Abstract
Ginseng roots are taken orally as adaptogens and nourishing stimulants in traditional Chinese medicine. Along with the rapid advancement of modern research technologies, ginseng’s effects in cancer treatment and prevention have been better elucidated both at molecular levels and on clinical aspects. Herein, we presented some techniques used for preparation for ginseng powders and separation of saponins, evidence from in vitro and in vivo tests and human studies to demonstrate effects of ginseng and its ingredients on cancer. Preparations of ginseng powders and saponins involve extensive use of extractions by methanol, water, hexane, n-BuOH, thin layer and high-performance liquid chromatography separations, depending on the constituents desired from the crudes. Ginseng is characterized by the presence of its active ingredients-ginsenosides, such as Rg3, Rg5, and Rh2, etc, which have been extensively studied in vitro and in vivo. Evidences from the in vitro studies demonstrated that ginsenosides can inhibit cancer cell invasion and metastasis, as well as induce apoptosis through different signaling pathways, which may substantiate the clinical use of ginseng as a preventing or supplementary therapy for cancer. In vivo animal studies further confirmed that Rg3 can improve the life quality and survival of the tumor-bearing mice and reduce their tumor weight. In addition, ginsenosides Rg3 and Rg5 showed statistically significant reduction in lung tumor incidence in mouse models, proving the chemo-preventive effects of red ginseng. In clinical studies, ginseng can improve the immune system activity of cancer patients and their appetites. Most importantly, regular ginseng consumption reduced the risk of development of all types of cancers, as observed in several randomized, double-blinded, placebo-controlled pilot trials. However, there is no conclusive evidence that ginseng itself can cure cancer. Its roles in cancer treatment should be viewed as a supplementary therapy to enhance host immune response to cancer and quality of patients’ life. Nonetheless, the beneficial effects of ginseng on cancer patients and high-risk populations (such as chronic immunosuppressive patients and the elders) have been well-demonstrated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ginseng Extract
- White Ginseng
- Human Lung Adenocarcinoma A549 Cell
- Asian Ginseng
- Bladder Transitional Cell Carcinoma
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
The term ginseng refers to several species of the genus Panax. The two most commonly used species are Asian ginseng (Panax ginseng C.A. Meyer), which is almost extinct in its natural habitat but is still cultivated, and American ginseng (Panax quinquefolius L), which is both harvested from the wild and cultivated. Ginseng has long been known as a multifunctional tonic and anti-fatigue agent as described in the oldest Chinese materia medica book—Shennong’s Classic of Materia Medica. The initial identification and description of ginseng can be dated back to about 5,500 years ago, when Shennong (a divine folk medical doctor) started to taste hundreds of herbs. Shennong’s work was then recorded by Tao Hongjing into a book entitled ‘Shennong’s Classic of Materia Medica’ during the Liang Dynasty (505–557 AD) (Zheng 1985).
The fundamentals of traditional Chinese medicine (TCM) stress the Yin-Yang balance. These theories apply the phenomena and laws of nature to the study of physiological activities and pathological changes of the human body and its interrelationships with other external elements. Yin and Yang are in conflict but at the same time mutually interdependent, with neither being able to exist in isolation. Under normal conditions, tumor and host immunosurveillance are kept within certain bounds. When the balance is broken, cancer grows. Chemo-therapeutic agents inhibit tumor growth in the tumor-bearing nude mice (Jia et al. 2008), but they often damage the host immunosurveillance simultaneously, resulting in a ruin of the physiological dynamic equilibrium. Such chemotherapy-derived imbalance could produce excess of Yin (cytotoxicity to all fast proliferative cells) and deficiency of Yang (quality of life and human’s nature power to fight diseases for full recovery). Consequently, cancer is only delayed for months to finally take a life away which would otherwise have been saved if the patient’s own recovery power was not damaged.
In general, the roles of ginseng in cancer treatment should be viewed as a supplementary therapy. Ginseng enhances host immune response (Scaglione et al. 1990), quality of patients’ life, patients’ appetite, and thus inhibiting further spreading of cancer. Generally, characteristics of the anticancer effects of ginseng may be summarized as follows: (1) it is observed only in slow-growing tumors such as Ehrlich and sarcoma 180 ascites tumors in vivo; (2) it is not observed in rapidly growing tumors such as L1210, P388 ascites tumors and Walker carcinosarcoma; and (3) there is no dose-response relationship and no cumulative effects (Yun et al. 2001a).
