoline (Lee et al. 2006). Farghaly et al. have shown that the Th2 cytokine IL-13 fails to induce hyper-responsiveness in isolated tracheal rings from PI3KdD910A mice. In this context, the reduced hyper-responsiveness may be attributed to a direct effect on airway structural cells rather than on infiltrating immune cells (Farghaly et al. 2008).
Overall, these studies have unveiled a key role for PI3Kd, in addition to PI3Kg, in the pathogenesis of allergic asthma. However, whether selective inhibition of PI3Kd might be protective in allergy is still controversial. In OVA-immunized mice, blockade of PI3Kd leads to a paradoxical increase of both total and OVA-specific IgE levels, despite diminished Th2 responses (Omori et al. 2006; Zhang et al. 2008). Hence, additional studies are needed to clarify the role of PI3Kd in regulating allergen-mediated IgE production, as well as the resulting clinical implications in the treatment of allergic disease. As PI3Kg and PI3Kd cooperate in the onset and progression of allergic conditions, a combined inhibition of these isoforms appears as a reasonable therapeutic strategy (Doukas et al. 2009).
6.2.5.4 Chronic Obstructive Pulmonary Disease
PI3Kd and PI3Kg cooperate in the pathogenesis of chronic obstructive pulmonary disease (COPD). COPD is a common respiratory disease which, unlike allergic asthma, involves CD8+ T cells releasing Th1-type cytokines, with the additional contribution of macrophages and neutrophils. In COPD, airflow limitation is progressive and may be steroid-resistant (Baraldo et al. 2007; Doherty 2004). The double selective inhibitor TG100-115, targeting both PI3Kd and PI3Kg, has been shown effective in controlling COPD in different mouse models. TG100-115 significantly reduces neutrophil accumulation as well as production of the classical Th1 cytokine TNFa in a LPS-induced model of COPD. Interestingly, TG100-115 is successful even in a steroid-resistant form of COPD induced in mice by cigarette smoke exposure (Doukas et al. 2009). Recent reports suggest that this beneficial effect is achieved through a selective involvement of PI3Kd, as genetic inactivation of this isoform, and not of PI3Kg, restores glucocorticoid responsiveness in smoke-induced airway inflammation (Marwick et al. 2009, 2010). Therefore, the complex interplay between PI3Kg and PI3Kd in the development of COPD needs further study. Currently, double selective compounds might represent the most promising response to the urgent need of new treatments for steroid-unresponsive inflammatory diseases.
6.3 PI3Ks in the Regulation of Glucose Metabolism and Insulin Sensitivity
The tight control of glucose metabolism represents a fundamental physiological mechanism regulated by a series of key metabolic hormones. The main player in this process is insulin, a peptide hormone secreted from pancreatic b-cells in response to elevated glucose concentrations. Insulin mainly acts by (i) inhibiting liver gluconeogenesis and glycogenolysis and (ii) stimulating glucose uptake in insulin-sensitive periferal tissues, mainly skeletal muscle and adipocytes. In addition, insulin affects lipid metabolism and stimulates the uptake of aminoacids, thus enhancing protein synthesis. Defects in insulin production and signaling underlie important pathological conditions such as diabetes mellitus and the metabolic syndrome. Type 2 diabetes represents a common disease and is characterized by impaired insulin-stimulated glucose uptake, increased hepatic glucose production and inadequate compensation by the pancreatic b-cells, ultimately leading to hyperglycemia (Kahn 1994).
The action of insulin is initiated by its binding to the insulin receptor (IR), a transmembrane glycoprotein with intrinsic protein tyrosine kinase activity. The adaptors IRS (insulin receptor substrate) are the first IR substrates that undergo tyrosine phosphorylation upon insulin stimulation (Myers and White 1993). Six IRS isoforms are expressed in mammals and play the role of linkers between the upstream tyrosine kinase and the downstream regulatory enzymes and adaptor molecules. PI3K was the first enzyme found to be associated with the IR/IRS signaling. Within this context, upon receptor activation, IRS serves as a docking site for the SH2 domains of the regulatory subunit of PI3K, which in turn recruit the p110 enzyme to the plasma membrane (Myers et al. 1992; Sun et al. 1993) (Fig. 6.2). Upon activation, the p110 catalytic subunit of PI3Ks produces the lipid second messenger PIP3, which in turn activates downstream PH domain-containing effectors that control various metabolic processes such as glucose uptake, lipolysis inhibition, triglyceride formation, and glycogen synthesis. Within these processes, a key molecule activated following PIP3 generation is PKB/Akt (Taniguchi et al. 2006). However, other phosphoinositide-activated kinases, such as atypical PKC, have also been implicated in insulin action (Farese et al. 2005).
PI3K function in glucose metabolism. Upon binding of insulin to its receptor (IR), the IRS adaptor proteins recruit class I PI3Ks, thus triggering PIP3 production and PKB/Akt activation. PKB, in turn, mediates the translocation of the glucose transporter GLUT4 to the plasma membrane, which leads to glucose uptake from the extracellular space. Furthermore, Akt mediates the inhibition of FOXO transcription, thereby negatively regulating the expression of gluconeogenic enzymes such as PEPCK and G6Pase. Insulin also modulates glycogen synthesis through PKB-mediated inhibition of GSK3 and the consequent activation of glycogen synthase. On the other hand, class II PI3K-C2a, following its TC10-dependent activation, contributes to GLUT4 translocation. However, the exact mechanisms linking IR activation to PI3K-C2a are yet to be defined
Amongst the downstream effectors of IR-PI3Ks, Akt represents the central node in the insulin signaling network, stimulating blood glucose disposal and glycogen synthesis, and inhibiting gluconeogenesis. Three different isoforms of Akt, encoded by different genes, are found in mammals. The study of knock-out mice have identified the specific functions of each Akt isoform. Of the three isoforms, Akt2 is in particular the main regulator of glucose homeostasis in vivo (Cho et al. 2001). Indeed, the disruption of Akt2 in mice results in impaired glucose uptake and in a complete failure of insulin to suppress hepatic glucose output (Cho et al. 2001). Akt2 plays an important role also in humans in the regulation of glucose homeostasis, since a germline mutation in Akt2 correlates with development of type 2 diabetes (George et al. 2004).
