Abstract
We examine the history and relative importance of marine crustacean invasions for North America. Nearly 400 non-native species of invertebrates and algae have established populations in marine and estuarine waters of North America. Of these documented invasions, 28% are crustaceans, contributing the largest number of species of any taxonomic group. Crustaceans also dominate non-native species richness on each coast of North America, but there are strong differences in the total number of non-native species and in their taxonomic distribution among coasts. Crustaceans contribute prominently to the current knowledge base about marine invasions, due both to the large number (proportion) of documented introductions and also the extent of research on the group; they are thus a potentially important model for understanding marine biological invasions in general. Using an analysis of available literature, we evaluate what is known about the impacts of 108 non-native crustaceans in North America. Ecological and economic impacts are reported for many (28%) of these species, but they are rarely well documented, resulting in low certainty about the magnitude, spatial scale, and temporal scale of effects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Biological invasions by crustaceans are a conspicuous feature of coastal marine ecosystems throughout the world. This prominence results from a combination of multiple factors, including the relatively large body size and good taxonomic (and biogeographic) resolution that exists for many species compared to some other taxonomic groups (Cohen and Carlton 1995; Carlton 1996; Ruiz et al. 2000). In addition, crustacean invaders can often achieve high abundances, further increasing the likelihood of detection and observation. All of these attributes are exemplified by many crab and barnacle species that are frequently recognized introductions, such as the European shore crab Carcinus maenas and the Chinese mitten crab Eriocheir sinensis.
It is therefore no surprise that crustaceans are among the most studied of all marine invaders and often comprise a large proportion of the documented non-native species in well-studied regions of the world (Cohen and Carlton 1995; Reise et al. 1999; Orensanz et al. 2002; Bouley and Kimmerer 2006; Hewitt et al. 2004; Galil 2008). Research on non-native species of crustaceans spans a wide range of topics, from population dynamics and evolutionary biology to community ecology and economic consequences (Vermeij 1982; Seeley 1986; Grosholz et al. 2000; Talley et al. 2001; Lohrer and Whitlach 2002; Floyd and Williams 2004; Hollebone and Hay 2008). Given the scope and extent of this past work, crustaceans provide an important model for understanding invasion patterns and processes.
In this chapter, we present a brief overview of the current state of knowledge about crustacean invasions for marine and estuarine waters of North America, excluding Mexico. We synthesized and analyzed occurrence records for marine species to identify non-native species that have been documented in North America, evaluating species considered to have established populations. The results were documented in a database (NEMESIS 2009) and used to characterize (a) the identity, distribution, and native region for non-native crustaceans documented among the Atlantic, Pacific, and Gulf coasts of North America, (b) the relative importance of crustaceans to overall non-native species richness documented for each coast, and (c) the impacts (effects) that have been reported for North America.
2 Taxonomic and Geographic Distribution of Non-native Crustaceans
We identified 108 non-native species crustaceans that were classified as having established populations in marine and estuarine (tidal) waters of North America, including the continental United States and Canada (Appendix 1). As noted in previous analyses (Ruiz et al. 2000), crustaceans make the single largest contribution of any taxonomic group to the number of documented non-native species in North America. In our current analysis, we classified 381 non-native species of invertebrates and algae (i.e., excluding vertebrates and vascular plants) as having established populations in tidal waters of North America. Thus, crustaceans comprised 28% of this total non-native species richness on a continental scale.
The largest contribution of non-native crustaceans came from amphipods, providing 30% of documented crustacean invasions in North America (Fig. 1). Three other groups each contributed strongly to the total species richness: isopods (21%), decapods (14%), and copepods (13%). Other groups of crustaceans each contributed less than 10% of the total species, with barnacles, mysids, and ostracods providing the most (7%, 6%, and 5%, respectively; Fig. 1).
2.1 Number of Species by Coast
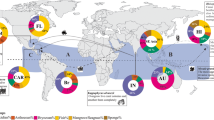
There are strong differences in the total number of non-native species among the three coasts of North America (Fig. 2). Far more non-native species have been documented for the West coast than the other two coasts, when considering established populations of invertebrates and algae. Despite such a disparity in overall numbers, the relative contribution of crustaceans to totals on each coast is similar: 42% of non-native species on the West coast, 33% on the Gulf coast, and 31% on the East coast (Fig. 2). Nonetheless, 75% of all non-native crustaceans are known from the West coast alone, and far fewer are documented on the Gulf coast (13%) and East coast (33%), with some species shared among coasts (Appendix 1).
Several factors may contribute to this disparity in the total number of non-native crustaceans and other taxa documented among coasts. One of these involves the quality of the historical record and knowledge about biogeography, both of which differ by coast. Marine biological studies on the East and Gulf Coasts began in the early-mid-1800s, centuries after the beginning of extensive European trade and shipping (1500s–1600s, Carlton 2003). Due to this long gap, many species may have invaded the Atlantic coast of North America before the biota were catalogued (Carlton 2003). In contrast, biological collections and studies began on the West Coast in the late 1800s, only few decades after the beginning of extensive settlement and shipping (mid-1800s, Carlton 1979), although we note that the West coast, too, experienced early contact (beginning in the 1500s) with European shipping. Such geographic differences in relative timing of events may greatly affect the ability to detect non-native species (Ruiz et al. 2000).
Specific characteristics of species transfer mechanisms have also affected observed differences in non-native species richness among coasts in at least two ways. Both the magnitude and geographic source(s) of species transferred by human means have clearly differed among coasts, affecting the species pool and the number of propagules (individuals) delivered. This variation is perhaps most pronounced for oyster transfers and their associated biota. Massive numbers of oysters were transferred to the West coast, first from eastern North America (the Eastern Oyster, Crassostrea virginica) and then from Asia (Pacific Oyster, C. gigas), resulting in a large number of invasions by associated biota (Carlton 1979; Cohen and Carlton 1995; Miller et al. 2007). In contrast, transplants of oysters to the East and Gulf coasts from other global regions have been very limited in scale, occurring later in time with more care to prevent movement of associated species (Loosanoff 1955; Hidu and Lavoie 1991). Some transfers of native oysters (C. virginica) along the Atlantic coast have resulted in introductions (e.g., Loxothylacus panopaei, from the Gulf of Mexico to Chesapeake Bay, Hines et al. 1997). Nonetheless, while oyster introductions have been a major source of invasions to the West Coast of North America, relatively few species are attributed to this mechanism on the East or Gulf Coast.
As with oysters, strong differences in commercial shipping have contributed to variation in non-native species richness among coasts, especially with respect to source region. For example, in recent time, most overseas ship arrivals to the West coast came from ports in Asia and other Pacific Rim countries, but those to the East and Gulf coasts have been mainly from Atlantic ports (NBIC 2009). Independent of potential differences in the magnitude (number of species and densities) of organism transfers among regions, the different origin of vessel traffic indicates that a different pool of species is delivered to each coast of North America. This said, exceptions to this general pattern occur, and may deliver notable species to the “opposite” coast, such as the Asian shore crab Hemigrapsus sanguineus that invaded the East coast in the 1980s (Lohrer and Whitlatch 2002).
Although there are conspicuous differences in the supply of propagules among coasts, it is also possible that these geographic regions differ in susceptibility to invasions. It has been suggested that West coast estuaries are more susceptible to invasions than those elsewhere because of disturbance or a depauperate native fauna (Cohen and Carlton 1998). This hypothesis remains to be tested in a way that controls for many of the other confounding factors or differences among locations (Ruiz et al. 1999, 2000).
It has also been suggested that the West coast is relatively susceptible to invasions, especially from Asia, due to climatic regime (Vermeij 1991; Chapman 2000). In the north-temperate zone, the eastern shores of oceans (Europe, western North America) have marine-dominated climates, with milder winters and summers, while the western shores (Asia, eastern North America) have continental-dominated climates, with a wider temperature range. Species that have evolved in the latter regions (Northwest Atlantic, Northwest Pacific) may be superior colonists, while species native to marine-dominated climates (Northeast Atlantic, Northeast Pacific) may have difficulty surviving the wide temperature range in continental climates. Chapman (2000) considers this an explanation for a west-to-east pattern of invasions for peracarid crustaceans in North America, and Carlton (1999) has found a similar pattern in molluscs.
2.2 Salinity Distribution of Species by Coast
For each coast, the majority of the non-native crustaceans in our analyses occur in marine waters, ranging from 65% to 78% (Appendix 1). A small subset of species is restricted to tidal freshwater reaches of estuaries on each coast, exhibiting a very narrow range (15–16% of the crustaceans) among coasts. The West coast has more than twice the frequency of brackish water specialists (18% of species) than the East or Gulf coasts (5% and 8%, respectively). This difference results largely from the greater number of copepods and mysids that have colonized brackish water on the West coast compared to other coasts, where these groups were relatively rare (see below for further discussion).
2.3 Taxonomic Distribution by Coast
A striking difference exists among coasts in the relative frequency of different taxonomic groups within the non-native crustaceans (Fig. 3). On the West coast, a disproportionately large percentage of the total is comprised of amphipods (35%) and copepods (17%) compared to the other coasts. In contrast, amphipods contribute 7% and 17% of the non-native crustaceans for the Gulf and East coast, and copepods were 0% and 3% of the totals for these respective coasts. Mysids are also more prevalent on the West coast (five species, 6% of crustaceans) compared to the Gulf coast (none) and East coast (one species or 3%).
For copepods and mysids, the differences among coasts are attributed in large part to invasions from Asia. The estuarine copepod and mysid fauna of the coast of Asia includes a substantial contribution from a diverse fauna, adapted to low-salinity brackish waters, many of which range into temperate waters. Fourteen of these species (ten copepods, four mysids) have been introduced to West Coast estuaries in ballast water (Cohen and Carlton 1995; Orsi and Ohtsuka 1999; Bollens et al. 2002; Modlin 2007; Cordell et al. 2008), representing 71% of copepod introductions and 80% of mysid introductions to this coast. In contrast, only one copepod and one mysid are known introductions to the Gulf and East coasts combined (Appendix 1).
The paucity of copepod invasions for the entire Atlantic coast is especially surprising, given the magnitude of shipping and ballast water discharge at major ports along this coast (NBIC 2009). Although ballast water is considered the source for most copepod invasion along the West coast, a similar signal is completely lacking for the East and Gulf coast, where the only reported invasion is a freshwater parasitic copepod on fish (Lernaea cyprinacea). Moreover, the only documented non-native marine zooplankton species established on the East Coast is the European mysid Praunus flexuosus (Wigley 1963).
