Abstract
Studies of dental development have reported conflicting results regarding whether Neanderthal growth and development was similar to that of modern humans. The discovery of a partial permanent maxillary juvenile dentition (OR-1) from the Obi-Rakhmat Grotto, Uzbekistan, provides the opportunity to assess dental development and age at death in a Paleolithic hominin with strong Neanderthal similarities using incremental dental features. Long-period lines on tooth crowns (perikymata) and roots (periradicular bands) were quantified, and crown formation, root development, and age at death were estimated. An anomalous upper molar was determined to be a left M2 with a rare developmental condition (gemination). Perikymata numbers for OR-1 were similar to modern southern African population means, but were less than modern northern European and Neanderthal means. Root extension rates were estimated to be similar to (or slightly higher than) modern human values, although few modern comparative data are available. Assuming the long-period line periodicity of this individual fell within a Neanderthal distribution (6–9 days), the maximum age at death of OR-1 is estimated at 8.1 years, but is more likely to have been 6.7–7.4 years (7 or 8 day periodicity). Modern European human developmental standards would suggest an age at death of approximately 8–9 years. These results are consistent with other studies suggesting that Neanderthal dental development overlaps with the low end of modern human populations, and demonstrates a greater range of variation in Middle Paleolithic hominins than previously reported. It is clear that perikymata number alone does not distinguish these taxa; data on long-period line periodicity and molar eruption would yield additional insight into Neanderthal life history.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Crown formation
- Root formation
- Perikymata
- Periradicular band
- Gemination
- Neanderthal
- Extension rate
- Life history
- Incremental feature
Introduction
Recent studies of hominin dental tissues have utilized incremental features to infer patterns of life history (the scheduling of development and the timing of reproductive events) (e.g., Bromage and Dean 1985; Dean et al. 2001; Ramirez Rozzi and Bermudez de Castro 2004; Guatelli-Steinberg et al. 2005; Smith et al. 2007a, b; reviewed in Smith 2008). Previous histological work on juvenile Neanderthal dentitions has been limited to studies of individuals from Devil’s Tower, Gibraltar (Dean et al. 1986; Stringer et al. 1990; Stringer and Dean 1997), Montgaudier Cave, France (Mann and Vandermeersch 1997); Hortus, France (Ramirez Rozzi 2005); Scladina, Belgium (Smith et al. 2007b); and Dederiyeh, Syria (Sasaki et al. 2002). These studies were primarily focused on determining age at death from counts of temporal lines in tooth enamel. The number and spacing of external long-period growth lines has also been studied in considerable samples of Neanderthals and Upper Paleolithic modern humans (Mann et al. 1991; Ramirez-Rozzi 1993a; Ramirez Rozzi and Bermudez de Castro 2004; Guatelli-Steinberg et al. 2005, 2007; Guatelli-Steinberg and Reid 2008; Reid et al. 2008). Histological studies of internal enamel development have been conducted on four permanent teeth from Tabun, Israel; La Chaise-de-Vouthon, France; Scladina, Belgium; and Lakonis, Greece (Dean et al. 2001; Macchiarelli et al. 2006; Smith et al. 2007b, 2009). Several of these studies have reported that the Neanderthal dentition developed in a shorter time than that of modern humans, although in some cases Neanderthals appear to overlap with the low or ‘rapid’ end of the human range.
