Abstract
The basic aim of this work was to motivate a realistic strategy to combat marine eutrophication in north-eastern Europe. Data from the Kattegat (located between Sweden and Denmark) were used to illustrate basic principles and processes related to nutrient fluxes. We have applied a process-based mass-balance model, CoastMab, to the Kattegat and quantified the nutrient fluxes to, within, and from the system. Several scenarios aiming to decrease eutrophication in the Kattegat have been modeled. By far the most dominating nutrient fluxes to the bioproductive surface-water layer in the Kattegat come from the south (from the Baltic Proper), which should be evident just by comparing the catchment area for the Baltic Sea, including the Baltic States, parts of Russia, Belarus and Germany, Poland, Finland, and Sweden in relation to the relatively small catchment area draining directly into the Kattegat (from SW Sweden and parts of Denmark). The dominating deep-water fluxes come from the north (from the Skagerrak). The strategy that one should ask for should concur with some evident practical constraints, e.g., it is not realistic to reduce all anthropogenic P or N discharges. For countries where major investments in nutrient reductions have already been made, it will become increasingly expensive to reduce the remaining tons. In the “optimal” scenario discussed in this work, about 10,000 t year–1 of P is being reduced and also N reductions that would lower the N concentration in the Baltic Proper by 10%. The cost for this “optimal” strategy was estimated at 200–420 million euro year–1 given that the focus will be on the most cost-effective P reductions connected to the most polluted estuaries and coastal areas. To achieve cost-effectiveness, one can assume that most of this would go to upgrading urban sewage treatment in the Baltic States, Poland, and other former East Bloc countries. The costs to reduce 15,016 t year–1 of P and 133,170 t year–1 of N according to the HELCOM strategy (agreed upon by the Baltic Sea states in November 2007) would be 3,100 million euro year–1. That is, 2,680–2,900 million euro year–1 higher than the “optimal” strategy advocated in this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Background and Aim of the Work
Validated process-based mass-balance models are – categorically – the only tool to quantify fluxes, concentrations, and amounts and to make predictions of how nutrient concentrations would change in response to reductions in nutrient loading where the given reduced flux is put into a context where all other fluxes influencing the given concentrations are quantified in an appropriate and realistic manner. The aim of this work has been to do the following:
-
Discuss fundamental aspects related to eutrophication in aquatic systems and using data from the Kattegat to illustrate basic principles and processes. The ultimate aim is to motivate the most realistic strategy to combat eutrophication. We have applied the CoastMab model (a process-based mass-balance model using ordinary differential equations giving monthly fluxes) to the Kattegat directly and without any “tuning” to quantify the nutrient fluxes to, within, and from system. This model has been described in detail in many other contexts (Håkanson and Bryhn 2008a, 2008b, Håkanson 2009) and the basic aim here is not to repeat the motivation and testing of the equations but to focus on the principles in more general terms and how to use the model in finding the best possible remedial strategy. We will, however, describe the basic structure of the model (i.e., how the water and sediment compartments are defined).
-
Present key driving variables related to salinity, water temperatures, water discharges, and nutrient concentrations and trend analysis for the study period (1995–2008) for the Kattegat system to stress that similar background information should be at hand for all aquatic systems in contexts where remediation of eutrophication is discussed from a mass-balance perspective. Boesch et al. (2008) has given a literature review related to the conditions in the Kattegat.
-
When the presuppositions have been defined, several remedial scenarios will also be given, which are meant to demonstrate how the given system would likely respond to changes in tributary P and N loading.
-
Finally, based on those results, recommendations will be given for a remedial strategy to reduce the eutrophication in the case study area, the Kattegat.
The transport processes in aquatic systems are general and apply for all substances in most aquatic systems, but there are also substance-specific parts (e.g., related to the particulate fraction, criteria for diffusion and denitrification). Note that the model used to quantify these transport processes in this work, CoastMab, is general so this is not a model where the user should make any tuning and calibrations or change model constants when the model is applied to a new aquatic system. The idea is to have a model based on general and mechanistically correct algorithms describing the monthly transport processes (sedimentation, resuspension, diffusion, mixing, etc.) at the ecosystem scale (i.e., for entire defined basins) and to calculate the role of the different transport processes and how a given system would react to changes in inflow related to natural changes and anthropogenic reductions of water pollutants.
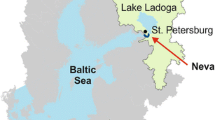
For persons not familiar with the Baltic Sea system, Fig. 2.1 gives a geographical overview and the names of the main basins. The salinity decreases from over 30 psu in Skagerrak to about 3 psu in the northern part of the Bothnian Bay. It is easy to imagine the enormous water dynamics of the system which is responsible for the inflow of salt water from the south (Kattegat and Skagerrak), the freshwater outflow and the rotation of the earth (the Coriolis force), the variations in winds and air pressures that cause the necessary mixing, and water transport casing this salinity gradient. These salinities demonstrate that the Baltic Sea system including the Kattegat is a very dynamic system. The catchment area of the entire Baltic Sea system is many times larger than the Swedish and Danish areas draining into the Kattegat, and the water from the entire Baltic Sea system will eventually also flow into the Kattegat. The basic structure of the work done and some of the main features of the CoastMab model are illustrated in Fig. 2.2. First (at level 1), the coastal mass-balance model for salt, which is explained in detail in Håkanson and Bryhn (2008a) for the Baltic Sea basins, will be used to quantify the water fluxes to, within, and from all the sub-basins and vertical layers in the Kattegat, including mixing and diffusion.
The main results will be given in Section 2.3. It should be stressed that the CoastMab modeling has been tested in many coastal areas and lakes and also discussed in Håkanson and Bryhn (2008a, 2008c). This model will calculate the water fluxes needed to explain the measured salinities. This means that data on salinities in the inflowing water to the Kattegat from the Baltic Proper and Skagerrak are needed to run the model and in the following simulations, data from the period 1995–2008 will be used. This modeling also needs morphometric data (mean depth, volume, form factor, dynamic ratio, etc.) and the hypsographic curve and those data are discussed in Section 2.2. The size and form of a given aquatic system, i.e., the morphometry, influences the way in which the system functions, since the depth characteristics influence resuspension and internal loading of nutrients, the nutrient concentrations regulate the primary production, which in turn regulates the secondary production, including zooplankton and fish (see Håkanson and Boulion 2002). At level 2, CoastMab for phosphorus is used (see Håkanson 2009). One should note that many of the algorithms to quantify the transport processes for phosphorus, salt, and nitrogen are also valid for other substances, e.g., inflow, sedimentation of particulate phosphorus and SPM, mixing, diffusion of salt and dissolved phosphorus and nitrogen, resuspension, and burial. There are also substance specific transport processes. For example, for nitrogen, atmospheric deposition, gas transport (nitrogen also appears in a gaseous phase), atmospheric N2 fixation, and denitrification. Nitrogen modeling is included in this work and data from Eilola and Sahlberg (2006) (see also Håkansson 2007) have been used for the atmospheric N deposition. At level 3, CoastMab for SPM (suspended particulate matter) is used. This means that the inflow, production, sedimentation, burial, and mineralization of suspended particulate matter are quantified on a monthly basis (Håkanson 2006). Sedimentation is important for the oxygen consumption and oxygen status of the system, especially for the oxygen conditions in the deep-water layer below the theoretical wave base and for the diffusion of phosphorus from sediments to water. At level 4, general regression models to predict how the two key bioindicators in eutrophication studies, the Secchi depth (a standard measure of water clarity and the depth of the photic zone) and the concentrations of chlorophyll-a (a key measure of both primary phytoplankton production and biomass and the driving variable for the foodweb model, CoastWeb; see Håkanson and Boulion 2002, Håkanson 2009), would likely change in relation to changing phosphorus and nitrogen concentrations, salinities, SPM values, temperature, and light conditions.
2.2 Basic Information
As a background to this work, Figs. 2.3 and 2.4 show maps related to the areal variations in two of the target bioindicators for eutrophication, the concentration of chlorophyll-a and the Secchi depth.
Areal distribution of chlorophyll-a concentrations in the Baltic Sea and parts of the North Sea during the growing season (May–September) in the upper 10 m water column for the period from 1990 to 2005 (from Håkanson and Bryhn 2008a)
Average annual Secchi depths in the Baltic Sea and parts of the North Sea in the upper 10 m water column for the period from 1990 to 2005 (from Håkanson and Bryhn 2008a)
These two maps provide an overview of the areal distribution patterns of two important variables and from maps such as these one can identify “hotspots,” i.e., areas with high algal biomasses expressed by the chlorophyll-a concentrations and areas with turbid water and low Secchi depths, which should be targeted in remedial contexts related to eutrophication. And vice versa, these maps also provide key information related to areas where reductions in anthropogenic nutrient input should not have a high priority. One can note that the conditions in the Kattegat are significantly better than in, e.g., the Gulf of Finland, the Gulf of Riga, and the estuaries of Oder and Vistula. However, this does not imply that nothing should be done to improve the eutrophication in the Kattegat. From Fig. 2.3, one can note typical chlorophyll-a concentrations in the Baltic Sea and parts of the North Sea. Values lower than 2 μg L–1 (oligotrophic conditions; see Table 2.1) are found in the northern parts of the Bothnian Bay and the outer parts of the North Sea, while values higher than 20 μg L–1 (hypertrophic conditions) are more often found in, e.g., the Vistula and Oder lagoons.
The hotspots shown in the map outside the British coast may be a result of data from situations when algal blooms are overrepresented. This map shows that at water depths smaller than 10 m, the Baltic Sea has typical chlorophyll concentrations between 2 and 6 μg L–1 during the growing season (May–September), which correspond to the mesotrophic class. Figure 2.4 shows that several areas with low Secchi depths can be observed, e.g., in the Gulf of Riga and along the North Sea coasts of Holland, Belgium, and Germany. However, some of the observed patchiness may be a result of the interpolation method rather than a true patchiness. In the following, the utilized morphometric data for the Kattegat will first be presented. It will also be explained why and how the given morphometrical parameters are important for the mass-balance calculations. This has been discussed in more detail for lakes by Håkanson (2004). The idea here is to provide a background illustrating how morphometric parameters are used in the CoastMab model.
Compilations of data on salinities, phosphorus, nitrogen, temperature, oxygen concentrations, Secchi depths, and concentrations of chlorophyll-a will also be given. The water fluxes will be presented in the next section. They are used for quantifying the transport of the nutrients. The dynamic mass-balance model for suspended particulate matter (CoastMab for SPM) quantifying sedimentation will also be used. SPM causes scattering of light in the water and influences the Secchi depth and hence the depth of the photic zone; SPM also influences the bacterial decomposition of organic matter, and hence also the oxygen situation and the conditions for zoobenthos, by definition an important food source for benthivorous prey fish. This section will give trend analyses concerning all the studied water variables for the period 1995–2008. An important aspect of this modeling (at the ecosystem scale) concerns the use of hypsographic curves (i.e., depth/area curves for defined basins) to calculate the necessary volumes of water of the defined vertical layers. This information is essential in the mass-balance modeling for salt, phosphorus, nitrogen, and SPM. If there are errors in the defined volumes, there will also be errors in the calculated concentrations since, by definition, the concentration is the mass of the substance in a given volume of water. This section also presents an approach to differentiate between the surface-water and the deep-water layers. Traditionally, this is done by water temperature data, which define the thermocline, or by salinity data, which define the halocline. CoastMab uses an approach which is based on the water depth separating areas where sediment resuspension of fine particles occurs from bottom areas where periods of sedimentation and resuspension of fine newly deposited material are likely to happen (the erosion and transportation areas, the ET areas). The depth separating areas with discontinuous sedimentation (the T areas) from areas with more continuous sediment accumulation (the A areas) of fine materials is called the theoretical wave base. This is an important concept in mass-balance modeling of aquatic systems (see Håkanson 1977, 1999, 2000). The theoretical wave base will also be used to define algorithms
-
to calculate concentrations of matter in the given volumes/compartments,
-
to quantify sedimentation by accounting for the mean depths of these compartments,
-
to quantify internal loading via advection/resuspension as well as diffusion (the vertical water transport related to concentration gradients of dissolved substances in the water),
-
to quantify upward and downward mixing between the given compartments, and
-
to calculate outflow of substances from the given compartments.
Empirical monthly values of the salinity for the period 1995–2008 have been used to calibrate the CoastMab model for salt and those calculations provide data of great importance for the mass balances for phosphorus, nitrogen, and SPM, namely
-
The fluxes of water to and from the defined compartments.
-
The monthly mixing of water between layers in the given basin.
-
The basic algorithm for diffusion of dissolved substances in water in each compartment.
-
The water retention rates influencing the turbulence in each compartment, and hence also
-
The sedimentation of particulate phosphorus, nitrogen, and SPM in the given compartments. So, this section will provide and discuss the data necessary to run the CoastMab model.
2.2.1 Morphometric Data and Criteria for the Vertical Layers
Basin-specific data are compiled in Table 2.2 for the case study area, the Kattegat, and will be briefly explained in this section. This table gives data on, e.g., total area, volume, mean depth, maximum depth and the depth of the theoretical wave base (D wb in m), the fraction of bottoms areas dominated by fine sediment erosion and transport (ET areas) above the theoretical wave base, the water transport between the Kattegat and the Baltic Proper (see Håkanson and Bryhn 2008a), sediment characteristics (water content and organic content = loss on ignition; mainly based on data supplied by Prof. Ingemar Cato, SGU, Uppsala), and latitude.
There are more than 15,000 measurements on water temperature, salinity, TN and TP concentrations, and chlorophyll and about 14,000 data on Secchi depths and oxygen concentrations for the period from 1995 to 2008 used in this work from the entire Kattegat. The theoretical wave base is defined from the ETA diagram (see Fig. 2.5; erosion–transport–accumulation; from Håkanson 1977), which gives the relationship between the effective fetch, as an indicator of the free water surface over which the winds can influence the wave characteristics (speed, height, length, and orbital velocity).
The ETA diagram (erosion–transportation–accumulation; redrawn from Håkanson 1977) illustrating the relationship between effective fetch, water depth, and potential bottom dynamic conditions. The theoretical wave base (D wb; 39.9 m in the Kattegat) may be used as a general criterion in mass-balance modeling to differentiate between the surface-water layer with wind/wave-induced resuspension and deeper areas without wind-induced resuspension of fine materials. The depth separating E areas with predominately coarse sediments from T areas with mixed sediments is at 25 m in the Kattegat
The theoretical wave base separates the transportation areas (T), with discontinuous sedimentation of fine materials, from the accumulation areas (A), with continuous sedimentation of fine suspended particles. The theoretical wave base (D wb in m) is, e.g., at a water depth of 39.9 m in the Kattegat. This is calculated from Eq. (2.1) (Area = area in km2):
It should be stressed that this approach to separate the surface-water layer from the deep-water layer has been used and motivated in many previous contexts for lakes (Håkanson et al. 2004), smaller coastal areas in the Baltic Sea (Håkanson and Eklund 2007), and the sub-basins in the Baltic Sea (Håkanson and Bryhn 2008a, 2008c). This approach gives one value for the theoretical wave base related to the area of the system. The validity of this approach for the Kattegat is demonstrated in Fig. 2.6a for the salinity, Fig. 2.6b for the oxygen concentration, and Fig. 2.7 for the TN/TP ratio (TN = total nitrogen; TP = total phosphorus).