Based on past and current shipping patterns, most overseas ship arrivals and ballast discharge to the East coast have come from Europe, whereas that to the West coast has been from Asia (NBIC 2009; Ruiz, 2005). It is noteworthy that the copepod fauna of Europe is considered relatively low in diversity, with many circumpolar and amphi-oceanic species and species complexes (Frost 1989; Bradford 1976; Lee 2000). While many of the copepods introduced to West coast estuaries from Asia are morphologically distinct and easily recognized (Orsi and Ohtsuka 1999), the species pool in Europe reaching the East Coast via ballast water of ships is more similar morphologically to the native fauna (Fofonoff and Ruiz, 2003). It is possible that some early introductions of European copepods have been overlooked, especially if species complexes disguise a richer biota than is presently recognized.
For amphipods, some of the same issues appear important in the observed disparity among coasts. Asia was an important source (native region) for amphipods on the West coast, and so was the Atlantic, resulting from transfers by oysters and ships (Cohen and Carlton 1995; NEMESIS 2009). Atlantic and Asian amphipods arriving on the West Coast appear to have often been morphological distinct and readily recognized from native biota (Carlton 1979; Chapman 1988, 2007). In contrast, a number of amphipod species, especially those building tubes on solid surfaces (e.g., Corophiinae, Jassa marmorata; Ampithoe valida; Erichthonius brasiliensis) are found on both sides of the Atlantic (Lincoln 1979; Conlan 1990; Bousfield and Hoover 1997). It is likely that some early introductions of European fouling-community amphipods to the East Coast have been overlooked, since they could have happened centuries before the onset of biological studies.
More generally, the frequency differences observed for taxonomic groups among coasts results from variation in (a) the historical supply of propagules, (b) susceptibility to invasions, and (c) detection of non-native species. The relative importance of each of these is still under debate for crustaceans and all other taxa, as discussed above (see Sect. 2.1). The patterns observed for copepods and other ballast-mediated invasions serve to further underscore the importance of propagule source in constraining the species pool and taxonomic resolution. There is also the suggestion that the biota from some regions may be superior colonists, such as species arriving from Asia to the West coast of North America (Vermeij 1991, 1996; Chapman 2000).
2.4 Native Region by Coast
In considering the native region of crustaceans on a broad-scale (Fig. 4), most species are from Asia and the western Pacific, ranging from 41% to 62% of all crustaceans per coast. North America itself is the second largest native region for crustacean invasions, contributing 21–32% of species with the vast majority on each coast from western Atlantic coast. The eastern Atlantic (including Eurasia and Africa) was the third largest native region for all three coasts of North America, being greatest for the East coast (19% of species) and lower for the Gulf and West coasts (8% and 4%, respectively). The native region for the residual species was classified as other, including other regions and those of unknown origin.
While the prominence of Asia and western Atlantic reflect the history of shipping and oyster transfers (as discussed above), the contribution of Asia across all regions is somewhat surprising when considering the historical and current trade patterns. For the East coast, this results from the combination of direct introductions as well as secondary introductions via Europe. The latter is illustrated by the recent introduction of the Chinese mitten crab Eriocheir sinensis to the East coast, where genetic data suggest Europe as the likely source population (Ruiz unpubl. data).
3 Impacts of Non-native Crustaceans
For all of North America, an impact was reported to occur in marine and estuarine waters for 30 of the 108 crustacean species, representing 28% of all species (Appendix 1). Here, we recorded an impact if there was a significant change attributed to the non-native species, causing either (a) a detectable change in composition or population size(s) of resident species or (b) economic impact, including effects on fisheries resources, agricultural products, infrastructure (e.g., docks, piers, dams, water supply), power plants, shipping, and recreation. As a first analysis, we considered impact as a binary condition, which was either reported or not reported, regardless of information type and impact magnitude (addressed separately below) or whether explicitly tested.
Considering only marine and estuarine waters of North America, some type of impact was reported most frequently for introduced barnacles (75% of species), copepods (57%), and decapods (33%) (Fig. 5; see also Appendix 1). Impacts were reported for 17–19% of the amphipod, isopod, and mysid species. No impacts were reported for cladocerans, ostracods, or other species of crustaceans.
Percent of non-native species in each crustacean group that are reported to have impacts in tidal waters of North America. See Fig. 1 for total number of species in each group
When expanding our analysis to include the same non-native species in non-tidal freshwater (i.e., lakes and rivers), another six species have been reported to have impacts, including five in North America and one on another continent (Appendix 1). These included three species of decapods, two species of amphipods, and one species of cladoceran. As such impacts were not reported for marine and estuarine waters, we have excluded them from further analyses.
3.1 Impact Type
Figure 6 shows the frequency of impact types reported for tidal waters of North America for all 108 non-native crustacean species. Slightly over 10% of species were reported to have competition or economic impacts. Approximately 5% of species were reported to have effects as a result of predation (including herbivory), providing a food/prey resource, or altering habitat. Effects on host populations by parasitism was reported for 3% of species, and effects on threatened or endangered (T & E) species were reported for 3% of species. These categories were not mutually exclusive, in that more than one impact type was reported for 13 of the 30 species with reported impacts (Appendix 1). Although we also considered changes in ecosystem processes (e.g., nutrient cycling) as a possible impact category, none of the crustaceans were reported to have a significant impact of this type.
3.2 Information Type and Effect Magnitude
Most of the impacts reported were based upon qualitative observation, correlated changes associated with the arrival or abundance of a non-native species, or changes inferred from understanding the ecology of a species (Appendix 2). For seven (23%) of the 30 species with reported impacts, impact assessments were based on laboratory or field experiments. The latter included three decapods (Carcinus maenas, Hemigrapsus sanguineus, Petrolisthes armatus) and a single species each of amphipod, isopod, barnacle, and copepod (Microdeutopus gryllotalpa, Sphaeroma quoianum, Loxothylacus panopaei, Tortanus dextrilobatus, respectively).
We estimated the effect size or magnitude for each of the species with reported impacts, and approximately half (14) of the 30 species were considered to cause change(s) in excess of 50% in other resident populations or to have economic effects. This was a coarse-level assessment, in which we estimated the magnitude of changes by impact type that were reported in the literature. Our goal was to gain some indication of the perceived effect size by classifying observed changes into one of five categories of increasing effect size (see Appendix 2; this is an initial application of methods being developed by Dahlstrom). Thus, this is not a precise measure but instead a rough index of reported effect size, on some spatial and temporal scale (see Conclusions).
For some species, the actual effect size was not explicitly stated. In some of these cases, we generated an estimate based on information provided. In other cases, there was simply too little information to obtain a reasonable estimate, and these were classified as “undescribed or unassessed”, occurring for 10 of the 30 species. This does not mean that the species is considered to have an insignificant impact, only that that effect magnitude was elusive. This situation is perhaps best illustrated by barnacles, many of which are known to have significant economic impact as biofouling organisms, affecting vessels (e.g., increased fuel consumption, hull maintenance) and water intake systems in North America and elsewhere. While the local effect of barnacles on an individual vessel or power plant can be severe, the impacts are often caused by a broader community of species that include barnacles, and effects are occurring on a global scale. Our ability to examine such partial contributions or cumulative effects was limited in the current classification scheme.
3.3 Certainty
We used a categorical index to assess the strength of inference about the magnitude of reported impacts. Our approach relied on information type (above), and certainty or confidence in reported estimates of effect magnitude was considered low in the absence of quantitative data or when the ability to partition effects among multiple (confounding) factors was limited. We assigned a certainty score from 1 to 3 (low to high) for each reported effect magnitude x impact type, for the 30 species in which an impact was reported (Appendix 3).
At the present time, the level of certainty or confidence about the effect magnitude of non-native crustaceans is very limited. We classified 8 (27%) of the 30 species as having a high level of certainty about the reported effect magnitude for at least one of the impact types. This included primarily species for which there were quantitative experimental data that evaluated ecological effects (three decapods, one copepod, one amphipod) and those which caused conspicuous erosion or destruction of docks (three isopods). All other species were classified as having low certainty about the reported effect magnitude for all impact types.
This analysis does not imply that these effect magnitudes are incorrect or did not occur, but only that the information available is too limited to have confidence in these reports. Assigning impacts to an individual invading species is especially difficult when environmental conditions, species assemblages, and foodwebs are all undergoing changes driven by multiple causes. Observed changes that are coincident or correlated with invasion dynamics are clearly useful, but the ability to attribute cause of a single factor is confounded by the many other changes that are occurring (Ruiz et al. 1999).
This complexity is illustrated in the fresh and brackish Delta regions of the San Francisco Bay estuary, where five non-native species of planktonic copepods (Acartiella sinensis, Pseudodiaptomus forbesi, P. marinus, Tortanus dextrilobatus, and Limnoithona tetraspina) and two non-native mysids (Hyperacanthomysis longirostris = Acanthomysis bowmani; Orientomysis aspera = A. aspera) appeared and became abundant or dominant over the course of approximately 7 years, from 1987 to 1993 (Orsi and Walter 1991; Cohen and Carlton 1995; Modlin and Orsi 1997; Orsi and Ohtsuka 1999). The calanoid copepod Eurytemora affinis, probably an early introduction to the estuary (Lee 2000; Orsi 2001), had been a dominant mesozooplankter (Ambler et al. 1985) and an important component of the diet of larval and planktivorous fishes in the Delta (Nobriga 2002; Nobriga and Feyrer 2008; Bryant and Arnold 2007). Within 2 years of its first detection, P. forbesi had become the most abundant calanoid in fresh and oligohaline regions of the Delta, while E. affinis had declined. Similarly, the native mysid Neomysis mercedis, another major prey item for fishes, had declined and was largely replaced by the two introduced mysids, H. longirostris and O. aspera, first collected in 1993 (Modlin and Orsi 1997).
The changes in species composition in the plankton coincided with declines in recruitment of two important fish species, the introduced Striped Bass, Morone saxatilis, a major game fish in the Bay, and the native, endangered Delta Smelt, Hypomesus transpacificus. Larvae of striped bass fed selectively on the copepod E. affinis in experiments, apparently because of superior escape abilities of P. forbesi (Meng and Orsi 1991). Eurytemora affinis was also considered to be a higher-quality prey, over P. forbesi, for all stages of the smelt (Moyle et al. 1992; Nobriga 2002). However, since P. forbesi has become dominant, it is now the primary copepod in the diet of both fish species (Hobbs et al. 2006; Bryant and Arnold 2007). The replacement of the mysid N. mercedis by the two exotic mysids is also considered to be adverse for juvenile striped bass, since the introduced mysids are smaller, and so require more search effort (Nobriga 2002; Feyrer et al. 2003).
At first glance, these drastic changes in species dominance and fish recruitment might be attributed to superior competitive abilities of the planktonic invaders. However, they have followed a drastic change in the estuary’s foodweb, caused by another invader, the Asian brackish-water clam Corbula amurensis, which appeared in the Bay in 1986, and quickly developed huge biomasses, whose suspension-feeding drastically decreased phytoplankton biomass and shifted energy and nutrients from the plankton to the benthos (Alpine and Cloern 1992; Nichols et al. 1990). The decrease in phytoplankton and zooplankton biomass, and decreased planktivorous fish recruitment, has persisted to the present, and has been termed ‘pelagic organism decline’ (Sommer et al. 2007; Baxter et al. 2008).