The discovery of a juvenile Middle Paleolithic hominin from the Obi-Rakhmat Grotto in Uzbekistan (Glantz et al. 2004, 2008) possessing several isolated, associated developing teeth presents the rare opportunity to assess dental development in a central Asian Paleolithic hominin. Metric and morphological analyses of the dentition suggest that this individual most closely resembles a Neanderthal (Glantz et al. 2004, 2008; Bailey et al. 2008). This study aims to assess whether the duration of crown formation and developmental stage at death in this individual supports the proposed ‘rapid developmental profile’ based on dental evidence from other Neanderthals. Surface manifestations of long-period incremental features on the tooth crowns and roots were quantified, the degree of root formation was assessed, and crown formation and root development prior to death were estimated using Neanderthal cuspal formation times and a range of likely long-period increment periodicity values. Age at death was estimated using Neanderthal initiation ages, which were added to the time of crown and root formation. These data were compared with data on incremental development in modern humans from northern England and southern Africa, as well as a large sample of Neanderthals (Macchiarelli et al. 2006; Reid and Dean 2006; Smith et al. 2007b, c; Guatelli-Steinberg and Reid 2008; Reid et al. 2008; Smith et al. 2010).
Material and Methods
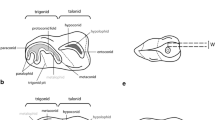
Six isolated teeth (left upper I2, C, P3, P4, M1, M2) were recovered from the site of Obi-Rakhmat Grotto (Fig. 13.1), which are considered to belong to a single individual due to their preservation, stage of development, and physical proximity (Glantz et al. 2004, 2008). The teeth have been described in several sources, which have noted that the most posterior molar tooth displays an anomalous morphology that has been interpreted differently by different scholars (Glantz et al. 2004; Bailey et al. 2008). During the course of this study, the anomalous posterior molar was judged to be a left M2 based on the orientation of the cusps and ridges, as well as root morphology. It is suggested that the major accessory cusp (and supporting root) represent an accessory cusp fused to the mesial aspect of the second molar (Fig. 13.2). The smaller accessory cusp on the lingual aspect of the protocone is interpreted as Carabelli’s cusp. The interpretation of this tooth as a left M2 is supported by comparison with the left M1 (Fig. 13.3), and when taken together, this row shows a consistent orientation of the crista obliqua as well as typical hypocone reduction from M1 to M2. This interpretation is similar to an illustration in Glantz et al. (2004: Figure 5, p. 87).
Interpretation of anomalous molar tooth as an upper left second molar. Left- maxillary cusps are indicated: par- paracone, pro- protocone, met- metacone, hyp- hypocone, ac- accessory cusp believed to represent molar gemination (fusion of a supernumary tooth - defined by dotted line). The solid line is the altered mesial border shown in Fig. 13.3. Right- underside of the developing anomalous molar, showing difference in root thickness (and root length by implication) likely due to pathology
The teeth were originally molded and cast using high-resolution impression materials (3 M Espe Imprint II, Vantico Araldite 2020), and computed tomographic (CT) scans of the original material were made at the Medical University of Innsbruck, Austria. However, the slice thickness of the resultant CT scans, as well as the image quality, were not adequate to yield accurate linear measurements of enamel thickness or quantification of tissues volumes (Olejniczak et al. 2007). Developmental times for postcanine teeth were estimated for individual cusps, which do not necessarily initiate and complete formation simultaneously (Ramirez-Rozzi 1993b; Reid et al. 1998). Crown formation time was determined as the sum of cuspal and imbricational formation time (methods reviewed in detail in Smith 2008). Cuspal formation times were taken from a histological study of several Neanderthals (Smith et al. 2010). Imbricational formation times were assessed from repeated counts of long-period lines (perikymata) on the surface of each crown/cusp (Fig. 13.4), which were made by two individuals (T.S. and D.R.) using stereomicroscopy at 50× magnification. A slight estimate was made for light wear on the M1 mesiolingual cusp. Perikymata number was multiplied by a range of probable periodicity values (discussed below).
Root length was assessed from casts and photographs of the original teeth, and corrections were made for minor amounts of missing root. Long-period lines known as periradicular bands, which are equal to internal long-period Andresen lines (Smith et al. 2007b; Smith and Reid, 2009), were counted from casts at 50× magnification (Fig. 13.4). Counts of perikymata and periradicular bands were multiplied by a range of Neanderthal periodicity values (6–9 days: Smith et al. 2010) to yield imbricational formation and root formation times, respectively. Root extension rate ranges were estimated for each intact root by dividing the total root length by the product of the respective number of long period lines multiplied by the minimum and maximum estimated periodicity values (6 and 9 days). Finally, age at death was estimated from each cusp by adding histologically derived initiation ages from the Scladina Neanderthal (Smith et al. 2007b) to the range of estimated crown and root formation times. Alternative models for initiation ages are considered in the discussion.