The relationship between (a) water depth and salinity in the Kattegat and (b) between water depth and oxygen concentration. The two figures also show the theoretical wave base at about 40 m in the Kattegat. Data from SMHI. The statistical analyses given in Fig. 2.6b demonstrate that the theoretical wave base at 40 m is also the threshold depth for the oxygen concentrations
From Fig. 2.6a, it may be noted that for the Kattegat the surface-water (SW) salinity is clearly different from the deep-water (DW) salinity. The mean SW salinity is 24.6 psu (see Table 2.3, which also gives monthly mean values and coefficients of variation, CV), whereas the mean DW salinity is 33.3 (the CV value is very low, 0.02; CV = coefficient of variation, CV = SD/MV; SD = standard deviation, MV = mean value). Tables 2.3 and 2.4 give mean monthly values and coefficients of variations not just for salinity but also for water temperatures, oxygen concentrations, phosphate, TP, nitrite, nitrate, ammonium, and TN, and Table 2.3 gives the corresponding data for PON (particulate organic nitrogen), POC, chlorophyll, and Secchi depth.
The aim of the modeling is to describe these empirical salinities as close as possible and to predict the given TP, TN, chlorophyll concentrations and Secchi depths so that the predicted values agree with the empirical data. Note that the basic aim is to predict the mean annual values rather than the monthly data because (1) annual and not monthly nutrient fluxes from the Baltic Proper are used in this modeling and (2) annual and not monthly nutrient fluxes from land (from HELCOM 2000) are used. So, in this modeling, the case study system (KA) has been divided into two depth intervals: (1) the surface-water layer (SW), i.e., the water above the theoretical wave base; (2) the deep-water layer (DW) defined as the volume of water beneath the theoretical wave base. It should be stressed that the theoretical wave base at around 40 m in the Kattegat describes average conditions. During storm events, the wave base will be at greater water depths (see Jönsson 2005) and during calm periods at shallower depths. The wave base also varies spatially within the studied area. From Figs. 2.6 and 2.7, it is evident that the depth of the wave base describes the conditions in the Kattegat very well. Figure 2.8 gives the hypsographic curve for the Kattegat and how the areas above and below the theoretical wave base are defined.
One can note that the area below the theoretical wave base (D wb) at 39.9 m in KA is 3,134 km2 and the total area is 21,818 km2. The volume of the SW layer is 487.5 km3 and of the DW layer only 35.3 km3; the entire volume is 522.7 km3. The maximum depth is 130 m, but from Fig. 2.8, one can see that the area below 91 m is very small so 91 m has been used as a functional maximum depth in this modeling. Among the morphometric parameters characterizing the studied sub-basin, three main groups can be identified (see Håkanson 2004):
-
Size parameters: different parameters in length units, such as the maximum depth, parameters expressed in area units, such as water surface area, and parameters expressed in volume units, such as water volume and SW volume.
-
Form parameters (based on size parameters) such as mean depth and the form factor.
-
Special parameters, for example, the dynamic ratio and the effective fetch.
The CoastMab model uses several of these variables. They are listed in Table 2.2. The volume development, also often called the form factor (V d, dimensionless), is defined as the ratio between the water volume and the volume of a cone, with a base equal to the water surface area (A in km2) and with a height equal to the maximum depth (D Max in m):
The form factor describes the form of the basin. The form of the basin is very important, e.g., for internal sedimentological processes. In basins of similar size but with different form factors, one can presuppose that the system with the smallest form factor would have a larger area above the theoretical wave base and more of the resuspended matter transported to the surface-water compartment than to the deep-water compartment below the theoretical wave base compared to a system with a higher form factor. This is also the way in which the form factor is used in the CoastMab model.
The dynamic ratio (DR; see Håkanson 1982) is defined by the ratio between the square root of the water surface area (in km2 not in m2) and the mean depth, D MV (in m; DR = √Area/D MV). DR is a standard morphometric parameter in contexts of resuspension and turbulence in entire basins. ET areas above the theoretical wave base (i.e., areas where fine sediment erosion and transport processes prevail) are likely to dominate the bottom dynamic conditions in basins with dynamic ratios higher than 3.8. Slope processes are known (see Håkanson and Jansson 1983) to dominate the bottom dynamic conditions on slopes greater than about 4–5%. Slope-induced ET areas are likely to dominate basins with DR values lower than 0.052.
One should also expect that in all basins there is a shallow shoreline zone where wind-induced waves will create ET areas, and it is likely that most basins have at least 15% ET areas. If a basin has a DR of 0.26, one can expect that in this basin the ET areas would occupy 15% of the area. If DR is higher or lower than 0.26, the percentage of ET areas is likely to increase. Basins with high DR values, i.e., large and shallow system, are also likely to be more turbulent than small and deep basins. This will influence sedimentation. During windy periods with intensive water turbulence, sedimentation of suspended fine particles in the water will be much lower than under calm conditions. This is accounted for in the CoastMab model and the dynamic ratio is used as a proxy for the potential turbulence in the monthly calculations of the transport processes. It should be stressed that the form factor and the dynamic ratio provide different and complementary aspects of how the form may influence the function of aquatic systems. The effective fetch (see the ETA diagram in Fig. 2.5) is often defined according to a method introduced by the Beach Erosion Board (1972). The effective fetch (L ef in km) gives a more representative measure of how winds govern waves (wave length, wave height, etc.) than the effective length, since several wind directions are taken into account. Using traditional methods, it is relatively easy to estimate the effective fetch by means of a map and a special transparent paper (see Håkanson 1977). The central radial of this transparent paper is put in the main wind direction or, if the maximum effective fetch is requested, in the direction which gives the highest L ef value. Then the distance (x in km) from the given station to land (or to islands) is measured for every deviation angle a i , where a i is ± 6, 12, 18, 24, 30, 36, and 42°. L ef may then be calculated from
-
∑cos(a i ) = 13.5, a calculation constant.
-
SC′ = the scale constant; if the calculations are done on a map in scale 1:250,000, then SC′ = 2.5.
The effective fetch attains the highest values close to the shoreline and the minimum values in the central part of a basin. This relationship is important in, e.g., contexts of shore erosion and morphology, for bottom dynamic conditions (erosion–transportation–accumulation), and hence also for internal processes, mass-balance calculations, sediment sampling, and evaluations of sediment pollution. For entire basins, the mean effective fetch may be estimated as √Area (see Fig. 2.5). In a round basin, the requested value should be somewhat lower than the diameter (d = 2r; r = the radius); the area is πr 2 and hence d = 1.13·√Area and the mean fetch approximately √Area.
2.2.2 Sediments and Bottom Dynamic Conditions
As stressed in Fig. 2.5, the theoretical wave base may also be determined from the ETA diagram. This approach focuses on the behavior of the cohesive fine materials settling according to Stokes’ law in laboratory vessels:
-
Areas of erosion (E) prevail in shallow areas or on slopes where there is no apparent deposition of fine materials but rather a removal of such materials; E areas are generally hard and consist of sand, consolidated clays, and/or rocks with low concentrations of nutrients.
-
Areas of transportation (T) prevail where fine materials (such as the carrier particles for water pollutants) are deposited periodically (areas of mixed sediments). This bottom type generally dominates where wind/wave action regulates the bottom dynamic conditions. It is sometimes difficult in practice to separate areas of erosion from areas of transportation. The water depth separating transportation areas from accumulation areas, the theoretical wave base, is, as stressed, a fundamental component in these mass-balance calculations.
-
Areas of accumulation (A) prevail where the fine materials (and particulate forms of water pollutants) are deposited continuously (soft bottom areas).
Generally hard or sandy sediments within the areas of erosion (E) often have a low water content, low organic content, and low concentrations of nutrients and pollutants. These are the areas (the “end stations”) where high concentrations of pollutants may appear (see Table 2.5). The conditions within the T areas are, for natural reasons, variable, especially for the most mobile substances, like phosphorus, manganese, and iron, which react rapidly to alterations in the chemical “micro-climate” (given by the redox potential) of the sediments. Fine materials may be deposited for long periods during stagnant weather conditions.
In connection with a storm or a mass movement on a slope, this material may be resuspended and transported up and away, generally in the direction toward the A areas in the deeper parts, where continuous deposition occurs. Thus, resuspension is a most natural phenomenon on T areas. It should also be stressed that fine materials are rarely deposited as a result of simple vertical settling in natural aquatic environments. The horizontal velocity is generally at least 10 times larger, sometimes up to 10,000 times larger, than the vertical component for fine materials or flocs that settle according to Stokes’ law (Bloesch and Burns 1980, Bloesch and Uehlinger 1986). An evident boundary condition for this approach to calculate the ET areas is that if the depth of the theoretical wave base D wb > D Max, then D wb = D Max.
In CoastMab, there are also two boundary conditions for ET (= the fraction of ET areas in the basin):
If ET > 0.99 then ET = 0.99 and if ET < 0.15 then ET = 0.15.
ET areas are generally larger than 15% (ET = 0.15) of the total area since there is always a shore zone dominated by wind/wave activities. For practical and functional reasons, one can also generally find sheltered areas, macrophyte beds, and deep holes with more or less continuous sedimentation, that is, areas which actually function as A areas, so the upper boundary limit for ET may be set at ET = 0.99 rather than at ET = 1. The value for the ET areas is used as a distribution coefficient in the CoastMab model. It regulates whether sedimentation of the particulate fraction of the substance (here phosphorus, nitrogen, or SPM) goes to the DW or ET areas. The sediment data are compiled in Table 2.6.
One can note the following:
Most TP values from the upper 2 cm of the accumulation area sediments below the theoretical wave base vary in the range from 0.7 to 1.1 mg TP g–1 dw (the mean value is close to 0.88 mg g–1 dw; dw = dry weight); the TN data from 2.1 to 2.8 mg g–1 dw (MV = 2.4 mg g–1 dw); the organic content is about 10–11% ww (ww = wet weight).
-
1.
Due to substrate decomposition by bacteria and compaction from overlying sediments, the TP, TN concentrations and the organic content (loss on ignition, IG) decrease with sediment depth in the accumulation areas (see Håkanson and Jansson 1983). In all of the following simulations, a sediment depth of 0–10 cm will be used and this means that the reference values for the water content, organic content, TP and TN concentrations will be adjusted to this. The reference values for the 0–10 cm layer are set to be 33% lower than the P and N values given in Table 2.4 for the 0–2 cm layer.
-
2.
The bulk density (d in g cm–3 ww) is between 1.1 and 1.3.
-
3.
The water content (W in % ww) has been set to 70% for the upper 10 cm accumulation area sediments in the Kattegat (0–10 cm) and to 85% for the newly deposited SPM on the ET areas.
-
4.
The organic content (= loss on ignition, IG in % dw) is set to 10% for the upper 10 cm accumulation area sediments in the Kattegat. The IG value in underlying clayey sediments is around 7.5% dw.
The area of erosion (AreaE) is calculated from the hypsographic curve and the corresponding depth given by the ETA diagram (Fig. 2.5). This means that the depth separating E areas from T areas is given by
Note that the area is given in km2 in Eq. (2.3) to get the depth in m.
2.2.3 Trends and Variations in Water Variables
This section will present and discuss empirical data in the Kattegat for the period 1995–2008 (data from SMHI) as a background to the subsequent modeling. Figure 2.9 first gives data on the target bioindicators, Secchi depth, oxygen concentrations, and concentrations of chlorophyll-a in the surface-water layer in Kattegat.
The temporal variation in (a) Secchi depths (m), (b) oxygen concentrations (O2), and (c) concentrations of chlorophyll-a (μg L–1) in the surface-water layer of the Kattegat in the years 1995–2008 (month 1 is January of 1995). The figure also gives statistical trend analyses (regression line; coefficient of determination, r 2, and number of data, n; data from SMHI)
This figure and the following figures also give statistical trend analyses (regression line, coefficient of determination, r 2, and number of data, n). From Fig. 2.9, one can note the following:
-
There is a very weak trend for these three bioindicators, as revealed by the small slope coefficients (–0.00776 for Secchi depth, –0.0021 for oxygen, and –0.0028 for chlorophyll) and the low r 2 values (0.21, 0.0052, and 0.0027). So, for this period, the conditions have been rather stable in the Kattegat for these three key variables.
-
One can also note the clear seasonal pattern for oxygen, no evident seasonal pattern for Secchi depth, and a fairly distinct pattern for chlorophyll. One might have expected a more evident seasonal pattern for chlorophyll with peak values in the spring and fall.
The corresponding information is given in Fig. 2.10 for surface-water temperatures, salinity, TP and TN concentrations, and the TN/TP ratio.
The temporal variation in (a) temperatures, (b) salinities (psu), (c) TP concentrations, (d) TN concentration, and (e) the TN/TP ratio in the surface-water layer of the Kattegat in the years 1995–2008 (month 1 is January of 1995). The figure also gives statistical trend analyses (regression line; coefficient of determination, r 2, and number of data, n). Data from SMHI
The TN/TP ratio addresses the question about “limiting” nutrient, which is certainly central in aquatic ecology and has been treated in numerous papers and textbooks (e.g., Dillon and Rigler 1974, Smith 1979, 2003, Riley and Prepas 1985, Howarth 1988, Evans et al. 1996, Wetzel 2001, Newton et al. 2003, Smith et al. 2006, Håkanson and Bryhn 2008a, 2008c). The average composition of algae (C106N16P) is reflected in the Redfield ratio (N/P = 7.2 by mass). So, by definition, algae need both nitrogen and phosphorus and one focus of coastal eutrophication studies concerns the factors limiting the phytoplankton biomass, often expressed by chlorophyll-a concentrations in the water. Note that the actual phytoplankton biomass at any given moment in a system is a function of the bioavailable nutrient concentrations, light, and predation on phytoplankton by herbivorous zooplankton minus the death of phytoplankton regulated by the turnover time of the phytoplankton (see Håkanson and Boulion 2002). From Fig. 2.10, one can note the following:
-
All trends are weak. The strongest is the decrease in TN concentrations; the increase in temperature is also interesting in these days when global warming is on the agenda; the changes in salinity, TP, and TN/TP are very small. It should be stressed that all these changes are statistically significant because the number of data is so large. These data support the conclusion that there have been no major changes in the Kattegat system during the last 18 years regarding the variables in Fig. 2.10.
-
Figure 2.11 gives the temporal (monthly) trend in tributary water discharge from Swedish rivers entering the Kattegat. Here, one can see a characteristic seasonal variation with high water discharge in spring, but also this trend is very weak.
-
Figure 2.12 illustrates another problem related to the concept of “limiting” nutrient. Using data from the Baltic Proper, this figure gives a situation where the chlorophyll-a concentrations show a typical seasonal “twin peak” pattern with a pronounced peak in April. The higher the primary production, the more bioavailable nitrogen (nitrate, ammonium, etc.) and phosphorus (phosphate) are being used by the algae (the spring bloom is mainly diatoms) and eventually the nitrate concentration drops to almost zero and the primary production decreases – but the important point is that the primary production, the phytoplankton biomass, and hence also the concentration of chlorophyll-a remain high during the entire growing season!
Trends in nutrient inputs to the Kattegat have to some extent been investigated by Carstensen et al. (2006). They found a significant decrease from 1989 to 2002 in TP inputs to Kattegat, Öresund, and the Belt Sea from the catchment but no changes in TN inputs from land or from the atmosphere during this period. Carstensen et al. (2006) also correlated changes in nutrient inputs from land with changes in nutrient concentrations of Kattegat waters, but failed to account for any trends in nutrient inputs from the Skagerrak and the Baltic Proper. Carstensen et al. (2006) dismissed the possibility of explaining nutrient trends in bottom waters of the Kattegat by nutrient trends in the Skagerrak on the grounds that nutrient concentrations in the Skagerrak are very low and scantly influenced by inputs from land.
