Abstract
In this chapter, the combined effects of eutrophication and of heavy metal contamination on the zooplankton community of a freshwater ecosystem are analyzed. Through biomonitoring, it was possible to study zooplanktonic attributes as indicators of environmental stress: species richness, species diversity, equity, and biomass. These attributes allowed the detection of structural and functional changes. There was an inverse relationship between stress situations and zooplankton body size with a proliferation of r-strategist species (rotifers) and opportunistic species (nauplii larvae), a dominance of tolerant species, and a decrease in the most sensitive ones, such as larger size crustaceans (copepods and cladocerans). The results of this study showed that zooplankton responds as a good descriptor of water quality, constituting an efficient tool to assess eutrophication and heavy metal contamination. A general diagram integrating possible effects of eutrophication and heavy metal contamination on the trophic webs of freshwater ecosystems is also included. Emphasis in biological control is suggested as a relevant control measure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Unfortunately, the most spread and generalized use of surface water courses is as a means of transport to evacuate urban and industrial residual wastes. However, under the paradigm of the “multiple use of water – the precious fluid and a basis of life on the Earth, sensu Khan and Ansari (2005)”, the use of a water body for a certain purpose should not damage other possible uses, as consumption, preservation of aquatic life, or recreation. As Moss (1999) pointed out, most freshwater systems have been seriously altered by human activities. We may wish to restore them to self-sustaining systems that provide conservation or amenity values or products such as poTable water or fish, which is completely impossible without profound understanding of their functioning. Water eutrophication in lakes, reservoirs, estuaries, and rivers is widespread all over the world and the severity is increasing, especially in developing countries like Argentina. The eutrophication of several water bodies leads to significant changes in the structure and functioning of the aquatic ecosystems (Khan and Ansari 2005). Eutrophication and various forms of pollution, which cause both foreseen and unforeseen problems, must be addressed and solutions must be found. However, this is a complex problem that cannot have a simple solution. In recent years it has become apparent that toxicity testing using single species is not adequate to assess the potential hazard of anthropogenic compounds and eutrophication. The studies of community-level impacts are a very useful tool for understanding the effects on the ecosystems. For example, Xu et al. (2001) proposed a set of ecological indicators for a lake ecosystem health assessment. The structural indicators included phytoplankton cell size and biomass, zooplankton body size and biomass, species diversity, macro- and microzooplankton biomass, the zooplankton–phytoplankton ratio, and the macrozooplankton–microzooplankton ratio. This case study demonstrated that this method provided results which corresponded with the lake’s actual trophic state. In general terms, the studies on the change in structure, function, and diversity of the ecosystems have been used as parameters to assess the effects of contamination and eutrophication.
The objective of this study was to contribute to the knowledge of heavy metal–zooplankton interactions and the factors that condition the levels of heavy metals in zooplankton, such as the degree of eutrophication of systems that, due to their complexity, continue without a solution. Urbanization and intensive agriculture exploitation produce excessive nutrient inputs to lentic and lotic bodies, promoting algal proliferation and other eutrophication symptoms. This process has an adverse effect in water quality, because of the decrease in oxygen, the increase in turbidity, and interferences in water potabilization processes. In this study, we approached this problem taking a freshwater system with problems of eutrophication and contamination by heavy metals as an example. The effects of tannery wastewater with high contents of heavy metals, nutrients, and sulfide along a pollution gradient on the zooplankton assemblage in the lower Salado River basin (Santa Fe, Argentina) were assessed.
The lower Salado River is one of the most important basins in Argentina. It receives inputs of heavy metals, mainly from tanneries and metallurgic industries, thus representing an important segment of the economy. The Salado River runs along 2,010 km from northeastern Argentina, to the Santa Fe Province, where it joins the Paraná River. In the lower basin, where this survey was performed, it also receives nutrients of different sources, especially from agricultural origin. The levels of organic matter, dissolved oxygen, nitrites, nitrates, and phosphates showed that the system is eutrophicated. DBO values allow us to classify the studied systems as meso or polisaprobial. The values of chromium, copper, cadmium, and sulfide were higher than standard ones. Zooplankton density, biomass, species richness, and species diversity diminished along the pollution gradient. Cladocerans were the less tolerant organisms and Eucyclops neumani dominated the copepods. This survey allowed the understanding of the contamination of the ecosystem in terms of eutrophication and heavy metal concentration and their effects on zooplanktonic attributes. The aim of this chapter was to identify problems in a polluted freshwater environment, find general patterns, and extract recommendations for successful biomanipulation. Emphasis in biological control is suggested as a relevant control measure.