Results
Developmental variables, crown formation time, and age at death are presented in Table 13.1. Estimated extension rates for intact roots were estimated as follows: 14.0 mm of I2 distal root formed at 8.0–12.1 μm/day (assuming a 9 or 6 day periodicity, respectively), 5.0 mm of mesial canine root formed at 4.9–7.4 μm/day, 4.6 mm of P3 buccal root formed at 4.2–6.3 μm/day, 5.4 mm of P3 lingual root formed at 4.4–6.7 μm/day, 5.0 mm of P4 buccal root formed at 4.7–7.1 μm/day, 4.8 mm of P4 lingual root formed at 4.6–6.9 μm/day, 15.4 mm of M1 mesiobuccal root formed at 6.0–9.1 μm/day, and 10.6 mm of M1 lingual root formed at 4.4–6.7 μm/day. The duration of M1 root formation was estimated to be between 4.6 and 7.0 years for the mesiobuccal root.
The mean maximum likely age at death was 8.1 years using a periodicity of 9 days. However, using the modal periodicity of 11 Neanderthals, 7 or 8 days, yields an age at death of 6.7–4 years.
Discussion
Anomalous Molar Morphology
As noted above, the morphology of the unusual posterior molar has been interpreted in a number of ways, resulting in different classifications as an upper right or upper left second or third molar. We believe this tooth shows a rare condition where either a supernumerary tooth has fused with the second molar during crown development, or the developing second molar underwent additional division during formation, resulting in additional cusps in a process known as gemination (e.g., Kronfeld 1939; Tsesis et al. 2003). Several clinical case studies describe these conditions in mandibular molars (e.g., Turell and Zmener 1999; Nunes et al. 2002; Tsesis et al. 2003), noting that the clinical distinction between tooth fusion and gemination is subtle. No evidence of separate pulp chambers was found in CT slices, which suggests that this represents an instance of gemination, although had the tooth completed formation it may have been easier to eliminate the possibility of fusion of a supernumerary tooth. Other aspects of this molar are also pathological, only a few millimeters of mesial and lingual roots are present, while the buccal root was estimated to be almost twice as long based on its thickness at the cervical margin (Fig. 13.2). It is quite possible that the presence of an accessory cusp/root affected the position of the tooth in the crypt, and may have caused the lingual root to develop later or slower than the buccal roots.
We also note that although it is uncommon to find a Carabelli’s cusp on an upper second molar that is larger than that of the first molar, the frequency of Carabelli’s cusp (grade 3 or larger) on the UM2 in Neanderthals is 58% (Bailey, unpublished data). Other Neanderthal upper second molars have been observed to show large Carabelli’s cusps (e.g., Ehringsdorf, Arago, Krapina DP#3).
Developmental Implications
Long-period line (perikymata) numbers for the Obi-Rakhmat tooth crowns are generally lower than mean values for modern humans from northern Europe, but are similar to southern African modern human values (Table 13.2). Values from OR-1 are also lower than Neanderthal mean perikymata numbers, but are similar to the juvenile Neanderthal from Hortus, with the exception of the canine (Fig. 13.5) (Ramirez Rozzi 2005; Guatelli-Steinberg and Reid 2008; Reid et al. 2008). It appears that perikymata number is variable in modern humans, and ranges encompass most Neanderthal values (Mann et al. 1991; Guatelli-Steinberg et al. 2005; Guatelli-Steinberg and Reid 2008; Reid et al. 2008; Smith et al. 2010). The individuals from Obi-Rakhmat Grotto and Hortus expand Neanderthal perikymata ranges for maxillary teeth reported by Guatelli-Steinberg and Reid (2008).