The replacement of E. affinis by P. forbesi appears to be partly due to the superior ability of its nauplii to escape the feeding currents of the Asian Clam (Kimmerer et al. 1994). The role of competition in the species replacement is not clear. However, P. forbesi did rapidly replace a previous invader, P. inopinus, in the Columbia River estuary (Sytsma et al. 2004; Cordell et al. 2008). Similarly, the mechanism of species replacement of the native mysid N. mercedis by the two exotic mysids has not been studied. Competition has been suggested, but the invading mysids may be better adapted to the altered foodweb, or more tolerant to other environmental changes in this highly disturbed estuary. The invading species maintain a much smaller biomass than N. mercedis, probably reflecting the overall plankton biomass decline (Feyrer et al. 2003).
Thus, ecological impacts of the introduced copepods and mysids in the San Francisco estuary are reported, and may have a large effect magnitude, but certainty remains low. The changes in species composition and fish recruitment associated with the invasions occurred not just in the context of the Corbula invasion, but also in an environment affected by many anthropogenic disturbances, including pollution, water diversions, fishing pressure, and natural drought/flood cycles, resulting in drastic changes in river flow, salinity, suspended sediment, and water quality (Hobbs et al. 2006; Sommer et al. 2007).
4 Conclusions
In North America, crustaceans constitute a dominant component of the documented non-native marine diversity, whether considered at the continental scale or on individual coasts. While we have summarized the relative importance (percent contribution) of crustaceans to the recorded non-native diversity, as well as the contributions of different crustacean groups to these spatial scales, we advise some caution in interpreting these results. Certainly many invasions have gone undetected, creating the potential for strong biases in the available record, and understanding the scale and effects of such biases remain a significant challenge for invasion biology (Carlton 1996; Ruiz et al. 2000; Hewitt et al. 2004). Nonetheless, crustaceans provide a significant amount of the available knowledge about invasions in coastal marine systems.
Our analysis indicated that crustaceans not only contribute to the overall spatial patterns observed but also provide an important barometer for invasion dynamics. The relative difference in total non-native species richness among coasts was also reflected in the crustaceans, contributing 31–42% of the total on each coast (Fig. 2). The invasion record for crustaceans indicates the operation and importance of different transfer mechanisms in space and time. This record appears to have been particularly sensitive for detection of ballast-mediated introductions on the West coast, signaling the increased trade with Asia and delivery of low-salinity ballast water over the past few decades. The observed pattern for ballast introductions raises interesting hypotheses about the importance of source(s) and why a similar spike in ballast water introductions is not evident along the East and Gulf coasts, especially for copepods and mysids. In short, the crustaceans provide a good model for testing ideas about coastal invasions.
This study found that significant impacts were reported within tidal marine and estuarine waters of North America for at least 28% of the 108 non-native crustacean species documented here, spanning a range of impact types. Importantly, this analysis does not imply that the other species have no impact or even that effects of species are restricted to those observed. We have merely attempted to characterize effects that were observed at some spatial and temporal scale.
While impacts from crustacean invasions occur in North America, and some are well documented (e.g., effects of predation, biofouling, boring, and erosion), it is particularly challenging to understand the magnitude and spatial scale of effects. For most cases (72% of species with reported impacts), there was a low level of certainty about the magnitude of effects reported at some scale, due largely to (a) the lack of quantitative data and (b) the potential confounding effects of many other factors associated with observed changes.
Use of quantitative experiments can help address the issue of certainty in many cases, but the spatial and temporal extent of impacts still remains difficult to estimate in the field. Most experiments or studies are conducted over relatively small spatial and temporal scales. However, the application of these results to broader scales is poorly understood, because effects are expected to vary in both space and time, due to changes in population characteristics, local conditions, and scale-dependent processes (Diamond 1986; Parker et al. 1999; Ruiz et al. 1999).
The issues of variation and scale are not new or unique to the analysis of invasion impacts. While various approaches can be considered to integrate or represent effects across a species range (e.g., Parker et al. 1999), each requires an adequate foundation of measures that appears to be limited at the present time, even for those species where impacts are known. This is illustrated by the European crab Carcinus maenas, one of the best studied invaders with well-documented impacts in multiple geographic regions. For example, it is evident that the crab has caused significant population and community effects as an introduced predator in eastern North America, western North America, and Australia in a wide range of habitats (Glude 1955; Grosholz et al. 2000; Walton et al. 2002; Ross et al. 2004; Trussell et al. 2004; Griffen and Byers 2009; Kimbro et al. 2009). There are unusually good quantitative estimates in many of these cases, but most are limited in spatial scale and do not assess geographic variation. Yet, such measures are needed to evaluate the full scope of identified effects across a geographic range.
Overall, increasing quantitative measures for non-native species impacts is a high priority for invasion ecology. While invaders are a conspicuous and growing component of marine and estuarine communities (Cohen and Carlton 1998; Ruiz et al. 2000), there are many critical gaps in our understanding of ecological and economic effects for most species. In advancing research to address these gaps, it would be particularly useful to implement approaches that address the issues of spatial and temporal variation. Selecting a few species for standardized measures and experiments across multiple locations would be most valuable and an obvious next step in this direction. Crustaceans offer an excellent model for this purpose, due to (a) clear impacts that have been documented for some species, (b) the widespread nature of many non-native species (both within North America and globally), and (c) the extensive background knowledge about the biology and ecology of many groups (e.g., crabs and barnacles). In addition, past studies demonstrate that many species are conducive to experimental manipulations that quantify the magnitude of effects and can be replicated in space and time.
References
Alvarez F, Hines AH, Reaka-Kudla ML (1995) Effects of parasitism by the barnacle Loxothylacus panopaei (Gissler) (Cirripedia: Rhizocephala) on growth and survival of the host crab Rhithropanopeus harrisii (Gould) (Brachyura: Xanthidae). J Exp Mar Biol Ecol 192:221–232
Alpine AE, Cloern JE (1992) Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnol Oceanogr 37:946–955
Ambler JW, Cloern JE, Hutchinson A (1985) Seasonal cycles of zooplankton from San Francisco Bay. Hydrobiologia 129:177–197
Barnard JL (1950) The occurrence of Chelura terebrans Philippi in Los Angeles and San Francisco Harbors. Bull Soc Calif Acad Sci 49:90–97
Barnard JL (1955) The wood boring habits of Chelura terebrans Philippi in Los Angeles harbor. In: Essays in the natural sciences in honor of Captain Allan Hancock. University of Southern California Press, Los Angeles
Barnard JL (1958) Amphipod crustaceans as fouling organisms in Los Angeles-Long Beach Harbors, with reference to the influence of seawater turbidity. Calif Fish Game 44:161–170
Baxter R, Brown L, Breuer R, Chotkowski M, Feyrer F, Gingras M, Herbold B, Mueller-Solger A, Nobriga M, Sommer T, Souza K (2008) Pelagic organism decline progress report: 2007 synthesis of results. Inter-Agency Ecological Program, Sacramento
Bollens SM, Cordell JR, Avent SHR (2002) Zooplankton invasions: a brief review, plus two case studies from the northeast Pacific Ocean. Hydrobiologia 480:87–110
Boudreaux ML, Walters LJ, Rittschof D (2009) Interactions between native barnacles, non-native barnacles, and the Eastern Oyster Crassostrea virginica. Bull Mar Sci 84:43–57
Bouley P, Kimmerer WJ (2006) Ecology of a highly abundant, introduced cyclopoid copepod in a temperate estuary. Mar Ecol Prog Ser 324:219–228
Bousfield EL, Hoover PM (1997) The amphipod superfamily Corophioidea on the Pacific coast of North America. Part V. Family Corophiidae: Corophiinae, new subfamily. Systematics and distributional ecology. Amphipacifica 2:67–139
Bradford JM (1976) Partial revision of the Acartia subgenus Acartiura (Copepoda:Calanoida: Acartiidae). NZ J Mar Freshw Res 10:159–202
Bros WE (1987) Effects of removing or adding structure (barnacle shells) on recruitment to a fouling community in Tampa Bay, Florida. J Exp Mar Bio Ecol 105:275–295
Bryant ME, Arnold JD (2007) Diet of age-0 striped bass in the San Francisco Estuary, 1973-2002. Calif Fish Game 93:1–22
Carlton JT (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific Coast of North America. Ecology. University of California, Davis
Carlton JT (1996) Marine bioinvasions: the alteration of marine ecosystems by nonindigenous species. Oceanography 9:36–43
Carlton JT (1999) Molluscan invasions in marine and estuarine communities. Malacologia 41:439–454
Carlton JT (2003) Community assembly and historical biogeography in the North Atlantic Ocean: the potential role of human-mediated dispersal vectors. Hydrobiologia 503:1–8
Chapman JW (1988) Invasions of the Northeast Pacific by Asian and Atlantic gammaridean amphipod crustaceans, including a new species of Corophium. J Crust Biol 8:364–382
Chapman JW (2000) Climate effects on the geography of nonindigenous peracarid crustaceans in estuaries. In: Pederson J (ed) Marine biological invasions. MIT Sea Grant, Cambridge