Although there is no conclusive evidence that ginseng itself can cure cancer, it makes sense that ginseng usage in cancer therapy should focus on synergistic combinations or palliative treatment. During active cancer therapy, ginseng should generally be administered in combination with chemotherapy and radiation. In this role, it acts as a biological response modifier and an adaptogen to synergistically enhance efficacy of the conventional therapy.
It should be mentioned that Chinese, Russian, Korean, and Japanese scientists did not explore the possibility of ginseng’s preventive effects on cancer until 1990 (Yun et al. 2001a). Before that, ginseng research was heavily concentrated on its general beneficial effects, including its aphrodisiac effect, cognitive effect, tonic effect, and cardiovascular effect (Jia and Zhao 2009; Jia et al. 2009).
4.2 Preparations of Ginseng Powders and Saponins
Isolation and separation of ginseng saponins start with extraction of these ingredients from ginseng with methanol. The methanolic suspension is then subjected to column chromatographic separation (column styles: amberlite XAD-2, Diaion MCI Gel HP20, or Kogel BG4600). After removing saccharides and amino acids with water, elution of the columns with methanol is applied to obtain a saponin fraction. Those ginseng saponins, ginsenoside Rx (Table 4.1), are further separated on thin layer and high-performance liquid chromatography. A water-containing silica gel column and the water-containing solvent systems (Pak Aquasil) yield a good separation of ginsenosides (Kaizuka and Takahashi 1983).
Ginsenosides are generally labile under acidic conditions: ordinary acidic hydrolysis is always accompanied by many side reactions, such as cyclization of side chains, glycosyl elimination, and epimerization of carbone-20 by SN1 reaction. Therefore, the chemical transformations of secondary metabolites may occur during preparation processes.
For separation of panaxadiol type and panaxatriol type saponins, powdered red ginseng (2 kg) of 6 years old could be extracted with water (2 l × 2) at 90°C and filtered. One-tenth of the combined filtrates were evaporated to give a “water extract” (104.4 g). The remaining combined filtrates were successively extracted with hexane (1 l × 3) and water saturated n-BuOH (700 ml × 3). The dried hexane fraction (1.2 g) was named panaxan A-U, consisting of glucose, arabinose, galatose, rhamnose, xylose, and/or uronic acid. The water layer was also evaporated to give water fraction (715.9 g). The n-BuOH fraction was chromatographed on silica gel column, and the gel was eluted with CHCl3-MeOH-H2O (10:3:1 → 7:3:1) system. Eluates were examined by thin layer chromatography together with authentic samples, and the panaxadiol type saponins (29.2 g) and panaxatriol type saponins (32.8 g) were obtained (Kasai et al. 1983; Yun et al. 2000).
For separation of the total saponins, fresh ginseng can be air-dried and powdered. The powdered fresh ginseng (1 kg) can be extracted with water (2 l × 2) at 90°C and filtered. One tenth of the combined filtrates can be evaporated to give a water extract (49.2 g), and nine-tenths of the combined filtrates are extracted with ethyl ether (1 l × 3) and water saturated n-BuOH (700 ml × 3), to give n-BuOH fraction. The combined n-BuOH fraction can be dried and evaporated under the reduced pressure to give total saponin (63.6 g).
For preparation of the ginsenoside Rg3 and Rg5 mixture, the ginsenoside Rb1 (10 g) obtained from Korean ginseng can be hydrolyzed with 50% acetic acid (500 ml) at 70°C for 3 hours. The reaction mixture, concentrated to appropriate volume, is left at 4°C for 1 day and filtered. The filtrate is diluted with water (500 ml) and extracted with n-BuOH (250 ml × 3). The combined n-BuOH fractions are washed with saturated NaHCO3 solution and evaporated under the reduced pressure. The residue is chromatographed on silica gel column, using CHCl3-MeOH-H2O (9:3:1) as eluting solvent, to obtain ginsenoside Rg3 and Rg5 mixture. Ginsenoside Rg3 and Rg5 mixtures can be subjected to high-performance liquid chromatography using acetonitrile-water (60:40) as mobile phase to analyze the ratio of ginsenoside Rg3 to Rg5 (2.6 g) (Kim et al. 1996).