Long-period line (perikymata) numbers in the Obi-Rakhmat individual (O), Hortus II-III individual (H), and a sample of Neanderthals (vertical range bars with mean values indicated by horizontal bars). Data for post-canine teeth are from buccal/mesiobuccal cusps. Tooth types and data are from Table 13.2, Guatelli-Steinberg and Reid (2008), and Ramirez Rozzi (2005)
If the periodicity of OR-1 fell at the lower end of the Neanderthal (or modern human) range (6–8 days), crown and root long-period line counts would be consistent with other studies that suggest that Neanderthals show a slightly more rapid period of dental development than some modern human populations. However, because an inverse relationship exists between Retzius line number and periodicity in modern humans (Reid and Ferrell 2006), it may not be the case that imbricational formation time is lower than in modern humans. If this individual had a periodicity of 10 or more days (seen in 31 of 365 modern humans: Smith et al. 2007c) the time represented by perikymata would be equal to or greater than modern human means. While we consider this quite unlikely, we cannot exclude this possibility.
Relatively little data exist regarding root extension rates in living or fossil hominins, particularly for human maxillary teeth (reviewed in Dean 2006; Smith 2008). The estimated rates of M1 root extension calculated for OR-1 (6.0–9.1 μm/day) are fairly similar to the overall extension rate (6.3 μm/day) reported for the mandibular first molar from La Chaise (Macchiarelli et al. 2006) and the maxillary first molar from Scladina (min rate 6.6 μm/day: Smith et al. 2007b) as well as longitudinal data from modern human first molars (Dean 2006; Macchiarelli et al. 2006). This result represents additional (albeit indirect) evidence that periradicular bands are equivalent to other long-period lines, and may therefore be used to assess the rate and duration of root development (also see Dean 1995; Smith et al. 2007b; Smith and Reid, 2009).
Histological analysis of the Obi-Rakhmat juvenile is dependent on several parameters that must be estimated, barring physical or virtual sectioning of the dentition, which may lead to some degree of uncertainty in the final age at death. These estimates include the cuspal formation time, long-period line periodicity, and initiation age. Because Neanderthal molars possess thinner cuspal enamel than modern humans (Smith et al. 2007b), which is a consistent pattern across the dentition (Smith et al. 2010), it is likely that estimated times from the Scladina Neanderthal are more accurate than those derived from modern humans. Similarly, modal long-period line values from other Neanderthals (7 or 8 days) are likely to be more accurate than values derived from other taxa. It has been demonstrated that the mean estimated age at death changes by 0.7 years in this individual when the periodicity is increased or decreased by 1 day. Finally, estimates of initiation age may represent an additional source of error. Very few histological estimates are available for the maxillary dentition, which include an individual of African origin (Dean et al. 1993), four or less European individuals (Reid et al. 1998), and several teeth from the Scladina Neanderthal (Smith et al. 2007b). The Scladina juvenile’s initiation ages differed by less than 2 months from the African individual for the tooth types available in this study (UI2, UC, UM1, UM2). Using European initiation ages from Reid et al. (1998) would increase the mean age at death by approximately 3–4 months, which is rather unlikely given numerous studies that have reported early tooth initiation in Neanderthals (reviewed above). In short, a range of possible ages is reported in this study that reflect the most accurate picture of Neanderthal dental development currently available, and the error associated with these ages is likely to be on the order of months rather than years.