However, although nutrient concentrations are low in the Skagerrak and the Baltic Proper compared to concentrations in many tributaries, nutrient fluxes from the Skagerrak and the Baltic Proper are very large in a mass-balance context, which has been noted by Eilola and Sahlberg (2006) and which will be further elaborated in this work. Comprehensive trends in TN and TP inputs to the Kattegat from land plus inputs from the atmosphere, the Skagerrak, and the Baltic Proper have to the best of our knowledge not been studied.
2.2.4 The Dilemma Related to Predictions of Cyanobacteria
Figure 2.13 illustrates this dilemma using data for the Kattegat. The figure gives the TN/TP ratio on the y-axis and the surface-water temperature on the x-axis. It has been demonstrated by analyses of empirical data from many systems that there exists a threshold value for blooms of cyanobacteria when the TN/TP ratio is lower than 15 and when the SW temperatures are higher than 15°C (see Håkanson et al. 2007).
Based on this, one should expect that the conditions in the Kattegat would favor cyanobacteria in about 20% of the time (Fig. 2.13). However, cyanobacteria do not seem to abound in Kattegat but they certainly abound in the Baltic Sea (see Håkanson and Bryhn 2008a, 2008c). In hypertrophic lakes, the biomass of cyanobacteria can be very high with concentrations of about 100 mg L–1 (Smith 1985). Howarth et al. (1988a, 1988b) found no data on N-fixing planktonic species in estuaries and coastal seas, except for the Baltic Sea and the Peel-Harvey estuary, Australia. Also results from Marino et al. (2006) support this general lack of N-fixing cyanobacteria in estuaries. There are more than 10 nitrogen-fixing cyanobacteria species in the Baltic Proper (Wasmund et al. 2001). A field study in the Baltic Sea (Wasmund 1997) indicated that in this brackish environment cyanobacteria have the highest biomass at 7–8 psu and that the blooms in the Kattegat and Belt Sea are more frequent if the salinity is below 11.5 psu (see also Sellner 1997). A laboratory experiment with cyanobacteria from the Baltic Sea supports the results that the highest growth rate was at salinities in the range between 5 and 10 psu (Lehtimäki et al. 1997). So, the scarcity of cyanobacteria in the Kattegat may be related to the relatively high salinity of about 25 psu in this system. This also means that in this mass-balance modeling for nitrogen, there is no atmospheric nitrogen fixation.
2.2.5 The Reasons Why This Modeling Is Not Based on Dissolved Nitrogen or Phosphorus
At short timescales (seconds to days), it is evident that the causal agent regulating/limiting primary production is the concentration of the nutrient in bioavailable forms, such as DIN (dissolved inorganic nitrogen) and DIP, nitrate, phosphate, and ammonia. Short-term nutrient limitation is often determined by measuring DIN and DIP concentrations or by adding DIN and/or DIP to water samples in bioassays. However, information on DIN and DIP from real coastal systems often provides poor guidance in management decisions because
-
DIN and DIP are quickly regenerated (Dodds 2003). For example, zooplankton may excrete enough DIN to cover for more than 100% of what is consumed by phytoplankton (Mann 1982). In highly productive systems, there may even be difficulties to actually measure nutrients in dissolved forms because these forms are picked up so rapidly by the algae. Dodds (2003) suggested that only when the levels of DIN are much higher than the levels of DIP (e.g., 100:1), it is unlikely that DIN is limiting and only if DIN/DIP < 1, it is unlikely that P is the limiting nutrient. He also concluded that DIN and DIP are poor predictors of nutrient status in aquatic systems compared to TN and TP.
-
Phytoplankton and other primary producers also take up dissolved organic N and P (Huang and Hong 1999, Seitzinger and Sanders 1999, Vidal et al. 1999).
-
DIN and DIP are highly variable in most aquatic systems including the Kattegat (see Håkanson and Bryhn 2008a, 2008c and Tables 2.3 and 2.4) and are, hence, very poor predictors of phytoplankton biomass and primary production (as measured by chlorophyll concentrations; see Fig. 2.14).
-
Primary production in natural waters may be limited by different nutrients in the long run compared to shorter time perspectives (see Redfield 1958, Redfield et al. 1963). Based on differences in nutrient ratios between phytoplankton and seawater, Redfield (1958) hypothesized that P was the long-term regulating nutrient, while N deficits were eventually counteracted by nitrogen fixation. Schindler (1977, 1978) tested this hypothesis in several whole-lake experiments and found that primary production was governed by P inputs and unaffected by N inputs, and that results from bioassays were therefore irrelevant for management purposes. Redfield’s hypothesis has also been successfully tested in modeling work for the global ocean (Tyrrell 1999) and the Baltic Proper (Savchuk and Wulff 1999). However, Vahtera et al. (2007) have used a “vicious circle” theory to suggest that both nutrients should be abated to the Baltic Sea since they may have different long-term importance at different times of the year.
Empirical data from the Baltic Sea, Kattegat, and Skagerrak on mean monthly chlorophyll-a concentrations (logarithmic data) versus empirical data (log) on DIN and DIP, respectively. The figure also gives the equations for the regressions and the corresponding r 2 values (from Håkanson and Bryhn 2008a)
So, the concentrations of the bioavailable fractions, such as DIN and DIP in μg L–1 or other concentration units, cannot as such regulate primary phytoplankton production in μg day–1 (or other units), since primary production is a flux including a time dimension and the nutrient concentration is a concentration without any time dimension. The central aspect has to do with the flux of DIN and DIP to any given system and the regeneration of new DIN and DIP related to bacterial degradation of organic matter containing N and P. The concentration of DIN and DIP may be very low and the primary phytoplankton production and biomass can be high as in Fig. 2.12 because the regeneration and/or inflow of DIN and DIP is high.
The regeneration of DIN and DIP concerns the amount of TN and TP available in the water mass, i.e., TN and TP represent the pool of the nutrients in the water, which can contribute with new DIN and DIP. It should be stressed that phytoplankton has a typical turnover time of about 3 days and bacterioplankton has a typical turnover time of slightly less than 3 days (see Håkanson and Boulion 2002). This means that within a month there can be 10 generations of phytoplankton, which would need both DIN and DIP in the approximate proportions given by the Redfield ratio (7.2 in grams).
2.2.6 The Reasons Why It Is Generally Difficult to Model Nitrogen
There are four highlighted spots with question marks in Fig. 2.15 indicating that for many coastal systems, it is very difficult to quantify some of the most important transport processes in a general manner for nitrogen. Three of them are denitrification, atmospheric wet and dry deposition, and nitrogen fixation, e.g., by certain forms of cyanobacteria.
Overview of important transport processes and mechanisms related to the concept of “limiting” nutrient (from an illustration for the Baltic Sea from Håkanson and Bryhn 2008a)
Figure 2.15 also highlights another major uncertainty related to the understanding of nitrogen fluxes in coastal systems, the particulate fraction, which is necessary for quantifying sedimentation. Atmospheric nitrogen fixation may be very important in contexts of mass-balance calculations for nitrogen (see Rahm et al. 2000) and in this modeling; the same value for atmospheric nitrogen deposition has been used as in the OSPAR model by SMHI. The data on atmospheric nitrogen deposition for the Kattegat should be reasonable in terms of order-of-magnitude values. Without empirically well-tested algorithms to quantify nitrogen fixation, crucial questions related to the effectiveness of the remedial measures to reduce nutrient discharges to aquatic systems cannot be properly evaluated, since costly nitrogen reductions may be compensated for by nitrogen fixation by cyanobacteria. However, this is a problem in many systems, such as the Baltic Sea, but not in the Kattegat where there seem to be no significant amounts of cyanobacteria.
2.2.7 Comments and Conclusions
Traditional hydrodynamic or oceanographic models to calculate water fluxes to, within, and out of coastal areas generally use water temperature data (the thermocline) or salinity (the halocline) to differentiate between different water layers. This section has motivated another approach, the theoretical wave base as calculated from process-based sedimentological criteria, to differentiate between the surface-water layer and lower vertical layers and this approach gives one characteristic value for each basin. Morphometric data for the Kattegat and the hypsographic curve have been used in the CoastMab modeling. The basic aim of this section has been to present empirical data from the Kattegat on total phosphorus (TP), total nitrogen (TN), chlorophyll, Secchi depth, water temperature, and salinity. The empirical data from the Kattegat show the following:
-
1.
All relevant water variables in the SW layer of the Kattegat have been fairly stable in the period between 1995 and 2008.
-
2.
There is a small increase in surface-water temperatures in the Kattegat (compare global warming).
-
3.
The salinities have also been fairly stable since 1995.
-
4.
The concentration of chlorophyll-a shows a very slowly decreasing trend in the surface-water layer of the Kattegat since 1975. The seasonal pattern in monthly median chlorophyll-a concentrations is relatively obscure.
-
5.
The water column has been divided into two layers, separated by the theoretical wave base. This describes the conditions very well.
The long-term trends in TN and TP inputs to the Kattegat from land plus inputs from the atmosphere, the Skagerrak, and the Baltic Proper are, however, largely unknown.
2.3 Water, SPM, Nutrient, and Bioindicator Modeling
2.3.1 Background on Mass Balances for Salt and the Role of Salinity
The salinity is of vital importance for the biology of coastal areas influencing, e.g., the number of species in a system (see Remane 1934) and also the reproductive success, food intake, and growth of fish (Rubio et al. 2005, Nissling et al. 2006). Furthermore, a higher salinity increases the flocculation and aggregation of particles (see Håkanson 2006) and hence affects the rate of sedimentation, which is of particular interest in understanding variations in water clarity within and among coastal areas. More salt in the water, greater the flocculation of suspended particles. This does influence not only the concentration of particulate matter, but also the concentration of any substance with a substantial particulate phase such as phosphorus and nitrogen. The salinity also affects the relationship between total phosphorus (TP), total nitrogen (TN), and primary production/biomass (chlorophyll-a; Håkanson and Bryhn 2008a, 2008c). These relationships are shown in Figs. 2.16 and 2.17 and they are used in this work to calculate chlorophyll-a concentrations from dynamically modeled salinities in the different sub-basins, from dynamically modeled phosphorus and nitrogen concentrations, and from information on the number of hours with daylight. The salinity is easy to measure and the availability of salinity data for the Kattegat is very good.
Scatter plot of all available data relating the ratio Cl/TN to salinity (psu). The figure also gives two regressions for salinities either below (crosses) or higher than the threshold value of 10 (circles) (from Håkanson and Bryhn 2008a). Note that for the Kattegat, the surface-water (SW) salinity is about 25 psu; if TN is 300 μg L–1, this gives Chl ≈ 3 μg L–1. The scatter around the given regression partly depends on light, uncertainties in data, and uncertainties in the particulate coefficient for nitrogen
Box-and-whisker plot (showing medians, quartiles, percentiles, and outliers) illustrating the Chl/TP ratio for 10 salinity classes. The statistics give the median values, the coefficients of variation (CV), and the number of data in each class (from Håkanson and Bryhn 2008a). Note that for the Kattegat, the surface-water (SW) salinity is about 25 psu; if TP is 20 μg L–1, this gives Chl ≈ 3 μg L–1. The scatter around the given regression partly depends on light, uncertainties in data, and uncertainties in the particulate coefficient for nitrogen
So, Figs. 2.16 and 2.17 illustrate the role of salinity in relation to the Chl/TP and Chl/TN ratios. The figures give the number of data in each salinity class; the box-and-whisker plots give the medians, quartiles, percentiles, and outliers; and the table below the diagram provides information on the median values, the coefficients of variation (CV = SD/MV; SD = standard deviation; MV = mean value), and the number of systems included in each class (n). These results are evidently based on many data from systems covering a wide salinity gradient. An interesting aspect concerns the pattern shown in the figure. One can note the following:
-
The median value for the Chl/TP ratio for lakes is 0.29, which is almost identical to the slope coefficient for the key reference model for lakes (0.28 in the OECD model; see OECD 1982).
-
The Chl/TP ratio changes in a wave-like fashion when the salinity increases. It is evident that there is a minimum in the Chl/TP ratio in the salinity range between 2 and 5. Subsequently, there is an increase up to the salinity range of 10–15 and then a continuous decrease in the Chl/TP range until a minimum value of about 0.012 is reached in the hypersaline systems. From the relationship between the Chl/TN ratio and the salinity, one can identify differences and similarities between the results presented for the Chl/TP ratio.
-
At salinities higher than 10–15, there is a steady decrease also in the Chl/TN ratio (note that there are no data on TN from the hypersaline Crimean lakes).
-
The Chl/TN ratio attains a maximum value for systems in the salinity range between 10 and 15 and significantly lower values in lakes and less saline brackish systems.
-
The table in Fig. 2.16 gives the median Chl/TN values and they vary from 0.0084 (for lakes), to 0.017 for brackish systems in the salinity range between 10 and 15, to very low values (0.0041) for marine coastal systems in the salinity range between 35 and 40.
The water exchange in the Kattegat is calculated using the CoastMab model for salt. This section will present monthly budgets for water and salt in the Kattegat. Mass-balance models have long been used as a tool to study lake eutrophication (Vollenweider 1968, OECD 1982) and also used in different coastal applications (see Håkanson and Eklund 2007, Håkanson and Bryhn 2008c). Mass-balance modeling makes it possible to predict what will likely happen to a system if the conditions change, e.g., a reduced discharge of a pollutant related to a remedial measure. Mass-balance modeling can be performed at different scales depending on the purpose of the study. A large number of coastal models do exist, all with their pros and cons. For example, the 1D nutrient model described by Vichi et al. (2004) requires meteorological input data with a high temporal resolution, which makes forecasting for time periods longer than 1 week ahead problematic.
The 3D model used by Schernewski and Neumann (2005) has a temporal resolution of 1 min and a spatial resolution of 3 nm (nautical miles), which means that it is difficult to find reliable empirical data to run and validate the model. Several water balance studies have also been carried out in the Kattegat and the Baltic Sea, see, e.g., Jacobsen (1980), HELCOM (1986, 1990), Bergström and Carlsson (1993, 1994), Omstedt and Rutgersson (2000), Stigebrandt (2001), Rutgersson et al. (2002), Omstedt and Axell (2003), Omstedt et al. (2004), and Savchuk (2005). The result of such mass-balance calculations for salt or for other substances depends very much on how the system is defined and how the model is structured.
Within the BALTEX program (BALTEX 2006, BACC 2008), the water and heat balances are major research topics and estimates on the individual terms in the water balance are frequently being revised (e.g., Bergström and Carlsson 1993, 1994, Omstedt and Rutgersson 2000, Rutgersson et al. 2002). The major water balance components in the Baltic Sea are the in- and outflows at the entrance area, river runoff, and net precipitation (Omstedt et al. 2004). Change in water storage needs also to be considered at least for shorter time periods. The different results depend on the time period studied and the length of the period. Several studies have also divided the Baltic Sea into sub-basins and from the water and salt balances estimated the flows (e.g., Omstedt and Axell 2003, Savchuk 2005).
The necessary empirical data on salinity (and other water variables) to run the CoastMab model have originally been obtained from SMHI (the Swedish Meteorological and Hydrological Institute) and data from the period 1995 to 2008 have been used in this work. There are inter-annual and seasonal variations in both net precipitation and riverine water input to the Kattegat (HELCOM 1986, Bergström and Carlsson 1993, 1994, Winsor et al. 2001) as well as in the exchange of water with the Kattegat and the salinity of this water (Samuelsson 1996). This work has focused on a period when there is access to comprehensive data for the mass balances for salt, but also for this period there are inherent uncertainties in the data. This is shown by the CV values in Tables 2.3 and 2.4.