4.3 Evidences of Ginseng as a Preventing or Supplementary Therapy for Cancer
4.3.1 In Vitro Experimental Evidences
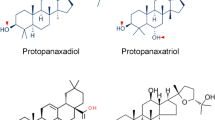
Kitagawa et al. (1995) demonstrated that ginsenosides (Fig. 4.1), especially 20(R)-ginsenoside Rg3, can specifically inhibit cancer cell invasion and metastasis in vitro. Kitagawa’s group developed an in vitro model of tumor cell invasion and metastasis. Tumor cells were seeded on a monolayer of mesothelial or endothelial host cells. The number of tumor cells that penetrated through the monolayer indicated their capacity of invasion. The capacity of the penetration of tumor cells in vitro was in parallel with that of the in vivo implantation experiment. In the in vitro invasion system, 10% fetal calf serum was essentially required for the culture medium. It was found that fetal calf serum could be replaced with 1-oleoyl-lysophosphatidic acid. By using this model, more than 10 kinds of ginsenosides Rx were tested for their inhibition activity of tumor cell invasion and metastasis, and ginsenoside Rg3 was found to be the most effective in preventing cancer cell invasion. In addition, Azuma and Mochizuki (1994) found that ginsenoside Rb2 inhibited tumor angiogenesis, and Kikuchi et al. (1991) reported that ginsenoside Rh2 inhibited the human ovarian cancer growth in nude mice model. Recently, ginsenoside Rg3 was produced as an anti-angiogenic anticancer drug in China.
Rg3 is one of the most effective cytostasis ginsenosides isolated from ginseng. Rg3 inhibits human prostate cancer cells and other androgen dependent cells from proliferating (Liu et al. 2000). The mechanisms of action of Rg3 include: (1) decreasing genetic expression of 5α-reductase; (2) inhibiting cell cycle evolution genes such as proliferating cell nuclear antigen gene and cell cycle protease D1 gene that would stop cells from proliferating; (3) increasing cyclinase suppressor genes such as, p21 and p27, to arrest cells at G1 stage; (4) down-regulating Bcl-2 (the anti-induction apoptosis gene); and (5) activating caspase-3 (the induction apoptosis gene) to induce cell death. Rg3 was discovered to inhibit tumor cell proliferation and induce cell apoptosis in mice with induced liver cancer (Li et al. 2005). In addition, Rg3 can affect the differential expression of cell signaling genes and other related genes in human lung adenocarcinoma cell line A549, and induce apoptosis in the A549 tumor cells and HUVEC 304 cell lines. It was found that Rg3 and Rg5 had significant inhibition activity on benzo[a]pyrene-induced adenocarcinoma and dimethylbenz[a]anthracene-induced lung tumor in mice (Yun et al. 2001a). Both ginsenosides had strong inhibitory effects on the development of rat mammary adenocarcinoma induced by methyl-N-nitrosourea and N-ethyl-N-nitrosourea administration, as well as on uterine and vaginal tumors induced by dimethylbenz[a]anthracene (Bespalov et al. 2001). Chen et al. (2007) reported that Rg3 could induce apoptosis in human bladder transitional cell carcinoma cell line EJ at the IC50 value of 125.5 µg/ml after 48 hours of incubation. When treated with 150 µg/ml of Rg3 for 24 hours and 48 hours, the cells showed significant DNA ladders and apoptotic morphological characteristics, including condensed chromatin, nuclear fragmentation, apoptotic bodies, and bright fluorescent granules, as well as a higher caspase-3 expression. When the cells were treated with 75 µg/ml of Rg3 for 24 hours and 48 hours, the percentage of cells in S phase and G2/M phase was increased, whereas the percentage of cells in G0/G1 was decreased.