Relatively little is known about maxillary dental development in modern human populations due to limitations in radiographic techniques and the time-consuming nature of histological studies. Published standards for modern humans of European origin with a mandibular developmental stage equivalent to the Obi-Rakhmat juvenile suggest an age at death of approximately 8–9 years (Smith 1991). The histological approach in this study yields a most likely age between 6.7 and 7.4 years, which is near the low end of modern European ranges. Global variation in crown formation times and eruption ages is still poorly understood. It is clear that African populations show more rapid anterior and premolar dental development than European populations (Dean et al. 1993; Reid and Dean 2006; Reid et al. 2008), younger initiation ages (Dean et al. 1993; Reid et al. 1998; Liversidge 2008), and younger ages at dental eruption for certain tooth positions (Liversidge 2003). Given the range of modern human variation documented to date, it appears that traditional assessments of age at death in juvenile Paleolithic hominins (e.g., Tillier 2000) should not be based on comparisons with modern European juveniles (Smith et al. 2007b, 2010). It is possible that an African developmental model is more accurate; should this be the case, the ‘rapid developmental profile’ reported for Neanderthals may be due, in part, to limited comparative samples. In conclusion, while the Obi-Rakhmat hominin shows slightly more rapid development than northern European modern humans, additional data are needed regarding initiation ages, long-period line periodicity, and eruption ages in order to resolve debates over life history differences between modern humans and Neanderthals.
References
Bailey, S. E., Glantz, M., Weaver, T., & Viola, B. (2008). The affinity of the dental remains from Obi-Rakhmat Grotto, Uzbekistan. Journal of Human Evolution, 55, 238–248.
Bromage, T. G., & Dean, M. C. (1985). Re-evaluation of the age at death of immature fossil hominids. Nature, 317, 525–527.
Dean, M. C. (1995). The nature and periodicity of incremental lines in primate dentine and their relationship to periradicular bands in OH 16 (Homo habilis). In J. Moggi-Cecchi (Ed.), Aspects of dental biology: Paleontology, anthropology and evolution (pp. 239–265). Florence: International Institute for the Study of Man.
Dean, M. C. (2006). Tooth microstructure tracks the pace of human life-history evolution. Proceedings of the Royal Society B, 273, 2799–2808.
Dean, M. C., Stringer, C. B., & Bromage, T. G. (1986). Age at death of the Neanderthal child from Devil’s Tower, Gibraltar and the implications for studies of general growth and development in Neanderthals. American Journal Physical Anthropology, 70, 301–309.
Dean, M. C., Beynon, A. D., Reid, D. J., & Whittaker, D. K. (1993). A longitudinal study of tooth growth in a single individual based on long- and short-period incremental markings in dentine and enamel. International Journal of Osteoarchaeology, 3, 249–264.
Dean, C., Leakey, M. G., Reid, D., Schrenk, F., Schwartz, G. T., Stringer, C., & Walker, A. (2001). Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature, 414, 628–631.
Glantz, M. M., Viola, T. B., & Chikisheva, T. (2004). New hominid remains from Obi-Rakhmat grotto. In A. P. Derevianko (Ed.), Obi-Rakhmat grotto (pp. 77–99). Novosibirsk: Institute of Archaeology and Ethnography SB RAS Press.
Glantz, M., Viola, B., Wrinn, P. J., Chikisheva, T., Derevianko, A., Krivoshapkin, A. I., Islamov, U., Suleimanov, R. H., & Ritzman, T. (2008). New hominin remains from Uzbekistan. Journal of Human Evolution, 55, 223–237.
Guatelli-Steinberg, D., & Reid, D. J. (2008). What molars contribute to an emerging understanding of lateral enamel formation in Neandertals vs. modern humans. Journal of Human Evolution, 54, 236–250.
Guatelli-Steinberg, D., Reid, D. J., Bishop, T. A., & Larsen, C. S. (2005). Anterior tooth growth periods in Neanderthals were comparable to those of modern humans. Proceedings of the National Academy of Sciences of the United States of America, 102, 14197–14202.
Guatelli-Steinberg, D., Reid, D. J., & Bishop, T. A. (2007). Did the lateral enamel of Neandertal anterior teeth grow differently from that of modern humans? Journal of Human Evolution, 52, 72–84.