10.2 Methodology
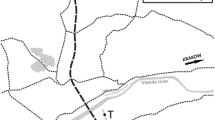
Five sampling sites, considered to be polluted, were established along approximately 40 km (Fig. 10.1). The section was selected according to a pollution gradient: Salado River at Manucho (MSR), two sites in Las Prusianas Stream (LP1 and LP2), and two sites in the North Channel (NCH) and the South Channel (SCH). The reference site was located in the Salado River, 153 km upstream from San Justo city (SJSR).
Map of the Salado River basin showing location of the sampling sites, species diversity (H), total species richness (S), and relative richness of Rotifera, Copepoda, and Cladocera, recorded at each sampling site (Modified from Gagneten and Paggi 2009)
In each sampling site, we measured pH, temperature, dissolved oxygen, turbidity, and conductivity. Sulfide and organic matter values, water hardness, chemical and biological oxygen demands (QOD, BOD), dissolved organic carbon (DOC), nitrates, nitrites, phosphates (NO3, NO2, P3O4), total suspended solids (TSS), and metal concentrations (Cr, Cr VI, Pb, Cu, and Cd) of river water samples were also recorded (see methodology details in Gagneten et al. 2007). To perform zooplankton analysis, five zooplankton samples (replicates) were taken at each site with a 20 L Schindler-Patalas trap of 45 μm mesh size, fixed and stained in situ. The quali-quantitative analysis of samples was carried out for mesozooplankton (adult copepoda and cladocerans) and for microzooplankton (rotifers and copepod nauplii). The attributes of the community selected as variables of response were total density (No ind L–1) and by-group density (Copepoda, Cladocera, and Rotifera), micro and mesozooplankton density and biomass (μg L–1). Species diversity through the Shannon–Weaver index and its components of richness (S) and equity (E) were also calculated.
One-way ANOVA with a significance level of p ≤ 0.05 was conducted to determine whether the differences among concentrations could be significant between contaminated and control sites and for the comparison of the composition of the zooplanktonic assemblage. Data were normally distributed (Kolmogorov–Smirnov test). Hierarchical cluster analysis (Euclidean measures, UPGMA method) was used to study the different sampling sites based on physicochemical records, concentrations of metals, and community attributes (Zar 1984, Hair et al. 1999) using the program InfoStat (2007). For details, see Gagneten and Paggi (2009).
10.3 Results
10.3.1 Environmental Context
The spatial distribution of some physical and chemical parameters recorded in the water of the sampling sites is shown in Figs. 10.2 and 10.3.
Physicochemical parameters of sampling sites. Values correspond to the mean of four samples at each sampling site and the error bars represent one standard deviation (Modified from Gagneten et al. 2007)
Chemical parameters of sampling sites. Values correspond to the mean of four samples at each sampling site and the error bars represent one standard deviation (Modified from Gagneten et al. 2007)
Temperature changed throughout the study period (16–29°C), showing a normal seasonal dynamic. Depth was <1 m in the channels and Las Prusianas, but larger in the Salado River (MSR=3.50 m, SJSR=5.70 m). Turbidity showed high variability and great differences between sampling sites: 3–54 NTUs (Nephelometric Turbidity Units). High concentrations of TSS (Fig. 10.3) were recorded in the South Channel (mean 3.662 mg L–1), intermediate values were found in Las Prusianas (mean 1.602–2.158 mg L–1), and minimal values were recorded in the Salado River (mean 1.848 mg L–1 in SJSR and 2.698 mg L–1 in MSR). The median pH range was 7.5–7.8 (Fig. 10.2), with higher values in winter and lower values in summer at all sampling sites. Conductivity was relatively high (>1,000 μS cm–1) at all study sites, a characteristic pattern of this river as it is suggested by its name (“salado” = salted). Highest values were recorded in Las Prusianas (3,000–7,100, mean 5,965 μS cm–1) and in Salado River, Manucho (3,900–5,300, mean 3,260 μS cm–1). Total hardness was high in the South Channel (mean 502.9 CaCO3 L–1, Fig. 10.3). This parameter showed minimal values in the North Channel and in the Salado River (mean 164 mg CaCO3 L–1). Very low values of dissolved oxygen were recorded, being extremely low in Las Prusianas in winter and spring (0.1–0.2 mg L–1). This parameter only showed high values at the reference site (SJSR 8 mg L–1) and in a few other cases, but mostly lower than 6 mg L–1. QOD values showed higher concentrations at Las Prusianas (mean 65.6 mg O2 L–1 at Las Prusianas 1 and 128 mg O2 L–1 at Las Prusianas 2, Fig. 10.3) and lower values at MSR (mean 30.8 mg O2 L–1). Nutrients (N and P) were higher at all sampling sites than at the reference site (Fig. 10.3), indicating an eutrophication process. The results of previous research indicate that the ratio 0.95:1 between ammonium and nitrate in the Salado River is definitely lower that those found in unpolluted water bodies included in the Paraná River floodplain. This fact could be interpreted as the product of an unlikely higher biological productivity or the consequences of pollution from human activities (Maglianesi and Depetris 1970).