In adipocytes and muscle cells, PI3K-dependent activation of Akt2 (and to a lesser extent of Akt1) controls insulin-mediated glucose uptake by stimulating GLUT4 translocation from an intracellular compartment to the plasma membrane (Bai et al. 2007; Stenkula et al. 2010). GLUT4 exocytosis is highly regulated by PIP3 production, although the exact mechanism is still unclear. A number of Akt substrates, including the Rab-GAP AS160 (Akt substrate of 160 kDa) (Chen et al. 2011; Sano et al. 2003) and PI5-kinase (PIKfyve) (Berwick et al. 2004), are involved in this process. Other PI3K-dependent mediators of glucose uptake are atypical PKC (aPKC) isoforms that regulate the kinesin and Rab4-dependent GLUT4 exocytosis (Imamura et al. 2003). Further support for this model is given by the muscle-specific knock-out of the aPKC PKC-l, which results in systemic insulin resistance and glucose intolerance (Farese et al. 2007).
The class IA PI3K pathway is also involved in insulin-mediated inhibition of hepatic gluconeogenesis (Agati et al. 1998; Kotani et al. 1999), a process indispensable in a starved condition and switched-off when external resources are available. In the latter condition, insulin negatively regulates gluconeogenesis by suppressing the expression of key gluconeogenic enzymes through the Akt-mediated phosphorylation of the transcription factor FoxO1. Phosphorylated FoxO1 is excluded from the nucleus and consequently fails to activate the transcription of genes required for the gluconeogenesis, such as phosphoenolpyruvate caboxykinase and glucose-6-phosphatase (Nakae et al. 2001).
Together with class IA PI3Ks, PI3Ks of other classes might be involved in insulin-mediated glucose disposal. Indeed, insulin stimulation triggers the production of PI3P, thus possibly involving either class II or III PI3K. PI3P binds the PX and FYVE domains of proteins involved in the control of vesicular trafficking. It is thus possible that PI3K other than class IA are involved in processes needed for plasma membrane fusion of cytoplasmic vesicles containing GLUT4 (Shepherd 2005). Interestingly, insulin-mediated PI3P production appears insensitive to the action of wortmannin, thus excluding a role for class III enzymes, while suggesting a role for the wortmannin-insensitive class II PI3K-C2 isoenzymes. In line with this view, PI3K-C2a is activated in response to insulin-dependent activation of the small GTPase TC10, thus triggering PI3P elevation necessary for full-scale GLUT4 plasma membrane translocation (Maffucci et al. 2003).
6.3.1 Class I PI3K Regulatory Subunits in Glucose Metabolism
The crucial role of PI3Ks in insulin signaling suggests that a dysfunction of the PI3K pathway may deeply affect insulin sensitivity. Indeed, several lines of evidence have indicated that PI3K signaling is compromised in the obese diabetic ob/ob mouse model (Folli et al. 1993; Kerouz et al. 1997), as well as in diabetic patients (Bjornholm et al. 1997; Goodyear et al. 1995).
Despite the key role of PI3K in the insulin signaling, gene deletion studies of adaptors of the p85 family have reported unexpected paradoxical effects. Indeed, mice lacking p85a develop hypoglycemia and increased insulin sensitivity (Fruman et al. 2000). This phenotype probably correlates with the up-regulation of the other Pik3r1 splicing variants, p50a and p55a, in fat and muscle, leading to an elevation of PIP3 levels and to facilitated GLUT4 translocation to the plasma membrane (Terauchi et al. 1999). A slight enhancement of insulin signaling is also present in mice lacking either p50a/p55a or p85b (Chen et al. 2004; Ueki et al. 2002). On the other hand, deletion of all Pik3r1 gene products (p85, p55, and p50), results in perinatal lethality, associated with a substantial decrease in the expression and activity of class IA PI3K catalytic subunits. Nonetheless, Pik3r1-deficient mice are hypoglycemic and more insulin sensitive because of a more active glucose transport in insulin-responsive tissues (Fruman et al. 2000). Similar findings have been obtained with a hepatic deletion of Pik3r1, which causes improved insulin sensitivity in liver, muscle, and fat, but leads to a 60% decrease in total hepatic PI3K activity (Taniguchi et al. 2006). The phosphorylation of Akt downstream PI3K is enhanced as a consequence of reduced activity of the phosphatase PTEN, suggesting a role of p85 in PTEN regulation (Taniguchi et al. 2006). Indeed, PTEN is a potent negative regulator of the insulin signaling and loss of PTEN in adipose tissue results in increased insulin sensitivity and GLUT4 recruitment (Kurlawalla-Martinez et al. 2005). A recent study has shown that p85 directly binds and activates PTEN, which explains the paradox of increased insulin sensitivity in p85-deficient animals (Chagpar et al. 2010).
These studies indicate that a critical molecular balance between regulatory and catalytic subunits determines the optimal response of the PI3K pathway to insulin signaling (Ueki et al. 2002). In physiological conditions, the p85 regulatory subunit is more abundant than the catalytic p110 subunit. p85 monomers can thus inhibit PIP3 production either by binding to phosphorylated IRS proteins (Ueki et al. 2002) or by altering subcellular localization of p110/p85 dimers (Inoue et al. 1998). Unbalanced p85 levels can compromise this regulatory mechanisms and lead to a paradoxical increase in PI3K activity. Finding of increased p85/55/50 protein expression, in mouse models of obesity as well as in type 2 diabetic patients further confirms this view (Bandyopadhyay et al. 2005) and suggests that p85 family members play a complex role in the regulation of PI3K-mediated insulin signaling.
6.3.2 Class I PI3K Catalytic Subunits in Glucose Metabolism
The specific role of single p110 isoforms in insulin signaling has recently begun to emerge, thanks to the development of selective pharmacological inhibitors and of mouse genetic models. Although the lethal phenotype of mice lacking either p110a or p110b hampers the study of their specific roles in insulin signaling, analysis of insulin-mediated responses in heterozygous animals suggests that both proteins might be involved. Heterozygous mice lacking either p110a or p110b show normal responses to insulin. However, mice heterozygous for both isoforms have decreased insulin sensitivity (Brachmann et al. 2005), suggesting that p110a and p110b play complementary roles in insulin signaling. Nonetheless, heterozygous mice expressing a catalytically inactive p110a become insulin resistant with aging, and develop glucose intolerance, hyperlipidemia, adiposity, as well as hyperglycemia and deregulate hepatic gluconeogenesis (Foukas et al. 2006). The use of isoform-selective p110 inhibitors has shown that p110a constitutes the major effector downstream of the IR, while p110b plays only a marginal role, by in providing a basal threshold of PIP3 production that potentiates p110a activity (Knight et al. 2006). Indeed, mice expressing a kinase-dead p110b or carrying a liver-specific ablation of this enzyme develop only mild impaired insulin sensitivity and glucose homeostasis (Ciraolo et al. 2008; Jia et al. 2008). On the contrary, the disruption of p110a in liver results in impaired insulin action and glucose homeostasis which cannot be rescued by p110b (Sopasakis et al. 2010). These findings demonstrate that p110a is the primary PI3K isoform required for the metabolic actions of insulin in the liver. Instead, p110b plays a minor but not negligible role.
