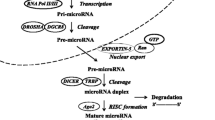
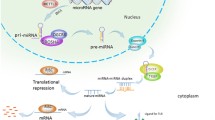
Abstract
MicroRNAs (miRNAs) are small regulatory RNAs involved in post-transcriptional gene regulation of molecular pathways related with differentiation, proliferation, and survival of cells. Normal lymphocytes are not an exception, and even relevant molecular pathways involved in the immunologic functions of these cells are also controlled by miRNAs. These important roles make miRNA alterations to be oncogenetic mechanisms with multiple targeting roles in lymphomagenesis. Among miRNA alterations, mutations in mature and pre-miRNA forms have been punctually described, but the most well-known miRNA alteration mechanisms are changes in their expression levels. The origin of these expression abnormalities seems to be diverse regarding the considered particular miRNA and lymphoma entity. Thus, some miRNAs have been described as targets of genomic instability affecting miRNA containing chromosomal loci. On the reverse side, other miRNA expression changes seem to involve epigenetic alterations or other molecular mechanisms not related with direct alterations of their loci. Regardless of their alteration mechanisms, miRNA expression profiles have been shown useful for improvement of clinical management of the patients, through their good performance in the diagnosis discrimination among types and subtypes of lymphomas. In addition to the biological features of these neoplasms, miRNA expression profiles have also proven to help in the identification of patient subsets with different prognosis outcomes. Taking together all these findings, we can foresee an increasing importance of the miRNA-based translational research in practical applications to the lymphoma clinical management and hopefully, even help in achieving more specific treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple Myeloma
- Chronic Lymphocytic Leukemia
- Mantle Cell Lymphoma
- Burkitt Lymphoma
- Chronic Lymphocytic Leukemia Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 MicroRNAs in the Normal Physiology of Lymphoid Cells
11.1.1 As Regulators of Lymphoid Cell Development in the Primary Lymphoid Organs
Systematic analyses on microRNA (miRNA) expression in mammalian hematopoiesis have revealed distinct profiles in the different related cell types, with specific sets of miRNAs dynamically regulated during T and B cell development (Chen et al. 2004; Chen and Lodish 2005; Ramkissoon et al. 2006; Sonkoly et al. 2008). Even before the stage of the common lymphoid (B/T) progenitor, several miRNAs are involved in the generation of these cells from hematopoietic stem cells in the bone marrow (as miR-221, miR-222, miR-196b, miR-126, and miR-10 family members) through their action on relevant targets as KIT, PLK2, and several HOX family members (O’Connell et al. 2010) (Fig. 11.1).
11.1.2 In B Cells
MiRNAs from the miR-17-92 cluster are essential for the pro-B to pre-B transition, enhancing the survival of the B cells at this stage by targeting transcripts of the pro-apoptotic protein BIM, and the tumor suppressor PTEN (Xiao et al. 2008). MiR-150 has also a role during the B cell development, but blocks the proB-preB transition if its expression is forced at this stage, and involving the targeting on the MYB transcription factor (Xiao et al. 2007; Zhou et al. 2007). Constitutive expression of miR-34a also have a blocking effect at this stage, as a result of targeting on FoxP1 transcription factor, that regulates the expression of the relevant immunoglobulin differentiation genes Rag1 and Rag2 (O’Connell et al. 2010).
11.1.3 In T Cells
MiR-17-92 cluster expression has been described to be essential for T cell proliferation and survival, sharing some targets as for B cell development (Xiao et al. 2008). MiR-181a has been also characterized to regulate relevant signal transduction pathways (DUSP5, DUSP6, SHP2, and PTPN22 genes involved in the TCR response and CD4+ T cell selection) in the T cell development (Li et al. 2007).
11.2 As Regulators of Immunologic Function of the Mature Lymphoid Cells
As mediators of the acquired immune response, the B and T cell immunological functions are also modulated by miRNA action in these cells (Tsitsiou and Lindsay 2009). Several relevant examples are highlighted below (Fig. 11.1).
11.2.1 In B Cells
MiR-155 participates in many aspects of the B cell functionality and thus, its expression in these cells is under the control of B cell receptor, CD40 and Toll-like receptors (Rodriguez et al. 2007; Thai et al. 2007; van den et al. 2003). In B cells, miR-155 regulate targets like c-MAF, PU.1/Sfpi1, and activation-induced cytidine deaminase (AID) (Dorsett et al. 2008; Rodriguez et al. 2007; Teng et al. 2008; Thai et al. 2007; van den et al. 2003; Vigorito et al. 2007). AID is essential in the B cell maturation of the antibody response through the induction of antigen-dependent hypermutations in the immunoglobulin heavy chain (IgH) gene during the secondary immunologic response. Thus, differentiation of naïve B cells to centroblasts in the germinal center (GC) of lymphoid follicles is characterized by marked changes in miRNA expression profile, while naïve and memory B cells showed marked similarities (Malumbres et al. 2009). Among these, miR-125b repress PRDM1 and IRF4, two essential factors of the post-GC, but not BCL6, a key transcription factor of this process (Malumbres et al. 2009). In addition, miR-223 represses LMO2, and miR-9/miR-30 family regulate PRDM1/BLIMP1 gene expression, a master gene in the GC-plasma cell transition (Calame 2008; Zhang et al. 2009). Finally, in the post-GC B cell maturation either to plasma cell as to memory B cell, it has been described a modulation of a number of miRNAs. Thus, plasma cells showed 31 miRNAs highly expressed and 27 in memory B cells compared to GC cells (Zhang et al. 2009).
11.2.2 In T Cells
MiR-155 is essential for the T cell dependent antibody response in the lymphoid germinal centers (Thai et al. 2007), and it is involved in T cell subpopulation (TH1/TH2) differentiation and Treg homeostasis through targets like c-MAF and SOCS1 in response to CD3/CD28 and FoxP3 signaling (Lu et al. 2009; Rodriguez et al. 2007). miR-326 has been described to be involved in the TH17 cell subpopulation through targeting of ETS1 gene (Du et al. 2009).
11.3 Oncomirs with Pathogenetic Implications in Lymphomas
Mechanisms of miRNA alteration:
11.3.1 Expression Deregulation as a Result of Genomic Instability
11.3.1.1 Involving Gains or Losses of MiRNA Chromosomal Loci
As a deregulation mechanism of miRNA expression levels, imbalances of chromosomal regions have been demonstrated to target miRNAs in several types of neoplasm. In addition, several examples also involving lymphomas are explained below.
MiR-15a/16-1 cluster is located at 13q14.3 locus, the most frequent chromosomic alteration (over 65%) in chronic lymphocytic leukemia (CLL), and this alteration was also present in 50% of mantle cell lymphoma (MCL) and 16–40% of multiple myeloma (MM). In fact, these miRNAs are the most consistent oncogenic targets inside this deletion, included in the genomic locus DLEU2 (Klein et al. 2010; Lerner et al. 2009), besides from another recently described cancer-related locus (DLEU7) in this chromosomic region (Palamarchuk et al. 2010).
MiR-17-92 cluster comprises six precursor miRNAs that are codified inside a non-protein-coding gene MIR17HG/MIRHG1 (formerly named as C13orf25) located at 13q31.3. This region is frequently amplified in several types of lymphomas, including Burkitt lymphoma (BL), diffuse large B cell lymphoma (DLBCL) and MCL (Bea et al. 2009; Ota et al. 2004; Tagawa and Seto 2005), where an associated over-expression of the involved miRNAs has also been found, suggesting that high-level amplification is the main source of oncogenic miR-17-92 cluster over-expression in these lymphomas (Navarro et al. 2009a; Tagawa et al. 2007) (Fig. 11.2).