Variable seasonal levels of Cr were recorded (Fig. 10.4), the highest ones being in the South Channel (11 μg L–1, mean 5.36 μg L–1), Las Prusianas (13.6 μg Cr L–1, mean 7.03 μg L–1), and Manucho (13 μg L–1, mean 8.32 μg L–1). Cr VI was high in the South Channel and Manucho (4.6 and 4.8 μg L–1, respectively) and in San Justo (2.5 μg L–1). Cr VI always showed values above the standard, even at the reference site. Pb was higher than the detection limit only in the South Channel (maximum 6.1 μg L–1, mean 4.74 μg L–1) and in Manucho (mean 5.1 μg L–1). Relatively high values of Cu were found in water at all sampling sites (maximum 22.9 μg L–1, mean 13.0 μg L–1 in the South Channel), even at the control site (14.1 μg L–1, mean 8.16 μg L–1). Cd in water showed higher values than standard values in Manucho (maximum 1.9 μg L–1, mean 0.85 μg L–1).
Cr, Cr VI, Pb, Cu, and Cd content in water. Values correspond to the mean of four samples at each sampling site and the error bars represent one standard deviation. The dark circles indicate maximum and minimum values (Modified from Gagneten et al. 2007)
Cr in water was sometimes higher than Canadian (8.9 μg L–1, CEPA 2003) but not Argentine (44 μg L–1, Subsecretaria de Recursos Hídricos de la Nación 2003) standards at sampling sites. On the other hand, Cr VI in water showed higher values than the Canadian standard (1.0 μg L–1) and sometimes than the Argentine standard (2.5 μg L–1) at all sampling sites. Standards for Cu were surpassed in the South Channel and that for Cd exceeded the standard value in Las Prusianas. We can see that the pollution of the lower Salado River shows a close relationship with adverse impact of heavy metal contaminants and eutrophication. The water of Las Prusianas system and of the North Channel is contaminated with heavy metals if compared to the control site. Organic matter values were high (200–256 mg L–1) although not very different between sampling sites (Table 10.1). BOD showed very high values in SCH and LP2 and high in NCH and LP1, corresponding to poly and mesosaprobial environments, respectively. Dissolved oxygen concentrations were very low (1.6 mgO2 L–1) in the sampling site closest to the effluent discharge (LP2), corresponding with the higher BOD (45.8).
Sulfide values (16–59.9 mg L–1) allowed to recognize two environmental groups (Table 10.1): the furthest sites in the pollution gradient, with comparatively lower values (16–16.3 mg L–1), and the closest sites in the pollution gradient, with higher values (59.5–59.9 mg L–1). At all sites, however, sulfide concentrations were much higher than the reference level for surface freshwater (<1 mg L–1). Total chromium concentration was highest at LP2 (215 μg L–1), the site closest to the pollution source. This value was also much higher than permitted standards: 2 μg L–1 for protection of phyto and zooplankton and 20 μg L–1 for protection of fish (CEPA 2002).