The fluxes and retention rates for the different sub-basins and compartments of the Kattegat, as defined in this mass-balance modeling for salt, will be used in the following mass-balance modeling for phosphorus, nitrogen, and SPM. The basic structuring of this model (CoastMab) enables extensions not just to substances other than salt, but also to systems other than the Baltic Sea and the Kattegat.
2.3.2 Water Fluxes
Figure 2.18 illustrates the basic structure of the model with its two water compartments (SW and DW in the Kattegat) and also results of the modeling for water fluxes. Note that this modeling is done on a monthly basis to achieve seasonal variations, which is important in the mass-balance models for phosphorus, nitrogen, and SPM.
All the water fluxes in Fig. 2.18 are given in km3 year–1 to get an overview. This figure also shows water fluxes from Swedish and Danish tributary rivers, precipitation, and evaporation. For the tributary fluxes data from SHMI for the period 1995–2008 have been used. The salinities in the inflowing water from Skagerrak have been calculated using data exemplified in Table 2.7 for the surface-water inflow.
The model quantifies the fluxes needed to achieve steady-state concentrations for the salinity that correspond as closely as possible to the empirical monthly salinities in the two compartments. All equations have been given by Håkanson and Bryhn (2008a), and they are compiled in Table 2.8.
One can note from Fig. 2.18 that the greatest water fluxes into the Kattegat are the deep-water (DW) flux from Skagerrak (SK) (2,165 km3 year–1), the surface-water (SW) flux from the Baltic Proper (BP) (960 km3 year–1); the tributary inflow, precipitation, and deep-water inflow from the Baltic Proper are relatively small (30, 51, and 47 km3 year–1, respectively). Since this is mass balance for salt, the fluxes out of the system should be equal to the inflow at steady state. These fluxes provide a very important interpretational framework for the other mass balances (for phosphorus, nitrogen, and SPM). From the fluxes of water, one can also define the associated retention times (T) and retention rates (1/T). The retention rates for water may be used in mass-balance models for, e.g., nutrients since these rates indicate the potential turbulence in the given compartment, and the turbulence regulates the settling velocity for suspended particles – the higher the potential turbulence, the lower the settling velocity for particulate phosphorus (Håkanson and Bryhn 2008a). The retention time for water in each compartment is defined from the total inflow of water (m3 year–1) and the volume of the compartment (m3). Empirical salinity data are compared to modeled values in Fig. 2.19a. The inherent empirical uncertainties in the mean monthly salinity values (the CV values) are small, about 0.28 in the SW layer and very small in the DW layer, 0.02 (see Tables 2.3 and 2.4).
Empirical data versus modeled values in the Kattegat. (a) Salinities (the two upper lines give the DW salinities, the two lower lines the SW salinities), (b) modeled TP concentrations in the surface-water (SW) layer versus ±1 standard deviation (SD) of the mean empirical value, (c) modeled TP concentrations in the deep-water (DW) layer versus ±1 SD, (d) modeled dissolved fractions of phosphorus in the SW layer versus PO4/TP ratio, (e) modeled dissolved fractions of phosphorus in the DW layer versus the PO4/TP ratio, (f) modeled TN in SW layer versus ± 1 SD, (g) modeled TN in DW versus ±1 SD, (h) modeled dissolved fractions of N in SW versus the DIN/TN ratio, (i) modeled dissolved fractions of N in DW versus DIN/TN
The excellent results shown in Fig. 2.19a are not a result of a blind test, rather a result achieved after many calibrations. To understand how the Kattegat system, or any aquatic system, responds to changes in, e.g., loading of toxins, salt, or nutrients, it is imperative to have a dynamic process-based perspective quantifying the factors and functions regulating inflow, outflow, and internal transport processes and retention rates. This section has demonstrated that this modeling using the theoretical wave base rather than traditional temperature data to define the surface-water and deep-water compartments can give excellent correspondence between empirical and modeled data for the salinity. It is often stressed in contexts of marine eutrophication that it is important to develop practically useful general dynamic mass-balance models based on the ecosystem perspective to be able to give realistic evaluations of how systems will respond to changes in nutrient loading or other remedial actions (Smith 2003). The basic aim of this section has been to present data on the fluxes of water and the theoretical retention times for water and salt since those values give fundamental information on how the system reacts to changes in, e.g., nutrient loading. The idea with this modeling is that these water fluxes, water retention rates, and the algorithms to quantify vertical mixing and diffusion among the defined layers should be structured in such a manner that the model can be used to quantify also fluxes of phosphorus, nitrogen, and SPM. This places certain demands on the structure of this model, which are different from oceanographic models, e.g., in quantifying resuspension, mixing, and diffusion and in the requirements regarding the accessibility of the necessary driving variables.
2.3.3 Mass Balances
2.3.3.1 Phosphorus Dynamics
To combat eutrophication, it is fundamental to try to identify the anthropogenic contributions to the nutrient loading. HELCOM (see Table 2.9) has presented very useful data regarding the natural, diffuse, and point source discharges of phosphorus and nitrogen to the Kattegat. Evidently, the natural nutrient fluxes should not be reduced, only a certain part of the anthropogenic fluxes from point sources and diffuse emissions.
As a background to the discussion to find the best possible remedial strategy to mitigate the eutrophication in the Baltic Sea, Table 2.10 shows central aspects of the strategy proposed by HELCOM (2007b), which was also accepted by the Baltic States in November 2007. Based on costs for building water treatment plants in the Baltic States and the St. Petersburg area (20,000 euro t–1 P; HELCOM and NEFCO 2007), the action alternative motivated in Håkanson and Bryhn (2008a; about 10,000 t phosphorus year–1) would cost 0.2–0.4 billion euro year–1, or about 10% of the cost of the Baltic Sea Action Plan.
In the requested budgets for nitrogen and phosphorus for the Kattegat, it is essential to include all major transport processes in order to understand the situation and especially to know how remedial measures reducing nutrient loading to the system will likely change nutrient concentrations in water and sediments. The importance of the internal fluxes and the transport between basins compared to the anthropogenic nutrient input from land has also been shown by Christiansen et al. (1997) in a study of parts of the Kattegat. The transport processes (sedimentation, resuspension, burial, diffusion, mixing, biouptake, etc.) for phosphorus, nitrogen, and SPM quantified in the CoastMab model are general and apply for all substances in all/most aquatic systems (see Fig. 2.20), but there are also substance-specific parts (mainly related to the particulate fraction, the criteria for diffusion from sediments, and the fact that nitrogen appears with a gaseous phase).
So, these processes have the same names for all systems and for all substances:
-
Sedimentation is the flux from water to sediments or to deeper water layers of suspended particles and nutrients attached to such particles.
-
Resuspension is the advective flux from sediments back to water, mainly driven by wind/wave action and slope processes.
-
Diffusion is the flux from sediments back to water or from water layers with high concentrations of dissolved substances to connected layers with lower concentrations. Diffusion is triggered by concentration gradients, which would often be influenced by small-scale advective processes; even after long calm periods, there are currents related to the rotation of the earth, the variations of low and high pressures, temperature variations between day and night, etc.; it should be noted that it is difficult to measure water velocities lower than 1–2 cm s–1 in natural aquatic systems.
-
Mixing (or large-scale advective transport processes) is the transport between, e.g., surface-water layers and deeper water layers related to changes in stratification (variations in temperature and/or salinity).
-
Mineralization (and regeneration of nutrients in dissolved forms) is the decomposition of organic particles by bacteria.
-
Primary production is creation of living suspended biomass from sunlight and nutrients.
-
Biouptake is the uptake of the substance in biota. In the CoastMab/CoastWeb model, one first calculates biouptake in all types of organisms with short turnover times (phytoplankton, bacterioplankton, benthic algae, and herbivorous zooplankton) and from this biouptake in all types of organisms with long turnover times (i.e., fish, zoobenthos, predatory zooplankton, jellyfish, and macrophytes) to account for the fact that phosphorus circulating in the system will be retained in these organisms and the retention times for phosphorus in these organisms are calculated from the turnover times of the organisms.
-
Burial is the sediment transport of matter from the biosphere to the geosphere often of matter from the technosphere.
-
Outflow is the flux out of the system of water and everything dissolved and suspended in the water.
Figure 2.19b, c gives the modeled annual TP concentrations in SW and DW water against the corresponding empirical data. The results in Fig. 2.19 are well within the uncertainty bands given by ±1 standard deviation for the empirical data and one cannot expect better results given the fact that there have been no calibrations and that the dominating transport from the Baltic Proper is based on the mean annual transport. The modeled mean annual TP concentrations in A-sediments (0–10 cm) are given in Fig. 2.21a and also these modeled values fall within the requested empirical range (0.5–0.66 mg TP g–1 dw). The annual fluxes of phosphorus are shown in Fig. 2.22. These fluxes give information of fundamental importance related to how the Kattegat reacts to changes in phosphorus loading. It should be noted that the phosphorus fluxes to and from organisms with short turnover times (BS) are very large compared to all other fluxes, but the amount of TP found in biota is small compared to what is found in some other compartments.
Empirical data versus modeled values in the Kattegat. (a) Modeled TP concentrations in the accumulation area sediments (0–10 cm) versus empirical maximum and minimum values, (b) modeled TN concentrations in the accumulation area sediments (0–10 cm) versus empirical maximum and minimum values and modeled TN concentrations in recently deposited matter on ET areas, (c) modeled Secchi depths versus ±1 standard deviation (SD) of the mean empirical value, (d) empirical mean concentrations of chlorophyll, modeled chlorophyll concentrations based on only TP, on only TN, and on both TP and TN (bold), (e) modeled sedimentation based on the water content of recently deposited matter and on the mean water content in sediments from the upper 10 cm sediment layer and compared to the mean annual sedimentation in the Baltic Proper, and (f) modeled SPM concentrations in the surface-water layer and in the deep-water layer in the Kattegat
Characteristic annual phosphorus fluxes to, from, and within the Kattegat for the period 1995–2008. Note that the net inflow of phosphorus from the Baltic Proper is 17.5 kt year–1, SMHI (Håkansson 2007, the OSPAR assessment) gives 14 kt year–1
This illustrates the classical difference between “flux and amount.” In the ranking of the annual fluxes for the Kattegat from Fig. 2.22, it is evident that the most dominating fluxes are the ones to and from biota with short turnover times (about 320 kt year–1), whereas the average monthly amount of TP in all types of plankton is just about 1.7 kt. Most phosphorus is found in A-sediments (104 kt), on ET areas (10 kt), and in the SW layer (5 kt). Looking at the TP fluxes to the Kattegat, the DW flux from Skagerrak is the dominating one (47 kt year–1), followed by the SW inflow from the Baltic Proper (20 kt year–1), DW inflow from the Baltic Proper (5.4 kt year–1), SW inflow from the Skagerrak (2.4 kt year–1), tributary inflow (2 kt year–1), and atmospheric precipitation (0.1 kt year–1). Sedimentation in the SW layer is also important, 3.1 kt year–1 to the DW layer and 19 kt year–1 to the ET sediments (Fig. 2.23).
Sedimentation in the DW layer is relatively small (4.2 kt year–1) since about 50% of the phosphorus in the SW layer and about 85% of the phosphorus in the DW layer (see Table 2.12 and Fig. 2.19d, e) are in dissolved forms, which do not settle out. Figure 2.19d, e gives a comparison between modeled dissolved fractions and empirical ratios between phosphate and total phosphorus. It should be stressed that the dissolved fraction (DF) as defined in the model from the particulate fraction (DF = 1 – PF) is not the same thing as phosphate.
There are several different dissolved forms of phosphorus often abbreviated as DP (DIP + DOP), and Fig. 2.19d, e illustrates that the overall correspondence between modeled DF and the ratio between phosphate and total phosphorus in the Kattegat is reasonable. Together with the relatively high oxygen concentrations in the entire Kattegat, this also implies that diffusion of phosphorus from the A-sediments is small in the Kattegat (only 0.008 kt year–1). The diffusive flux in the water from the DW compartment to the SW compartment is also small (0.01 kt year–1). Burial, i.e., the transport of TP from the sediment biosphere to the sediment geosphere, is 5.1 kt year–1.
2.3.4 SPM Dynamics
The dynamic SPM model (CoastMab for SPM) has been described by Håkanson (2006, 2009). The model gave very good results for the tested 17 different Baltic Sea coastal areas. The mean error when empirical data on sedimentation (from sediment traps) were compared to modeled values was 0.075, the median error was –0.05, the standard deviation was 0.48, and the corresponding error/uncertainty for the empirical data was 1.0, as given by the coefficient of variation. This means that the uncertainties in the empirical data set the limit for further improvements of model predictions. The error for the modeled values was defined from the ratio between modeled and empirical data minus 1, so that the error is zero when modeled values correspond to empirical data. There are different sources for SPM:
-
1.
Primary production, which causes increasing biomasses for all types of plankton (phytoplankton, bacterioplankton, and herbivorous zooplankton) influencing SPM in the water.
-
2.
Inflow of SPM to the surface-water layer in the Kattegat from the Baltic Proper and Skagerrak.
-
3.
Inflow of SPM to the deep-water layer (i.e., from Baltic Proper and/or Skagerrak).
-
4.
Tributary inflow.
Table 2.11 gives the panel of driving variables for the dynamic SPM model. These are the site-specific data on variables needed to run the dynamic SPM model. No other parts of the model should be changed. Figure 2.22 shows the annual SPM fluxes to, within, and from the Kattegat. It is evident that the most dominating abiotic SPM inflow is DW inflow from the Skagerrak (about 12,000 kt year–1), followed by tributary inflow (2,000 kt year–1), SW inflow from the Baltic Proper (1,850 kt year–1), SW inflow from the Skagerrak (800 kt year–1), and DW inflow from the Baltic Proper (100 kt year–1). Sedimentation in the SW layer is also important with 5,600 kt year–1. Sedimentation of SPM from the SW to the DW layer is 950 kt year–1. The flux related to internal loading (resuspension) is 915 kt year–1 from ET areas to the SW layer and 325 kt year–1 to the DW layer. Burial, i.e., the transport of SPM from the sediment biosphere to the sediment geosphere, is 1,500 kt year–1. The total SPM production is 9,000 kt year–1.
Previous knowledge regarding the SPM concentration, its variation, and the factors influencing variations among and within sites was very limited for the Kattegat. The results discussed here represent a step forward in understanding and predicting SPM in the Kattegat and also in other similar systems. Evidently, it would have been preferable to have access to a large database on SPM, but it is very demanding (in terms of costs, manpower, ships, etc.) to collect such data, especially under storms. It should also be noted that bioturbation, fish movements (Meijer et al. 1990), currents (Lemmin and Imboden 1987), and slope processes (Håkanson and Jansson 1983), as well as boat traffic, trawling, and dredging, might all influence the SPM concentrations and how SPM varies among and within sites. These factors have, however, not been accounted for in this modeling, which does not concern sites but entire basins.
2.3.5 Nitrogen Fluxes
The dynamic modeling of the nitrogen fluxes uses the same CoastMab model and the same water fluxes (to, within, and from the Kattegat) and the same mixing rates and diffusion rates, as given by the CoastMab model for salinity; it uses the same algorithms for sedimentation, resuspension, biouptake, and retention in biota as the CoastMab model for phosphorus. However, for nitrogen, the following substance-specific modifications have been applied:
-
1.