Ginsenoside Rh2 induces apoptosis in various tumor cells by different signaling pathways. In general, Rh2 cause cell apoptosis by interfering with Bcl-2 (B cell lymphocyte/leukemia-2) family proteins and caspase-3/PKC (protein kinase C) signaling transduction. Rh2 induced apoptosis in rat C6Bu-1 glioma cells and human SK-N-BE(2) neuroblastoma cells through PKC pathway. It was also found to induce apoptosis in human malignant melanoma, which was partially dependent on caspase-8 and caspase-3 (Fei et al. 2002). Cheng et al. (2005) demonstrated that ginsenoside Rh2 can induce apoptosis and inhibit cell growth in C6 glioma cells, human lung adenocarcinoma A549 cells, and various ovarian cancer cell lines. Mediating G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells appeared to be the molecular mechanisms of Rh2 in this research. Rh2 could also inhibit human hepatoma Bel-7404 cell lines via arresting cell cycle, up-regulating Bax protein expression, and down-regulating mutated p53 protein expression. In addition, Rh2 inhibited the growth of MCF-7 cells by inducing p21 protein expression and reducing cyclin D levels. As a result, cyclin/Cdk complex kinase activity, pRb phosphorylation, and E2F release could be inhibited.
Another ginsenoside, Compound K (or IH901), was shown to induce apoptosis in human hepatoblastoma HepG2 cells and KMS-11 cells through a cytochrome C-mediated activation of caspase-3 and caspase-8 proteases and inhibition of the FGFR3 (fibroblast growth factor receptor 3) expression (Oh and Lee 2004). Incubation of leukemic cells HL260 with Compound K produced apoptosis in the cells in a concentration- and time-dependent manner with morphologic changes in chromatic agglutination, atrophy, and nuclear fragmentation (Suda et al. 2000). Compound K suppressed melanoma cell proliferation in BI6-BL6 mice by activating PKC and releasing cytochrome C in mitochondria into cytoplasm. Western blot test revealed that Compound K could elevate p27Kipl expression, degrade expression of c-Myc and cyclin D1, and induce cell LLC apoptosis through activating caspase-3 protein kinase at the same time. Recently, IH-901 has been reported to induce both G1 arrest and apoptosis, and the apoptosis could be inhibited by COX-2 induction (Yim et al. 2005).
The combinational use of ginseng extracts or active ginsenosides with clinical anticancer drugs has also been studied. For example, the effect of panaxytriol (isolated from Asian ginseng) on the cytotoxicity of mitomycin C (MMC) against a human gastric carcinoma cell line, MK-1, was investigated, and a synergistic effect was observed when MK-1 cells were treated with the mixture of MMC and panaxytriol or treated with MMC followed by panaxytriol. Further research suggested that these synergistic effects may be induced by acceleration of the effect of MMC on cellular accumulation by panaxytriol (Matsunaga et al. 1994). In another research, quasipanaxatriol, ginsenoside Rh2, and compound K was found to greatly enhance the cytotoxicity of anticancer drugs daunomycin (DAU) and vinblastine in multidrug-resistant P388 leukemia cells (P388/ADM). The extent of enhancement ranged from 4- to 46-fold in DAU cytotoxicity, and 2- to 37-fold in vinblastine cytotoxicity. The reversal of DAU resistance in P388/ADM by quasipanaxatriol could be explained by the effective accumulation of the drugs mediated by the DAU-efflux blockage (Hasegawa et al. 1995).
4.3.2 In Vivo Animal Test Evidences
Using athymic mice transplanted with ovarian SKOV-3 cancer cells, Xu et al. (2007) showed that intraperitoneal injection of ginsenoside Rg3 alone, or Rg3 combined with cyclophosphamide, to the mice for 10 days improved the life quality and survival of the mice and reduced the tumor weight of the mice in comparison to that of the untreated control mice.
The inhibitory effect of ginsenoside Rh2 alone or together with cisplatin on the growth of human ovarian cancer cells (HRA) in nude mouse was studied, and it was discovered that po administration of Rh2 has a dose-dependent inhibitory effect on the tumor growth, while ip administration showed little activity. In addition, Rh2 significantly potentiated the inhibitory effect of cisplatin against ovarian cancer cell growth in the mice model, and these mice survived longer when given the ginsenoside preparation (Nakata et al. 1998).