Chapman JW (2007) Gammaridea. In: Carlton JT (ed) The Light and Smith manual. Intertidal invertebrates from Central California to Oregon. University of California Press, Berkeley
Chinese Mitten Crab Working Group (2003) National management plan for the genus Eriocheir (mitten crabs). Aquatic Nuisance Species Task Force, Washington
Cohen AN, Carlton JT (1995) Nonindigenous aquatic species in a United States estuary: a case study of the biological invasions of the San Francisco Bay and Delta. US Fish and Wildlife Service and National Sea Grant College Program (Connecticut Sea Grant), Washington
Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science NY 279:555–558
Conlan KE (1990) Revision of the crustacean amphipod genus Jassa (Corophioidea: Ischyroceridae). Can J Zool 68:2031–2075
Conover DO, Reid GK (1975) Distribution of the boring isopod Sphaeroma terebrans in Florida. Fla Sci 38:65–72
Cordell J, Bollens SM, Draheim R, Sytsma M (2008) Asian copepods on the move: recent invasions in the Columbia-Snake River system, USA. ICES J Mar Sci 65:753–758
Coughlan J (1977) Marine borers in Southampton Water. Proc Hampshire Field Club Archaeol Soc 33:5–15
Davidson TM (2006) The invasion of the Australasian burrowing isopod (Sphaeroma quoianum) in Coos Bay, Oregon. Biology. University of Oregon, Eugene
Diamond J (1986) Overview: laboratory experiments, field experiments, and natural experiments. In: Diamond J, Case TJ (eds) Community ecology. Harper and Row, New York
Dürr S, Wahl M (2004) Isolated and combined impacts of the blue mussels Mytilus edulis and barnacles, Balanus improvisus, on structure and diversity of a fouling community. J Exp Mar Bio Ecol 306:181–195
Eltringham SK, Hockley AR (1967) Migration and reproduction of the wood-boring isopod, Limnoria, in Southampton Water. Limnol Oceanogr 6:467–282
Estevez ED (1994) Inhabitation of tidal salt marshes by the estuarine wood-boring isopod Sphaeroma terebrans in Florida. In: Thompson MF, Nagabhushanam R, Sarojini R, Fingerman M (eds) Recent developments in biofouling control. Oxford & IBH, New Delhi
Feyrer F, Herbold B, Matern SA, Moyle P (2003) Dietary shifts in a stressed fish assemblage: consequences of a bivalve invasion in the San Francisco Estuary. Environ Biol Fish 67:277–288
Fleminger A, Kramer SH (1988) Recent introductions of an Asian estuarine copepod, Pseudodiaptomus marinus (Copepoda: Calanoida), in southern California embayments. Mar Biol 98:535–541
Floyd T, Williams J (2004) Impact of green crab (Carcinus maenas L.) predation on a population of soft-shell clams (Mya arenaria L.) in the Southern Gulf of St. Lawrence. J Shellfish Res 23:457–462
Frost BW (1989) A taxonomy of the marine calanoid copepod genus Pseudocalanus. Can J Zool 67:525–551
Galil BS (2008) Alien species in the Mediterranean Sea- which, when, where, why? Hydrobiologia 606:105–116
Glude JB (1955) The effects of temperature and predators on the abundance of the soft-shell clam, Mya arenaria, in New England. Trans Am Fish Soc 84:13–26
Griffen BD, Byers JE (2006) Intraguild predation reduces redundancy of predator species in multiple-predator assemblage. J Anim Ecol 75:959–966
Griffen BD, Byers JE (2009) Community impacts of two invasive crabs: the interactive roles of density, prey recruitment, and indirect effects. Biol Invas 11:927–940
Griffen BD, Delaney DG (2007) Species invasion shifts the importance of predator dependence. Ecology 88:3012–3021
Griffen BD, Guy T, Buck JC (2008) Inhibition between invasives: a newly introduced predator moderates the impacts of a previously established invasive predator. J Anim Ecol 77:32–40
Grimaldo LF, Stewart AR, Kimmerer W (2009) Dietary segregation of pelagic and littoral fish assemblages in a highly modified tidal freshwater estuary. Mar cst Fish 1:200–217
Grosholz ED, Ruiz GM, Dean CA, Shirley KA, Maron JL, Connors PG (2000) The impacts of a nonindigenous marine predator in a California Bay. Ecology 81:1206–1224
Grosholz ED, Lovell S, Besedin E, Katz M (in press) Modeling the impacts of the European Green Crab on commercial shellfisheries. Ecol Applications
Haley AJ, Winn HE (1959) Observations on a lernaean parasite of freshwater fishes. Trans Am Fish Soc 88:128–129
Hauxwell J, McClelland J, Behr PJ, Valiela I (1998) Relative importance of grazing and nutrient controls of macroalgal biomass in three temperate shallow estuaries. Estuaries 21:347–360
Hewitt CL, Campbell ML, Thresher RE, Martin RB, Boyd S, Cohen BF, Currie DR, Gamon MF, Keough MJ, Lewis JA, Lockett MM, Mays N, McArthur MA, O’Hara TD, Poore GCB, Ross DJ, Storey MJ, Watson JE, Wilson R (2004) Introduced and cryptogenic and species in Port Phillip Bay, Victoria, Australia. Mar Biol 144: 183–202
Hidu H, Lavoie RE (1991) The European oyster, Ostrea edulis, in Maine and Eastern Canada. In: Menzel W (ed) Estuarine and marine bivalve mollusk culture. CRC Press, Boca Raton
Hieb K (2006) Common shrimp of the San Francisco Estuary. InterAgency Ecol Program Newslett 20:14–18
Hines AH, Alvarez F, Reed SA (1997) Introduced and native populations of a marine parasitic castrator: variation in prevalence of the rhizocephalan Loxothylacus panopaei in xanthid crabs. Bull Mar Sci 61:197–214
Hobbs JA, Bennett WA, Burton JE (2006) Assessing nursery habitat quality for native smelts (Osmeridae) in the low-salinity zone of the San Francisco Estuary. J Fish Biol 69:907–922
Hoffman GL (1967) Parasites of North American freshwater fishes. University of California Press, Berkeley
Hollebone AL, Hay ME (2008) An invasive crab alters interaction webs in a marine community. Biol Invas 10:347–358
Hooff RC, Bollens SM (2004) Functional response and potential predatory impact of Tortanus dextrilobatus, a carnivorous copepod recently introduced to the San Francisco Estuary. Mar Ecol Prog Ser 277:167–179
Jensen GC, McDonald PS, Armstrong DA (2002) East meets west: competitive interactions between green crab Carcinus maenas, and native and introduced shore crab Hemigrapsus spp. Mar Ecol Prog Ser 225:151–262
Johnson JL, Havel JE (2001) Competition between native and exotic Daphnia: in situ experiments. J Plankton Res 23:373–387
Katkansky SC, Sparks AK, Chew KK (1967) Distribution and effects of the endoparasitic copepod, Mytilicola orientalis on the Pacific oyster, Crassostrea gigas, on the Pacific coast. Proc natl Shellfish Ass 57:50–58
Khalifa A, Post G (1976) Histopathological effect of Lernaea cyprinacea (a copepod parasite) on fish. Prog Fish Cult 38:110–113
Kimbro DL, Grosholz ED, Baukus AJ, Nesbitt NJ, Travis NM, Attoe S, Coleman-Hulbert C (2009) Invasive species cause large-scale loss of native California oyster habitat by disrupting trophic cascades. Oecologia 160:563–575
Kimmerer W, Gartside E, Orsi JJ (1994) Predation by an introduced clam as the likely cause of substantial declines in zooplankton of San Francisco Bay. Mar Ecol Prog Ser 113:81–93
Kolar CS, Wahl DH (1998) Daphnid morphology deters fish predators. Oecologia 116:556–564
Kotta J, Kotta I, Simm M, Lankov A, Lauringson V, Pöllumäe A, Ojaveer H (2007) Ecological consequences of biological invasions: three invertebrate case studies in the north-eastern Baltic Sea. Helgoland Mar Res 60:106–112
Kruse I, Hare MP (2007) Genetic diversity and expanding nonindigenous range of the rhizocephalan Loxothylacus panopaei parasitizing mud crabs in the western North Atlantic. J Parasitol 93:575–582
Kuhne H, Becker G (1964) Der holz-flohkrebs Chelura terebrans Philippi (Amphipoda, Cheluridae). Beih Z Angew Zool 1:1–141
Lee CE (2000) Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate populations. Evolution 54:2014–2027
Lincoln RJ (1979) British marine amphipoda: gammaridea. British Museum (Natural History), London
Lohrer AM, Whitlatch R (2002) Relative impacts of two exotic brachyuran species on blue mussel populations in Long Island Sound. Mar Ecol Prog Ser 227:135–144
Loosanoff VL (1955) The European oyster in American waters. Science NY 121:119–121
Meng L, Orsi JJ (1991) Selective predation by larval striped bass on native and introduced copepods. Trans Am Fish Soc 120:187–192
Miller AW, Minton RGM, MS ARF (2007) Differentiating successful and failed molluscan invaders in estuarine ecosystems. Mar Ecol Prog Ser 332:41–51
Modlin RF (2007) Mysidacea. In: Carlton JT (ed) The Light and Smith manual. Intertidal invertebrates from Central California to Oregon. University of California Press, Berkeley
Modlin RF, Orsi JJ (1997) Acanthomysis bowmani, a new species, and A. aspera li, Mysidacea newly reported from the Sacramento-San Joaquin Estuary, California (Crustacea: Mysidae). Proc Biol Soc Wash 110:439–446
Moore HB, Albertson HD, Miller SM (1974) Long-term changes in the settlement of barnacles in the Miami area. Bull Mar Sci 24:86–100
Moore HB, Frue AC (1959) The settlement and growth of Balanus improvisus, B. eburneus, and B. amphitrite in the Miami area. Bull Mar Sci Gulf Caribb 9:421–440
Moyle PB, Herbold B, Stevens DE, Miller LW (1992) Life history and status of the delta smelt in the Sacramento-San Joaquin Estuary, California. Trans Am Fish Soc 121:67–77
NBIC (2009) National Ballast Information Clearinghouse. Available at http://invasions.si.edu/nbic/search.html
NEMESIS (2009) National exotic marine and estuarine species information system. Available at http://invasions.si.edu/nemesis/
Nichols FH, Thompson JK (1985) Persistence of an introduced mudflat community in South San Francisco Bay, California. Mar Ecol Prog Ser 24:83–97
Nichols FH, Thompson JK, Schemel L (1990) Remarkable invasion of San Francisco Bay by the Asian clam Potamocorbula amurensis. II. Displacement of a former community. Mar Ecol Prog Ser 66:95–101
Nobriga ML (2002) Larval delta smelt diet composition and feeding incidence: environmental and ontogenetic influences. Calif Fish Game 88:149–164
Nobriga ML, Feyrer F (2008) Diet composition in San Francisco Estuary striped bass: does trophic adaptability have its limits? Environ Biol Fish 83:495–503
Odlaug TO (1946) The effect of the copepod, Mytilicola orientalis upon the Olympia Oyster, Ostrea lurida. T Am Microsc Soc 65:311–317
Onbe T (1966) Observations on the tubicolous amphipod, Corophium acherusicum, in Fukuyama harbor area. J Fac Fish Anim Husbandry Hiroshima Univ 6:323–338
Orensanz JM, Schwindt E, Pastorino G, Bortolus A, Casas G, Darrigran G, Elías R, López Gappa J, Obenat S, Pascual M, Penchaszadeh P, Piriz M, Scarabino F, Spivak E, Vallarino E (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biol Invas 4:115–143
Orsi JJ (2001) Eurytemora affinis is introduced. InterAgency Ecol Program Newslett 14:12