Box plot representing the relative expression levels in relative units (RU) of several miRNAs at 13q31 and their copy number changes in examined nodal mantle cell lymphoma cases. All 5 miRNAs showed a significant correlation between the expression levels quantified by reverse transcriptase-looped quantitative PCR and the gene dosage of this locus according to comparative genomic hybridization results. Loss (LOSS), wild-type (WT), and amplification (AMP). Circles and asterisks, extreme values and outliers, respectively. Reprinted with permission from Navarro et al. (2009a) (corresponding to the original Fig. 4)
Finally, several miRNAs have been described in MM to be associated with loss of heterozygosity, or copy number alterations at their chromosomal loci (Lionetti et al. 2009).
11.3.1.2 Involving Epigenetic Mechanisms Acting on MiRNA Genes
A proportion of miRNA promoters seem to be regulated by epigenetic mechanisms and related alterations could lead to oncogenic effects (Guil and Esteller 2009). One example is miR-203, that it has been found down-regulated in T cell lymphomas, among other hematological disorders, and targeting the ABL oncogene (Bueno et al. 2008). The expression down-regulation mechanism found was the deletion of one allele (at 14q32) and CpG hypermethylation in the promoter of the remaining allele (Bueno et al. 2008). Another example is miR-124a that it has been shown to be regulated by CpG methylation in several human tumors, including lymphomas (Lujambio et al. 2007). Other examples are also further explained in the text.
11.3.1.3 Involving Mutation of MiRNA Genes
Mutations in miRNA loci could result in alterations of sequence-specific recognition of their targets. Germ-line and somatic miRNA mutations were found in 12% of sequenced miRNAs and in 15% of CLL patients analyzed. Noticeably, no such mutations could be found in a large series of normal controls, and the majority of patients showing these mutations in the mR-15a/16-1 locus had a personal or family history of CLL or other hematopoietic and solid tumors, suggesting a possible role of this miRNA cluster in familial CLL (Calin et al. 2005). Nevertheless, mutations of this miRNA have not been found in later studies on sporadic CLL among other neoplasms (Yazici et al. 2009).
11.3.2 Expression Deregulation as a Result of Virus Infection
Epstein-Barr virus (EBV) infects B cells and after an initial phase establishes an asymptomatic latent infection. In addition, EBV encoded factors are related with the oncogenesis of several lymphoid models, although the factors that are conditioning their development are not fully understood (De Falco et al. 2009). MiRNA expression is involved in this process by EBV-encoded miRNAs through which cellular mRNAs could be manipulated, and also more complex molecular mechanisms including the interference with endogenous RNAi machinery (Scaria et al. 2007). The resulting effect of the EBV infection is a global down-regulation of many cellular miRNAs, although re-expression of some of them occurs when the cells are immortalized, as described for miR-155 (Godshalk et al. 2008; Lu et al. 2008). This miRNA is also up-regulated by the EBV-encoded latent membrane protein 1 (LMP1) in infected Burkitt lymphoma cell lines (Rahadiani et al. 2008; Yin et al. 2008), and it has been shown that contributes to EBV immortalization and its oncogenic potential by modulation of the NF-κB signaling (Lu et al. 2008). It also seems to have a cytostatic activity in BL cells. The underlying molecular mechanisms of the growth inhibitory property of LMP1 seems to involve its ability to down-regulate a major oncogene, TCL1 in DLBCL and BL cells by inducing miR-29b transcript expression (Anastasiadou et al. 2009). The underlying mechanism for this effect involves p38 mitogen-activated protein kinase-activating function of LMP1. As LMP1 is also important for B cell transformation, the final effect on lymphomagenesis may depend on a combination of levels of its expression, lineage and differentiation of the target cells and also of the miRNA expression context of the host cells. Another described miRNA induced by LMP1 in BL cell lines is miR-146a, and the authors proposed that this miRNA should plays a role in EBV latency by modulating innate immune responses to the virus-infected cell (Motsch et al. 2007). As it has been described that miR-146a negatively regulates IRF7, an activator of the LMP1 promoter, this miRNA may function as a negative feedback control to modulate the transforming potential of EBV (Cameron et al. 2008; Li et al. 2010). In addition, miR-21 is induced by EBV infection in human B cells (Mrazek et al. 2007), and it has been found over-expressed in a significant proportion of EBV-positive natural killer (NK)/T cell lymphomas, where targeting over the anti-apoptotic transcript PTEN was also demonstrated (Yamanaka et al. 2009). Finally, EBV influence in the expression of a 10 miRNA host subset could be demonstrated in classical Hodgkin’s lymphoma (cHL) (Navarro et al. 2008). Regarding EBV-mediated effects on lymphocyte differentiation program, it has been described altered levels of the transcription factors BLIMP1 and XBP1 by miR-127 in EBV-positive BL.
Regarding miRNA influence of other lymphoma-related viruses, the reticuloendotheliosis virus strain T (REV-T) induces B cell lymphomas through up-regulation of miR-155 resulted in a prosurvival effect by down-regulation of JARID2/Jumonji, a cell cycle regulator part of a histone methyltransferase complex (Bolisetty et al. 2009; Yin et al. 2008). In addition, human herpesvirus 8/Kaposi’s sarcoma-associated herpesvirus (KSHV) has been characterized as the etiologic agent associated, among others, with primary effusion lymphoma (PEL). This virus encodes orthologue miRNAs for the control of relevant host targets, as KSHV miR-K12-11 that has a common sequence and targets compared to the cellular miR-155 (Gottwein et al. 2007; Skalsky et al. 2007).
11.4 As Regulators of Known Oncogenes and Tumor Suppressor Genes
Several examples of both miRNA types are explained below and summarized in Table 11.1.
11.4.1 Tumor Suppressor MiRNAs
MiRNAs encoded by the miR-15a/16-1 cluster are known to act as tumor suppressors and, in fact, it were the first miRNAs to be found experimentally related to mammalian carcinogenesis. Down-regulation of these miRNAs has been reported in CLL and other neoplasms (Aqeilan et al. 2010). In normal cells, the expression of these miRNAs inhibits cell proliferation, promotes apoptosis of cancer cells, and suppresses tumorigenicity through their suppressive action on cancer-related targets like BCL2, WT1, WNT3A, CCND1, MCL1, ETS1, RAB9B, and PDCD6IP (Aqeilan et al. 2010; Calin et al. 2008). It has been proposed that the high relevance of miR-15a/16-1 in CLL lymphomagenesis could be mainly resumed by its causal role in BCL2 over-expression in a proportion of these lymphomas that harbor 13q14 deletions (Cimmino et al. 2005). The experimental evidence showed that the expression of BCL2 and these miRNAs are inversely correlated in CLL tumors, and in vitro apoptosis could be induced by BCL2 repression through these miRNAs (Cimmino et al. 2005).
As above mentioned, CCND1 is a validated miR-16-1 target. The over-expression of this oncogene is the hallmark of MCL. The t(11;14)(q13:q23) is the primary genetic alteration that induced the CCND1 over-expression by means of the promoting influence of the IgH enhancer onto the CCND1 promoter. It has been also described in MCL other molecular alterations in additional layers of CCND1 expression control. One of these mechanisms involves the post-transcriptional regulation of CCND1 by means of several miRNAs like miR-19a, miR-155, miR-503, miR-424, miR-195, miR-34a, miR-15a, and miR-16-1 (Jiang et al. 2009). It has been described that point mutations and genomic deletions affecting the 3′UTR of CCND1 resulted in increased proliferation and shorter survival (Wiestner et al. 2007). A miR-16-1 orientated study has demonstrated in MCL cell lines that these truncated CCND1 forms lack the miR-16-1 binding sequences in the CCND1 3′UTR (Chen et al. 2008). The proposed oncogenic effect of this alteration is similar to other described examples involving different mechanisms that also generate shorter CCND1 transcript forms which are associated with a higher mRNA stability (Wiestner et al. 2007).