Korean investigators carried out extensive long-term anti-carcinogenicity experiments with 2,000 newborn mice to investigate whether Asian ginseng could inhibit carcinogenesis induced by several chemical carcinogens. By using the so-called Yun’s model, they confirmed significant anti-carcinogenic effects of powders and extracts of the 6-year-old dried fresh ginseng, 5- and 6-year old white ginsengs, and 4-, 5-, and 6-year old red ginseng. They also demonstrated that the anti-carcinogenicity of ginseng was more prominent in aged or heat treated extracts of ginseng and in red ginseng made by steaming (Yun et al. 2001b). The Yun’s 9–12 weeks medium-term anti-carcinogenicity test mouse model was created as follows: The N:GP(S) newborn mice less than 24 hours old are subcutaneously injected once in the scapular region with 0.02 ml of benzo[a]pyrene (0.5 mg suspension of benzo[a]pyrene in aqueous gelatin) (Yun et al. 1995). After weaning, anti-carcinogenicity test materials are administered for 6 weeks through drinking water or diets. All mice are usually sacrificed at the 9th week after birth. The procedures to score the index of lung tumor incidence are then conducted.
To investigate the active components for cancer prevention, several fractions of 6-year old fresh ginseng and red ginseng, four semi-synthetic ginsenoside Rh1, Rh2, Rg3, and Rg5, major saponin components in red ginseng, were prepared. Among the ginsenosides, Rg3 and Rg5 showed statistically significant reduction of lung tumor incidence and Rh2 had a tendency of decreasing the incidence. Ginsenoside Rg3, Rg5, and Rh2 were found to be active anti-carcinogenic compounds. Rg3, Rg5, and Rh2 are active components in red ginseng, and they prevent cancer either singularly or synergistically (Yun et al. 2001a).
4.3.3 Evidence-based on Human Studies
Ginseng can improve immune system activity of cancer patients and their appetite, and function as a supplementary agent of chemotherapy. For instance, in Scaglione’s human studies (1990), they examined effects of ginseng extract on following parameters of healthy volunteers: chemotaxis, phagocytosis index, phagocytosis fraction, intracellular killing, total lymphocytes (T3), T helper (T4) subset, suppressor cells (T8) subset, blastogenesis of circulating lymphocytes, natural killer cell activity. Each group (n = 20) was given 100 mg of the ginseng extract every 12 hours daily for up to 8 weeks. Blood samples were withdrawn before beginning the treatment, at the 4th week and at the 8th week. They found that the activity of these immune biomarkers was significantly enhanced at the end of the 8-week study in comparison with the placebo group (P < 0.05 or 0.001).
Xing et al. (2001) treated 35 rectal cancer patients with retention enema containing 85% ginsenoside for 4–6 hours per day for 6–8 consecutive days before surgical operation. The control group (n = 15) received retention enema containing saline in the same way. They reported that after ginsenoside treatment, symptoms such as frequent defecation, hematochezia, and tenesmus were palliated in most patients (25 out of 35), and abdominal pain was relieved in 7 patients with incomplete intestinal obstruction. Electron microscopic examination showed apoptosis in 23 treated patients. In comparison, the above-mentioned changes were not observed in the control group. Pre-clinical studies have also showed some immune-stimulating activity of ginseng and ginsenosides (Xing et al. 2001; Block and Mead 2003).
Suh et al. (2002) reported that the red ginseng powder from Asian ginseng inhibits the recurrence of AJCC stage III gastric cancer. The CD4 and CD3 levels in patients during postoperative chemotherapy were restored to the initial preoperative values after red ginseng powder ingestion demonstrated by the flow cytometric analyses for peripheral T lymphocyte subsets, suggesting some immunomodulatory properties of ginseng in patients with advanced gastric cancer during postoperative chemotherapy. This study further demonstrated that the 5-year disease free survival and overall survival rate in patients taking the red ginseng powder during postoperative chemotherapy was significantly higher versus control (68.2% versus 33.3%, 76.4% versus 38.5%, respectively, P < 0.05). In spite of the limitation of a small number of patients (n = 42), these findings suggest that red ginseng powder may help to improve postoperative survival in these gastric cancer patients. In another study, a commercial combination product that contained ginseng was administered to 126 cachectic cancer patients. Improvements were also observed in fatigue, pain tolerance, mental concentration, physical activity, and appetite (Chang et al. 2003).