Orsi JJ, Ohtsuka S (1999) Introduction of the Asian copepods Acartiella sinensis, Tortanus dextrilobatus (Copepoda:Calanoida), and Limnoithona tetraspina (Copepoda: Cyclopoida) to the San Francisco Estuary, California, USA. Plankton Bio Ecol 46:128–131
Orsi JJ, Walter TC (1991) Pseudodiaptomus forbesi and P. marinus (Copepoda: Calanoida), the latest copepod immigrants to California’s Sacramento-San Joaquin Estuary. Bull Plankton Soc Jap 20:553–562
Page HM, Dugan JE, Schroeder DM, Nishimoto MM, Love MS, Hoesterey JC (2007) Trophic links and condition of a temperate reef fish: comparisons among offshore oil platform and natural reef habitats. Mar Ecol Prog Ser 344:245–256
Panning A (1939) The Chinese mitten crab. Rep Smithson Instn [1938] 3508:361–375
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invas 1:3–19
Quayle DB (1992) Marine wood borers in British Columbia. Can Spec Publ Fish Aquat Sci 115:1–55
Rehm A, Humm HJ (1973) Sphaeroma terebrans: a threat to the mangroves of southwestern Florida. Science NY 182:173–174
Reise K, Gollasch S, Wolff WJ (1999) Introduced marine species of the North Sea coasts. Helgol Meeres 52:219–234
Ribi G (1982) Differential colonization of roots of Rhizophora mangle by the wood boring isopod Sphaeroma terebrans as a mechanism to increase root density. Mar Ecol 3:13–19
Roche DG, Torchin ME (2007) Established population of the North American Harris mud crab, Rhithropanopeus harrisii (Gould 1841) (Crustacea: Brachyura: Xanthidae) in the Panama Canal. Aquat Invas 2:155–161
Ross DJ, Johnson CR, Hewitt CL, Ruiz GM (2004) Interaction and impacts of two introduced species on a soft-sediment marine assemblage in SE Tasmania. Mar Biol 144:747–756
Rudnick D, Hieb K, Grimmer KF, Resh VH (2003) Patterns and process of biological invasion: the Chinese mitten crab in San Francisco Bay. Basic Appl Ecol 4:249–262
Rudnick DA, Chan V, Resh V (2005) Morphology and impacts of the burrows of the Chinese mitten crab Eriocheir sinensis H. Milne Edwards (Decapoda, Grapsoidea), in south San Francisco Bay, California, USA. Crustaceana 78:787–807
Ruiz GM, Fofonoff P, Hines AH, Grosholz ED (1999) Non-indigenous species as stressors in estuarine and marine communities: assessing invasion impacts and interactions. Limnol Oceanogr 44:950–972
Ruiz GM, Fofonoff P, Wonham MJ, Hines AH, Carlton JT (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Ann Rev Ecol Syst 31:481–531
Schwartz FJ, Rubelmann R, Allison J (1963) Ecological population expansion of the introduced crayfish Orconectes virilis. Ohio J Sci 63:266–273
Seeley RH (1986) Intense natural selection caused a rapid morphological transition in a living marine snail. Proc Natl Acad Sci USA 83:6897–6901
Shucksmith R, Cook EJ, Hughes DJ, Burrows MT (2009) Competition between the non-native amphipod Caprella mutica and two native species of caprellids Pseudoprotella phasma and Caprella linearis. J Mar Biol Ass UK 89:1125–1132
Simberloff D, Brown BJ, Lowrie S (1978) Isopod and insect borers may benefit Florida mangroves. Science NY 201:630–632
Sommer T, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, Culberson S, Feyrer S, Gingras M, Herbold B, Kimmerer W, Mueller-Solger A, Nobriga M, Souza K (2007) The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries 32:270–277
Steele S, Mulcahy MF (2006) Impact of the copepod Mytilicola orientalis on the Pacific oyster Crassostrea gigas in Ireland. Dis Aquat Organ 47:145–149
Sutherland JP, Karlson RH (1977) Development and stability of the fouling community at Beaufort, North Carolina. Ecol Monogr 47:425–446
Swaffar SM, O’Brien WJ (1996) Spines of Daphnia lumholtzi create feeding difficulties for juvenile bluegill sunfish (Lepomis macrochirus). J Plankton Res 18:1055–1061
Sytsma M, Cordell JR, Chapman JW, Waldeck RD (2004) Lower Columbia River aquatic nonindigenous species survey 2001–2004: final technical report. Center for lakes and reservoirs. Portland State University, Portland
Talley TS, Crooks JA, Levin LA (2001) Habitat utilization and alteration by the invasive burrowing isopod, Sphaeroma quoyanum, in California salt marshes. Mar Biol 138:561–573
Talman S, Bite SJ, Holloway M, McArthur M, Ross DJ, Storey M (1999) Impacts of some introduced marine species found in Port Phillip Bay. In: Hewitt CL, Campbell ML, Thresher RE, Martin RB (eds) Marine biological invasions of Port Phillip Bay, Victoria, center for research on introduced marine pests. CSIRO Marine Research, Hobart
Taugbøl T, Johnsen SI (2006) Invasive alien species fact sheet – Pacifastacus leniusculus. In: Online Database of the North European and Baltic Network on Invasive Alien Species – NOBANIS Available at: www.nobanis.org/files/factsheets/Pacifastacus_leniusculus.pdf. Cited 10 Dec 2009
Tidd WM, Shields RJ (1963) Tissue damage inflicted by Lernaea cyprinacea Linnaeus, a copepod parasitic on tadpoles. J Parasitol 49:693–696
Trussell GC, Ewanchuk PJ, Bertness M, Silliman BR (2004) Trophic cascades in rocky shore tide pools: distinguishing lethal and nonlethal effects. Oecologia 139:427–432
Tyrrell M, Guarino PA, Harris LG (2006) Predatory impacts of two introduced crab species: inferences from microcosms. Northeast nat 13:375–390
Utinomi H (1970) Studies on the cirripedian fauna of Japan.IX., Distributional survey of thoracic cirripeds in the southeastern part of the Japan Sea. Publ Seto Mar Biol Lab 17:339–372
Van Riel MC, Healy EP, van der Velde G, bij de Vaate A (2007) Interference competition among native and invader emphipods. Acta Oecol 31:282–289
Vermeij GJ (1982) Phenotypic evolution in a poorly dispersing snail after arrival of a predator. Nature 299:349–350
Vermeij GJ (1991) When biotas meet: understanding biotic interchange. Science NY 253:1099–1104
Vermeij GJ (1996) An agenda for invasion ecology. Biol Conserv 78:3–9
Villalobos CR (1985) Notas sobre la biologia de Sphaeroma terebrans Bate. 1866 (Sphaeromatidae: Isopoda) en el manglar de Pochote, Provincia de Puntarenas. Costa Rica Brenesia 24:287–296
Visscher JP (1927) Nature and extent of fouling of ship’s bottoms. Bull Bur Fish 43:193–252
Vuorinen I, Laihonen P, Lietzén E (1986) Distribution and abundance of invertebrates causing fouling in power plants on the Finnish coast. Mem Soc Fauna Flora Fennica 62:123–125
Wallour DB (1960) Thirteenth progress report on marine borer activity in test boards operated during 1959. William F. Clapp Laboratories, Duxbury
Walton WC, MacKinnon C, Rodriguez LF, Proctor C, Ruiz GM (2002) Effect of an invasive crab upon a marine fishery: green crab, Carcinus maenas, predation upon a venerid clam, Katelysia scalarina, in Tasmania (Australia). J Exp Mar Bio Ecol 272:171–189
West JM, Williams GD, Madon SP, Zedler JB (2003) Integrating spatial and temporal variability into the analysis of fish food web linkages in Tijuana Estuary. Environ Biol Fish 67:297–309
Whitcraft CR, Levin LA, Talley D, Crooks JA (2008) Utilization of invasive tamarisk by salt marsh consumers. Oecologia 158:259–272
Wigley RL (1963) Occurrence of Praunus flexuosus (O. F. Müller) (Mysidacea) in New England waters. Crustaceana 6:158
Zaitsev Y, Öztürk B (2001) Exotic species in the Aegean, Marmara, Black, Azov and Caspian Seas. Turkish Marine Research Foundation, Istanbul
Zevina GB (1988) Underwater scraping of ship foulings in tropical waters. Sov J Mar Biol 1988:41–46
Zvyagintsev AY (2003) Introduction of species into the northwestern Sea of Japan and the problem of marine fouling. Russ J Mar Biol 29:10–21
Acknowledgements
We thank Bella Galil and Paul Clark for the opportunity to contribute to this volume and also for their encouragement and patience in production. We thank Jim Carlton for critical insights in compiling NEMESIS and exploring these data over many years. We thank Stacey Havard for assistance with the references. Portions of this research were supported by the Maryland Sea Grant Program, National Sea Grant Program, and Smithsonian Institution.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Appendix 1
Classification of distribution and impact type for non-native species of crustaceans considered established in tidal (marine and estuarine) waters of North America. Information included is as follows:
-
(a)
Taxonomic Group and Species is provided for each crustacean classified as non-native and established in North America.
-
(b)
Coast indicates which of the three North American coasts (P)acific, (A)tlantic, or (G)ulf; asterisk (*) indicates native to part of the coast indicated; (Cr)yptogenic indicates cryptogenic to Atlantic and Gulf coasts.
-
(c)
Salinity Range characterizes known salinity distribution of the species as either Marine (M), Brackish Specialist (B), Freshwater (F), or Catadromous (C).
-
(d)
Impact Type indicates the type of impact reported separately for each species in each of three habitat regions, including (1) marine and estuarine waters of North America, (2) freshwater inland lakes and rivers in North America, and (3) other global regions where the species has been introduced (i.e., not including the native region). For each of these habitat regions, the impact type is classified broadly into the following categories: P = effect(s) through predation including herbivory, C = effects through competition, X = effects through parasitism, F = effects as food or prey, H = effects through habitat alteration, T = effects on threatened or endangered species, and E = economic effects on fisheries resources, agricultural products, infrastructure (e.g., docks, piers, dams, water supply), powerplants, shipping, and recreation. Occurrence of each impact type is indicated in individual columns for the marine and estuarine region and combined into one column for the other two habitat regions. An impact was recorded for any of these categories only if it was reported to result in a significant change(s) in a target population, community, or economic resource. The final column (SUM) indicates the number of impact types reported in North America.
For a-c above, data and associated references are summarized in our database (available in NEMESIS 2009). For references on impact type on each species, see Appendix 3. Note that impact references are not an exhaustive list for all species; in cases where multiple sources demonstrated the same type of impact, a subset was selected that included quantitative measures with highest levels for effect magnitude and certainty (see text and Appendices 2 and 3). Additional references are available in NEMESIS (2009) and upon request.