Kronfeld, R. (1939). Histopathology of the teeth and their surrounding structures. Philadelphia: Lea & Febiger.
Liversidge, H. (2003). Variation in modern human development. In J. L. Thompson, G. E. Krovitz, & A. J. Nelson (Eds.), Patterns of growth and development in the Genus Homo (pp. 73–113). Cambridge: Cambridge University Press.
Liversidge, H. (2008). Timing of human mandibular third molar formation. Annals of Human Biology, 35, 294–321.
Macchiarelli, R., Bondioli, L., Debénath, A., Mazurier, A., Tournepiche, J.-F., Birch, W., & Dean, C. (2006). How Neanderthal molar teeth grew. Nature, 444, 748–751.
Mann, A., & Vandermeersch, B. (1997). An adolescent female Neandertal mandible from Montgaudier Cave, Charente, France. American Journal of Physical Anthropology, 103, 507–527.
Mann, A. E., Monge, J. M., & Lampl, M. (1991). Investigation into the relationship between perikymata counts and crown formation times. American Journal of Physical Anthropology, 86, 175–188.
Nunes, E., de Moraes, I., Novaes, P., & de Sousa, S. (2002). Bilateral fusion of mandibular second molars with supernumerary teeth: Case report. Brazilian Dental Journal, 13, 137–141.
Olejniczak, A. J., Grine, F. E., & Martin, L. B. (2007). Micro-computed tomography of primate molars: Methodological aspects of three-dimensional data collection. In S. E. Bailey & J.-J. Hublin (Eds.), Dental perspectives on human evolution: State of the art research in dental paleoanthropology (pp. 103–116). Dordrecht: Springer.
Ramirez Rozzi, F. (2005). Age at death of the Neanderthal child from Hortus. Bulletins et mémoires de la Société d’anthropologie de Paris, 17, 47–55.
Ramirez-Rozzi, F. V. (1993a). Microstructure and development of the enamel tooth of the Neanderthal from Zafarraya, Spain. Comptes Rendus de l’Academie des Sciences, 316, 1635–1642.
Ramirez-Rozzi, F. V. (1993b). Tooth development in East African Paranthropus. Journal of Human Evolution, 24, 429–454.
Ramirez Rozzi, F. V., & Bermudez de Castro, J. M. (2004). Surprisingly rapid growth in Neanderthals. Nature, 428, 936–939.
Reid, D., & Dean, M. C. (2006). Variation in modern human enamel formation times. Journal of Human Evolution, 50, 329–346.
Reid, D. J., & Ferrell, R. (2006). The relationship between number of striae of Retzius and their periodicity in imbricational enamel formation. Journal of Human Evolution, 50, 195–202.
Reid, D. J., Beynon, A. D., & Ramirez-Rozzi, F. V. (1998). Histological reconstruction of dental development in four individuals from a medieval site in Picardie, France. Journal of Human Evolution, 35, 463–477.
Reid, D. J., Guatelli-Steinberg, D., & Walton, P. (2008). Variation in modern human premolar enamel formation times: Implications for Neanderthals. Journal of Human Evolution, 54, 225–235.
Sasaki, C., Suzuki, K., Mishima, H., & Kozawa, Y. (2002). Age determination of the Dederiyeh 1 Neanderthal child using enamel cross-striations. In T. Akazawa & S. Muhesen (Eds.), Neanderthal burials: Excavations of the Dederiyeh Cave Afrin, Syria (pp. 263–267). Kyoto: International Research Center for Japanese Studies.
Smith, B. H. (1991). Standards of human tooth formation and dental age assessment. In M. A. Kelley & C. S. Larsen (Eds.), Advances in dental anthropology (pp. 143–168). New York: Wiley-Liss.
Smith, T. M. (2008). Incremental dental development: methods and applications in hominoid evolutionary studies. Journal of Human Evolution, 54, 205–224.