Ginseng consumption reduced the risk of development of all types of cancer. Yun proposed that all non-toxic chemo-preventive agents should be classified into three categories: organ-specific, multiorgan-specific, and non-organ-specific agents (Yun 2001). He suggested that the first priority should be clinical studies of non-organ-specific cancer preventives. This approach would be justified in terms of time and cost-effectiveness and raise the possibility of providing a real chemo-preventive medicine for general use in cancer. In a case-control study, Yun and Choi (1990, 1995) performed epidemiological studies among Korean people and the results demonstrated the non-organ-specific cancer-preventive activity of ginseng extracts. In their case-control study on the cancer-preventive effect of ginseng, the number of the subjects was extended from the original 905 pairs to later 1,987 pairs. In both the epidemiological studies, odds ratios (OR) of white ginseng powder intake were 0.44 (for 905 pairs) and 0.30 (for 1,987 pairs), and odds ratios of red ginseng extracts intake were 0.45 (for 905 pairs) and 0.20 (for 1,987 pairs). The OR indicates the possibility of being falsely significant. The smaller the number is, the lesser the possibility of being falsely significant is. Yun’s studies stated that the preventive effects of ginseng on cancers were non-organ-specific. The consumption of fresh ginseng slices, fresh ginseng juice, and white ginseng tea did not decrease the risk for cancer. However, as the odds ratios show, the risk for cancer was rather unexpectedly low in the cases of intake of 1–3 times/year (OR = 0.62), 4–11 times/year (OR = 0.48), and 1 time/month or more (OR = 0.31). Overall, the risk decreased as the frequency and duration of ginseng consumption increased. With respect to the site of cancer, the ORs for cancers of the lip, oral cavity, pharynx, esophagus, stomach, colorectum, liver, pancreas, larynx, lung, and ovary were significantly reduced by ginseng usage. Smokers with ginseng intake showed lower ORs for cancers of lung, lip, oral cavity, pharynx, and liver than those without ginseng intake.
The cancer preventive effect was also observed in a prospective study conducted on 4,634 people living in the ginseng cultivation area (Yun and Choi 1998). Subjects born before 1947 (over 40 years) were selected. A cohort of 4,634 persons over 40 years of age residing in Kangwha-eup (the ginseng cultivation area) was interviewed and examined between August 1987 and December 1989. Each study subject was interviewed by means of a standard questionnaire about demographic characteristics, life-long occupation, smoking and drinking habits, and past history of diseases, etc. In an attempt to obtain detailed information on ginseng intake, they used the same questionnaire as used in the previous two case-control studies (Yun and Choi 1990, 1995). The interviewers had been instructed and trained beforehand to ensure uniformity in the method of inquiry. The follow-up studies were carried out on all cohort members to document the development of cancer and other illnesses and to update exposure information. The length of the follow-up was calculated for each individual in the study as the number of days elapsed since completion of the questionnaire until death from cancer or other diseases. Deaths among the cohort from August 1987 to December 1997 were traced by population registration cards with no follow-up loss. A cohort member was classified as a cancer case if they had any disease code of cancer in hospital records, death certificates of the provincial government, privileged data of Korea Medical Insurance Corporation, etc. In the 5-year follow-up cohort study cancer risk significantly decreased among consumers of fresh ginseng extract, alone or together with other ginseng products. Among 24 red ginseng consumers, no cancer death occurred during the follow-up period. The risk for stomach and lung cancers was significantly reduced by ginseng intake, showing a statistically significant dose-response relationship in each follow-up year. These results suggest that ginseng exerted a preventive effect against the development of cancers of all organs, i.e. non-organ-specific.
4.4 Perspectives and Challenges
Ginseng is used in traditional Chinese medicine and is of interests to cancer patients in many other countries chiefly for its reputed anti-fatigue properties. However, ginseng is chemically complex, with more than 30 ginsenosides, polysaccharides or glycans, enzymes, organic acids and ester, amino acids, sugars, essential oils, minerals, etc. The active ingredients are believed to be the ginsenosides, and the contents of individual ginsenosides are more important than the total amount, since the individual efficacy or/and potency of each ginsenoside differs and will affect the pharmacological effects overall. However, as other botanic products, the percentage of the active ginsenosides, such as Rg3, Rg5, Rb1, and Rh2, varies from species to species and from extraction preparation methods, making the quality control difficult and challenging.