Another lymphoid neoplasm showing down-regulation of miR-15a/miR-16 is MM (Roccaro et al. 2009). In this model, the mentioned miRNAs regulate proliferation and induced angiogenesis, and the gene targets that could be related with these miRNAs were AKT3, S6, MAP kinases and the NF-κB-activator MAP3KIP3 (Roccaro et al. 2009). Although, these miRNAs were located in 13q14, a region frequently deleted in MM, their low expression was also detectable in cases without this chromosome imbalance.
Expression levels of miR-143 and miR-145 have been found decreased in CLL, DLBCL, EBV-transformed B cell lines, and BL cell lines (Akao et al. 2007). In this study, introduction of precursor forms of these miRNAs into Raji cells resulted in a significant growth inhibition that occurred in a dose-dependent manner. One target gene of miR-143 was determined to be the extracellular signal-regulated kinase 5 (ERK5), involved in mitogen-activated protein kinase proliferation pathways, and also previously found to be targeted by this miRNA in colorectal carcinomas and colon cancer cell lines (Akao et al. 2006; 2007). Another work has showed that DNA methyltransferase 3 (DNMT3A) is another target of miR-143 in colorectal cancer (Ng et al. 2009), suggesting that the down-regulation of miR-143 could impair the activation of tumor suppressor genes by demethylation. Thus, it could be very interesting to confirm the existence of this oncogenic mechanism in the miR-143-down-regulated lymphomas.
Down-regulation of miR-29 family members (miR-29-a2, miR-29-b2, and miR-29c) have been described associated with the unmutated-IgH poor prognosis CLL subgroup (Calin et al. 2005). This association was also confirmed in other CLL series (Fulci et al. 2007; Marton et al. 2008; Stamatopoulos et al. 2009). Two demonstrated oncogenes that are target of several miR-29 family members are TCL1 (as also showed for miR-181b) and MCL1, acting both in PI3K/Akt survival pathways (Mott et al. 2007; Pekarsky et al. 2006). In addition, TCL1 transgenic mice over-expressing this gene in B cells developed phenotypic changes very similar to that seen in CLL, supporting the pathogenetic role of this gene, and thus of miR-29b and miR-181b in CLL (Bichi et al. 2002). Expression down-regulation of miR-29a and miR-29b-1 were also described to be associated with 7q32 deletion in splenic marginal zone lymphoma (SMZL). Finally, it has been shown that miR-29a/b/c down-regulation in MCL involved their targeting on CDK6 (Zhao et al. 2010).
MiR-34 family is composed by miR-34a and miR-34b/c that are tumor suppressor miRNAs induced by p53 (Hermeking 2009). Thus, p53 alterations are one of the known miR-34 family inactivating mechanisms. Demonstrated target genes for these miRNAs included many oncogenes (CCND1, CDK4/6, CCNE, E2F3, and c-Myc among others) (Hermeking 2010). In addition, chromosomal losses including the coding region of these miRNAs (miR-34a at 1p36 and miR-34b/c at 11q23) are another possible mechanism of alteration of their expression. In CLL, it has been shown that miR-34a is a part of the p53 response network, thus low levels of this miRNA caused by p53 abnormalities (17p deletion and point mutations) are associated with an impaired DNA damage response and chemotherapy resistance (Zenz et al. 2009). In addition, some CLL cases also showed low basal miR-34a levels in absence of p53 mutations (Zenz et al. 2009). A possible repression mechanism for these cases could be mediated by promoter hypermethylation. This mechanism has been also previously described in many tumor types, also including non-Hodgkin’s lymphomas like extranodal NK/T (44%) as the most frequent non-Hodgkin’s lymphoma with this alteration (Chim et al. 2010). As mentioned before, miR-34b/c are located at 11q23, a chromosomal region deleted in 10–32% of CLL cases and that it is associated with patients with a poor outcome (Seiler et al. 2006). ATM gene has been previously characterized as a relevant target of this chromosomal alteration in CLL. Nevertheless, miR-34b/c locus has also been found frequently deleted in 11q- CLL cases (Cardinaud et al. 2009; Lehmann et al. 2008). This is a remarkable observation taking in account that the same authors showed that miR-34b-5p regulates the TCL1 expression that is, as already mentioned, a very relevant gene in CLL pathogenesis (Cardinaud et al. 2009). In addition to gene deletion, it has been also described that expression of pre-miR-34b/c is also regulated at methylation level, suggesting an alternative inactivation mechanism of these miRNAs in CLL that deserve further exploration (Toyota et al. 2008).
MiR-135a down-regulation in cHL has been showed to be involved in the JAK2 expression regulation (Navarro et al. 2009b). Concordantly with the pathogenetic role of this gene in cHL, chromosomal gains and amplifications involving JAK2 gene have been also described in these lymphomas, leading to the same prosurvival effect of this miRNA alteration.
MiR-26a has been identified to be repressed by c-Myc in primary BL cells and in a murine B cell c-Myc-over-expressing mouse model (O’Donnell et al. 2005; Sander et al. 2008). This miRNA has been showed to directly regulate EZH2 expression (Sander et al. 2008). EZH2 is member of the Polycomb repressive complex 2 that resulted in epigenetic silencing of genes involved in tumor suppression and differentiation (Sander et al. 2009). Thus, c-Myc-dependent repression of this post-transcriptional repressor of EZH2 constitutes another transforming mechanism of c-Myc in BL. This kind of transforming mechanism involving c-Myc-dependent repression of miRNA expression has been also described involving miR-23a/b, a negative regulator of GLS2 that codifies for mitochondrial glutaminase, and resulting in a convenient enhancement of energetic metabolism for the proliferating B cells (Gao et al. 2009).
MiR-127 was shown to regulate BCL6 expression in a BL cell line, suggesting a possible pathogenetic role in lymphomas of germinal center origin (Saito et al. 2006). Interestingly, the expression of this miRNA has described to have clinical impact in DLBCL (Roehle et al. 2008). Epigenetic changes are also described to regulate the expression of this miRNA (Saito et al. 2006). Finally, its expression seem to be modulated in different anatomic locations of DLBCL, being higher in testicular than in central nervous system or nodal DLBCL (Robertus et al. 2009).
11.4.2 Oncomirs
The oncogenic role of miR-155 was discovered from the precursor transcript corresponding to the BIC locus, that was first observed to cooperate with c-Myc in chicken B cell lymphomas induced by avian leukosis proviral integrations (Clurman and Hayward 1989). Its over-expression in transgenic lymphoma prone mice accelerates the death rate for malignancy (Costinean et al. 2006). In human B-lymphomas, miR-155 expression is very reduced in BL (Kluiver et al. 2006), but it is highly over-expressed in cHL and another non-Hodgkin’s lymphomas such primary mediastinal B cell lymphoma (PMBCL) and DLBCL of poor prognosis activated B cell (ABC) subtype (Eis et al. 2005; Kluiver et al. 2005). ABC subtypes of DLBCL are characterized by constitutive activation of NF-κB pathway. Concordantly, this pathway is also targeted by miR-155 through SMAD5 down-regulation, although the detailed mechanism by which this alteration contributes to DLBCL pathogenesis it is not fully understood (Rai et al. 2008; 2010). In addition, miR-155 is also frequently over-expressed in NK/T cell lymphomas, where this miRNA targets the inositol phosphatase transcript SHIP1, that it is involved in Akt signaling, and affecting the apoptotic activity of these cells (Yamanaka et al. 2009). This targeting on SHIP1 was also described in DLBCL, where it could be demonstrated that high levels of miR-155 were caused by autocrine stimulation by TNFα (Pedersen et al. 2009). Other potential targets of miR-155 have been demonstrated, and many of them are relevant either for T and B cell functionality.