Smith, T. M., & Reid, D. J. (2009). Temporal nature of periradicular bands (“striae periradicales”) on mammalian tooth roots. In: T. Koppe, G. Meyer, & K.W. Alt (Eds.), Comparative Dental Morphology (pp. 86–92). Basel: Karger.
Smith, T. M., Tafforeau, P. T., Reid, D. J., Grün, R., Eggins, S., Boutakiout, M., & Hublin, J.-J. (2007a). Earliest evidence of modern human life history in North African early Homo sapiens. Proceedings of the National Academy of Sciences of the United States of America, 104, 6128–6133.
Smith, T. M., Toussaint, M., Reid, D. J., Olejniczak, A. J., & Hublin, J.-J. (2007b). Rapid dental development in a Middle Paleolithic Belgian Neanderthal. Proceedings of the National Academy of Sciences of the United States of America, 104, 20220–20225.
Smith, T. M., Reid, D. J., Dean, M. C., Olejniczak, A. J., Ferrell, R. J., & Martin, L. B. (2007c). New perspectives on chimpanzee and human dental development. In S. E. Bailey & J.-J. Hublin (Eds.), Dental perspectives on human evolution: State of the art research in dental paleoanthropology (pp. 177–192). Dordrecht: Springer.
Smith, T. M., Tafforeau, P., Reid, D. J., Pouech, J., Lazzari, V., Zermeno, J. P., Guatelli-Steinberg, D., Olejniczak, A. J., Hoffman, A., Radovcic, J., Masrour, M., Toussaint, M., Stringer, C. & Hublin, J-J. (2010). Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proceedings of the National Academy Sciences of the United States of America 107, 20923–20928.
Smith, T. M., Harvati, K., Olejniczak, A. J., Reid, D. J., Hublin, J.-J., & Panagopoulou, E. (2009). Brief communication: Dental development and enamel thickness in the Lakonis Neanderthal molar. American Journal of Physical Anthropology, 138, 112–118.
Stringer, C. B., & Dean, M. C. (1997). Age at death of Gibraltar 2 – a reply. Journal of Human Evolution, 32, 471–472.
Stringer, C. B., Dean, M. C., & Martin, R. D. (1990). A comparative study of cranial and dental development within a recent British sample and among Neandertals. In C. J. De Rousseau (Ed.), Primatelife history and evolution (pp. 115–152). New York: Wiley-Liss.
Tillier, A. M. (2000). Neanderthal ontogeny: a new source for critical analysis. Anthropologie, XXXVIII(1), 109–120.
Tsesis, I., Steinbock, N., Rosenberg, E., & Kaufman, A. Y. (2003). Endodontic treatment of developmental anomalies in posterior teeth: Treatment of geminated/fused teeth- report of two cases. International Endodontic Journal, 36, 372–379.
Turell, I., & Zmener, O. (1999). Endodontic management of a mandibular third molar fused with a fourth molar. International Endodontic Journal, 32, 229–231.
Acknowledgements
The authors acknowledge the excavators of Obi-Rakhmat: Andrei Krivoshapkin, Patrick Wrinn, Anatoly Derevianko, and the rest of the Obi-Rakhmat team. We appreciate the comments of two reviewers, as well as the invitation to contribute to this volume by Silvana Condemi. Debbie Guatelli-Steinberg also provided helpful assistance by making comparative data available. Funding was provided by the Max Planck Society, the EVAN Marie Curie Research Training Network MRTN-CT-019564, and Harvard University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Smith, T.M. et al. (2011). Dental Development and Age at Death of a Middle Paleolithic Juvenile Hominin from Obi-Rakhmat Grotto, Uzbekistan. In: Condemi, S., Weniger, GC. (eds) Continuity and Discontinuity in the Peopling of Europe. Vertebrate Paleobiology and Paleoanthropology. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0492-3_13
Download citation
DOI: https://doi.org/10.1007/978-94-007-0492-3_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0491-6
Online ISBN: 978-94-007-0492-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)