Numerous studies have examined cytotoxicity of ginseng and its fractions in vitro against a wide variety of cancer cell lines or in vivo in neoplasm models, with mixed results, probably due to the difference in constituents. Several of the isolated ginsenosides have demonstrated interesting anticancer activities, including induction of apoptosis, inhibition of cell cycle progression, and anti-angiogenic activity. Yet, the presences of these active ingredients are in trace amount in the crude extracts, which further necessitate the quality control guideline of ginseng products.
Studies on ginseng and cancer therapy in humans are rare. More reports focus on cancer prevention, which showed beneficial preventive effects of ginseng towards the risk of cancers. However, more carefully designed clinical studies are still needed to further address issues concerning the immunomodulation, anti-stress, anticancer, and anti-fatigue properties of ginseng, which are not statistically conclusive based on current random studies.
References
Azuma, I., & Mochizuki, M. (1994). Inhibition of tumor cell invasion and metastasis by ginsenosides. Ginseng Review, 18, 37–39.
Bespalov, V. G., Alexandrov, V. A., Limarenko, A. Y., et al. (2001). Chemoprevention of mammary, cervix and nervous system carcinogenesis in animals using cultured Panax ginseng drugs and preliminary clinical trials in patients with precancerous lesions of the esophagus and endometrium. Journal of Korean Medical Science, 16(Suppl), S42–S53.
Block, K. I, & Mead, M. N. (2003). Immune system effects of echinacea, ginseng, and astragalus: A review. Integrative Cancer Therapies, 2, 247–267.
Chang, Y. S., Seo, E. K., Gyllenhaal, C., et al. (2003). Panax ginseng: A role in cancer therapy? Integrative Cancer Therapies, 2, 13–33.
Chen, J. X., Peng, H. M., Pu, S. P., et al. (2007). Inducement effect of ginsenoside Rg3 on apoptosis of human bladder transitional cell carcinoma cell line EJ. Zhongguo Zhong Yao Za Zhi, 32, 1680–1684.
Cheng, C. C., Yang, S. M., Huang, C. Y., et al. (2005). Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemotherapy and Pharmacology, 55, 531–540.
Fei, X. F., Wang, B. X., Tashiro, S., et al. (2002). Apoptotic effects of ginsenoside Rh2 on human malignant melanoma A375-S2 cells. Acta Pharmacologica Sinica, 23, 315–322.
Hasegawa, H., Sung, J. H., Matsumiya, S., et al. (1995). Reversal of daunomycin and vinblastine resistance in multidrug-resistant P388 leukemia in vitro through enhanced cytotoxicity by triterpenoids. Planta Medica, 61, 409–413.
Jia, L., & Zhao, Y. (2009). Current evaluation of the millennium phytomedicine—Ginseng (I): Etymology, pharmacognosy, phytochemistry, market and regulations. Current Medicinal Chemistry, 16, 2475–2484.
Jia, L., Noker, P. E., Piazza, G. A., et al. (2008). Pharmacokinetics and pharmacodynamics of Phor21-betaCG(ala), a lytic peptide conjugate. The Journal of Pharmacy and Pharmacology, 60, 1441–1448.
Jia, L., Zhao, Y., & Liang, X. J. (2009). Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Current Medicinal Chemistry, 16, 2924–2942.
Kaizuka, H., & Takahashi, K. (1983). High-performance liquid chromatographic system for a wide range of naturally occurring glycosides. Journal of Chromatography, 258, 135–146.
Kasai, R., Bess, H., Tanaka, O., et al. (1983). Saponin of red ginseng. Chemical Pharmaceutical Bulletin, 31, 2120–2125.
Kikuchi, Y., Sasa, H., Kita, T., et al. (1991). Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside Rh2 and adjuvant effects to cisplatin in vivo. Anti-Cancer Drugs, 2, 63–67.
Kim, S. I., Park, J. H., Ryu, J. H., et al. (1996). Ginsenoside Rg5, a genuine dammarane glycoside from Korean red ginseng. Archives of Pharmacal Research, 19, 551–553.
Kitagawa, I., Kobayashi, M., Akedo, H., et al. (1995). Inhibition of tumor cell invasion and metastasis by ginsenoside Rg-3. Ginseng Review, 20, 41–46.
Li, X., Guan, Y. S., Zhou, X. P., et al. (2005). Anticarcinogenic effect of 20(R)-ginsenoside Rg3 on induced hepatocellular carcinoma in rats. Sichuan Da Xue Xue Bao Yi Xue Ban, 36, 217–220.