It has been hypothesized that AID miss-expression could be a source of oncogenic mutations, and the fact that miR-155 inhibits the expression of AID suggested a tumor suppressor activity of this miRNA in B cells. In fact, a relationship of miR-155 levels with AID-induced oncogenic reciprocal chromosomal translocations between IgH and c-Myc genes has been described (Dorsett et al. 2008). Nevertheless, as AID activity is essential for B cell maturation, it seems that the relationship of miR-155 levels with AID or even other targets should be controlled in a certain range to avoid oncogenic effects (Tili et al. 2009). Noticeably, induced miR-155 levels are higher and transient in physiological situations than those found in lymphomas. The time limited dynamics of miR-155 up-regulation found in normal lymphocytes is overcame in lymphoid neoplasms probably due to the insufficient miRNA levels to generate a putative inhibitory feedback loop (as the blocking of its own transcription) (Tili et al. 2009).
MiR-17-92 cluster includes six mature miRNAs (miR-17-5p, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92-1). Transfection of the vertebrate-specific miR-17-miR-19b-1 section showed oncogenic cooperation with c-Myc over-expression in transgenic mice models (He et al. 2005; Tagawa et al. 2007). Further experiments have demonstrated that the rationale for this cooperation consisted in the requirement of miR-17-92 activity to suppress apoptosis in c-Myc-driven B cell lymphomas (Mu et al. 2009). Moreover, miR-19a and miR-19b are necessary and sufficient for this oncogenic prosurvival activity, targeting proapoptotic genes as PTEN (Mu et al. 2009). In addition, BIM proapoptotic gene has also been showed to be targeted by miR-19 and miR-92 in experimental lymphoid mice models and in primary samples of MM (Pichiorri et al. 2008; Xiao et al. 2008). In fact, the relationship of miR-17-92 cluster with c-Myc is very complex. First, c-Myc oncogene induces the expression of miR-17-92 cluster by direct binding to regulatory sequences therein (O’Donnell et al. 2005). Second, miR-17-5p and miR-20 negatively regulate the c-Myc -target gene E2F1. In addition, E2F3 and, in a lesser extent, E2F1 can also activate the transcription of the miR-17-92 cluster (Sylvestre et al. 2007; Woods et al. 2007), in a complex circuitry with an important role in the control of proliferation and apoptosis (Aguda et al. 2008). Moreover, the alteration of a miR-17-92 cluster-mediated E2F1 regulation seem to affect the expression timing of this protein along the cell cycle, and leading to accumulation of DNA double-strand breaks by disruption of the E2F1-dependent G1-cell cycle arrest (Pickering et al. 2009). Finally, among other specific targets of the miR-17-92 components, it could be mentioned that the G1-S checkpoint cyclin-dependent kinase inhibitor CDKN1A has been found to be repressed by miR-17-5p and miR-20a (Cloonan et al. 2008; Pickering et al. 2009). In fact, this target was considered the main oncogenic mechanism underlying the miR-17-92 over-expression in a MCL cell line (Inomata et al. 2009). miR-17-5p and miR-20a expression were also found to show oncogenic cooperation with c-Myc in primary samples of MCL (Navarro et al. 2009a) (Fig. 11.3). In MM, miR-19a and miR-19b have been shown to target SOCS1, a negative regulator of IL-6R/STAT3 pathway giving a role of this miRNA within the anti-apoptotic signal of IL-6 in these lymphomas (Pichiorri et al. 2008).
Overall survival of 50 patients with nodal MCL according to the combined expression of c-Myc and miR-17-5p/miR-20a. Mantle cell lymphoma patients with concomitant high expression of both c-Myc and miR-17-5p/miR-20a (n = 8; line A) have a significant shorter overall survival than patients with high expression of only one (n = 17; fail 5; line B) or none (n = 25; fail 10; line C) of these factors (p = 0.025, log-rank test). Reprinted with permission from Navarro et al. (2009a) (corresponding to the original Fig. 5)
Heat map representation of the significant miRNA expression differences in paired samples of purified tumor cells from simultaneous matched peripheral blood (PB) and tumor lymph nodes (NODE) of two patients (A and B). Eleven miRNAs were found differentially expressed in the paired significance analysis of microarrays of expression levels between the two tumor cell populations from these two patients. Relative expression was obtained in reference to CD5+ control cells but, additionally for each miRNA, its expression in the nodal samples has been normalized in reference to the peripheral blood values for a more clear representation. Reprinted with permission from Navarro et al. (2009a) (corresponding to the original Fig. 2)
MiR-181a over-expression was found to be associated with the pathogenesis of several lymphoid neoplasms. First, it was described to belong to a miRNA signature over-expressed in a subset of CLL patients with a faster development of clinical features that made need to start treatment, thus suggesting an oncogenic action of miR-181a in this lymphoma (Calin et al. 2005). However, the oncogenic zinc-finger transcription factor PLAG1 was found to be one of the repressed targets of this miRNA in CLL (Pallasch et al. 2009). Marton et al. (2008) also found down-regulation of this miRNA in CLL compared to normal B cells. These results stressed the complex role of this and other miRNAs for which oncogenic and tumor suppressor features seem to coexist, even in a same neoplastic entity. This phenomenon could also be reflecting the different molecular context in which the miRNA expression must be considered. Thus, miR-181a expression levels have opposite trends in the progression of CLL depending on the karyotype, as high expression of this miRNA was associated with disease progression in CLL patients with trisomy 12, whereas aggressive CLL forms with 17p deletion corresponded to cases with lower levels of miR-181a expression (Visone et al. 2009). Over-expression of this miRNA was also found to be present in MM and oncogenic features could be demonstrated for this alteration (Pichiorri et al. 2008). In this work, a positive regulator of p53 was established to be an oncogenic target of miR-181a together with other as miR-181b, miR-32, miR-106b, miR-25, and miR-93 (Pichiorri et al. 2008). This target was the p300-CBP-associated factor (PCAF) that is a histone acetyl-transferase involved in acetylation-dependent p53 regulation and that, in addition, affect the levels of p53 through its intrinsic ubiquitination activity controlling Hdm2 protein levels (Linares et al. 2007; Schiltz and Nakatani 2000). MiR-181a expression levels have also been elevated in a distinct biological subset of MCL showing unmutated IgH genes, higher proliferation status and higher levels of chromosomal instability (Navarro et al. 2009a). The mechanisms of miR-181a expression modulation in all these lymphomas are not fully understood. For instance, CpG methylation status seem to be very different among individual of CLL cases, at least for the miR-181a2 subtype (Pallasch et al. 2009).
MiR-221 and miR-222 have been found to regulate CDKN1B in CLL in vitro (Frenquelli et al. 2010). In primary CLL samples, the levels of these miRNAs and the CDKN1B were inversely correlated and, interestingly, high miR-221/222 levels were found in lymph nodes/bone marrow compared to peripheral blood CLL samples from the same patients. These findings suggest a relevant role of the miR-dependent post-transcriptional regulation of CDKN1B in the proliferation status of CLL (Frenquelli et al. 2010).
MiR-9 and let-7a have been described to down-regulate PRDM1/BLIMP1 gene (Nie et al. 2008), the previously mentioned master regulator of plasma cell differentiation, and that it has been often found inactivated by other mechanisms in DLBCL (Parekh et al. 2007; Pasqualucci et al. 2006). It has been proposed for cHL, that precursors of the Reed-Sternberg cells (RSC) are initiating a plasma cell differentiation program through initial action of PRDM1/BLIMP1 but the up-regulation of these miRNAs aborted that genetic program by blocking the accumulation of these transcripts (Nie et al. 2008).