6.4 Class I PI3Ks in Cardiac Physiology and Disease
Three different class I PI3Ks are expressed in the myocardial tissue (p110a, p110b and p110g). However, a growing amount of evidence indicates that different PI3K isoforms participate in distinct physiological and pathological processes within cardiomyocytes. In particular, class IA p110a mostly functions as a critical regulator of cardiomyocyte viability and growth downstream tyrosine kinase receptors such as IGF-I and insulin. Based on available studies, p110a activity can be generally considered as beneficial and protective for heart function. In contrast, class IB p110g is uniquely controlled by GPCRs such as b-adrenergic receptors. Of note, robust activation of p110g is essentially detected in the context of maladaptive remodeling and during the natural history of heart failure, where p110g contributes to myocardial dysfunction and impaired contractility. Hence, pharmacological inhibition of p110g has emerged as a novel potential therapeutic strategy in cardiac disease. Finally, our understanding of p110b function in the myocardium is still very initial and is awaiting for further and detailed study.
6.4.1 PI3Ka Regulates Cardiomyocyte Growth
Studies performed on gain and loss-of function models have consistently shown that PI3Ka activity represents a master switch leading to cardiomyocyte growth and survival (Fig. 6.3, upper panel). Systemic knockout of p110a is embryonic lethal without obvious cardiac abnormalities, but a general impairment in cell growth is observed (Bi et al. 1999). In line with this finding, over-expression of a myocardial-selective dominant-negative p110a results in smaller cardiomyocytes and reduced heart mass, without affecting tissue architecture and contractility (Crackower et al. 2002; McMullen et al. 2003; Shioi et al. 2000). On the other hand, over-expression of a cardiac-specific and constitutively active p110a leads to myocardial hypertrophy and increased heart/body weight, but without progression to hypertrophic cardiomyopathy or cardiac dysfunction (Shioi et al. 2000). These findings phenocopy the genetic loss of myocardial PTEN, which leads to PIP3 accumulation (Crackower et al. 2002). In both over-expression and dominant negative models, the changes in p110a activity are paralleled by corresponding changes in Akt, Gsk3b and p70S6K activity, indicating that p110a boosts myocardial growth through the activation of canonical PIP3-dependent anabolic pathways. Indeed, genetic manipulations of Akt lead to similar cardiac phenotypes: Akt1 knockout mice show reduced heart size, while different models of constitutively active Akt1 result in different degrees of cardiac hypertrophy (Condorelli et al. 2002; DeBosch et al. 2006; Matsui et al. 2002; McMullen et al. 2003).
PI3K signaling in normal and failing myocardium. Upper panel. In the myocardium, PI3Ka is activated by tyrosine kinase receptors (RTKs) such as IGF-I, EGF and insulin receptor. Downstream p110a, Akt and other classical PIP3-dependent anabolic pathways promote normal myocardial development and growth. Moreover, PI3Ka is required for physiological hypertrophy. On the other hand, p110b represents the master PI3K isoform producing PIP3 and activating Akt upon b-adrenergic stimulation. In physiological conditions, p110g is expressed at low levels and contributes residually to PIP3 production upon b-adrenergic stimulation. p110g, along with the p87 regulatory subunit, is part of a macromolecular complex mediating the cAMP-dependent activation of PDEs, while the lipid kinase activity of p110g is blunted by PKA phosphorylation. Lower panel. The natural history of heart failure is characterized by the progressive increase in p110g lipid kinase activity, due to p110g upregulation, regulatory isoform switch (from p87 to p101) and loss of inhibition by PKA. By interacting with GRK2, in this context p110g leads to AP2 and b-arrestin mediated downregulation and desensitization of b-adrenergic receptors. In the presence of active myocardial damage, p110a contributes to cardiomyocyte survival and provides protection for adverse remodeling and failure
Beyond cardiac development, the activity of p110a appears to control essentially physiological hypertrophy, as mice expressing a dominant-negative p110a are resistant to left ventricular hypertrophy induced by exercise training. Nonetheless, loss of p110a leaves the animals prone to compensatory hypertrophy following pressure overload by aortic constriction, indicating that p110a represents a master switch of physiological but not of pathological hypertrophy (McMullen et al. 2003). Importantly, cardiac p110a is functionally relevant in conditions of myocardial damage, where p110a protects cardiomyocytes from cell death and dysfunction caused by various pathological noxae (Fig. 6.3, lower panel). Indeed, it has been shown that in several heart disease models such as dilative myocardiopathy, myocardial infarction, chronic adrenergic stimulation and pressure overload, the loss of p110a is highly detrimental and accelerates adverse ventricular remodeling, while on the other hand constitutive p110a activity exerts cardioprotective effects (Lin et al. 2010; McMullen et al. 2007). Taken together, our understanding of myocardial PI3Ka suggests that any treatment targeting myocardial PI3Ks should leave p110a activity unchanged to circumvent a negative impact on cardiac function, especially in the context of cardiotoxicity (e.g. tumor chemotherapy) and heart failure.
6.4.2 PI3Kg Regulates Cardiac Contractility and Remodeling
PI3Kg is expressed at high levels in hematopoietic cells, especially in leukocytes. However, p110g is also present in the myocardium, including cardiomyocytes, endothelial cells and possibly myofibroblasts. Although p110g levels are relatively low in cardiomyocytes, this class IB PI3K isoform has important functions both in heart physiology and disease. In particular, p110g is a key player in the regulation of cardiac contractility, through combined kinase-dependent and kinase-independent molecular mechanisms interlacing cyclic AMP (cAMP) and PIP3 signaling.