The particulate fraction of nitrogen (PN) in the SW layer is calculated using the same basic algorithm as used for phosphorus except that for the dissolved fraction of nitrogen in the SW compartment, the monthly correction factors given in Table 2.12 have been used (i.e., the (DIN/TP)/(PO4/TP) data have been multiplied with the monthly modeled DF value for phosphorus). These modeled values are compared to the empirical DIN/TN values in Fig. 2.19 h and there is a good general agreement.
-
2.
The particulate fraction of nitrogen (PN) in the DW layer in the Kattegat is calculated using the same approach. Table 2.13 gives the monthly correction factors [i.e., (DIN/TP)/(PO4/TP)]. The modeled values are compared to the empirical DIN/TN values in Fig. 2.19 l and also these values are in relative good agreement with the measured DIN/TN values.
-
3.
Since there are no or very small amounts of nitrogen-fixing cyanobacteria in the Kattegat, N2 fixation is not accounted for in this modeling.
-
4.
The nitrogen inflow from Skagerrak is based on the same water fluxes as the ones used for the salinity, phosphorus, and SPM, the empirical data for the SW layer in Skagerrak.
-
5.
The nitrogen inflow from the Baltic Proper is based on the same empirical data (TN in μg L–1) for the SW layer (from HELCOM 2007a, 2007b) as presented and used by Håkanson and Bryhn (2008a), i.e.,
Jan.
298.7
Jul.
270.4
Feb.
292.1
Aug.
266.9
Mar.
292.8
Sep.
265.5
Apr.
280.5
Oct.
283.7
May
264.7
Nov.
278.8
Jun.
273.2
Dec.
305.7
For the DW inflow from the Baltic Proper to the Kattegat, the following mean annual value has been used (also from HELCOM 2007a, 2007b): 314 μg L–1.
-
6.
The tributary inflow of nitrogen to the Kattegat is based on the values from HELCOM given in Table 2.10.
-
7.
The denitrification in the Kattegat (in water and sediments) has been calculated as a residual term to satisfy the mass balance for nitrogen. This means that denitrification in the SW layer has been calculated by
where 0.01 is a calibration constant (a denitrification rate for the water with the dimension 1 month–1); denitrification is assumed to be temperature dependent (SWT) and 9.33 is the mean annual temperature and SWT/9.33 is a dimensionless temperature moderator; M TNSW is the mass (amount) of TN in the SW layer (g); V SW is the SW volume; and V is the total volume (m3) so V SW/V is a dimensionless moderator for the SW layer.
Denitrification in the DW layer is given by
For the ET sediments, denitrification has been calculated from
where 3 is a calibration constant (a denitrification rate for the ET sediments with the dimension 1 month–1); M TNET is the mass (amount) of TN in the ET sediments (g).
Denitrification in the A-sediments (0–10 cm) is given by
It should be stressed again that all the denitrification constants are determined from calibrations to satisfy the mass balance for nitrogen and they are not based on general, tested, algorithms which have been proven to work well in many coastal systems. This means that the predictions using the mass-balance model for nitrogen are more uncertain than the predictions of salt, phosphorus, and SPM. The diffusion of dissolved nitrogen from the deep-water layer to the surface-water layer is small in the Kattegat because the concentration gradient is small; the diffusion is calculated with the same algorithm as used for salinity and phosphorus. The predictions for the TN concentrations in the SW and DW layers in the Kattegat are compared to empirical monthly data in Fig. 2.19. Since these modeled values are based on calibrated denitrification rates, the modeled values are close to the empirical data. Annual fluxes of nitrogen are shown in Fig. 2.24. These fluxes give important information of how the Kattegat system likely reacts to changes in nitrogen loading. It should be noted that also the nitrogen fluxes to and from organisms with short turnover times (BS) are very large compared to all other fluxes, but the amounts of TN found in biota are small compared to what is found in other compartments.
Characteristic annual nitrogen fluxes to, from, and within the Kattegat for the period 1995–2008. Note that the net inflow of nitrogen from the Baltic Sea is 207 kt year–1, SMHI (Håkansson 2007, the OSPAR assessment) gives 190 kt year–1
In the ranking of the annual fluxes to the Kattegat, the most dominating abiotic fluxes are the TN flux to DW layer from the Skagerrak (435 kt year–1), followed by the SW inflow from the Baltic Proper (270 kt year–1), tributary inflow (82 kt year–1), SW inflow from the Skagerrak (30 kt year–1), atmospheric precipitation (18 kt year–1), and DW inflow from the Baltic Proper (15 kt year–1). Sedimentation in the SW layer is 30 kt year–1 to the DW layer and 180 kt year–1 to the ET sediments. Sedimentation in the DW layer is 17 kt year–1; about 25% of the nitrogen in the SW layer and about 50% in the DW layer (see Fig. 2.19 h, i) of the nitrogen appear in dissolved form. Figure 2.19 h, i gives a comparison between modeled dissolved fractions and empirical ratios between DIN and TN. It should be stressed that the dissolved form (DF), as defined in the model from the particulate fraction (DF = 1 – PF), is not the same thing as DIN. Figure 2.19 shows that the overall correspondence between modeled DF and the ratio between DIN and TN in the Kattegat is quite good, especially for the SW layer. From Fig. 2.24 one can note that the diffusion of nitrogen from sediments to water and from the DW layer to the SW layer is very small. Denitrification, on the other hand, is large: 13 kt year–1 from SW, 170 kt year–1 from ET, 8.3 kt year–1 from A-sediments, and 0.1 kt year–1 from the DW layer. Burial of TN from the A-sediments is 12 kt year–1.
2.3.6 Predicting Chlorophyll-a Concentrations
Values of chlorophyll-a concentrations in the surface-water layer drive the secondary production (including the production of zooplankton and fish), which means that it is very important to model chlorophyll as accurately as possible. This section will first describe the approach used to model chlorophyll and then present results describing how well modeled values correspond to measured data. Typical chlorophyll-a concentrations for the Kattegat and parts of the North Sea are shown in Fig. 2.3. Values lower than 2 μg L–1 (oligotrophic conditions) are found in the northern parts of the Bothnian Bay and the outer parts of the North Sea, while values higher than 20 μg L–1 (hypertrophic conditions) are often found in, e.g., the Vistula and Oder lagoons.
Concentrations of chlorophyll-a represent one of the most important bioindicators related to eutrophication. Håkanson and Bryhn (2008a, 2008c) discussed several approaches to predict chlorophyll in the surface-water layer:
-
1.
From regressions based on empirical TN concentrations and light conditions (see, e.g., Fig. 2.25)
-
2.
From regressions based on modeled or empirical TP concentrations (see, e.g., Fig. 2.26), light, salinity, and boundary conditions related to surface-water temperatures
Scatter plot between chlorophyll and TN. The figure also gives regressions for the actual data and log-transformed data for the 618 data points (from Håkanson and Bryhn 2008a)
Scatter plot between median surface-water concentrations of chlorophyll and total P (TP) for the growing season from 10 sub-groups constituting a salinity gradient. The figure also gives regressions for the actual data and log-transformed data for the 533 data points. How much of the scatter in this diagram depends on variations in salinity is discussed in the running text (from Håkanson and Bryhn 2008a)
Approaches applied in this work are also given in Table 2.14.
To obtain seasonal/monthly variations, the following calculations will use three approaches, which will be compared to empirical data:
-
1.
Chl from TP, TN, and salinity. This is the approach given in Table 2.14a, which has provided an r 2 value (r 2 = coefficient of determination) of 0.76 and is based on data from 493 systems from many parts of the world. The relationship between TN and TP concentrations for these data is shown in Fig. 2.26 and the results shown in this figure are important in contexts of remedial strategies, since the figure demonstrates that there is generally a significant co-variation between TN and TP concentrations and this indicates that one would often reduce also TP concentrations in receiving water systems if remedial measures focus on nitrogen reductions, and vice versa. To achieve realistic seasonal patterns, the dimensional moderator (Y DayL = HDL/12) based on the number of hours with daylight each month (from Table 2.6) has also been applied in all the following predictions using the regression in Table 2.14a.
-
2.
Chl from TP and salinity. This approach used the results shown in Fig. 2.27 and also modeled values on the dissolved fraction of phosphorus, since this is the only fraction that can be taken up by phytoplankton and since values of the dissolved fraction of phosphorus in the SW layer (DFSW; dim. less) are automatically calculated by the CoastMab model for phosphorus and are thus available for predicting chlorophyll.
This modeling also uses a boundary condition related to low water temperatures given by
$$\begin{aligned}&{\textrm{If}}\;{\textrm{SWT}} > 4^\circ {\textrm{C,}}\;{\textrm{then}}\;Y_{{\textrm{SWT}}} = 1 \nonumber \\ &\qquad{\textrm{else}}\;Y_{{\textrm{SWT}}} = ({\textrm{SWT}} + 0.1)/4 \end{aligned} $$((2.9))This water temperature moderator will not influence modeled chlorophyll values when the surface-water temperature is higher than 4°C, but it will lower predicted chlorophyll values during the winter time, and since there is also primary production under ice, the constant 0.1 is added. This moderator has been used and motivated before (see Håkanson and Eklund 2007). This means that using this approach Chl (μg L–1) is predicted from
$${\textrm{Chl}}_{{\textrm{Mod}}} = {\textrm{TP}}_{{\textrm{SW}}} \cdot{\textrm{DF}}_{{\textrm{SW}}} \cdot Y_{{\textrm{DayL}}} \cdot Y_{{\textrm{Sal}}} \cdot Y_{{\textrm{SWT}}} $$-
TPSW = TP concentration in SW water in μg L–1.
-
Y Sal = Y4 a dimensionless moderator for the influence of salinity on chlorophyll calculated from:
-
Y1 = if Sal < 2.5 psu then (0.20–0.1·(Sal/2.5–1)) else (0.20+0.02·(Sal/2.5–1))
-
Y2 = if Sal< 12.5 then Y1 else (0.28–0.1·(Sal/12.5–1))
-
Y3 = if Sal > 40 then (0.06–0.1·(Sal/40–1)) else Y2
-
Y4 = if Y3 < 0.012 then 0.012 else Y3.
-
-
3.
Chl from TN. This approach is similar to the algorithm given in Eq. (2.9) but the basic relationship between Chl, TN, and salinity is the one given in Fig. 2.16.
Scatter plot between concentrations of total P (TP) and total N (TN) for the growing season from nine sub-groups constituting a salinity gradient. The figure also gives regressions for the actual data and log-transformed data for the 495 data points (from Håkanson and Bryhn 2008a)
Figure 2.21d compares the modeled values using the three approaches with the mean monthly empirical chlorophyll values from the Kattegat for the period 1995–2008. There is generally relatively good correspondence between the modeled values and the empirical data and in all following simulations, the regression based on both TP and TN will be used. It should be stressed that the empirical chlorophyll values are quite uncertain; the average monthly CV value is as high as 1.08, so all model predictions are well within ±1 standard deviation of the empirical mean values.
2.3.7 Predicting Water Clarity and Secchi Depth
The Secchi depth is an important variable since the water clarity defines the depth of the photic zone. In all the following calculations, the depth corresponding to two Secchi depths is used to define the entire depth of the photic zone (see Håkanson and Peters 1995). There exists a close relationship between SPM, Secchi depth, and salinity (see Håkanson 2006) – the higher the salinity, the higher the aggregation of suspended particles, the larger the particles, and the higher the water clarity. An SPM concentration of 10 mg L–1 would imply turbid conditions in a freshwater system, but relatively clearer water in a saline system. The relationship between Secchi depth (Sec in m), SPMSW (mg L–1), and salinity (SalSW in psu) is given by
The SPM concentrations in the SW layer (SPMSW in mg L–1) are predicted from the dynamic SPM model. It should be noted that this approach is also used to predict SPM concentrations in the SW layer in the Skagerrak from empirical data on Secchi depth in Skagerrak (and from empirical salinities, as already explained). The results of these model predictions for the Secchi depth in the Kattegat are compared to measured data in Fig. 2.21c. The modeled values are close to the empirical values and within the uncertainty band given by ±1 standard deviation for the empirical data. These results give further empirical support to the general validity and predictive power of the CoastMab modeling.
2.3.8 Conclusions
To understand how the Kattegat system, or any aquatic system, responds to changes in, e.g., loading of toxins or nutrients, it is imperative to have a dynamic process-based perspective, quantifying the factors and functions regulating inflow, outflow, and internal transport processes and retention rates. This section has demonstrated that this modeling approach, using the theoretical wave base rather than traditional temperature and salinity data to define the surface-water and deep-water compartments, can give excellent correspondence between empirical and modeled data on the salinity. This section has presented budgets for water, salt, TP, TN, and SPM in the Kattegat. This process-based mass-balance modeling has used empirical data (from SMHI) for the period 1995–2008. An aim of the first part of this section was to present data on the fluxes of water and the theoretical retention times for water and salt in the defined sub-basins of the Kattegat since those values give fundamental information on how the system reacts to changes in, e.g., nutrient loading. This places certain demands on the structure of this model, which are different from oceanographic models, e.g., in quantifying resuspension, mixing, and diffusion and in the requirements regarding the accessibility of the necessary driving variables. This section has also discussed empirically based models, which have been added to the process-based dynamic CoastMab model. These are the sub models for Secchi depth and chlorophyll-a concentrations. When tested against empirical data for the Kattegat, there was good overall correspondence between predicted values for Secchi depth and chlorophyll-a concentrations and the dynamic SPM model predicts sedimentation, SPM concentrations, and burial in accordance with existing, but rather scattered, data.
2.4 Management Scenarios
This section will present several scenarios, which are meant to focus on key problems related to a sustainable management of the trophic state in the Kattegat. The same principles and questions discussed in this section should apply to most systems in contexts of remediation of eutrophication. The last scenario will put the results together and discuss an “optimal” management plan for the Kattegat related to realistic nutrient reductions to lower the eutrophication. The first scenario is logical in the sense that the main focus is on the largest nutrient flux to the surface water in the Kattegat. If very costly remedial actions reducing 10,000–100,000 t nutrients (P and N, respectively) annually to the Baltic Sea including the Kattegat are needed at a yearly cost in the range of 1,000–30,000 million euro year–1, the model should be able to predict the expected changes in the surface-water layer (the bioproductive layer) not just for the nutrient concentrations but also for key bioindicators of eutrophication, such as the Secchi depth and the concentration of chlorophyll-a. So, scenario 1 is the first logical step in an attempt to find an “optimal” abatement plan to reduce eutrophication. Comprehensive analyses based on very large data sets on the conditions in the Kattegat have shown (in Section 2.2) that the anthropogenic nutrient emissions have not altered the eutrophication in the Kattegat markedly during the last 15–20 years. It is, however, well documented (see, e.g., a compilation of data and literature references in Håkanson and Bryhn 2008a, 2008c) that the eutrophication in the Baltic Sea increased significantly in the period between 1920 and 1980. The second and third scenarios will focus specifically on phosphorus and nitrogen reductions in the catchments of the rivers entering the Kattegat from Sweden. The Baltic Sea Action Plan (see Table 2.10), which the governments of the Baltic countries agreed upon in November 2007, implies that 15,000 t of phosphorus and 133,000 t of nitrogen of the total riverine nutrient fluxes to the entire Baltic Sea (including the Kattegat) should be reduced annually, including 290 t year–1 of phosphorus and 20,780 t year–1 of nitrogen from Sweden. The second and third scenarios will address how such reductions would likely influence the Kattegat. The fourth scenario will be based on the results from the first three scenarios and on the results presented in this work on the water fluxes, salt fluxes, nutrient fluxes, and fluxes of suspended particulate matter to, within, and from the Kattegat as well as the results related to how the two key bioindicators (Secchi depth and chlorophyll) would likely respond to changes in nutrient concentrations in surface water of the Kattegat. The basic idea is that this scenario should motivate an “optimal” remedial strategy to improve the eutrophication in the Kattegat. Nutrient reductions are ultimately related to political decisions. One can safely assume that it is practically impossible to remediate all human emissions of nutrients to the Baltic Sea. The 15,000 t year–1 suggested by HELCOM (2007b) represent a reduction of 50% of the 30,000 t year–1 of phosphorus transported via rivers/countries to the Baltic Sea. From countries that have already carried out costly measures to reduce nutrient discharges to the Baltic Sea, only a smaller part of the remaining anthropogenic nutrient fluxes can realistically and cost-effectively be reduced. The costs for nutrient reductions are essential to quantify for optimizing the cost-effectiveness of nutrient abatement strategies. Cost-effectiveness is not only a means for saving money, but also a means for increasing the chances that the selected strategy will be fully implemented. Less expensive measures are easier to undertake than expensive measures (Bryhn 2009). One point made in this section is that there are major differences in cost-effectiveness among the different options. Comparing cost-effectiveness between options is really important and the CoastMab model can be a useful complementary tool in such contexts to address the “benefit” side of the cost–benefit analysis.