11.5 Oncomirs with Biomarker Implications in Lymphomas
11.5.1 Using MiRNA Expression for Lymphoma Diagnosis Improvement
11.5.1.1 In B/T Lymphomas
DLBCL is considered to be a highly heterogeneous disease that includes several subgroups either at biological and pathological levels (Friedberg and Fisher 2008). In addition, FL could also progress to a more aggressive DLBCL form. Gene-expression profiling allows differentiation into 3 major subgroups: ABC-DLBCL, germinal center B cell (GCB) DLBCL, and PMBCL. Several studies have shown that miRNA expression profiling is also different among these entities. Initially, several miRNAs were found to be differentially expressed between DLBCL and normal reactive lymph nodes, and between these tumors and FL (Roehle et al. 2008). The authors conclude that a decision tree using only 4 miRNAs (miR-330, miR-17-5p, miR-106a, and miR-210) is enough to differentiate between these three types of lymphoid tissues, although without a comprehensive consideration of the subtypes that exists inside these lymphomas. In this way, a later study also included DLBCL cases transformed from FL, in addition to the de novo DLBCL and other FL cases that did not progressed (Lawrie et al. 2009). Thus, the comparison between DLBCL and FL was then performed only considering de novo DLBCL and non-transformed FL. Twenty-six miRNAs were found to be differentially expressed between these two types of lymphoma, including 6 miRNAs encoded by the miR-17-92 and/or homologous clusters (Table 11.2). In addition, other miRNAs were also found to be differentially expressed between de novo and transformed DLBCL (14 miRNAs), and between non-transformed FL and the cases of this lymphoma type that underwent transformation (6 miRNAs) (Table 11.2) (Lawrie et al. 2009). Regarding DLBCL subtypes, current differentiation between GCB and non-GCB subtype without using gene expression profiling has been proposed by immuno-phenotyping with CD10, BCL6, and MUM1 antibodies (Hans et al. 2004), and this classification method was the used in the miR-related studies. Thus, differential expression of 26 miRNAs was found between the immuno-phenotyped groups (as GCB or non-GCB-like) was also found in the above mentioned work (Table 11.2) (Lawrie et al. 2009). However, even less miRNAs seem to be enough to separate these DLBCL subtypes, as has been showed in DLBCL-derived cell lines where 9 miRNAs where differentially expressed between the mentioned DBCL subtypes (Malumbres et al. 2009), including miR-155 and miR-21 that were also described to be highly expressed in the ABC-DLBCL subtypes in other previous works with cell lines (Table 11.2) (Lawrie et al. 2007; 2009; Rai et al. 2008). Moreover, these miRNAs could be also detected in the serum of DLBCL patients at significant different levels compared to healthy controls, suggesting that these types of non-invasive analysis are also possible in human lymphomas, or at least in DLBCL (Lawrie et al. 2008).
PEL is a unique type of DLBCL, and it has been shown to express a 26 miRNA signature compared to normal lymphoid tonsils (Table 11.2) (O’Hara et al. 2008). Among them, it could be found B cell lineage and B cell-lymphoma specific miRs. Although some genomic alterations were found to be associated with the miRNA expression changes, no one miRNA locus was consistently lost or amplified in all cases, thus suggesting that the PEL-specific miRNA signature arise from other mechanisms.
The oncogenic hallmark of BL is the c-Myc over-expression. As previously mentioned, c-Myc induces miR-17-92 expression and it is involved with this miRNA cluster in a complex regulatory loop that determines the deregulation of E2F1 normal expression with oncogenic impact in proliferation and apoptosis regulation (Sylvestre et al. 2007). In BL, c-Myc over-expression is mainly originated from translocations with immunoglobulin genes, but in a proportion of cases over-expression of this oncogene was present in c-Myc-translocation negative BL cases. Interestingly, there was a strong correlation of miR-34b down-regulation only in these c-Myc translocation-negative cases (Leucci et al. 2008). This result suggested a possible c-Myc expression control by this miRNA that was confirmed by in vitro increasing of a synthetic miR-34b leading to c-Myc expression modulation (Leucci et al. 2008). Noticeably, miR-34b down-regulation in the c-Myc translocation-negative BL cases was independent of p53 status, a previously demonstrated transcriptional inducer. This observation suggested that other mechanisms must be causing the miR-34b down-regulation in this subset of BL, probably by epigenetic mechanisms, as previously suggested (Toyota et al. 2008). In addition, there was a down-regulation of the let-7c miRNA in all BL cases analyzed, either with or without the c-Myc translocation, although it is not clear its pathogenetic meaning.
In CLL, it has been described several miRNAs that are differentially expressed in comparison with normal B cells (Calin et al. 2004). Using cloning approaches, miR-150, miR-21, and miR-155 were described to be significantly up-regulated and miR-92 down-regulated in this neoplasm compared to normal CD19+ blood cells (Fulci et al. 2007). A later study using a similar quantification technique also confirmed the over-expression of miR-155 compared to normal peripheral blood B cells but it could not confirm the remaining mentioned miRNAs (Marton et al. 2008). Another interesting published data about miR-150 and miR-155 in CLL is their inverse expression pattern in the proliferation centers of the CLL affected lymph nodes, revealed by in situ hybridization, and with miR-155 expressed in the proliferation centers whereas miR-150 is expressed outside these areas (Wang et al. 2008).
In MM, several studies have showed differentially expressed miRNAs between the tumor and normal plasma cells, although with some variations between them. Thus, over-expression of miR-181a/b in MM was described in two reports (Pichiorri et al. 2008; Roccaro et al. 2009), similarly to miRNAs of the paralog clusters miR-17-92, miR-106a-92, and miR-106b-25 (Pichiorri et al. 2008; Unno et al. 2009). Other miRNAs also showed to be over-expressed are miR-221, miR-222, miR-382 (Roccaro et al. 2009), as well as miR-21 and miR-32, among others (Pichiorri et al. 2008). On the other side, miR-328 (Pichiorri et al. 2008) as well as miR-15a and miR-16 down-regulation were also observed (Roccaro et al. 2009). In addition, other down-regulated miRNAs in MM have been also described (Unno et al. 2009). Finally, the miRNA cluster miR-193b-365 was identified to be expressed exclusively in MM (Unno et al. 2009).
Also in MM, association of different miRNA expression profiles have been described in relation with cytogenetic tumor subtypes (TC classification (Hideshima et al. 2004)), including over-expression of the clustered miR-99b, let-7e, and miR-125a-5p in the t(4;14) positive TC4 group, over-expression of miR-133b in t(14;16)/t(14;20) positive TC5 group, and the miR-582-5p over-expression in the t(11;14) TC1 group (Lionetti et al. 2009). Finally, in another work, miRNA expression profiles were also studied in different cytogenetic tumor subtypes of MM, but focusing at their differences regarding which miRNAs are differentially expressed compared to normal plasma cells (Gutierrez et al. 2010).
In MCL, there are two studies characterizing the miRNA expression in comparison to normal B cells. In the study of Zhao et al. the comparison was performed between lymph node MCL samples and peripheral blood normal B cells, and only a few miRNAs were found to be deregulated in more than 50% of cases, including decrease of miR-150, miR-142-3p/5p, miR-29 family, and increase of miR-124a and miR-155 (Zhao et al. 2010). However, a previous study using a selected 86 miRNA set demonstrated significant differences in a longer number of miRNAs either from peripheral blood or lymph node MCL samples compared with CD5+ or CD5− tonsillar normal B cells (Navarro et al. 2009a). These results showed that, as in other neoplasms, MCL has a characteristic altered miRNA expression profile compared to normal counterparts, although normal or transformed B cells could also show expression profiles affected by the microenvironment of these cells. This later consideration was further extended in the MCL scope, as it was shown that tumoral cells from the same patient showed differential expression of some miRNAs in different anatomic environments (peripheral blood and lymph nodes, Fig. 11.4) (Navarro et al. 2009a). This finding suggests that anatomic source of tumoral samples is a relevant parameter to take in account for consideration of certain miRNAs as potential biomarkers clinically useful, at least in MCL.