Taxonomic group and species | Coast | Salinity range | Impact type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
North America | Other global regions | Sum | |||||||||||
Marine and estuarine | Fresh | ||||||||||||
P | C | X | F | H | T | E | |||||||
Cladocera | |||||||||||||
Daphnia lumholtzi | A, G, P | F | F,C | 0 | |||||||||
Ilyocryptus agilis | A | F | 0 | ||||||||||
Copepoda-Harpacticoida | |||||||||||||
Harpacticella paradoxa | P | F | 0 | ||||||||||
Copepoda-Calanoidea | |||||||||||||
Acartiella sinensis | P | B | 0 | ||||||||||
Sinocalanus doerrii | P | F | 1 | 1 | 2 | ||||||||
Pseudodiaptomus forbesi | P | B | 1 | 1 | 1 | 3 | |||||||
Pseudodiaptomus inopinus | P | B | 0 | ||||||||||
Pseudodiaptomus marinus | P | M | 1 | 1 | |||||||||
Eurytemora affinis (A subclade) | P | M | 1 | 1 | 1 | 3 | |||||||
Tortanus dextrilobatus | P | B | 1 | 1 | |||||||||
Copepoda-Cyclopoida | |||||||||||||
Lernaea cyprinacea | A, P | F | 1 | X | 1 | ||||||||
Mytilicola orientalis | P | M | 1 | 1 | |||||||||
Pseudomyicola ostreae | P | M | 0 | ||||||||||
Limnoithona sinensis | P | F | 0 | ||||||||||
Limnoithona tetraspina | P | B | 1 | 1 | |||||||||
Oithona davisae | P | M | 0 | ||||||||||
Cirripedia-Rhizocephala | |||||||||||||
Loxothylacus panopaei | A | M | 1 | 1 | |||||||||
Cirripedia-Thoracica | |||||||||||||
Amphibalanus amphitrite | A; G; P | M | 1 | 1 | C,E | 2 | |||||||
Amphibalanus eburneus | P | M | 1 | E | 1 | ||||||||
Amphibalanus improvisus | P | B | 1 | C,H,E | 1 | ||||||||
Amphibalanus reticulatus | A; G | M | 1 | E | 1 | ||||||||
Balanus trigonus | A; G | M | 1 | E | 1 | ||||||||
Chthamalus fragilis | A* | M | 0 | ||||||||||
Megabalanus coccopoma | A | M | 0 | ||||||||||
Ostracoda | |||||||||||||
Eusarsiella zostericola | P | M | 0 | ||||||||||
Aspidoconcha limnoriae | P | M | 0 | ||||||||||
Redekea californica | P | M | 0 | ||||||||||
Spinileberis quadriaculeata | P | M | 0 | ||||||||||
Kotoracythere inconspicua | G | M | 0 | ||||||||||
Leptostraca | |||||||||||||
Epinebalia sp A. | P | M | 0 | ||||||||||
Mysidacea | |||||||||||||
Deltamysis holmquistae | P | B | 0 | ||||||||||
Hyperacanthomysis longirostris | P | B | 1 | 1 | 2 | ||||||||
Neomysis japonica | P | M | 0 | ||||||||||
Orientomysis aspera | P | B | 0 | ||||||||||
Orientomysis hwanhaiensis | P | M | 0 | ||||||||||
Praunus flexuosus | A | M | 0 | ||||||||||
Cumacea | |||||||||||||
Nippoleucon hinumensis | P | M | 0 | ||||||||||
Isopoda | |||||||||||||
Asellus hilgendorfi | P | F | 0 | ||||||||||
Caecidotea racovitzai | P | F | 0 | ||||||||||
Caecijaera horvathi | P | M | 0 | ||||||||||
Dynoides dentisinus | P | M | 0 | ||||||||||
Eurylana arcuata | P | M | 0 | ||||||||||
Gnorimosphaeroma rayi | P | M | 0 | ||||||||||
Iais californica | P | M | 0 | ||||||||||
Iais floridana | A; G | M | 0 | ||||||||||
Ianiropsis sp. | A | M | 0 | ||||||||||
Ligia exotica | A; G | M | 0 | ||||||||||
Ligia oceanica | A | M | 0 | ||||||||||
Limnoria pfefferi | A; G | M | 0 | ||||||||||
Limnoria quadripunctata | P | M | 1 | E | 1 | ||||||||
Limnoria tripunctata | P (Cr) | M | 1 | E | 1 | ||||||||
Paracerceis sculpta | A | M | 0 | ||||||||||
Paradella dianae | A; G | M | 0 | ||||||||||
Paranthura japonica | P | M | 0 | ||||||||||
Pseudosphaeroma campbellensis | P | M | 0 | ||||||||||
Sphaeroma quoianum | P | M | 1 | 1 | 2 | ||||||||
Sphaeroma terebrans | A; G | M; F | 1 | 1 | 2 | ||||||||
Sphaeroma walkeri | A; G; P | M | 0 | ||||||||||
Synidotea laevidorsalis | A; P | M | 0 | ||||||||||
Uromunna sp. A | P | B | 0 | ||||||||||
Tanaidacea | |||||||||||||
Sinelobus cf. stanfordi | P | M | 0 | ||||||||||
Amphipoda-Gammaridea | |||||||||||||
Abludomelita rylovae | P | M | 0 | ||||||||||
Ampelisca abdita | P | M | 1 | 1 | 2 | ||||||||
Ampithoe longimana | P | M | 0 | ||||||||||
Ampithoe valida | P | M | 0 | ||||||||||
Aoroides secunda | P | M | 0 | ||||||||||
Chelura terebrans | P (Cr) | M | 1 | 1 | |||||||||
Crangonyx floridanus | P | F | 0 | ||||||||||
Crangonyx pseudogracilis | P | F | C | 0 | |||||||||
Echinogammarus ischnus | A | F | C | C | 0 | ||||||||
Eochelidium miraculum | P | M | 0 | ||||||||||
Eochelidium sp. A | P | M | 0 | ||||||||||
Gammarus daiberi | P | B | 1 | 1 | |||||||||
Gammarus tigrinus | A | B | 0 | ||||||||||
Gitanopsis sp. | A | M | 0 | ||||||||||
Grandidierella japonica | P | M | 0 | ||||||||||
Incisocalliope derzhavini | P | M | 0 | ||||||||||
Jassa marmorata | P | M | 1 | 1 | |||||||||
Melita nitida | P | M | 0 | ||||||||||
Microdeutopus gryllotalpa | A; P | M | 1 | 1 | |||||||||
Monocorophium acherusicum | P (Cr) | M | 1 | 1 | 1 | C | 3 | ||||||
Monocorophium insidiosum | P (Cr) | M | 0 | ||||||||||
Monocorophium uenoi | P | M | 0 | ||||||||||
Paracorophium lucasi | P | M | 0 | ||||||||||
Paradexamine sp. | P | M | 0 | ||||||||||
Corophium alienense | P | M | 0 | ||||||||||
Corophium heteroceratum | P | M | 0 | ||||||||||
Stenothoe valida | P | M | 0 | ||||||||||
Transorchestia enigmatica | P | M | 0 | ||||||||||
Amphipoda-Caprellidea | |||||||||||||
Caprella drepanochir | P* | M | 0 | ||||||||||
Caprella mutica | A; P | M | C | 0 | |||||||||
Caprella scaura | A; G; P | M | 0 | ||||||||||
Caprella simia | P | M | 0 | ||||||||||
Decapoda-Anomura | |||||||||||||
Petrolisthes armatus | A* | M | 1 | 1 | 1 | 3 | |||||||
Upogebia affinis | A* | M | 0 | ||||||||||
Decapoda-Astacoidea | |||||||||||||
Orconectes virilis | A; P | F | C,E | 0 | |||||||||
Pacifastacus leniusculus | P* | F | C,T,E | 0 | |||||||||
Procambarus clarkii | A;G*;P | F | H,E | 0 | |||||||||
Decapoda-Caridea | |||||||||||||
Exopalaemon modestus | P | F | 1 | 1 | |||||||||
Macrobrachium olfersii | A; G | C | 0 | ||||||||||
Palaemon macrodactylus | P | M | 0 | ||||||||||
Decapoda-Brachyura | |||||||||||||
Carcinus maenas | A; P | M | 1 | 1 | 1 | P,C,E | 3 | ||||||
Charybdis hellerii | A; G | M | 0 | ||||||||||
Eriocheir sinensis | P; A | C | 1 | 1 | 1 | H,E | 3 | ||||||
Eurypanopeus depressus | A* | M | 0 | ||||||||||
Hemigrapsus sanguineus | A | M | 1 | 1 | 2 | ||||||||
Platychirograpsus spectabilis | G | C | 0 | ||||||||||
Rhithropanopeus harrisii | P | B | 0 | ||||||||||
Appendix 2
Classification of information type and effect magnitude for non-native species of crustaceans considered established in tidal (marine and estuarine) waters of North America. Information included is as follows:
-
(a)
Taxonomic Group and Species is provided for each crustacean classified as non-native and established in North America.
-
(b)
Information Type indicates the quality of information source that reported an impact. Information type was classified as one of the following: 1 = qualitative information (including best professional judgment); 2 = presence/absence data that indicate a measureable change associated with the occurrence of the non-native species; 3 = mechanistic or autoecological data that infer impact has occurred; 4 = correlative data that demonstrates a quantitative relationship between the invader (presence or abundance) and an effect; 5 = controlled laboratory experiment(s); 6 = controlled field experiment(s). Information type is shown for the same categories and format as outlined above (see Impact Type); MAX indicates the highest numerical value recorded for Information Type for North America.
(continued on page xx)
-
(c)
Effect Magnitude indicates an estimate of the reported effect size or magnitude. The magnitude was estimated from the original source(s) for each Impact Type and classified as one of the following: 0 = undescribed or unassessed (in cases where a significant impact is reported); 1 = an effect of <10% change; 2 = an effect of 10–50% change; 3 = an effect of 50–75% change; 4 = an effect of >75% change. Information type is shown for the same categories and format as outlined above (see Impact Type); MAX indicates the highest numerical value recorded for Effect Magnitude for North America.
For references associated with impact characteristics of each species, see Appendix 3. Note that impact references are not an exhaustive list for all species; in cases where multiple sources demonstrated the same type of impact, a subset was selected that included quantitative measures with highest levels for effect magnitude and certainty (see text and Appendices 2 and 3). Additional references are available in NEMESIS (2009) and upon request.