Target variables which should be used for measuring benefits should not be the reductions in nutrient input from countries or tributaries related to a given remedial action, neither the reductions in nutrient concentrations in the Kattegat system, but rather the change in the target bioindicators in the system: How would a certain remedial strategy for reducing X tons of phosphorus for Y euro in river Z change the water clarity, the Secchi depth; reduce the risks of blooming of cyanobacteria (e.g., in the Baltic Proper); and reduce the maximum concentration of chlorophyll-a in the Baltic Proper and/or the Kattegat? To address such issues, one needs a validated, process-based mass-balance model. No such model is at present available for nitrogen, but the CoastMab model presented in this work may be used to address the target issues related to how the Kattegat would respond to changes in phosphorus input and also, with the given reservations, for nitrogen in the Kattegat and for the key bioindicators, and this will be demonstrated in this section.
2.4.1 Reductions in Tributary Phosphorus Loading to the Baltic Sea
This scenario is based on the following two key arguments:
-
The focus is set on the dominating fluxes to the surface-water layer in the Kattegat. That is, on the nutrient fluxes from the Baltic Proper (see the annual budgets presented in Fig. 2.22 for phosphorus and in Fig. 2.24 for nitrogen). By far the most dominating nutrient loading to the bioproductive surface-water layer in the Kattegat comes from the Baltic Proper, which should be evident just by looking at the catchment area for the entire Baltic Sea, including the Baltic States, parts of Russia, Belarus, and Germany, Poland, Finland, and Sweden in relation to the relatively small catchment area draining directly into the Kattegat (from south-western Sweden and parts of Denmark).
The focus will also be set on phosphorus and not on nitrogen because
-
It is not possible to provide scientifically relevant predictions how the Baltic Sea system would respond to reductions in nitrogen loading since there are many major uncertainties related to the quantification of nitrogen fixation, wet and dry deposition of nitrogen, the algorithm regulating the particulate fraction for nitrogen and hence also sedimentation of particulate nitrogen and denitrification. For the Kattegat, on the other hand, atmospheric nitrogen fixation is neglected in this modeling because there are no significant amounts of N-fixing cyanobacteria in this system; the atmospheric deposition used in this modeling for the Kattegat comes from the OSPAR model (see Eilola and Sahlberg 2006, Håkansson 2007) and should be reliable in terms of order-of-magnitude values. Quantifying the denitrification is uncertain also in the Kattegat and it has been treated as a residual term in the mass balance for nitrogen so that the modeled concentrations in the surface-water layer, the deep-water layer, in the ET sediments, and the A-sediments should correspond to empirical data. No such calibrations have been done in the mass-balance calculations for phosphorus (i.e., the basic, validated CoastMab model is used directly without any tuning) or for the mass-balance calculations for SPM.
-
In the Baltic Sea, and especially in the Baltic Proper, nitrogen reductions are likely to favor the blooming of harmful algae (cyanobacteria), and such events should be avoided. This means that reductions in tributary nitrogen loading to the Baltic Sea may, in fact, even increase the nitrogen concentration in the water (see Håkanson and Bryhn 2008a).
-
So, there are no general, process-based mass-balance models for nitrogen, neither for the Baltic Sea basins, the Kattegat, or for any other coastal areas in the world, which have been tested (validated) for independent coastal systems and been demonstrated to yield good predictive power.
-
In spite of the fact that costly measures have been implemented to reduce nitrogen transport from agriculture, urban areas (e.g., from water purification plants), and industries, the nitrogen concentrations in the surface water in the Kattegat have remained largely constant for the last 15–20 years.
So, the focus is set on the mass-balance modeling of phosphorus in scenario 1.
Figure 2.28 gives the results from three simulations:
-
When half of the total phosphorus reductions have been carried out (i.e., a reduction of 7,500 t TP year–1) for the tributaries to the Baltic Proper (as if 7,500 t TP year–1 was suddenly reduced from Polish rivers entering the Baltic Proper). Evidently, it is not realistic to implement such large and sudden reductions. These simulations illustrate the dynamic response of the Kattegat system to such a sudden P reduction into the Baltic Proper delivering its water to the Kattegat.
-
When 9,775 t TP year–1 from the tributaries entering the Baltic Sea have been (suddenly) reduced. Many tests have been presented by Håkanson and Bryhn (2008a) to try to find an optimal strategy for Baltic Sea management. Such a strategy should also concur with some evident practical constraints. For example, it may be very difficult and costly and maybe damaging for agriculture, urban development, and industry to reduce more than 60–70% of the anthropogenic point source and diffuse discharges of TP in Russia, Poland, and the Baltic states. There was also a focus on the conditions in the “hotspots,” i.e., the Gulf of Finland, the Gulf of Riga, and the Baltic Proper, and not on smaller coastal areas and not on the oligotrophic basins (i.e., the Bothnian Bay and the Bothnian Sea). The total phosphorus reduction of 9,775 t year–1 advocated in this management strategy was allocated accordingly: inputs to the Baltic Proper would be reduced by 6,625 t year–1 (48% of anthropogenic emissions), in addition to reductions of 2,725 t year–1 from the rivers entering the Gulf of Finland (corresponding to 60% of the anthropogenic input) and 425 t year–1 of TP to the Gulf of Riga (or 46% of the anthropogenic input to this basin). Effective and cost-effective measures available to meet such reductions will be discussed in Section 2.4.5.
-
This would give an average Secchi depth of 7 m in the Gulf of Finland and this is what the Secchi depth was in the Gulf of Finland before 1920. It would also give a Secchi depth of almost 10 m (9.7 m) in the Bothnian Sea, of about 8 m in the Bothnian Bay, 5.6 m in the Gulf of Riga, and almost 8 m (7.9 m) in the Baltic Proper.
-
When 15,000 has been reduced according to the Baltic Sea Action Plan. One can estimate that this would create a mean annual TP concentration in the surface water of the Baltic Proper of about 12 μg L–1, as compared to the default value today of about 20 μg L–1. Case 1 (a reduction of 7,500 t TP year–1) would give an annual mean TP concentration of 15.2 μg L–1; case 2 (when 9,775 t TP year–1 is being reduced as described) would give a mean annual value of 13.6 μg L–1 in the surface-water layer of the Baltic Proper (see Håkanson and Bryhn 2008a).
Scenario 1 – changes in nutrient concentration in the Baltic Proper (BP). Curve 1 gives the default conditions, when the mean TP concentration in the Baltic Proper is 20 μg L–1; curve 2 when the value is 15 μg L–1 (in SWBP) corresponding to a reduction in TP loading of 7,500 t year–1 to the Baltic Proper; curve 3 when the value is 13.6 μg L–1 corresponding to a reduction in TP loading of 9,775 t year–1 (the optimal scenario according to Håkanson and Bryhn 2008); curve 4 when the value is 12 μg L–1. (a) Corresponding modeled TP concentrations in the surface water (SW) of the Kattegat (KA). (b) Corresponding TN concentrations in the surface water (SW) of Baltic Proper (BP). (c) Probable changes in Secchi depth in the Kattegat. (d) Corresponding likely changes in chlorophyll-a concentrations in the Kattegat
From Fig. 2.28, one can note that one should expect major reductions in the TP concentration (Fig. 2.28a) in the SW layer in the Kattegat if these remedial actions were carried out; see also Table 2.15, which gives the corresponding mean annual values for the Secchi depth and the chlorophyll, TP, and TN concentrations in the surface-water layer in the Kattegat. In these simulations, it is assumed that reductions in TP loading would also imply reductions in SPM loading. This may not be the case if the TP reductions would mainly relate to the building of water treatment plant, which could target specifically on phosphorus removal.
So, if that would be the case, the improvements in SPM concentrations and the related improvements in water clarity (Fig. 2.28b, c) would be smaller. This would also affect the predicted changes in chlorophyll-a concentrations, but to a lesser extent. So, the results would depend on the way in which the remedial actions are carried out and the results shown in Fig. 2.28 are meant to represent what one would “normally” expect. One can also note from Table 2.15 that the TN concentrations should increase slightly (from 281 to 290 μg L–1) as a consequence of the reductions in SPM concentrations and the related increases in Secchi depths (from 6.5 to 8.4 m); the lower SPM concentrations would decrease the settling velocities for particulate nutrient forms (nitrogen and phosphorus). One can conclude from this scenario (and the following scenarios) that no other realistic actions will improve the eutrophication in the Kattegat more than reductions in phosphorus loading to the Baltic Sea. This is, in fact, evident from looking at the phosphorus fluxes (Fig. 2.22) into the surface-water layer in the Kattegat, since this action addresses the largest TP flux into the surface-water layer in the Kattegat.
2.4.2 Reductions in Tributary Phosphorus Loading to the Kattegat from Sweden
From Fig. 2.22, one can also see that the total Swedish contribution from diffuse sources corresponds to 500 t year–1 or 0.65% of the total TP inflow to the Kattegat; the TP contribution from Swedish point source emissions amounts to 180 t year–1, or 0.23% of the total annual TP inflow to the Kattegat (76,900 t year–1). So, what could one expect if half the Swedish BSAP quota of 145 t year–1 or if all of the Swedish quota (290 t year–1) would be directed (rather unrealistically) to the catchment areas of the Swedish rivers entering the Kattegat. It is evident from Fig. 2.29 that this is not an effective strategy to improve the eutrophication in the Kattegat. It should be stressed that more or less the same results as shown in Fig. 2.29 would be obtained if 145 or 290 t phosphorus year–1 would be reduced from any inflow to the Kattegat system, whether this is from Sweden, Denmark, the Skagerrak, or the Baltic Proper.
Scenario 2 – curve 1 gives the default conditions; curve 2 the modeled response when 145 t year–1 (half the Swedish BSAP quota) of the tributary TP inflow to the Kattegat have been removed; and curve 3 the modeled response when 290 t year–1 of the tributary TP inflow to the Kattegat has been removed. (a) TP concentrations in the surface water (SW) of the Kattegat (KA). (b) The corresponding SPM concentrations in the surface water (SW) of the Kattegat. (c) Probable changes in Secchi depth in the Kattegat. (d) Corresponding likely changes in chlorophyll-a concentrations in the Kattegat
2.4.3 Reductions in Tributary Nitrogen Loading to the Kattegat from Sweden
Figure 2.24 gives the annual budget for nitrogen and Fig. 2.30 three simulations in analogy with the results for phosphorus in Fig. 2.29. As an important background, one can note that the total contribution from Swedish diffuse sources corresponds to 29,100 t TN year–1 or 3.4% of the total nitrogen inflow to the Kattegat; the TN contribution from point sources amounts to 3,500 t year–1, or 0.41% of the total annual TN inflow to the Kattegat (850,000 t year–1). If half of the Swedish BSAP quota of 10,390 t year–1 or the entire Swedish quota (20,780 t year–1) were (hypothetically) reduced from the tributaries or other inflows to the Kattegat, the environmental gain would be very small, as shown in Fig. 2.30a.
Scenario 3 – curve 1 gives the default conditions; curve 2 the modeled response when 10,390 t year–1 (half the Swedish BSAP quota) of the tributary TN inflow to the Kattegat have been removed; and curve 3 the modeled response when 20,780 t year–1 of the tributary TN inflow to the Kattegat have been removed. (a) TN concentrations in the surface water (SW) of the Kattegat (KA). (b) The corresponding TP concentrations in the surface water (SW) of the Kattegat. (c) Probable changes in Secchi depth in the Kattegat. (d) Corresponding likely changes in chlorophyll-a concentrations in the Kattegat
The improvements for the Secchi depth and for the phytoplankton biomass (the chlorophyll-a concentration) would also be very small indeed. This is also evident by looking at the nitrogen fluxes to the Kattegat in Fig. 2.24.
2.4.4 An “Optimal” Management to Reduce the Eutrophication in the Kattegat
How would a more “optimal” remedial scenario for the Kattegat look? Many alternatives have been tested and it seems clear from the results already given that the first focus should be on phosphorus reductions in the rivers entering the Baltic Proper. The second focus could be on remedial actions for phosphorus that would also reduce the nitrogen transport to the Baltic Proper, although it is difficult to predict how such nitrogen reductions would actually change the nitrogen concentrations in the Baltic Proper. It is also, evidently, very important to seek remedial measures that would reduce phosphorus and nitrogen emissions in a cost-effective manner; the costs per removed kilogram nutrient may vary with a factor of 10–100 depending on the selected approach; and if the same approach is carried out in different Baltic Sea countries and whether the reduction concerns the “first kg” or the “last kg” in a long-term remedial strategy removing 10,000–100,000 t year–1. It should also be stressed that nutrient reductions in the Baltic Proper would be beneficial for the entire Baltic Sea systems, where there are several “hotspots” (e.g., the Gulf of Finland, the Gulf of Riga, the area outside Kaliningrad, the Oder and Vistula estuaries) with significantly worse conditions than in the Kattegat system (see Figs. 2.3 and 2.4). Reductions in the “upstream” Baltic Sea system would also clearly benefit the Kattegat system. Figure 2.31 gives results from simulations when 9,775 t TP year–1 has been reduced (as described and motivated by Håkanson and Bryhn 2008a) and when also the average nitrogen concentration in the Baltic Proper has been hypothetically lowered by 10% (from 281 μg L–1 on an annual basis to 253 g L–1). This would significantly lower the TP concentrations in the SW layer in the Kattegat (Fig. 2.31a) and also reduce the TN concentrations in the SW layer in the Kattegat (Fig. 2.31b) and if those measures would be carried out in a manner that would also reduce SPM emissions to the Baltic Proper (in a “normal” way), then there would also be clear reductions in the SPM concentrations in the SW layer in the Kattegat and corresponding increases in water clarity and lower chlorophyll-a concentrations, as shown in Fig. 2.31.