11.5.1.2 In Other Lymphomas
In cHL, miRNA expression profiling allows to differentiate these tumors from reactive lymph nodes through a 25-miRNA signature (Table 11.2) (Navarro et al. 2008). Moreover, major histological subtypes (nodular sclerosis and mixed cellularity) could also be differentiated by the expression of a reduced number of miRNAs (Navarro et al. 2008). Noticeably, this study was performed without tumor cell purification. On the contrary, a study using quantitative PCR on microdissected RSC cells described a signature of 12 over-expressed and 3 down-regulated miRNAs in comparison to normal CD77+ progenitor cells, and showing little overlap with the previous work on whole biopsies (Table 11.2) (Van Vlierberghe et al. 2009). In addition, absolute miRNA abundance was also measured by direct cloning in two HL cell lines, and among the most abundant miRNAs were found miR-16, miR-21, miR-155, and miR-9, concordantly with the previous results of Van Vlierberghe et al. (Nie et al. 2008).
11.5.2 Using MiRNA Expression for Lymphoma Prognosis Stratification Improvement
11.5.2.1 In B/T Lymphomas
In DLBCL, the expression of 7 miRNAs was found to be associated with event-free or overall survival, but only miR-127 expression was associated with both parameters, being the expression of this miRNA lower in poor prognosis cases (Roehle et al. 2008). In another study, de novo DLBCL cases were split into two groups with low (0–2) or high (3–5) international prognostic index (IPI), and 13 miRNAs correctly predicted 85% of the cases (Table 11.2) (Lawrie et al. 2009). Event-free survival (EFS) was analyzed in the de novo DLBCL uniformly treated with the chemotherapy combination of rituximab-cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone/prednisolone (R-CHOP) and 8 miRNAs were found to be associated with EFS. These including high expression of miR-142, miR-302b, miR-519d, and down-regulation of miR-330, miR-425, miR-27a, miR-222, and miR-199b, being the last one the most significantly associated with poor outcome. Nevertheless, in a later study using a longer series of R-CHOP uniformly treated DLBCL patients, only high miR-222 expression (also associated with ABC subtype) showed a significant association with overall survival and progression-free survival (Malumbres et al. 2009). Finally, it must be stressed that the differential expression that has been shown for several miRNAs between ABC and GCB subtypes of DLBCL could also have prognostic relevance as ABC subtypes are more aggressive. Noticeably, this was not the case of miR-155, because it was not found related to prognosis of the whole series (Rai et al. 2008; Roehle et al. 2008) and surprisingly, it was associated with a subset of ABC-DLBCL with a less aggressive behavior (Jung and Aguiar 2009).
CLL is characterized by highly variable clinical behavior, with a survival range from 1 to more than 15 years. A plethora of factors have been showed to influence survival of CLL patients (Dal Bo et al. 2009). The expression of several miRNAs has also described to have prognostic value. Thus, loss of 13q14.3, including miR-16-1 and miR-15a, is not only the most frequent genomic alteration of this neoplasm but it is also associated to a good prognosis if it is the sole aberration. Noticeably, the deletion is often interstitial and is homozygous in up to 15% of the cases (Haferlach et al. 2007). In addition, other miRNAs related with prognosis were found by Calin et al. when analyzing a total of 190 miRNAs (Calin et al. 2005). The prognostic signature found consisted in 13 miRNAs that also included the above mentioned miRNAs down-regulated in the group of patients with good prognosis (also characterized by low expression of ZAP-70 and hypermutation of IgH genes) (Table 11.2). The fact that miR-15a/16-1 levels were higher in the poor prognosis group points out that these miRNAs are involved in complex regulatory webs of both oncogenes and tumor suppressor genes and it is difficult to predict the final effect in vivo. Experimental data also support this complex regulatory effects of this miRNA cluster in CLL (Calin et al. 2008). In addition, other miRNAs included in this signature with the same prognosis association that the above mentioned were miR-195, miR-221, miR-23b, miR-155, miR-24-1, miR-146, and miR-16-2. On the other hand several miRNAs were down-regulated in the poor prognosis CLL group, including miR-223 and miR-29a-2/b-2/c. In another study, using a different technology for miRNA expression quantification in CLL, lower expression levels of miR-150, miR-223, and miR29b/c were found to be associated with the aggressive patient subgroup (Fulci et al. 2007). The majority of these miRNAs were also found differentially expressed between the two defined CLL prognostic groups in another miRNA cloning study, although also finding miR-30d and miR-191 as differentially expressed in the poor prognosis group (Marton et al. 2008). In addition, miR-29c and miR-223 were later also showed to be down-regulated in patients with poor prognosis independently of the prognostic marker classification used (Stamatopoulos et al. 2009). Moreover, the expression of these miRNAs were used in an index through which a clear separation of patients into 5 groups with different median treatment free-survival and into 3 groups with different median overall survival was obtained (Stamatopoulos et al. 2009). Noticeably, it has been shown that down-regulation of miR-29a/b/c is also associated with poor prognosis in MCL (Zhao et al. 2010).
Another relevant clinical parameter of CLL in which expression of several miRNA has also been associated is the time to initial therapy. Thus, treatment of CLL begins with the development of symptomatic or progressive disease, and patients with related shorter interval have been characterized to show high expression of miR-181a, miR-155, miR-146, miR-24-2, miR-23a/b, miR-222, and miR-221, and low expression of miR-29c.
Finally, low expression of miR-15a and over-expression of miR-181a/b were associated with worse prognosis in MM (Roccaro et al. 2009).
11.5.2.2 In Other Lymphomas
In cHL, high expression levels of miR-138 were found to be related with Ann Arbor stage I-II of these tumors (Navarro et al. 2008), and low expression of miR-135a was associated with a higher probability of relapse and shorter disease-free survival (Navarro et al. 2009b).
11.6 Oncomirs as Possible Candidates for Therapeutic Targeting: A Promise of More Specific Lymphoma Therapies?
As we have seen in the previous subheadings, miRNAs are relevant regulatory molecules in the lymphomagenesis. Altered miRNA expression levels through different causal mechanisms constitute the main final pathogenetic effect. In relation with treatment, it is possible that miRNA expression changes could be contributing to the cytotoxic effect of some chemotherapeutics. Thus, inhibition of histone deacetylases induces cell death in CLL cells through up-regulation of miR-106b by a characterized molecular pathway controlled by this miRNA (Sampath et al. 2009). Nevertheless, an even more interesting approach is the finding of treatments based on specific expression modulation of relevant miRs, allowing the correction of pathogenic molecular imbalances. In this sense, different molecular systems have been experimentally tested to induce specific miRNA expression changes including locked nucleic acids (LNA) or 2′-O-methyl-antisense RNA. These molecules have been used in different tumor models because of their ability to specifically inhibit the miRNA to target by complementary sequence binding (Frieden and Orum 2008). In addition, it is possible to synthesize pre-miRNAs that could be introduced in lymphoma cells to replace low expressed or silenced endogenous miRNAs. Nevertheless, there are at least two issues that should be resolved for a real clinical application: (1) To reach an effective tumor cytotoxicity either alone or combined with existing chemotherapy; and (2) To obtain specific delivering to the tumor cells avoiding side effects on other cell populations, although at enough dose to obtain a physiological effect. Neither of these issues has been resolved yet, including lymphomas. Nevertheless, the known role of miRNAs in apoptosis regulation of the lymphoid neoplasms offers interesting candidates that will be surely targeted in lymphomas as soon as the above mentioned issues will be overcame with new technological improvements.