Taxonomic group & species | Information type | Effect magnitude | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
North America | Other global regions | Max | North America | Other global regions | Max | ||||||||||||||||
Marine and estuarine | Fresh | Marine and estuarine | Fresh | ||||||||||||||||||
P | C | X | F | H | T | E | P | C | X | F | H | T | E | ||||||||
Cladocera | |||||||||||||||||||||
Daphnia lumholtzi | 4,5 | 0 | 0 | ||||||||||||||||||
Ilyocryptus agilis | 0 | 0 | |||||||||||||||||||
Copepoda-Harpacticoida | |||||||||||||||||||||
Harpacticella paradoxa | 0 | 0 | |||||||||||||||||||
Copepoda-Calanoidea | |||||||||||||||||||||
Acartiella sinensis | 0 | 0 | |||||||||||||||||||
Sinocalanus doerrii | 4 | 3,4 | 4 | 4 | 3 | 4 | |||||||||||||||
Pseudodiaptomus forbesi | 4 | 3,4 | 4 | 4 | 4 | 3 | 4 | 4 | |||||||||||||
Pseudodiaptomus inopinus | 0 | 0 | |||||||||||||||||||
Pseudodiaptomus marinus | 2 | 2 | 4 | 4 | |||||||||||||||||
Eurytemora affinis (A subclade) | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | |||||||||||||
Tortanus dextrilobatus | 3,5 | 5 | 3 | 3 | |||||||||||||||||
Copepoda-Cyclopoida | |||||||||||||||||||||
Lernaea cyprinacea | 2 | 2 | 0 | 0 | |||||||||||||||||
Mytilicola orientalis | 2 | 2 | 2 | 2 | |||||||||||||||||
Pseudomyicola ostreae | 0 | 0 | |||||||||||||||||||
Limnoithona sinensis | 0 | 0 | |||||||||||||||||||
Limnoithona tetraspina | 4 | 4 | 4 | 4 | |||||||||||||||||
Oithona davisae | 0 | 0 | |||||||||||||||||||
Cirripedia-Rhizocephala | |||||||||||||||||||||
Loxothylacus panopaei | 3,5 | 5 | 0 | 0 | |||||||||||||||||
Cirripedia-Thoracica | |||||||||||||||||||||
Amphibalanus amphitrite | 4 | 2,3,4 | 2 | 4 | 0 | 0 | 4 | 0 | |||||||||||||
Amphibalanus eburneus | 2,3 | 2,3 | 3 | 0 | 0 | 0 | |||||||||||||||
Amphibalanus improvisus | 2,3 | 2,3,6 | 3 | 0 | 0–3 | 0 | |||||||||||||||
Amphibalanus reticulatus | 2,3 | 2,3 | 3 | 0 | 0 | 0 | |||||||||||||||
Balanus trigonus | 2,3 | 2,3 | 3 | 0 | 0 | 0 | |||||||||||||||
Chthamalus fragilis | 0 | 0 | |||||||||||||||||||
Megabalanus coccopoma | 0 | 0 | |||||||||||||||||||
Ostracoda | |||||||||||||||||||||
Eusarsiella zostericola | 0 | 0 | |||||||||||||||||||
Aspidoconcha limnoriae | 0 | 0 | |||||||||||||||||||
Redekea californica | 0 | 0 | |||||||||||||||||||
Spinileberis quadriaculeata | 0 | 0 | |||||||||||||||||||
Kotoracythere inconspicua | 0 | 0 | |||||||||||||||||||
Leptostraca | |||||||||||||||||||||
Epinebalia sp A. | 0 | 0 | |||||||||||||||||||
Mysidacea | |||||||||||||||||||||
Deltamysis holmquistae | 0 | 0 | |||||||||||||||||||
Hyperacanthomysis longirostris | 4 | 3,4 | 4 | 4 | 4 | 4 | |||||||||||||||
Neomysis japonica | 0 | 0 | |||||||||||||||||||
Orientomysis aspera | 0 | 0 | |||||||||||||||||||
Orientomysis hwanhaiensis | 0 | 0 | |||||||||||||||||||
Praunus flexuosus | 0 | 0 | |||||||||||||||||||
Cumacea | |||||||||||||||||||||
Nippoleucon hinumensis | 0 | 0 | |||||||||||||||||||
Isopoda | |||||||||||||||||||||
Asellus hilgendorfi | 0 | 0 | |||||||||||||||||||
Caecidotea racovitzai | 0 | 0 | |||||||||||||||||||
Caecijaera horvathi | 0 | 0 | |||||||||||||||||||
Dynoides dentisinus | 0 | 0 | |||||||||||||||||||
Eurylana arcuata | 0 | 0 | |||||||||||||||||||
Gnorimosphaeroma rayi | 0 | 0 | |||||||||||||||||||
Iais californica | 0 | 0 | |||||||||||||||||||
Iais floridana | 0 | 0 | |||||||||||||||||||
Ianiropsis sp. | 0 | 0 | |||||||||||||||||||
Ligia exotica | 0 | 0 | |||||||||||||||||||
Ligia oceanica | 0 | 0 | |||||||||||||||||||
Limnoria pfefferi | 0 | 0 | |||||||||||||||||||
Limnoria quadripunctata | 2,3 | 2,3 | 3 | 4 | 4 | 4 | |||||||||||||||
Limnoria tripunctata | 2,3 | 2,3 | 3 | 4 | 4 | 4 | |||||||||||||||
Paracerceis sculpta | 0 | 0 | |||||||||||||||||||
Paradella dianae | 0 | 0 | |||||||||||||||||||
Paranthura japonica | 0 | 0 | |||||||||||||||||||
Pseudosphaeroma campbellensis | 0 | 0 | |||||||||||||||||||
Sphaeroma quoianum | 3,5,6 | 3,5,6 | 6 | 4 | 4 | 4 | |||||||||||||||
Sphaeroma terebrans | 3 | 3 | 3 | 0 | 1 | 1 | |||||||||||||||
Sphaeroma walkeri | 0 | 0 | |||||||||||||||||||
Synidotea laevidorsalis | 0 | 0 | |||||||||||||||||||
Uromunna sp. A | 0 | 0 | |||||||||||||||||||
Tanaidacea | |||||||||||||||||||||
Sinelobus cf. stanfordi | 0 | 0 | |||||||||||||||||||
Amphipoda-Gammaridea | |||||||||||||||||||||
Abludomelita rylovae | 0 | 0 | |||||||||||||||||||
Ampelisca abdita | 4 | 4 | 4 | 2 | 2 | 2 | |||||||||||||||
Ampithoe longimana | 0 | 0 | |||||||||||||||||||
Ampithoe valida | 0 | 0 | |||||||||||||||||||
Aoroides secunda | 0 | 0 | |||||||||||||||||||
Chelura terebrans | 3 | 3 | 1 | 1 | |||||||||||||||||
Crangonyx floridanus | 0 | 0 | |||||||||||||||||||
Crangonyx pseudogracilis | 4 | 0 | 2 | 0 | |||||||||||||||||
Echinogammarus ischnus | 5,6 | 5,6 | 0 | 2 | 2 | 0 | |||||||||||||||
Eochelidium miraculum | 0 | 0 | |||||||||||||||||||
Eochelidium sp. A | 0 | 0 | |||||||||||||||||||
Gammarus daiberi | 1 | 1 | 0 | 0 | |||||||||||||||||
Gammarus tigrinus | 0 | 0 | |||||||||||||||||||
Gitanopsis sp. | 0 | 0 | |||||||||||||||||||
Grandidierella japonica | 0 | 0 | |||||||||||||||||||
Incisocalliope derzhavini | 0 | 0 | |||||||||||||||||||
Jassa marmorata | 1 | 1 | 0 | 0 | |||||||||||||||||
Melita nitida | 0 | 0 | |||||||||||||||||||
Microdeutopus gryllotalpa | 6 | 6 | 3 | 3 | |||||||||||||||||
Monocorophium acherusicum | 4 | 1 | 1 | 1 | 4 | 2 | 0 | 0 | 0 | 2 | |||||||||||
Monocorophium insidiosum | 0 | 0 | |||||||||||||||||||
Monocorophium uenoi | 0 | 0 | |||||||||||||||||||
Paracorophium lucasi | 0 | 0 | |||||||||||||||||||
Paradexamine sp. | 0 | 0 | |||||||||||||||||||
Corophium alienense | 0 | 0 | |||||||||||||||||||
Corophium heteroceratum | 0 | 0 | |||||||||||||||||||
Stenothoe valida | 0 | 0 | |||||||||||||||||||
Transorchestia enigmatica | 0 | 0 | |||||||||||||||||||
Amphipoda-Caprellidea | |||||||||||||||||||||
Caprella drepanochir | 0 | 0 | |||||||||||||||||||
Caprella mutica | 5 | 0 | 4 | 0 | |||||||||||||||||
Caprella scaura | 0 | 0 | |||||||||||||||||||
Caprella simia | 0 | 0 | |||||||||||||||||||
Decapoda-Anomura | |||||||||||||||||||||
Petrolisthes armatus | 6 | 5 | 6 | 6 | 2 | 2 | 2 | 2 | |||||||||||||
Upogebia affinis | 0 | 0 | |||||||||||||||||||
Decapoda-Astacoidea | |||||||||||||||||||||
Orconectes virilis | 4 | 0 | 3 | 0 | |||||||||||||||||
Pacifastacus leniusculus | 1 | 0 | 1 | 0 | |||||||||||||||||
Procambarus clarkii | 1 | 0 | 1 | 0 | |||||||||||||||||
Decapoda-Caridea | |||||||||||||||||||||
Exopalaemon modestus | 4 | 4 | 4 | 4 | |||||||||||||||||
Macrobrachium olfersii | 0 | 0 | |||||||||||||||||||
Palaemon macrodactylus | 0 | 0 | |||||||||||||||||||
Decapoda-Brachyura | |||||||||||||||||||||
Carcinus maenas | 1–6 | 1–6 | 3 | 1–6 | 6 | 3 | 3 | 2 | 1–3 | 3 | |||||||||||
Charybdis hellerii | 0 | 0 | |||||||||||||||||||
Eriocheir sinensis | 1–3 | 1–3 | 1–3 | 1–3 | 3 | 1 | 0 | 4 | 1–4 | 4 | |||||||||||
Eurypanopeus depressus | 0 | 0 | |||||||||||||||||||
Hemigrapsus sanguineus | 1–6 | 1–6 | 6 | 4 | 4 | 4 | |||||||||||||||
Platychirograpsus spectabilis | 0 | 0 | |||||||||||||||||||
Rhithropanopeus harrisii | 0 | 0 | |||||||||||||||||||
Appendix 3
Classification of certainty that observed effects were caused by the respective non-native species of crustaceans considered established in tidal (marine and estuarine) waters of North America. Information included is as follows:
-
(a)
Taxonomic Group and Species is provided for each crustacean classified as non-native and established in North America.
-
(b)
Certainty provides a qualitative and categorical index of the strength of inference about impact magnitude reported. This is derived from the information type, where certainty or confidence in available impact estimates is considered low in the absence of quantitative estimates or the ability to partition effects among multiple (confounding) factors. Values are: 1 = low, 2 = medium, 3 = high. Information type is shown for the same categories and format as outlined above (see Impact Type); MAX indicates the highest numerical value recorded for Certainty for North America.
-
(c)
Impact References shows the source(s) of information used to generate the above information matrix. Note that impact references are not an exhaustive list for all species; in cases where multiple sources demonstrated the same type of impact, a subset was selected that included quantitative measures with highest levels for effect magnitude and certainty (see text and Appendices 2 and 3). Additional references are available in NEMESIS (2009) and upon request.