Hearts derived from p110g knock-out mice are hyper-contractile compared to wild-type controls both in basal and stimulated conditions, due to functional activation of their contractile machinery (Crackower et al. 2002; Patrucco et al. 2004). This phenotype is caused by increased cellular levels of cAMP, a key second messenger controlling myocardial contractility in response to b-adrenergic stimulation. b-adrenergic receptors (b-ARs, which include b1, b2 and b3 subtypes) are GPCRs physiologically activated by circulating or sympathetic nerve catecholamines (mostly epinephrine). All b-AR isoforms are coupled to Gs subunits, while b2 and b3 receptors also associate to Gi subunits (Brodde et al. 2006; Skeberdis et al. 2008). Catecholamine engagement of b-ARs leads to Gs-triggered stimulation of adenylyl cyclase, which in turn generates the second messenger cAMP. The main signaling effector of cAMP is protein kinase A (PKA), which is constituted by regulatory and catalytic subunits and whose function is compartmentalized and controlled by different proteins known as A-kinase anchor proteins (AKAPs) (Scott and Pawson 2009). In cardiomyocytes, PKA phosphorylates key players orchestrating myocardial contractility, such as phospholamban, the ryanodine receptor and troponin I (Chu et al. 2000; Marx et al. 2000; Stelzer et al. 2007).
Our group and others have shown that p110g plays a key role in the regulation of cAMP signaling, as p110g is required for the activation of cellular phosphodiesterases (PDEs) which hydrolyse cAMP to 5’-AMP and terminate signaling, such as PDE3s and PDE4s (Conti and Beavo 2007; Ghigo and Hirsch 2011; Kerfant et al. 2007; Patrucco et al. 2004; Perino et al. 2011). The regulation of PDE3B by p110g is operated within a macromolecular complex which includes p110g, the PI3K adaptor subunit p84/87 and PKA (Patrucco et al. 2004; Perino et al. 2011; Voigt et al. 2006). In this complex, p110g functions as an AKAP, as it directly binds the RIIa subunit of PKA and thus allows the activation of PDE3B by PKA (Fig. 6.3, upper panel). Of note, this functional interaction does not require the kinase activity of p110g, which instead operates as a scaffold (Hirsch et al. 2009; Perino et al. 2011). In mice lacking p110g (and not in mice expressing a kinase-inactive p110g), disassembly of this critical signaling complex leads to a major reduction in the phosphodiesterase activity, thus resulting in abnormal levels of myocardial cAMP. When p110g-null mice are subjected to cardiac pressure overload, cAMP further raises uncontrolled and causes myocardial damage rapidly progressing towards overt cardiomyopathy and heart failure (Crackower et al. 2002; Patrucco et al. 2004). Similar to PDE3B, also PDE4 activity is controlled by p110g, thus modulating cAMP signaling within cardiomyocyte sub-cellular compartments containing SR Ca2+ ATPase (Kerfant et al. 2007). Taken together, these findings picture a scenario where PI3Kg orchestrates a complex modulation of multiple phosphodiesterases, deeply affecting the intracellular compartmentalization of cAMP signaling in cardiomyocytes. Furthermore, we have recently shown that p110g-associated PKA not only influences phosphodiesterase activity, but also modulates the lipid kinase activity of p110g itself, as the proximity of PKA and p110g within the same macromolecular complex allows active PKA to phosphorylate also p110g on T1024. As a result of PKA phosphorylation, p110g lipid kinase activity is reduced (Perino et al. 2011) (Fig. 6.3, upper panel).
A strict interplay exists between b-ARs and PI3Ks, as the stimulation of myocardial b-ARs not only mobilizes adenylyl cyclase and cAMP, but also PI3Kb, PI3Kg and thus PIP3 (Rockman et al. 2002). By using mice expressing a kinase inactive p110g or p110b, our group has recently shown in vivo that in physiological conditions, b-adrenergic stimulation of the myocardium essentially activates p110b, which is required to activate Akt. Instead, p110g activation only produces a minor fraction of b-adrenergic engaged PIP3, as in the normal myocardium p110g is expressed at low levels and is strictly controlled by PKA-mediated inhibition (Perino et al. 2011). During the natural history of heart failure, the signaling scenario changes deeply, as p110g levels and activity increase due to (i) up-regulation of p110g, (ii) regulatory subunit switch (from p84/87 to p101) of p110g and (iii) reduction of PKA-dependent inhibition of p110g. As a result, b-adrenergic stimulation of the failing myocardium engages unbalanced p110g activity, which contributes to adverse remodeling and left ventricular failure (Patrucco et al. 2004; Perino et al. 2011).
A key pathophysiological feature of heart failure is represented by uncontrolled catecholamine release, which produces a situation of chronic and abnormal stimulation of b-adrenergic receptors. Amongst the maladaptive effects of chronic b-adrenergic engagement is the progressive loss of the ability of b-ARs to transduce signals, a process called desensitization. Furthermore, continuous adrenergic stimulation culminates in b-AR endocytosis and cell surface downregulation. Due to desensitization and downregulation, adrenergic signaling is therefore progressively impaired in the failing myocardium, which loses tonic and phasic contractile responses to catecholamine stimulation (Bristow et al. 1982; Rockman et al. 2002). Both b-adrenergic desensitization and internalization processes depend on the phosphorylation of b-ARs, which permits the interaction between b-ARs and b-arrestins, cytoplasmic proteins that block coupling to G-proteins and start the internalization process. Once in early endosomes, phosphorylated b-ARs can be either dephosphorylated and recycled to the cell surface or subjected to degradation in late endosomes (Rockman et al. 2002). Nonetheless, b-ARs can induce maladaptive signaling pathways at the level of the early endosomes, before being degraded (Lefkowitz and Whalen 2004). Several studies have shown that p110g and PIP3 are key players in these detrimental processes. In particular, p110g preferentially cooperates with G protein-coupled receptor kinase-2 (GRK-2, also known as b-adrenergic receptor kinase-1, b-ARK-1) via its PIK domain (Naga Prasad et al. 2001; Nienaber et al. 2003). GRK-2 constitutes a cytoplasmic complex with p110g and, upon b-AR stimulation, translocates to the activated receptor, where p110g is activated by G-proteins (Naga Prasad et al. 2000). Herein, PIP3 produced by p110g is then required for AP-2 adaptor recruitment at the plasma membrane, which leads to the consequent organization of clathrin-coated pits orchestrating the internalization of the activated receptor (Naga Prasad et al. 2002). In vivo studies have confirmed these mechanistic insights. In mice, over-expression of the PIK domain of PI3K reduces GRK-2-associated PI3K activity and consequently b-AR internalization, without affecting Akt or MAPK pathways (Naga Prasad et al. 2002; Nienaber et al. 2003). In pigs subjected to ventricular pacing-induced heart failure, overexpression of the PIK domain derived from failing hearts rapidly reverts contractility to normal levels (Perrino et al. 2005b). Furthermore, in a model of murine cardiomyopathy (calsequestrin over-expression), over-expression of a catalytically inactive p110g is protective from b-adrenergic perturbation in heart failure, leading to reduced mortality (Perrino et al. 2005a). Our group has further shown that b-AR density remains unchanged after pressure overload in mice expressing a kinase-inactive p110g and that in wild type mice suffering from pressure overload-induced heart failure, administration of a p110g-specific pharmacological inhibitor can significantly improve both b-AR density and left ventricular contractility (Perino et al. 2011). Taken together, these findings picture a scenario where p110g deregulation leads to maladaptive b-adrenergic perturbation during heart failure, by coupling to GRK-2 and AP-2 (Fig. 6.3, lower panel).