References
Aguda BD, Kim Y, Piper-Hunter MG, et al. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci USA. 2008;105:19678–83.
Akao Y, Nakagawa Y, Kitade Y, et al. Downregulation of microRNAs-143 and -145 in B-cell malignancies. Cancer Sci. 2007;98:1914–20.
Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep. 2006;16:845–50.
Anastasiadou E, Boccellato F, Vincenti S, et al. Epstein-Barr virus encoded LMP1 downregulates TCL1 oncogene through miR-29b. Oncogene. 2009;29:1316–28.
Aqeilan RI, Calin GA, Croce CM. MiR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010;17:215–20.
Bea S, Salaverria I, Armengol L, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009;113:3059–69.
Bichi R, Shinton SA, Martin ES, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA. 2002;99:6955–60.
Bolisetty MT, Dy G, Tam W, et al. Reticuloendotheliosis virus strain T induces miR-155, which targets JARID2 and promotes cell survival. J Virol. 2009;83:12009–17.
Bueno MJ, Perez DCI, Gomez DC, et al. Genetic and epigenetic silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene expression. Cancer Cell. 2008;13:496–506.
Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20:259–64.
Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA. 2008;105:5166–71.
Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–801.
Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA. 2004;101:11755–60.
Cameron JE, Yin Q, Fewell C, et al. Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008;82:1946–58.
Cardinaud B, Moreilhon C, Marcet B, et al. MiR-34b/miR-34c: a regulator of TCL1 expression in 11q- chronic lymphocytic leukaemia? Leukemia. 2009;23:2174–7.
Chen RW, Bemis LT, Amato CM, et al. Truncation in CCND1 mRNA alters miR-16-1 regulation in mantle cell lymphoma. Blood. 2008;112:822–9.
Chen CZ, Li L, Lodish HF, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6.
Chen CZ, Lodish HF. MicroRNAs as regulators of mammalian hematopoiesis. Semin Immunol. 2005;17:155–65.
Chim C, Wong K, Qi Y, et al. Epigenetic inactivation of the miR-34a in hematological malignancies. Carcinogenesis. 2010;31:745–50.
Cimmino A, Calin GA, Fabbri M, et al. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–9.
Cloonan N, Brown MK, Steptoe AL, et al. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol. 2008;9:R127.
Clurman BE, Hayward WS. Multiple proto-oncogene activations in avian leukosis virus-induced lymphomas: evidence for stage-specific events. Mol Cell Biol. 1989;9:2657–64.
Costinean S, Zanesi N, Pekarsky Y, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci USA. 2006;103:7024–9.
Dal Bo M, Bertoni F, Forconi F, et al. Intrinsic and extrinsic factors influencing the clinical course of B-cell chronic lymphocytic leukemia: prognostic markers with pathogenetic relevance. J Transl Med. 2009;7:76.
De Falco G, Antonicelli G, Onnis A, et al. Role of EBV in microRNA dysregulation in Burkitt lymphoma. Semin Cancer Biol. 2009;19:401–6.
Dorsett Y, McBride KM, Jankovic M, et al. MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity. 2008;28:630–8.
Du C, Liu C, Kang J, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–9.
Eis PS, Tam W, Sun L, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA. 2005;102:3627–32.
Frenquelli M, Muzio M, Scielzo C, et al. MicroRNA and proliferation control in chronic lymphocytic leukemia: functional relationship between miR-221/222 cluster and p27. Blood. 2010;115:3949–59.
Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941–52, ix.
Frieden M, Orum H. Locked nucleic acid holds promise in the treatment of cancer. Curr Pharm Des. 2008;14:1138–42.
Fulci V, Chiaretti S, Goldoni M, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–51.
Gao P, Tchernyshyov I, Chang TC, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5.
Godshalk SE, Bhaduri-McIntosh S, Slack FJ. Epstein-Barr virus-mediated dysregulation of human microRNA expression. Cell Cycle. 2008;7:3595–600.
Gottwein E, Mukherjee N, Sachse C, et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–9.
Guil S, Esteller M. DNA methylomes, histone codes and miRNAs: tying it all together. Int J Biochem Cell Biol. 2009;41:87–95.
Gutierrez NC, Sarasquete ME, Misiewicz-Krzeminska I, et al. Deregulation of microRNA expression in the different genetic subtypes of multiple myeloma and correlation with gene expression profiling. Leukemia. 2010;24:629–37.
Haferlach C, Dicker F, Schnittger S, et al. Comprehensive genetic characterization of CLL: a study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia. 2007;21:2442–51.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82.
He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33.
Hermeking H. MiR-34a and p53. Cell Cycle. 2009;8:1308.
Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–9.
Hideshima T, Bergsagel PL, Kuehl WM, et al. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–18.
Inomata M, Tagawa H, Guo YM, et al. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402.
Jiang Q, Feng MG, Mo YY. Systematic validation of predicted microRNAs for cyclin D1. BMC Cancer. 2009;9:194.
Jung I, Aguiar RC. MicroRNA-155 expression and outcome in diffuse large B-cell lymphoma. Br J Haematol. 2009;144:138–40.
Klein U, Lia M, Crespo M, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40.
Kluiver J, Haralambieva E, de Jong D, et al. Lack of BIC and microRNA miR-155 expression in primary cases of Burkitt lymphoma. Genes Chromosomes Cancer. 2006;45:147–53.
Kluiver J, Poppema S, de Jong D, et al. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207:243–9.
Lawrie CH, Chi J, Taylor S, et al. Expression of microRNAs in diffuse large B cell lymphoma is associated with immunophenotype, survival and transformation from follicular lymphoma. J Cell Mol Med. 2009;13:1248–60.
Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–5.
Lawrie CH, Soneji S, Marafioti T, et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007;121:1156–61.
Lehmann S, Ogawa S, Raynaud SD, et al. Molecular allelokaryotyping of early-stage, untreated chronic lymphocytic leukemia. Cancer. 2008;112:1296–305.
Lerner M, Harada M, Loven J, et al. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16-1. Exp Cell Res. 2009;315:2941–52.
Leucci E, Cocco M, Onnis A, et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol. 2008;216:440–50.
Li QJ, Chau J, Ebert PJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–61.
Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand J Immunol. 2010;71:227–31.
Linares LK, Kiernan R, Triboulet R, et al. Intrinsic ubiquitination activity of PCAF controls the stability of the oncoprotein Hdm2. Nat Cell Biol. 2007;9:331–8.
Lionetti M, Biasiolo M, Agnelli L, et al. Identification of microRNA expression patterns and definition of a microRNA/mRNA regulatory network in distinct molecular groups of multiple myeloma. Blood. 2009;114:e20–6.
Lu LF, Thai TH, Calado DP, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91.
Lu F, Weidmer A, Liu CG, et al. Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J Virol. 2008;82:10436–43.
Lujambio A, Ropero S, Ballestar E, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–9.
Malumbres R, Sarosiek KA, Cubedo E, et al. Differentiation stage-specific expression of microRNAs in B lymphocytes and diffuse large B-cell lymphomas. Blood. 2009;113:3754–64.
Marton S, Garcia MR, Robello C, et al. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008;22:330–8.
Motsch N, Pfuhl T, Mrazek J, et al. Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol. 2007;4:131–7.
Mott JL, Kobayashi S, Bronk SF, et al. Mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–40.
Mrazek J, Kreutmayer SB, Grasser FA, et al. Subtractive hybridization identifies novel differentially expressed ncRNA species in EBV-infected human B cells. Nucleic Acids Res. 2007;35:e73.
Mu P, Han YC, Betel D, et al. Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23:2806–11.