Taxonomic group & species | Certainty | Impact references (N. American marine-estuarine in bold) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
North America | Other global regions | Max | |||||||||
Marine and estuarine | Fresh | ||||||||||
P | C | X | F | H | T | E | |||||
Cladocera | |||||||||||
Daphnia lumholtzi | 0 | Swaffar and O’Brien 1996; Kolar and Wahl 1998; Johnson and Havel 2001 | |||||||||
Ilyocryptus agilis | 0 | ||||||||||
Copepoda-Harpacticoida | |||||||||||
Harpacticella paradoxa | 0 | ||||||||||
Copepoda-Calanoidea | |||||||||||
Acartiella sinensis | 0 | ||||||||||
Sinocalanus doerrii | 1 | 1 | 1 | Meng and Orsi 1991 | |||||||
Pseudodiaptomus forbesi | 1 | 1 | 1 | 1 | Meng and Orsi 1991 ; Orsi and Walter 1991 ; Baxter et al. 2008 ; Sytsma et al. 2004 ; Cordell et al. 2008 | ||||||
Pseudodiaptomus inopinus | 0 | ||||||||||
Pseudodiaptomus marinus | 1 | 1 | Fleminger and Kramer 1988 | ||||||||
Eurytemora affinis (A subclade) | 1 | 1 | 1 | 1 | Ambler et al. 1985 ; Meng and Orsi 1991 ; Bryant and Arnold 2007 | ||||||
Tortanus dextrilobatus | 3 | 3 | Hooff and Bollens 2004 | ||||||||
Copepoda-Cyclopoida | |||||||||||
Lernaea cyprinacea | 1 | 1 | Haley and Winn 1959; Tidd and Shields 1963, Hoffman 1967; Khalifa and Post 1976 | ||||||||
Mytilicola orientalis | 1 | 1 | 1 | Odlaug 1946 ; Katkansky et al. 1967; Steele and Mulcahy 2006 | |||||||
Pseudomyicola ostreae | 0 | ||||||||||
Limnoithona sinensis | 0 | ||||||||||
Limnoithona tetraspina | 1 | 1 | |||||||||
Oithona davisae | 0 | ||||||||||
Cirripedia-Rhizocephala | |||||||||||
Loxothylacus panopaei | 1 | 1 | Alvarez et al. 1995 ; Hines et al. 1997 ; Kruse and Hare 2007 | ||||||||
Cirripedia-Thoracica | |||||||||||
Amphibalanus amphitrite | 1 | 1 | 1 | 1 | Visscher 1927 ; Moore and Frue 1959 ; Sutherland and Karlson 1977 ; Bros 1987 ; Zvyagintsev 2003; Boudreaux et al. 2009 ; | ||||||
Amphibalanus eburneus | 1 | 1 | 1 | ||||||||
Amphibalanus improvisus | 1 | 1–3 | 1 | Vuorinen et al. 1986; Zvyagintsev 2003; Dürr and Wahl 2004; Kotta et al. 2007 | |||||||
Amphibalanus reticulatus | 1 | 1 | 1 | ||||||||
Balanus trigonus | 1 | 1 | 1 | Zevina 1988 | |||||||
Chthamalus fragilis | 0 | ||||||||||
Megabalanus coccopoma | 0 | ||||||||||
Ostracoda | |||||||||||
Eusarsiella zostericola | 0 | ||||||||||
Aspidoconcha limnoriae | 0 | ||||||||||
Redekea californica | 0 | ||||||||||
Spinileberis quadriaculeata | 0 | ||||||||||
Kotoracythere inconspicua | 0 | ||||||||||
Leptostraca | |||||||||||
Epinebalia sp A. | 0 | ||||||||||
Mysidacea | |||||||||||
Deltamysis holmquistae | 0 | ||||||||||
Hyperacanthomysis longirostris | 1 | 1 | 1 | ||||||||
Neomysis japonica | 0 | ||||||||||
Orientomysis aspera | 0 | Nobriga and Feyrer 2008 | |||||||||
Orientomysis hwanhaiensis | 0 | ||||||||||
Praunus flexuosus | 0 | ||||||||||
Cumacea | |||||||||||
Nippoleucon hinumensis | 0 | ||||||||||
Isopoda | |||||||||||
Asellus hilgendorfi | 0 | ||||||||||
Caecidotea racovitzai | 0 | ||||||||||
Caecijaera horvathi | 0 | ||||||||||
Dynoides dentisinus | 0 | ||||||||||
Eurylana arcuata | 0 | ||||||||||
Gnorimosphaeroma rayi | 0 | ||||||||||
Iais californica | 0 | ||||||||||
Iais floridana | 0 | ||||||||||
Ianiropsis sp. | 0 | ||||||||||
Ligia exotica | 0 | ||||||||||
Ligia oceanica | 0 | ||||||||||
Limnoria pfefferi | 0 | ||||||||||
Limnoria quadripunctata | 3 | 3 | 3 | Eltringham and Hockley 1967; Coughlan 1977; Carlton 1979 ; Cohen and Carlton 1995 | |||||||
Limnoria tripunctata | 3 | 3 | 3 | Eltringham and Hockley 1967; Coughlan 1977; Carlton 1979 ; Quayle 1992 ; Cohen and Carlton 1995 | |||||||
Paracerceis sculpta | 0 | ||||||||||
Paradella dianae | 0 | ||||||||||
Paranthura japonica | 0 | ||||||||||
Pseudosphaeroma campbellensis | 0 | ||||||||||
Sphaeroma quoianum | 3 | 3 | 3 | Talley et al. 2001 ; Higgins (cited by Davidson 2006 ); Davidson 2006 | |||||||
Sphaeroma terebrans | 1 | 1 | 1 | Rehm and Humm 1973 ; Conover and Reid 1975 ; Simberloff et al. 1978 ; Ribi 1982; Villalobos 1985; Estevez 1994 | |||||||
Sphaeroma walkeri | 0 | ||||||||||
Synidotea laevidorsalis | 0 | ||||||||||
Uromunna sp. A | 0 | ||||||||||
Tanaidacea | |||||||||||
Sinelobus cf. stanfordi | 0 | ||||||||||
Amphipoda-Gammaridea | |||||||||||
Abludomelita rylovae | 0 | ||||||||||
Ampelisca abdita | 1 | 1 | 1 | Nichols and Thompson 1985 | |||||||
Ampithoe longimana | 0 | ||||||||||
Ampithoe valida | 0 | ||||||||||
Aoroides secunda | 0 | ||||||||||
Chelura terebrans | 1 | 1 | Barnard 1950 ; Barnard 1955; Wallour 1960 ; Kuhne and Becker 1964 | ||||||||
Crangonyx floridanus | 0 | ||||||||||
Crangonyx pseudogracilis | 1 | 0 | |||||||||
Echinogammarus ischnus | 3 | 0 | |||||||||
Eochelidium miraculum | 0 | ||||||||||
Eochelidium sp. A | 0 | ||||||||||
Gammarus daiberi | 1 | 1 | |||||||||
Gammarus tigrinus | 3 | 0 | Van Riel et al. 2007 | ||||||||
Gitanopsis sp. | 0 | ||||||||||
Grandidierella japonica | 0 | ||||||||||
Incisocalliope derzhavini | 0 | ||||||||||
Jassa marmorata | 1 | 1 | |||||||||
Melita nitida | 0 | ||||||||||
Microdeutopus gryllotalpa | 3 | 3 | Hauxwell et al. 1998 | ||||||||
Monocorophium acherusicum | 1 | 1 | 1 | 1 | 1 | ||||||
Monocorophium insidiosum | 0 | ||||||||||
Monocorophium uenoi | 0 | ||||||||||
Paracorophium lucasi | 0 | ||||||||||
Paradexamine sp. | 0 | ||||||||||
Corophium alienense | 0 | ||||||||||
Corophium heteroceratum | 0 | ||||||||||
Stenothoe valida | 0 | ||||||||||
Transorchestia enigmatica | 0 | ||||||||||
Amphipoda-Caprellidea | |||||||||||
Caprella drepanochir | 0 | ||||||||||
Caprella mutica | 2 | 0 | |||||||||
Caprella scaura | 0 | ||||||||||
Caprella simia | 0 | ||||||||||
Decapoda-Anomura | |||||||||||
Petrolisthes armatus | 3 | 3 | 3 | 3 | Hollebone and Hay 2008 | ||||||
Upogebia affinis | 0 | ||||||||||
Decapoda-Astacoidea | |||||||||||
Orconectes virilis | 1 | 0 | Schwartz et al. 1963 | ||||||||
Pacifastacus leniusculus | 1 | 0 | |||||||||
Procambarus clarkii | 1 | 0 | Cohen and Carlton 1995 | ||||||||
Decapoda-Caridea | |||||||||||
Exopalaemon modestus | 1 | 1 | |||||||||
Macrobrachium olfersii | 0 | ||||||||||
Palaemon macrodactylus | 0 | ||||||||||
Decapoda-Brachyura | |||||||||||
Carcinus maenas | 3 | 2 | 2 | 1–3 | 3 | Vermeij 1982 ; Grosholz et al. 2000 ; Jensen et al. 2002 ; Walton et al. 2002; Trussell et al. 2004 ; Floyd and Williams 2004 ; Griffen and Byers 2006 ; Grosholz et al (in review) | |||||
Charybdis hellerii | 0 | ||||||||||
Eriocheir sinensis | 1 | 1 | 3 | 1–3 | 3 | Panning 1939; Rudnick et al. 2003 ; Chinese mitten crab Working Group 2003 ; Rudnick et al. 2005 | |||||
Eurypanopeus depressus | 0 | ||||||||||
Hemigrapsus sanguineus | 3 | 3 | 3 | Jensen et al. 2002 ; Lohrer and Whitlatch 2002 ; Griffen and Byers 2006 ; Tyrrell et al. 2006 ; Griffen and Delaney 2007 ; Griffen et al. 2008 | |||||||
Platychirograpsus spectabilis | 0 | ||||||||||
Rhithropanopeus harrisii | 0 | Cohen and Carlton 1995; Zaitsev and Öztürk 2001, cited by Roche and Torchin 2007 | |||||||||
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Ruiz, G., Fofonoff, P., Steves, B., Dahlstrom, A. (2011). Marine Crustacean Invasions in North America: A Synthesis of Historical Records and Documented Impacts. In: Galil, B., Clark, P., Carlton, J. (eds) In the Wrong Place - Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature - Springer Series in Invasion Ecology, vol 6. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0591-3_6
Download citation
DOI: https://doi.org/10.1007/978-94-007-0591-3_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0590-6
Online ISBN: 978-94-007-0591-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)