Our group has further shown that mice expressing a kinase-inactive p110g are protected from adverse remodeling following pressure overload by transverse aortic constriction. By using bone marrow chimeras, Damilano et al. have provided evidence that interestingly, the detrimental effects of p110g on the myocardium are multifactorial. In particular, cardiomyocyte p110g plays a role in the long term deterioration of left ventricular contractility and diameter, as mice expressing a kinase-inactive p110g in cardiomyocytes are protected from left ventricular dilation and failure at later time points (Damilano et al. 2011). Nonetheless, leukocyte p110g is also key to myocardial infiltration by inflammatory cells, orchestrating local cytokine release and ultimately leading to cardiac fibrosis and diastolic dysfunction (Damilano et al. 2011). Indeed, the loss or inhibition of p110g activity strongly reduces tissue inflammation in the context of cardiovascular diseases such as myocardial infarction and aortic atherosclerosis (Fougerat et al. 2008; Siragusa et al. 2010). These findings are in line with our broader understanding of PI3Kg as a cornerstone signaling enzyme in the context of inflammatory and immune diseases.
In conclusion, these data indicate that p110g signaling occupies a central spot in the molecular pathophysiology of heart failure, which is dominated by abnormal b-adrenergic stimulation and p110g deregulation. In this context, p110g escapes physiological feedback control mechanisms and orchestrates key aspects of myocardial damage and remodeling, such as b-adrenergic desensitization and downregulation, myocardial inflammation and fibrosis. As several proof-of-concept studies have suggested, p110g inhibition by pharmacological inhibitors appears therefore as a promising strategy for the treatment of heart disease and calls for further translational and pharmacological studies.
6.5 Concluding Remarks
In the last decade, efforts from basic science have uncovered PI3K signaling as a fundamental biological process of eukaryotics. The high number of different isoforms grouped in the PI3K family might envisage a complex regulation of this signal transduction pathway, based on intricate interplays between distinct isoenzymes. Instead, there is growing evidence that specific cellular functions are peculiar to distinct PI3K isoforms. While the ubiquitous PI3Ka and PI3Kb are mainly involved in glucose metabolism and cancer, the hematopoietic-restricted PI3Kd and PI3Kg play a major role in immunity, with specific functions of the two isoforms in selected subpopulations of immune cells. Furthermore, PI3Ka and PI3Kg have emerged as master regulators of cardiac function, although further efforts are needed to clarify the role of other isoforms in this context. Overall, selective targeting of specific PI3K isoforms can be predicted to guarantee maximum of efficacy in the treatment of specific diseases, with minimal side effects. In some circumstances, however, different PI3K isoforms function in a synergistic manner in regulating specific biological process. Thus, some pathologic conditions might be more effectively treated with combined therapies targeting more than one selected PI3K. New isoforms-selective compounds are becoming now available, although their potency and isoform selectivity need further improvement before entering clinical trials. Only joint efforts of basic research on genetically engineered animals, pharmaceutical investigation and clinical trial will finally pave our way towards the clinical use of a PI3K inhibitor.
References
Agati JM, Yeagley D, Quinn PG (1998) Assessment of the roles of mitogen-activated protein kinase, phosphatidylinositol 3-kinase, protein kinase B, and protein kinase C in insulin inhibition of cAMP-induced phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 273:18751–18759
Alcazar I, Marques M, Kumar A, Hirsch E, Wymann M, Carrera AC, Barber DF (2007) Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J Exp Med 204:2977–2987
Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E et al (2004) Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature 431:1007–1011
Ali K, Camps M, Pearce WP, Ji H, Ruckle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B (2008) Isoform-specific functions of phosphoinositide 3-kinases: p110 delta but not p110 gamma promotes optimal allergic responses in vivo. J Immunol 180:2538–2544
Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C et al (2004) The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther 3:772–775
Bader AG, Kang S, Vogt PK (2006) Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci USA 103:1475–1479
Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T (2007) Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab 5:47–57
Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM (2005) Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 54:2351–2359
Baraldo S, Lokar Oliani K, Turato G, Zuin R, Saetta M (2007) The role of lymphocytes in the pathogenesis of asthma and COPD. Curr Med Chem 14:2250–2256
Barber DF, Bartolome A, Hernandez C, Flores JM, Fernandez-Arias C, Rodriguez-Borlado L, Hirsch E, Wymann M, Balomenos D, Carrera AC (2006) Class IB-phosphatidylinositol 3-kinase (PI3K) deficiency ameliorates IA-PI3K-induced systemic lupus but not T cell invasion. J Immunol 176:589–593
Barberis L, Pasquali C, Bertschy-Meier D, Cuccurullo A, Costa C, Ambrogio C, Vilbois F, Chiarle R, Wymann M, Altruda F et al (2009) Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur J Immunol 39:1136–1146
Benistant C, Chapuis H, Roche S (2000) A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene 19:5083–5090
Berenjeno IM, Vanhaesebroeck B (2009) PI3K regulatory subunits lose control in cancer. Cancer Cell 16:449–450
Berwick DC, Dell GC, Welsh GI, Heesom KJ, Hers I, Fletcher LM, Cooke FT, Tavare JM (2004) Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J Cell Sci 117:5985–5993
Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL (1999) Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem 274:10963–10968
Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B (2006) Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood 107:642–650
Bjornholm M, Kawano Y, Lehtihet M, Zierath JR (1997) Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes 46:524–527
Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann MP (2009) PI3Kgamma adaptor subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools in mast cells. Sci Signal 2:ra27
Borlado LR, Redondo C, Alvarez B, Jimenez C, Criado LM, Flores J, Marcos MA, Martinez AC, Balomenos D, Carrera AC (2000) Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. Faseb J 14:895–903
Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC (2005) Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol 25:1596–1607
Brennan FM, Feldmann M (1996) Cytokines in autoimmunity. Curr Opin Immunol 8:872–877
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB (1982) Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211
Brodde OE, Bruck H, Leineweber K (2006) Cardiac adrenoceptors: physiological and pathophysiological relevance. J Pharmacol Sci 100:323–337
Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B et al (2005) Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nature medicine 11:936–943
Carvalho S, Milanezi F, Costa JL, Amendoeira I, Schmitt F (2010) PIKing the right isoform: the emergent role of the p110beta subunit in breast cancer. Virchows Arch 456:235–243