Scenario 4 – the “optimal” management scenario. Curve 1 gives the default conditions, when the mean TP concentration in the surface-water layer in the Baltic Proper is 20 μg L–1; curve 2 when the value is 13.6 μg L–1 corresponding to a reduction in TP loading of 9,775 t year–1; curve 3 when also the TN concentration in the surface-water layer in the Baltic Proper has been reduced by 10% (from 281 to 253 μg L–1). (a) TP concentrations in the surface water (SW) of the Kattegat (KA). (b) The corresponding TN concentrations in the surface water (SW) of the Kattegat. (c) Probable changes in Secchi depth in the Kattegat. (d) Connected changes in SPM in the surface-water layer in the Kattegat. (e) Corresponding likely changes in chlorophyll-a concentrations in the Kattegat
It should be noted again that the modeled changes in TP concentrations are more reliable than the other changes shown in Fig. 2.31 and that the reductions in the TN concentrations in the Baltic Proper in this scenario are hypothetical. If the reductions in TN concentrations in the Baltic Sea would be even lower than 10% (which is suggested in the Baltic Sea Action Plan) this would create even smaller changes than the already small changes related to this scenario. “Optimal” in this scenario means that this is probably the best results one could realistically hope for.
2.4.5 Effective and Cost-Effective Nutrient Reductions
The “optimal” strategy advocated in Fig. 2.31 should appear more attractive when presented in combination with substantiated measures which could meet this strategy in an effective (decreasing the loading with a sufficient number of tons) and cost-effective (at the lowest possible cost) manner. An initial benchmark may be the Baltic Sea Action Plan, described in Section 2.3.3.1 and Table 2.10, whose full implementation would require a wide array of measures, including construction of wetlands, improved sewage treatment, and decreased agricultural production (Swedish 2008). The plan also includes measures for the Kattegat and the yearly cost of the plan has been estimated at 3.1 billion euro t year–1 (in 2008 prices; HELCOM and NEFCO 2007). According to calculations by the Swedish Department of Agriculture, N reductions, which Sweden has agreed to undertake in the Baltic Sea Action Plan, cannot be fulfilled unless a large part of the agricultural sector in the country would be permanently shut down, an option which would eliminate tens of thousands of jobs. Sweden, which is presently a net exporter of grain, could have to become a yearly net importer of millions of tons of grain (Swedish 2008), which would be associated with additional environmental pressure and transportation costs. Two particularly cost-effective measures for decreasing P inputs to the Baltic Sea are improved urban sewage treatment in former East Bloc countries and a ban on phosphates in detergents (Gren and Elofsson 2008, Bryhn 2009). Regarding the latter measure, however, attention must also be paid to the regional differences. On the one hand, in former East Bloc countries where urban sewage treatment is poor, a ban on phosphates would be very cost-effective, at least in the short run (Bryhn 2009). In Sweden, on the other hand, where sewage treatment has been implemented with relatively ambitious standards, marginal costs for P abatement are higher than those of many projects regarding urban sewage treatment in Poland, Russia, and the Baltic states, and a phosphates ban would probably have much lower cost-effectiveness in more countries if they would first upgrade their sewage treatment to Swedish standards (Bryhn 2009). It should also be noted that alternatives to phosphates in detergents may have their own adverse environmental effects. One of the most viable alternatives, Zeolite A, produces greater volumes of sludge which cannot be recycled in the same manner as phosphorus in sewage sludge can be used as a fertilizer in agriculture. Thus, with effective sewage treatment in place, phosphates may actually be the most environmentally friendly option in a life-cycle perspective (Köhler 2006), which would imply that many available marginal cost estimates for a phosphate ban may be greatly underestimated. Wetland construction and agricultural measures often have higher marginal P abatement costs than improvements in urban sewage treatment, and in some cases the cost difference may be a factor of 100 (Bryhn 2009). So, how much P can be removed by means of upgraded urban sewage treatment and how much would this cost? Helcom (2007a) estimated that advanced (tertiary) treatment was performed on sewage from 34% of the Estonian and Polish population, from 18% of Latvians and Lithuanians, and from 0% of Czechs, Russians, and Belarusians. Corresponding figures for Sweden, Finland, Germany, and Denmark were 86, 80, 85, and 81%, respectively. By upgrading urban sewage treatment in the former East Bloc countries, Helcom estimated that 12,400 t year–1 of phosphorus may be removed, which actually exceeds the TP abatement goal according to the “optimal” strategy motivated in Section 2.4.4. Thus, it appears to be possible to decrease the TP loading to the Baltic Sea with 10,000 t year–1 by means of upgrading urban sewage treatment. The cost is highly dependent on the available sewage pipe system in urban areas. According to Bryhn (2009), improved urban sewage treatment including the pipe system in former East Bloc countries had a typical marginal cost of 42 euro kg–1 P (2008 prices) while the typical marginal cost was only 20 euro kg–1 P when pipes were in an acceptable shape. This would mean that the TP abatement goal in Section 2.4.4 would cost 200–420 million euro year–1, an estimate which corresponds to 6.5–14% of the cost of the eutrophication part of the Baltic Sea Action Plan. As previously stressed in this chapter, it is not yet possible to predict the extent to which TN loading reductions would be needed to decrease TN concentrations in the Baltic Proper and so the cost of unknown reductions in TN loading is therefore likewise difficult to estimate. However, since curves 2 (TP reductions) and 3 (TN+TP reductions) in Fig. 2.31c (Secchi depth) and Fig. 2.31e (chlorophyll) are quite close to each other, the cost-effectiveness of separate N treatment in addition to P treatment should be quite low in any case. Nevertheless, it is worth mentioning that decreased TP inputs to the Baltic Proper may also decrease nitrogen fixation in surface waters (Savchuk and Wulff 1999), and upgrading P treatment in sewage treatment plants may in addition retain some of the N in the sewage effluent.
2.4.6 Comments and Conclusions
In this section, the wisdom of the HELCOM strategy to reduce the eutrophication in the Baltic Sea (including the Kattegat) has been challenged. Nitrogen reductions may fail to give lower N concentration in the water because of compensatory increases in the nitrogen fixation by cyanobacteria, especially in the Baltic Proper. The results presented in this section indicate that a reduction of 15,000 t year–1 of phosphorus would likely create what may well be an undesired oligotrophication of the Baltic Sea system in the sense that the trophic status, as revealed by the operational bioindicators (Secchi depth and chlorophyll), would approach a lower level than Baltic Sea managers should realistically ask for. An alternative remedial strategy to reduce the eutrophication in the Kattegat based on the following cornerstones has been presented and motivated:
-
Many remedial measures in agriculture, urban areas, or industry would remove both nutrients and when substance-specific methods are available, they should target on phosphorus removal; less substance-specific methods may reduce both phosphorus and nitrogen and if such remedial measures could be carried out in a cost-effective manner, it would be advantageous. The effects of nitrogen reductions cannot be predicted with any certainty in the Baltic Proper, but with some certainty in the Kattegat.
-
A remedial strategy where 3,180 t year–1 of the phosphorus to the Gulf of Finland, 550 t year–1 to the Gulf of Riga, and 5,000 t year–1 to the Baltic Proper (and no reductions at all to the Bothnian Sea and the Bothnian Bay) has been motivated as the most effective approach to reduce also the eutrophication in the Kattegat system. Evidently, it would take a long time to implement such reductions in the Baltic Sea system (including the Kattegat). The Baltic Sea system could face several changes in that time (e.g., related to climatic variations such as increased water temperatures and reductions in ice cover). This means that these recommendations should be taken with due reservations and that they should be adjusted to such possible future changes. The CoastMab model applied in this work could be a useful tool in such contexts.
2.5 Summary and Recommendations
To develop scientifically warranted programs of conservation, management, and remediation is a great challenge. In this situation, quantitative models are essential to predict, to guide assessment, and to direct intervention. The CoastMab model used in this work may be regarded as a tool for water management. It is also an approach to handle “trade-offs” and test working hypotheses concerning aquatic transport processes and interactions. The fact that the CoastMab model, in spite of its breadth and complexity , may be driven by relatively few readily accessible variables and that it is based on a general algorithm which may be repeated for different substances gives a certain robustness and attractiveness to the model and provides a framework for its practical usefulness and predictive power, which are essential components in models for aquatic management.
Section 2.2 gave basic information on the conditions in the case study area, the Kattegat, e.g., on the morphometry including the criteria to define the limit for the surface-water layer from the theoretical wave base. Section 2.3 presented the water fluxes to, within, and from the Kattegat system. These water fluxes are important for the quantification of all fluxes of salt, phosphorus, nitrogen, and SPM regulating all monthly concentrations. Section 2.3 also gave approaches to predict chlorophyll-a concentrations and Secchi depths from dynamically modeled values of phosphorus, nitrogen, SPM, and salinity and monthly light conditions.
These approaches are of fundamental importance in the Coast Web modeling because the food web model is driven by chlorophyll-a concentrations and the Secchi depth is a measure of the depth of the photic layer. The water fluxes determined from the CoastMab model for salinity are used throughout this modeling. It has been demonstrated that the CoastMab model for phosphorus, which prior to this work has been validated for many independent aquatic systems and been demonstrated to predict very well, also predicts TP concentrations in the Kattegat very well. It has been shown how the CoastMab model predicts TP and TN concentrations in water and sediments and also the target bioindicators. In fact, the inherent uncertainties in the available empirical data used to run and test the model for salt, phosphorus, SPM, and the two target bioindicators set the limit to the predictive power of the model.
It should, however, be noted that it is not possible to provide scientifically relevant predictions how the Baltic Sea system would respond to reductions in nitrogen loading since there are major uncertainties related to the quantification of nitrogen fixation, wet and dry deposition of nitrogen, the algorithm regulating the particulate fraction for nitrogen, and hence also sedimentation of particulate nitrogen and denitrification. For the Kattegat, on the other hand, atmospheric nitrogen fixation has been neglected in this modeling because there are no significant amounts of N-fixing cyanobacteria in this system; the atmospheric deposition used in this modeling for the Kattegat comes from the OSPAR model (SMHI) and should be reliable in terms of order-of-magnitude values; however, the denitrification is uncertain also in the Kattegat and it has been treated as a residual term in the mass balance for nitrogen so that the modeled concentrations in the surface-water layer, the deep-water layer, the ET sediments, and the A-sediments should correspond to empirical data. No such calibrations have been done in the mass-balance calculations for phosphorus (i.e., the basic, validated CoastMab model is used directly without any tuning) or for the mass-balance calculations for SPM. It is sub-optimal to give reduction quotas to different countries (such a strategy is based on political considerations rather than science). A more scientific strategy should be based on the identified “hotspots,” and so the strategy should rather be to target on basins (generally estuaries) with a high degree of eutrophication and reduce nutrient input to such systems. From the maps given in Section 2.1, one can identify the Gulf of Riga, the Gulf of Finland, the Oder and Vistula estuaries, and the coastal area outside of Kaliningrad as hotspots. Because of major changes in population structure, agriculture, species composition, fishing/trawling, etc., it is not possible to carry out measures that would bring the Baltic Sea ecosystem including key structural and functional characteristics, functional groups, and species back to the conditions as they were, say 100 years ago, but it would be possible to reduce nutrient inputs so that the Secchi depth in the Gulf of Finland could return to about 7 m as it was between 1900 and 1920. To reach such a specific goal, there must also be major reductions not just in the rivers entering the Gulf of Finland, but also in the rivers entering the Baltic Proper, since the water and nutrient exchange between the Baltic Proper and the Gulf of Finland is intense (which can be seen from the salinity maps for the entire Baltic Sea including the Kattegat). In this work, a realistic remedial scenario has been presented that would considerably improve the conditions not just in the Kattegat but also in the Gulf of Riga and the Gulf of Finland as well as the Baltic Proper and the entire Baltic Sea. The default conditions using the CoastMab model have been described in detail for water fluxes, salinity, phosphorus, SPM, chlorophyll, Secchi depth and it has been demonstrated that the general approaches used here (without any tuning or calibrations for the Kattegat system) also generally showed good correspondence between modeled values and empirical data. The nitrogen modeling also showed good results, but the CoastMab model for nitrogen includes calibrations related to denitrification so the results related to the mass balance for nitrogen are not as reliable as the other predictions. Many tests have been carried out to find a strategy to reach the goal that the eutrophication in the Kattegat system could be reduced. By far the most dominating nutrient loading to the bioproductive surface-water layer in the Kattegat comes from the Baltic Proper, which should be evident just by looking at the catchment area for the entire Baltic Sea, including the Baltic States, parts of Russia, Belarus, Germany, Poland, Finland, and Sweden in relation to the relatively small catchment area draining directly into the Kattegat (from south-western Sweden and parts of Denmark). The final results are given on a monthly basis in Fig. 2.31. Evidently, it is not realistic to implement such major reductions in nutrient P loading suddenly, and these curves are meant to illustrate the relatively fast dynamic response of the Kattegat system in this hypothetical remediation scenario.
One can note from these tests, and also from Håkanson and Bryhn (2008a), that a reduction of 15,000 t year–1 of phosphorus to the Baltic Sea, as suggested by HELCOM (see Table 2.10) and agreed upon by the Baltic Sea states in November 2007, would likely increase the Secchi depth in the Gulf of Finland beyond the mean or median values around the year 1900. One hundred years ago, the nutrient loss from human activity was already substantial in the Baltic Sea catchment (Savchuk et al. 2008). Natural fertilizers were used in agriculture, and horses were intensively used for transportation in urban and rural areas. Sewage systems were constructed to prevent outbreaks of cholera and other diseases in the cities but sewage treatment was absent or very ineffective in many areas until after the Second World War. This indicates that a reduction by 15,000 t year–1 is likely “overkill.” A lowering of the primary production in the Baltic Sea and the Kattegat will imply also a reduction in the secondary production, including zooplankton and fish; it would increase the acidification (since this is related to the primary production); it would also increase the concentration of organic toxins in fish – “in the clearest waters swim the most toxic fish.” This is a well-established fact called biological dilution (see Håkanson 1999, 2000). It relates to the definition of the average concentration of toxins in fish, C = M/BM, where M is the total mass of a given toxin in fish (in g; e.g., total PCB, total dioxins, methyl mercury) and BM is the total biomass of the fish (e.g., prey or predatory fish, or a given species of fish, such as cod; in kg). If BM decreases as it does in this oligotrophication scenario, C should increase if there are no simultaneous reductions in the loading of toxins to the system. There is evidently no point to lower the trophic status of the Baltic Sea or the Kattegat system to levels where the environmental drawbacks become larger than the benefits, and every action could potentially include benefits as well as drawbacks.
The strategy that one should ask for should also concur with some evident practical constraints. For example, it is not really realistic to reduce all anthropogenic TP or TN discharges. And for countries where major investments in nutrient reductions have already been made, it will become increasingly expensive to reduce the remaining tons. So, by a search for an optimal strategy, one could, for example, limit TP reductions to 60–70% of the anthropogenic emissions in coastal systems where few costly remedial actions have been implemented, and to much less in countries such as Sweden, Finland, and Germany. So, the wisdom of the HELCOM strategy to reduce eutrophication in the Baltic Sea may be challenged.
It should also be stressed that given the conditions in the Baltic Proper, nitrogen reductions may fail to give lower N/P ratios in the water because of compensatory increases in the nitrogen fixation by cyanobacteria (see Håkanson and Bryhn 2008a, 2008c). If nitrogen reductions lower the N/P ratios in the surface water, this could increase the competitiveness of cyanobacteria in relation to other algae even more, which is a clearly negative consequence of an expensive remedial strategy implemented to improve rather than worsen the conditions in the Baltic Sea. Conversely, P reductions may increase N/P ratios, thereby decreasing both the competitiveness of cyanobacteria and the fixation of atmospheric nitrogen (Savchuk and Wulff 1999, Tyrrell 1999).