Liu, W. K., Xu, S. X., & Che, C. T. (2000). Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sciences, 67, 1297–1306.
Matsunaga, H., Katano, M., Saita, T., et al. (1994). Potentiation of cytotoxicity of mitomycin C by a polyacetylenic alcohol, panaxytriol. Cancer Chemotherapy and Pharmacology, 33, 291–297.
Nakata, H., Kikuchi, Y., Tode, T., et al. (1998). Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Japanese Journal of Cancer Research, 89, 733–740.
Oh, S., & Lee, B. H. (2004). A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicology and Applied Pharmacology, 194, 221–229.
Scaglione, F., Ferrara, F., Dugnani, S., et al. (1990). Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Under Experimental and Clinical Research, 16, 537–542.
Suda, K., Murakami, K., Hasegawa, H., et al. (2000). Induction of apoptosis in Lewis lung carcinoma cells by an intestinal bacterial metabolite produced from orally administered ginseng protopanaxadiol saponins. Journal of Traditional Medicine, 17, 236–244.
Suh, S. O., Kroh, M., Kim, N. R., et al. (2002). Effects of red ginseng upon postoperative immunity and survival in patients with stage III gastric cancer. The American Journal of Chinese Medicine, 30, 483–494.
Tanaka, O., Kasai, R., & Morita, T. (1986). Chemistry of Ginseng and related plants: Recent advances. Chinese University of Hong Kong: Abstract of Chinese Medicines, 1, 130–152.
Xing, J. H., Chen, Y. Q., & Ji, M. X. (2001). Clinical study on effect of ginsenoside in inducing rectal cancer cell apoptosis. Zhongguo Zhong Xi Yi Jie He Za Zhi, 21, 260–261.
Xu, T. M., Xin, Y., Cui, M. H., et al. (2007). Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chinese Medical Journal, 120, 584–588.
Yim, H. W., Jong, H. S., Kim, T. Y., et al. (2005). Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Research, 65, 1952–1960.
Yun, T. K. (2001). Panax ginseng—A non-organ-specific cancer preventive? The Lancet Oncology, 2, 49–55.
Yun, T. K., & Choi, S. Y. (1990). A case-control study of ginseng intake and cancer. International Journal of Epidemiology, 19, 871–876.
Yun, T. K., & Choi, S. Y. (1995). Preventive effect of ginseng intake against various human cancers: A case-control study on 1987 pairs. Cancer Epidemiology Biomarkers Prevention, 4, 401–408.
Yun, T. K., & Choi, S. Y. (1998). Non-organ specific cancer prevention of ginseng: A prospective study in Korea. International Journal of Epidemiology, 27, 359–364.
Yun, T. K., Kim, S. H., & Lee, Y. S. (1995). Trial of a new medium-term model using benzo(a)pyrene induced lung tumor in newborn mice. Anticancer Research, 15, 839–845.
Yun, T. K., Lee, Y. S., Choi, K. J., et al. (2000). Anticarcinogenicity of various ginseng fractions and components in red ginseng using Yun’s anticarcinogenicity test model. Journal of the Korean Association Cancer Prevention, 5, 186–192.
Yun, T. K., Lee, Y. S., Lee, Y. H., et al. (2001a). Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. Journal of Korean Medical Science, 16(Suppl), S6–S18.
Yun, T. K., Lee, Y. S., Lee, Y. H., et al. (2001b). Cancer chemopreventive compounds of red ginseng produced from Panax ginseng C.A. Meyer. Journal of Ginseng Research, 25, 107–111.
Zheng, B. C. (1985). Shennong’s herbal—One of the world’s earliest pharmacopoeia. Journal of Traditional Chinese Medicine, 5, 236.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Jia, L., Qian, K. (2011). An Evidence-based Perspective of Panax Ginseng (Asian Ginseng) and Panax Quinquefolius (American Ginseng) as a Preventing or Supplementary Therapy for Cancer Patients. In: Cho, W. (eds) Evidence-based Anticancer Materia Medica. Evidence-based Anticancer Complementary and Alternative Medicine. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0526-5_4
Download citation
DOI: https://doi.org/10.1007/978-94-007-0526-5_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0525-8
Online ISBN: 978-94-007-0526-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)