Chagpar RB, Links PH, Pastor MC, Furber LA, Hawrysh AD, Chamberlain MD, Anderson DH (2010) Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 107:5471–5476
Chaussade C, Cho K, Mawson C, Rewcastle GW, Shepherd PR (2009) Functional differences between two classes of oncogenic mutation in the PIK3CA gene. Biochem Biophys Res Commun 381:577–581
Chen D, Mauvais-Jarvis F, Bluher M, Fisher SJ, Jozsi A, Goodyear LJ, Ueki K, Kahn CR (2004) p50alpha/p55alpha phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol Cell Biol 24:320–329
Chen S, Wasserman DH, MacKintosh C, Sakamoto K (2011) Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab 13:68–79
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728–1731
Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG (2000) A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta-agonists. J Biol Chem 275:38938–38943
Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C Wu H et al (2008) Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal 1:ra3
Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E et al (2002) A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med 196:753–763
Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, Vanhaesebroeck B, Turner M, Webb L, Wymann MP et al (2005) Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106:1432–1440
Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C et al (2002) Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 99:12333–12338
Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511
Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O, Neilsen PO, Ciraolo E, Altruda F, Prestwich GD et al (2007) Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci USA 104:14354–14359
Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J et al (2002) Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110:737–749
Damilano F, Franco I, Perrino C, Schaefer K, Azzolino O, Carnevale D, Cifelli G, Carullo P, Ragona R, Ghigo A et al (2011) Distinct effects of leukocyte and cardiac phosphoinositide 3-kinase {gamma} activity in pressure overload-induced cardiac failure. Circulation 123:391–399
DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ (2006) Akt1 is required for physiological cardiac growth. Circulation 113:2097–2104
De Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, Van Gils JM, Hendriks RW, Pals ST, Spaargaren M (2007) Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity 26:93–104
Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP (1999) Impaired Fas response and autoimmunity in Pten +/− mice. Science 285:2122–2125
Doherty DE (2004) The pathophysiology of airway dysfunction. Am J Med 117(Suppl 12A):11S–23S
Doukas J, Eide L, Stebbins K, Racanelli-Layton A, Dellamary L, Martin M, Dneprovskaia E, Noronha G, Soll R, Wrasidlo W et al (2009) Aerosolized phosphoinositide 3-kinase gamma/delta inhibitor TG100–115 [3-[2,4-diamino-6-(3-hydroxyphenyl)pteridin-7-yl]phenol] as a therapeutic candidate for asthma and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 328:758–765
Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS (2005) An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol 5:495–502
Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H, Innocenti P, Kocher HM, Falasca M (2010) Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clin Cancer Res 16:4928–4937
Edwards SW, Hallett MB (1997) Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunology today 18:320–324
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619
Ezeamuzie CI, Sukumaran J, Philips E (2001) Effect of wortmannin on human eosinophil responses in vitro and on bronchial inflammation and airway hyperresponsiveness in Guinea pigs in vivo. Am J Respir Crit Care Med 164:1633–1639
Farese RV, Sajan MP, Standaert ML (2005) Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans 33:350–353
Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR Jr, Nimal S, Choi CS, Kim S, Shulman GI et al (2007) Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117:2289–2301
Farghaly HS, Blagbrough IS, Medina-Tato DA, Watson ML (2008) Interleukin 13 increases contractility of murine tracheal smooth muscle by a phosphoinositide 3-kinase p110delta-dependent mechanism. Molecular pharmacology 73:1530–1537
Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S et al (2007) PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nature Cell Biol 9:86–91
Folli F, Saad MJ, Backer JM, Kahn CR (1993) Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest 92:1787–1794
Fougerat A, Gayral S, Gourdy P, Schambourg A, Ruckle T, Schwarz MK, Rommel C, Hirsch E, Arnal JF, Salles JP et al (2008) Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation 117:1310–1317
Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B (2006) Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441:366–370
Fruman DA, Cantley LC (2002) Phosphoinositide 3-kinase in immunological systems. Sem Immunol 14:7–18
Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC (2000) Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet 26:379–382
George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL et al (2004) A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304:1325–1328
Ghigo A, Hirsch E (2011) PI3Kgamma mediates cardiac cAMP compartmentalization through scaffloding of distinct phosphodiesterases. In Heart Failure Winter Meeting (Les Diablerets)
Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL (1995) Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95:2195–2204
Gymnopoulos M, Elsliger MA, Vogt PK (2007) Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci USA 104:5569–5574
Harris ED Jr (1990) Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med 322:1277–1289
Hayer S, Pundt N, Peters MA, Wunrau C, Kuhnel I, Neugebauer K, Strietholt S, Zwerina J, Korb A, Penninger J et al (2009) PI3K{gamma} regulates cartilage damage in chronic inflammatory arthritis. Faseb J 23:4288–4298
Henley T, Kovesdi D, Turner M (2008) B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur J Immunol 38:3543–3548
Hill KM, Kalifa S, Das JR, Bhatti T, Gay M, Williams D, Taliferro-Smith L, De Marzo AM (2010) The role of PI 3-kinase p110beta in AKT signally, cell survival, and proliferation in human prostate cancer cells. Prostate 70:755–764
Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP (2000) Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287:1049–1053
Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, Barberis L (2006) Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb Haemost 95:29–35
Hirsch E, Braccini L, Ciraolo E, Morello F, Perino A (2009) Twice upon a time: PI3K’s secret double life exposed. Trends Biochem Sci 34:244–248
Imamura T, Huang J, Usui I, Satoh H, Bever J, Olefsky JM (2003) Insulin-induced GLUT4 translocation involves protein kinase C-lambda-mediated functional coupling between Rab4 and the motor protein kinesin. Mol Cell Biol 23:4892–4900
Inoue G, Cheatham B, Emkey R, Kahn CR (1998) Dynamics of insulin signaling in 3T3-L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J Biol Chem 273:11548–11555
Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring P et al (2009) Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16:463–474
Ji H, Rintelen F, Waltzinger C, Bertschy Meier D, Bilancio A, Pearce W, Hirsch E, Wymann MP, Ruckle T, Camps M et al (2007) Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood 110:2940–2947