In the “optimal” scenario, about 10,000 t year–1 of phosphorus is being reduced and also nitrogen reductions that would lower the TN concentration in the Baltic Proper by 10%. The costs for this would likely be about 200–420 million euro t year–1 if this is done in a cost-effective manner, which means a focus on improved phosphorus removal in urban sewage which is discharged into the most polluted estuaries and coastal areas. The costs to reduce 15,016 t year–1 of TP and 133,170 t year–1 of nitrogen according to the HELCOM strategy would be 3,100 million euro year–1. That is, 2,680–2,900 million euro year–1 higher than the “optimal” strategy discussed in this work.
References
BACC (2008) Assessment of climate change for the Baltic Sea Basin. Springer, Heidelberg, 474 p
BALTEX (2006) Baltex Phase II 2003–2012. Science Framework and Implementation Strategy. International BALTEX Secretariat Publication, No. 34, GKSS, Geestacht, p 92
Beach Erosion Board (1972) Waves in inland reservoirs. Technical Memoir 132. Beach Erosion Corps of Engineers, Washington, DC
Bergström S, Carlsson B (1993) Hydrology of the Baltic basin. SMHI Rep Hydrol 7:32
Bergström S, Carlsson B (1994) River runoff to the Baltic Sea: 1950–1990. Ambio 23:280–287
Boesch DF, Carstensen J, Paerl H, Skjoldal R, Voss M (2008) Eutrophication of the Seas along Sweden’s West Coast. Naturvårdsverket, Report 5898, Stockholm, 78 p
Bloesch J, Burns NM (1980) A critical review of sedimentation trap technique. Schweiz Z Hydrol 42:15–55
Bloesch J, Uehlinger U (1986) Horizontal sedimentation differences in a eutrophic Swiss lake. Limnol Oceanogr 31:1094–1109
Bryhn AC (2009) Sustainable phosphorus loadings from effective and cost-effective phosphorus management around the Baltic Sea. PLoS ONE 4:5417
Carstensen J, Conley DJ, Andersen JH, Ærtebjerg G (2006) Coastal eutrophication and trend reversal: A Danish case study. Limnol Oceanogr 51:398–408
Christiansen C, Gertz F, Laima MJC, Lund-Hansen LC, Vang T, Jürgensen C (1997) Nutrient (P, N) dynamics in the southwestern Kattegat, Scandinavia: sedimentation and resuspension effects. Environ Geol 29:66–77
Dillon PJ, Rigler FH (1974) The phosphorus-chlorophyll relationship in lakes. Limnol Oceanogr 19:767–773
Dodds WK (2003) Misuse of inorganic N and soluble reactive P concentrations to indicate nutrient status of surface waters. J North Am Benthol Soc 22:171–181
Eilola K, Sahlberg J (2006) Model assessment of the predicted environmental consequences for OSPAR problem areas following nutrient reductions. SMHI, Reports Oceanography No. 83
Evans MS, Arts MT, Robarts RD, et al (1996) Algal productivity, algal biomass, and zooplankton biomass in a phosphorus-rich, saline lake: deviations from regression model predictions. Can J Fish Aquat Sci 53:1048–1060
Gren IM, Elofsson K (2008) Costs and benefits from nutrient reductions to the Baltic Sea. Swedish EPA Report 5877, Stockholm, 67 p
Håkanson L (1977) The influence of wind, fetch, and water depth on the distribution of sediments in Lake Vänern, Sweden. Can J Earth Sci 14:397–412
Håkanson L (1982) Lake bottom dynamics and morphometry – the dynamic ratio. Water Resour Res 18:1444–1450
Håkanson L (1999) Water pollution – methods and criteria to rank, model and remediate chemical threats to aquatic ecosystems. Backhuys, Leiden, 299 p
Håkanson L (2000) Modelling radiocesium in lakes and coastal areas – new approaches for ecosystem modellers. A textbook with Internet support. Kluwer Academic, Dordrecht, 215 p
Håkanson L (2004) Lakes – form and function. Blackburn Press, Caldwell, NJ, 201 p
Håkanson L (2006) Suspended particulate matter in lakes, rivers and marine systems. Blackburn Press, New Jersey, 331 p
Håkanson L (2009) Modeling nutrient fluxes to, within and from the Kattegat to find an optimal, cost-efficient Swedish remedial strategy. Uppsala University, Geotryckeriet, Uppsala, 122 p
Håkanson L, Blenckner T, Malmaeus JM, et al (2004) New, general methods to define the depth separating surface water from deep water, outflow and internal loading for mass-balance models for lakes. Ecol Modell 175:339–352
Håkanson L, Boulion V (2002) The Lake Foodweb – modelling predation and abiotic/biotic interactions. Backhuys, Leiden, 344 p
Håkanson L, Bryhn AC (2008a) Eutrophication in the Baltic Sea – present situation, nutrient transport processes, remedial strategies. Springer, Berlin, Heidelberg, 261 p
Håkanson L, Bryhn AC (2008b) Modeling the foodweb in coastal areas – a case study of Ringkobing Fjord, Denmark. Ecol Res 23:421–444
Håkanson L, Bryhn AC (2008c) Tools and criteria for sustainable coastal ecosystem management – with examples from the Baltic Sea and other aquatic systems. Springer, Heidelberg, 300 p
Håkanson L, Bryhn AC, Hytteborn JK, et al (2007) On the issue of limiting nutrient and predictions of cyanobacteria in aquatic systems. Sci Total Environ 379:89–108
Håkanson L, Eklund JM (2007) A dynamic mass-balance model for phosphorus fluxes and concentrations in coastal areas. Ecol Res 22:296–320
Håkanson L, Jansson M (1983) Principles of lake sedimentology. Springer, Berlin, 316 p
Håkanson L, Kulinski I, Kvarnäs H, et al (1984) Water dynamics and bottom dynamics in coastal areas (in Swedish, Vattendynamik och bottendynamik i kustzonen). SNV PM 1905, Solna, 228 p
Håkanson L, Peters RH (1995) Predictive limnology. Methods for predictive modelling. SPB Academic, Amsterdam, 464 p
Håkansson B (2007) Swedish National Report on Eutrophication Status in the Kattegat and the Skagerrak. OSPAR Assessment 2007. SHMI, Oceanography No. 36, 54 p
HELCOM (1986) Water balance of the Baltic Sea. Baltic Sea Environment Proceedings 16. HELCOM, Helsinki
HELCOM (1990) Second periodic assessment of the state of the marine environment of the Baltic Sea, 1984–1988; Background document. Baltic Sea Environment Proceedings 35B. HELCOM, Helsinki, 432 p
HELCOM (2000) Baltic Sea Environment Proceedings 100. HELCOM, Helsinki
HELCOM (2007a) Towards a Baltic Sea unaffected by eutrophication. HELCOM, Helsinki, 35 p
HELCOM (2007b) HELCOM Baltic Sea Action Plan – HELCOM Ministerial Meeting, Krakow, Poland, 15 Nov 2007, 101 pp
HELCOM, NEFCO (2007) Economic analysis of the BSAP with focus on eutrophication. HELCOM, Helsinki, 112 p
Howarth RW (1988) Nutrient limitation of net primary production in marine ecosystems. Annu Rev Ecol Syst 19:89–110
Howarth RW, Marino R, Lane J, Cole JJ, et al (1988a) Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnol Oceanogr 33:669–687
Howarth RW, Marino R, Cole JJ, et al (1988b) Nitrogen fixation in freshwater, estuarine, and marine ecosystems: 2. Biogeochemical controls. Limnol Oceanogr 33:688–701
Huang B, Hong H (1999) Alkaline phosphatase activity and utilization of dissolved organic phosphorus by algae in subtropical coastal waters. Mar Poll Bull 39:205–211
Jacobsen TS (1980) The Belt Project. Sea water exchange of the Baltic-measurements and methods. Report from the National Agency of Environmental Protection, Denmark, 106 pp
Jönsson A (2005) Model studies of surface waves and sediment resuspension in the Baltic Sea. Dr thesis No 332, Linköping Univ, Linköping
Köhler J (2006) Detergent phosphates: an EU policy assessment. J Bus Chem 3:15–30
Lehtimäki J, Sivonen K, Luukainen R, Niemelä SI, et al (1997) The effects of incubation time, temperature, light, salinity, and phosphorus on growth and hepatotoxin production by Nodularia strains. Arch Hydrobiol 130:269–282
Lemmin U, Imboden DM (1987) Dynamics of bottom currents in a small lake. Limnol Oceanogr 32:62–75
Mann KH (1982) Ecology of coastal waters. A systems approach. Blackwell, Oxford, 322 p
Marino R, Chan F, Howarth RW, Pace ML, Likens GE, et al (2006) Ecological constraints on planktonic nitrogen fixation in saline estuaries. I. Nitrogen and trophical controls. Mar Ecol Prog Ser 309:25–39
Meijer ML, Dehaan MW, Breukelaar AW, Buiteveld H, et al (1990) Is reduction of benthivorous fish an important cause of high transparency following biomanipulation in shallow lakes?. Hydrobiologia 200–201:303–315
Newton A, Icely JD, Falcao M, et al (2003) Evaluation of eutrophication in the Ria Formosa coastal lagoon, Portugal. Cont Shelf Res 23:1945–1961
Nissling A, Johansson U, Jacobsson M (2006) Effects of salinity and temperature conditions on the reproductive success of turbot (Scophthalmus maximus) in the Baltic Sea. Fish Res 80:230–238
OECD (1982) Eutrophication of waters. Monitoring, assessment and control. OECD, Paris, 154 p
Omstedt A, Axell LB (2003) Modeling the variations of salinity and temperature in the large Gulfs of the Baltic Sea. Cont Shelf Res 23:265–294
Omstedt A, Elken J, Lehmann A, Piechura J, et al (2004) Knowledge of the Baltic Sea Physics gained during the BALTEX and related programmes. Prog Oceanogr 63:1–28
Omstedt A, Rutgersson A (2000) Closing the water and heat cycles of the Baltic Sea. Meteorol Z 9:57–64
Rahm L, Jönsson A, Wulff F (2000) Nitrogen fixation in the Baltic proper: an empirical study. J Mar Syst 25:239–248
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–222
Redfield AC, Ketchum BH, Richards FA, et al (1963) The influence of organisms on the composition of sea-water. In: Hill, N (ed) The Sea 2. Interscience, New York, NY, pp 26–77
Remane A (1934) Die Brackwasserfauna. Verh Detsch Zool Ges 36:34–74
Riley ET, Prepas EE (1985) Comparison of the phosphorus-chlorophyll relationships in mixed and stratified lakes. Can J Fish Aquat Sci 42:831–835
Rubio VC, Sánchez-Vázques FJ, Madrid JA, et al (2005) Effects of salinity on food intake and macronutrient selection in European sea bass. Physiol Behav 85:333–339
Rutgersson A, Omstedt A, Räisänen J, et al (2002) Net precipitation over the Baltic Sea during present and future climate conditions. Clim Res 22:27–39
Samuelsson M (1996) Interannual salinity variations in the Baltic Sea during the period 1954–1990. Cont Shelf Res 16:1463–1477
Savchuk OP (2005) Resolving the Baltic Sea into seven subbasins: N and P budgets for 1991–1999. J Mar Syst 56:1–15
Savchuk OP, Wulff F (1999) Modeling the Baltic Sea eutrophication in a decision support system. Ambio 2–3:141–148
Savchuk OP, Wulff F, Hille S, Humborg C, Pollehne F, et al (2008) The Baltic Sea a century ago – a reconstruction from model simulations, verified by observations. J Mar Syst 74:485–494
Schernewski G, Neumann T (2005) The trophic state of the Baltic Sea a century ago: a model simulation study. J Mar Syst 53:109–124
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 195:260–262
Schindler DW (1978) Factors regulating phytoplankton production and standing crop in the world’s freshwaters. Limnol Oceanogr 23:478–486
Seitzinger SP, Sanders RW (1999) Atmospheric Inputs of Dissolved Organic Nitrogen Stimulate Estuarine Bacteria and Phytoplankton. Limnol Oceanogr 44:721–730
Sellner KG (1997) Physiology, ecology and toxic properties of marine cyanobacteria blooms. Limnol Oceanogr 42:1089–1104
Smith VH (1979) Nutrient dependence of primary productivity in lakes. Limnol Oceanogr 24:1051–1064
Smith VH (1985) Predictive models for the biomass of blue-green algae in lakes. Water Resour Bull 21:433–439
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environ Sci Pollut Res Int 10:126–139
Smith VH, Joye SB, Howarth RW, et al (2006) Eutrophication of freshwater and marine ecosystems. Limnol Oceanogr 51:351–355
Stigebrandt A (2001) Physical oceanography of the Baltic Sea. In: Wulff L, Rahm, L, Larsson, P(eds) A Systems analysis of the Baltic Sea. Springer, Berlin, pp 19–74
Swedish EPA (2008) Ingen övergödning (No over-enrichment), revised version. Swedish EPA report 5840, Stockholm, 123 p
Tyrrell T (1999) The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 688:525–531
Vahtera E, Conley DJ, Gustafsson BG, Kuosa H, Pitkänen H, Savchuk OP, Tamminen T, Viitasalo M, Voss M, Wasmund N, Wulff F (2007) Internal Ecosystem Feedbacks Enhance Nitrogen-fixing Cyanobacteria Blooms and Complicate Management in the Baltic Sea. Ambio 36:186–193
Vichi M, Ruardij P, Baretta JW (2004) Link or sink: a modelling interpretation of the open Baltic biogeochemistry. Biogeosci Discuss 1:79–100
Vidal M, Duarte CM, Agusti S (1999) Dissolved organic nitrogen and phosphorus pools and fluxes in the Central Atlantic Ocean. Limnol Oceanogr 44:106–115
Vollenweider RA (1968) The scientific basis of lake eutrophication, with particular reference to phosphorus and nitrogen as eutrophication factors, Technical Report. OECD, Paris, 159 p
Wallin M, Håkanson L, Persson J (1992) Nutrient loading models for coastal waters -especially for the assessment of environmental effects of marine fish farms. Nordiske Seminar - og Arbejdsrapporter 502. Nordic Council of Ministers, Copenhagen, 207 p
Wasmund N (1997) Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Int Rev Gesamten Hydrobiol 82:169–184
Wasmund N, Voss M, Lochte K, et al (2001) Evidence of nitrogen fixation by non-heterocystous cyanobacteria in the Baltic Sea and re-calculation of a budget of nitrogen fixation. Mar Ecol Prog Ser 214:1–14
Wetzel RG (2001) Limnology. Academic, London, 1006 p
Winsor P, Rodhe J, Omstedt A, et al (2001) Baltic Sea ocean climate: an analysis of 100 yr of hydrographic data with focus on the freshwater budget. Clim Res 18:5–15
Acknowledgments
Ingemar Cato, SGU, has been very helpful and freely supplied sediment data on nutrient concentrations. Pia Andersson, SHMI, has also been most helpful in supplying the necessary water chemical data, data on tributary discharges, and atmospheric nitrogen deposition.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Håkanson, L., Bryhn, A.C. (2010). Controlling Eutrophication in the Baltic Sea and the Kattegat. In: Ansari, A., Singh Gill, S., Lanza, G., Rast, W. (eds) Eutrophication: causes, consequences and control. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9625-8_2
Download citation
DOI: https://doi.org/10.1007/978-90-481-9625-8_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9624-1
Online ISBN: 978-90-481-9625-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)