Navarro A, Bea S, Fernandez V, et al. MicroRNA expression, chromosomal alterations, and immunoglobulin variable heavy chain hypermutations in Mantle cell lymphomas. Cancer Res. 2009a;69:7071–8.
Navarro A, Diaz T, Martinez A, et al. Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood. 2009b;114:2945–51.
Navarro A, Gaya A, Martinez A, et al. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood. 2008;111:2825–32.
Ng EK, Tsang WP, Ng SS, et al. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. Br J Cancer. 2009;101:699–706.
Nie K, Gomez M, Landgraf P, et al. MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in Hodgkin lymphomas. Am J Pathol. 2008;173:242–52.
Ota A, Tagawa H, Karnan S, et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–95.
O’Connell RM, Rao DS, Chaudhuri AA, et al. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–22.
O’Donnell KA, Wentzel EA, Zeller KI, et al. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–43.
O’Hara AJ, Vahrson W, Dittmer DP. Gene alteration and precursor and mature microRNA transcription changes contribute to the miRNA signature of primary effusion lymphoma. Blood. 2008;111:2347–53.
Palamarchuk A, Efanov A, Nazaryan N, et al. 13q14 deletions in CLL involve cooperating tumor suppressors. Blood. 2010;115:3916–22.
Pallasch CP, Patz M, Park YJ, et al. MiRNA deregulation by epigenetic silencing disrupts suppression of the oncogene PLAG1 in chronic lymphocytic leukemia. Blood. 2009;114:3255–64.
Parekh S, Polo JM, Shaknovich R, et al. BCL6 programs lymphoma cells for survival and differentiation through distinct biochemical mechanisms. Blood. 2007;110:2067–74.
Pasqualucci L, Compagno M, Houldsworth J, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203:311–7.
Pedersen IM, Otero D, Kao E, et al. Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of B cell lymphomas. EMBO Mol Med. 2009;1:288–95.
Pekarsky Y, Santanam U, Cimmino A, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–3.
Pichiorri F, Suh SS, Ladetto M, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA. 2008;105:12885–90.
Pickering MT, Stadler BM, Kowalik TF. MiR-17 and miR-20a temper an E2F1-induced G1 checkpoint to regulate cell cycle progression. Oncogene. 2009;28:140–5.
Rahadiani N, Takakuwa T, Tresnasari K, et al. Latent membrane protein-1 of Epstein-Barr virus induces the expression of B-cell integration cluster, a precursor form of microRNA-155, in B lymphoma cell lines. Biochem Biophys Res Commun. 2008;377:579–83.
Rai D, Karanti S, Jung I, et al. Coordinated expression of microRNA-155 and predicted target genes in diffuse large B-cell lymphoma. Cancer Genet Cytogenet. 2008;181:8–15.
Rai D, Kim SW, McKeller MR, et al. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc Natl Acad Sci USA. 2010;107:3111–6.
Ramkissoon SH, Mainwaring LA, Ogasawara Y, et al. Hematopoietic-specific microRNA expression in human cells. Leuk Res. 2006;30:643–7.
Robertus JL, Harms G, Blokzijl T, et al. Specific expression of miR-17-5p and miR-127 in testicular and central nervous system diffuse large B-cell lymphoma. Mod Pathol. 2009;22:547–55.
Roccaro AM, Sacco A, Thompson B, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood. 2009;113:6669–80.
Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11.
Roehle A, Hoefig KP, Repsilber D, et al. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br J Haematol. 2008;142:732–44.
Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–43.
Sampath D, Calin GA, Puduvalli VK, et al. Specific activation of microRNA106b enables the p73 apoptotic response in chronic lymphocytic leukemia by targeting the ubiquitin ligase Itch for degradation. Blood. 2009;113:3744–53.
Sander S, Bullinger L, Klapproth K, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–12.
Sander S, Bullinger L, Wirth T. Repressing the repressor: a new mode of MYC action in lymphomagenesis. Cell Cycle. 2009;8:556–9.
Scaria V, Hariharan M, Pillai B, et al. Host-virus genome interactions: macro roles for microRNAs. Cell Microbiol. 2007;9:2784–94.
Schiltz RL, Nakatani Y. The PCAF acetylase complex as a potential tumor suppressor. Biochim Biophys Acta. 2000;1470:M37–53.
Seiler T, Dohner H, Stilgenbauer S. Risk stratification in chronic lymphocytic leukemia. Semin Oncol. 2006;33:186–94.
Skalsky RL, Samols MA, Plaisance KB, et al. Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. 2007;81:12836–45.
Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–40.
Stamatopoulos B, Meuleman N, Haibe-Kains B, et al. MicroRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood. 2009;113:5237–45.
Sylvestre Y, De G, V, Querido E, et al. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–43.
Tagawa H, Karube K, Tsuzuki S, et al. Synergistic action of the microRNA-17 polycistron and Myc in aggressive cancer development. Cancer Sci. 2007;98:1482–90.
Tagawa H, Seto M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia. 2005;19:2013–6.
Teng G, Hakimpour P, Landgraf P, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–9.
Thai TH, Calado DP, Casola S, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–8.
Tili E, Croce CM, Michaille JJ. MiR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol. 2009;28:264–84.
Toyota M, Suzuki H, Sasaki Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68:4123–32.
Tsitsiou E, Lindsay MA. MicroRNAs and the immune response. Curr Opin Pharmacol. 2009;9:514–20.
Unno K, Zhou Y, Zimmerman T, et al. Identification of a novel microRNA cluster miR-193b-365 in multiple myeloma. Leuk Lymphoma. 2009;50:1865–71.
van den BA, Kroesen BJ, Kooistra K, et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–8.
Van Vlierberghe P, De Weer A, Mestdagh P, et al. Comparison of miRNA profiles of microdissected Hodgkin/Reed-Sternberg cells and Hodgkin cell lines versus CD77+ B-cells reveals a distinct subset of differentially expressed miRNAs. Br J Haematol. 2009;147:686–90.
Vigorito E, Perks KL, Abreu-Goodger C, et al. MicroRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–59.
Visone R, Rassenti LZ, Veronese A, et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood. 2009;114:3872–9.
Wang M, Tan LP, Dijkstra MK, et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J Pathol. 2008;215:13–20.
Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood. 2007;109:4599–606.
Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J Biol Chem. 2007;282:2130–4.
Xiao C, Calado DP, Galler G, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–59.
Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–14.
Yamanaka Y, Tagawa H, Takahashi N, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114:3265–75.
Yazici H, Zipprich J, Peng T, et al. Investigation of the miR16-1 (C > T) + 7 substitution in seven different types of cancer from three ethnic groups. J Oncol. 2009;2009:827532.
Yin Q, McBride J, Fewell C, et al. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J Virol. 2008;82:5295–306.
Zenz T, Mohr J, Eldering E, et al. MiR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–8.
Zhang J, Jima DD, Jacobs C, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;113:4586–94.
Zhao JJ, Lin J, Lwin T, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–9.
Zhou B, Wang S, Mayr C, et al. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci USA. 2007;104:7080–5.
Acknowledgments
The authors were supported (in part) by a grant (RD06/0020/0039) from Red Temática de Investigación Cooperativa en Cáncer (RTICC), Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science and Innovation and European Regional Development Fund (ERDF). In addition, L. Hernández is supported by FIS and “programa d’estabilització d’investigadors” of Direcció d’Estrategia i Coordinació del Departament de Salut (Generalitat de Catalunya).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Netherlands
About this chapter
Cite this chapter
López, A.N., Pous, L.H. (2011). MicroRNAs in Lymphoma. In: Cho, W. (eds) MicroRNAs in Cancer Translational Research. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0298-1_11
Download citation
DOI: https://doi.org/10.1007/978-94-007-0298-1_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0297-4
Online ISBN: 978-94-007-0298-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)