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM et al (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454:776–779
Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Carpino N, Wang D, Ihle JN (2002) Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol 22:8580–8591
Kahn CR (1994) Banting lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43:1066–1084
Kang S, Denley A, Vanhaesebroeck B, Vogt PK (2006) Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 103:1289–1294
Kerfant BG, Zhao D, Lorenzen-Schmidt I, Wilson LS, Cai S, Chen SR, Maurice DH, Backx PH (2007) PI3Kgamma is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticular calcium ATPase in cardiomyocytes. Circ Res 101:400–408
Kerouz NJ, Horsch D, Pons S, Kahn CR (1997) Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J Clin Invest 100:3164–3172
Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B et al (2006) A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125:733–747
Knobbe CB, Reifenberger G (2003) Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3'-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol 13:507–518
Kotani K, Ogawa W, Hino Y, Kitamura T, Ueno H, Sano W, Sutherland C, Granner DK, Kasuga M (1999) Dominant negative forms of Akt (protein kinase B) and atypical protein kinase Clambda do not prevent insulin inhibition of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 274:21305–21312
Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H (2005) Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol 25:2498–2510
Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP (2002) Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity 16:441–451
Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD (2006) Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. Faseb J 20:455–465
Lee SH, Poulogiannis G, Pyne S, Jia S, Zou L, Signoretti S, Loda M, Cantley LC, Roberts TM (2010) A constitutively activated form of the p110beta isoform of PI3-kinase induces prostatic intraepithelial neoplasia in mice. Proc Natl Acad Sci USA 107:11002–11007
Lefkowitz RJ, Whalen EJ (2004) beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol 16:162–168
Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D (2000) Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science 287:1046–1049
Lim DH, Cho JY, Song DJ, Lee SY, Miller M, Broide DH (2009) PI3K gamma-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling. Am J Physiol 296:L210–L219
Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP et al (2010) PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol 30:724–732
Link W, Rosado A, Fominaya J, Thomas JE, Carnero A (2005) Membrane localization of all class I PI 3-kinase isoforms suppresses c-Myc-induced apoptosis in Rat1 fibroblasts via Akt. J Cell Biochem 95:979–989
Liu K, Wakeland EK (2001) Delineation of the pathogenesis of systemic lupus erythematosus by using murine models. Adv Exp Med Biol 490:1–6
Liu L, Puri KD, Penninger JM, Kubes P (2007) Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood 110:1191–1198
Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, Uzonna JE (2009) The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol 183:1921–1933
Locke NR, Patterson SJ, Hamilton MJ, Sly LM, Krystal G, Levings MK (2009) SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol 183:975–983
Maffucci T, Brancaccio A, Piccolo E, Stein RC, Falasca M (2003) Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J 22:4178–4189
Marwick JA, Caramori G, Stevenson CS, Casolari P, Jazrawi E, Barnes PJ, Ito K, Adcock IM, Kirkham PA, Papi A (2009) Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med 179:542–548
Marwick JA, Caramori G, Casolari P, Mazzoni F, Kirkham PA, Adcock IM, Chung KF, Papi A (2010) A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 125:1146–1153
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101:365–376
Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A (2002) Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277:22896–22901
McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S (2003) Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100:12355–12360
McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T et al (2007) Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 104:612–617
Mishra RK, Scaife JE, Harb Z, Gray BC, Djukanovic R, Dent G (2005) Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K). Allergy 60:1204–1207
Morrow CJ, Gray A, Dive C (2005) Comparison of phosphatidylinositol-3-kinase signalling within a panel of human colorectal cancer cell lines with mutant or wild-type PIK3CA. FEBS Lett 579:5123–5128
Myers MG Jr, White MF (1993) The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes 42:643–650
Myers MG Jr, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF (1992) IRS-1 activates phosphatidylinositol 3'-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA 89:10350–10354
Naga Prasad SV, Esposito G, Mao L, Koch WJ, Rockman HA (2000) Gbetagamma-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J Biol Chem 275:4693–4698
Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA (2001) Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem 276:18953–18959
Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA (2002) Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J Cell Biol 158:563–575
Nakae J, Kitamura T, Silver DL, Accili D (2001) The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108:1359–1367
Nienaber JJ, Tachibana H, Naga Prasad SV, Esposito G, Wu D, Mao L, Rockman HA (2003) Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest 112:1067–1079
Nombela-Arrieta C, Lacalle RA, Montoya MC, Kunisaki Y, Megias D, Marques M, Carrera AC, Manes S, Fukui Y, Martinez AC et al (2004) Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity 21:429–441
Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, Fruman DA (2006) Sjogren’s syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci USA 103:16882–16887
Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M (1994) Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J Biol Chem 269:3563–3567
Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD et al (2002) Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297:1031–1034
Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B (2006) The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol 177:5122–5128
Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC (2006) Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity 25:545–557
Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM (1998) Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med 188:1621–1632
Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD et al (2004) PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118:375–387
Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B et al (2006) Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4 + CD25 + Foxp3 + regulatory T cells. J Immunol 177:6598–6602
Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie GS, Damilano F, Dunlop AJ, Pawson C, Walser R et al (2011) Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110γ. Mol Cell 42(1):84–95
Perrino C, Naga Prasad SV, Patel M, Wolf MJ, Rockman HA (2005a) Targeted inhibition of beta-adrenergic receptor kinase-1-associated phosphoinositide-3 kinase activity preserves beta-adrenergic receptor signaling and prolongs survival in heart failure induced by calsequestrin overexpression. J Am Coll Cardiol 45:1862–1870
Perrino C, Naga Prasad SV, Schroder JN, Hata JA, Milano C, Rockman HA (2005b) Restoration of beta-adrenergic receptor signaling and contractile function in heart failure by disruption of the betaARK1/phosphoinositide 3-kinase complex. Circulation 111:2579–2587
Phu T, Haeryfar SM, Musgrave BL, Hoskin DW (2001) Phosphatidylinositol 3-kinase inhibitors prevent mouse cytotoxic T-cell development in vitro. J Leukoc Biol 69:803–814
Pinho V, Souza DG, Barsante MM, Hamer FP, De Freitas MS, Rossi AG, Teixeira MM (2005) Phosphoinositide-3 kinases critically regulate the recruitment and survival of eosinophils in vivo: importance for the resolution of allergic inflammation. J Leukoc Biol 77:800–810
Puri KD, Doggett TA, Douangpanya J, Hou Y, Tino WT, Wilson T, Graf T, Clayton E, Turner M, Hayflick JS et al (2004) Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood 103:3448–3456
Puri KD, Doggett TA, Huang CY, Douangpanya J, Hayflick JS, Turner M, Penninger J, Diacovo TG (2005) The role of endothelial PI3Kgamma activity in neutrophil trafficking. Blood 106:150–157
Randis TM, Puri KD, Zhou H, Diacovo TG (2008) Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol 38:1215–1224
Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG (2004) Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol 173:2236–2240
Rockman HA, Koch WJ, Lefkowitz RJ (2002) Seven-transmembrane-spanning receptors and heart function. Nature 415:206–212
Rommel C, Camps M, Ji H (2007) PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7:191–201
Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J et al (2005) PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65:2554–2559
Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE (2003) Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol 170:2647–2654
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ et al (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304:554
Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE (2003) Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278:14599–14602
Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I et al (2000) Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287:1040–1046
Scott JD, Pawson T (2009) Cell signaling in space and time: where proteins come together and when they’re apart. Science 326:1220–1224
Shepherd PR (2005) Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol Scand 183:3–12
Shi J, Cinek T, Truitt KE, Imboden JB (1997) Wortmannin, a phosphatidylinositol 3-kinase inhibitor, blocks antigen-mediated, but not CD3 monoclonal antibody-induced, activation of murine CD4 + T cells. J Immunol 158:4688–4695
Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S (2000) The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J 19:2537–2548
Silvestris N, Tommasi S, Petriella D, Santini D, Fistola E, Russo A, Numico G, Tonini G, Maiello E, Colucci G (2009) The dark side of the moon: the PI3K/PTEN/AKT pathway in colorectal carcinoma. Oncology 77(Suppl 1):69–74
Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA (2008) Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol 9:513–521
Siragusa M, Katare R, Meloni M, Damilano F, Hirsch E, Emanueli C, Madeddu P (2010) Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res 106:757–768
Skeberdis VA, Gendviliene V, Zablockaite D, Treinys R, Macianskiene R, Bogdelis A, Jurevicius J, Fischmeister R (2008) beta3-adrenergic receptor activation increases human atrial tissue contractility and stimulates the L-type Ca2 + current. J Clin Invest 118:3219–3227
Smith LD, Hickman ES, Parry RV, Westwick J, Ward SG (2007) PI3Kgamma is the dominant isoform involved in migratory responses of human T lymphocytes: effects of ex vivo maintenance and limitations of non-viral delivery of siRNA. Cell Signal 19:2528–2539
Sopasakis VR, Liu P, Suzuki R, Kondo T, Winnay J, Tran TT, Asano T, Smyth G, Sajan MP, Farese RV et al (2010) Specific roles of the p110alpha isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab 11:220–230
Stelzer JE, Patel JR, Walker JW, Moss RL (2007) Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res 101:503–511
Stenkula KG, Lizunov VA, Cushman SW, Zimmerberg J (2010) Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal. Cell Metab 12:250–259
Sujobert P, Bardet V, Cornillet-Lefebvre P, Hayflick JS, Prie N, Verdier F, Vanhaesebroeck B, Muller O, Pesce F, Ifrah N et al (2005) Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood 106:1063–1066
Sun XJ, Crimmins DL, Myers MG Jr, Miralpeix M, White MF (1993) Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol 13:7418–7428
Szekanecz Z, Kim J, Koch AE (2003) Chemokines and chemokine receptors in rheumatoid arthritis. Sem Immunol 15:15–21
Takeda M, Ito W, Tanabe M, Ueki S, Kato H, Kihara J, Tanigai T, Chiba T, Yamaguchi K, Kayaba H et al (2009) Allergic airway hyperresponsiveness, inflammation, and remodeling do not develop in phosphoinositide 3-kinase gamma-deficient mice. J Allergy Clin Immunol 123:805–812
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96
Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K et al (1999) Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet 21:230–235
Tigani B, Hannon JP, Mazzoni L, Fozard JR (2001) Effects of wortmannin on airways inflammation induced by allergen in actively sensitised Brown Norway rats. Eur J Pharmacol 433:217–223
Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM (2003) The phospholipase C gamma 1-dependent pathway of Fc epsilon RI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem 278:48474–48484
Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC (2002) Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci USA 99:419–424
Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY et al (2009) AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 16:21–32
Velasco A, Bussaglia E, Pallares J, Dolcet X, Llobet D, Encinas M, Llecha N, Palacios J, Prat J, Matias-Guiu X (2006) PIK3CA gene mutations in endometrial carcinoma: correlation with PTEN and K-RAS alterations. Hum Pathol 37:1465–1472
Voigt P, Dorner MB, Schaefer M (2006) Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinase gamma that is highly expressed in heart and interacts with PDE3B. J Biol Chem 281:9977–9986
Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M (2005) Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol 175:2783–2787
Wee S, Wiederschain D, Maira SM, Loo A, Miller C, DeBeaumont R, Stegmeier F, Yao YM, Lengauer C (2008) PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 105:13057–13062
Wong KK, Engelman JA, Cantley LC (2010) Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 20:87–90
Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, Vanhaesebroeck B, Marshall AJ (2008) Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. J Allergy Clin Immunol 122:811–819 e812
Zhao L, Vogt PK (2008) Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci USA 105:2652–2657
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Ghigo, A., Morello, F., Perino, A., Hirsch, E. (2012). Phosphoinositide 3-Kinases in Health and Disease. In: Balla, T., Wymann, M., York, J. (eds) Phosphoinositides I: Enzymes of Synthesis and Degradation. Subcellular Biochemistry, vol 58. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3012-0_6
Download citation
DOI: https://doi.org/10.1007/978-94-007-3012-0_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-3011-3
Online ISBN: 978-94-007-3012-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)