In Table 10.2, correlation values between environmental variables and concentrations of chromium and sulfide are shown. Positive correlations were found between Cr concentrations and BOD (0.989), pH and Cr concentrations (0.948), and pH and BOD (0.893). Negative correlations were registered between concentrations of Cr and O2 (–0.714), O2 and pH (–0.839), O2 and BOD (–0.614), O2 and organic matter (–0.724), and O2 and Cr (–0.714). On the one hand, sulfide concentrations were negatively correlated to O2 (–0.587) and QOD (–0.659). On the other hand, COD values were much higher than those of DO. This would mean an accumulation of organic matter, i.e., eutrophication as dominating condition.
10.3.2 Zooplankton Structure
10.3.2.1 Abundance
Total density of organisms was higher at the reference site (Salado River at San Justo, 0.86 ind L–1) than at the more contaminated sites (0.31, 0.07, 0.03, 0.61, and 0.62 at the North Channel, South Channel, Las Prusianas 2, Las Prusianas 1, and Manucho, respectively). Copepods dominated the community in numbers. However, adult copepods were poorly represented quantitatively and qualitatively. The dominance observed at LP1, MSR, and SJSR is due to the great proliferation of larvae and juveniles (nauplii and copepodites). Nauplii reached densities of 6.9, 1.9, and 3.0 ind L–1 at LP1, MSR, and SJSR, respectively. In general terms, adult crustaceans were not as numerous as rotifers; the presence of cladocerans was very low or null at NCH, SCH, and LP2. The most frequent genera were Bosmina, Ceriodaphnia, and Moina. The most abundant species at LP1, MSR, and SJSR were M. minuta, B. hagmani, Diaphanosoma spinulosum, and Macrothrix squamosa. Among copepods, the most frequent genera were Eucyclops and Metacyclops, Acanthocyclops being represented in a lower proportion. The most frequent and abundant species was E. neumani, which was recorded in all environments and with a relatively high abundance, except at LP1.
Mesozooplankton was only well represented at San Justo, being scarce at Manucho and very scarce or null in the tributaries. Microzooplankton reached comparatively high values at Las Prusianas 1 (3.48 ind L–1), caused by the abundance of nauplii, and was lower in the Salado River (1.10 and 1.55 ind L–1) at Manucho and San Justo, respectively.
The high microzooplankton values are also explained by the abundance of rotifers, which were the best represented group, both qualitatively and quantitatively. The most frequent rotifer genera in relation to the number of species were Brachionus (10 species), Lecane (7 species), and Keratella (3 species). The most numerous species of the genus Brachionus, or with a more constant presence, were B. quadridentatus, B. calyciflorus, B. plicatilis, and B. caudatus. The latter, most of all abundant and frequent in the Salado River, was represented by different “varieties”: insuetus, provectus, and vulgatus. B. austrogenitus and B. alhstromi were also frequent at Manucho and San Justo. The most numerous and frequent species of the genus Lecane were L. lunaris and L. pyriformis, and K. americana and K. cochlearis prevailed from the genus Keratella. The genus Polyarthra was recorded in the Salado River, with P. vulgaris showing a high density at San Justo (3.5 ind L–1). Bdelloid rotifers (among them, Philodina sp.) were also frequent and abundant. Among the rotifer species of higher frequency, although represented with low density values, we can mention Monostyla lunaris, Lepadella acuminata, Asplanchna sp., and Epiphanes spp. Gagneten and Ceresoli (2004) showed significant negative correlations between zooplanktonic density with sulfide concentration (r = –0.841) and with Cr concentration (r = –0.512). These results show that both contaminants, and not only chromium, have important negative effects on the studied assemblage. Density showed significant positive correlations (p < 0.05) with depth, transparency, and temperature (r = 0.941; r = 0.955, and r = 0.541, respectively).
10.3.2.2 Biomass
Absolute biomass (B) was 17 μg L–1 for copepods (9.41, 4.24, 2.92, and 9.42 μg L–1 for Cyclopoida, Calanoida, Harpacticoida, and copepodites + nauplii, respectively), 4.2 μg L–1 for cladocerans, and 0.4 μg L–1 for rotifers. At Manucho and San Justo, zooplankton was constituted by the three main zooplanktonic groups: copepods, cladocerans, and rotifers, with high values of biomass. Biomass of copepods was high and constant (near 3 μg L–1) at SJSR. It was somewhat lower at MSR. Biomass of Copepoda, concentrated in the river and at LP1, was distributed as follows: 55% Cyclopoida, 25% Calanoida, and 17% Harpacticoida. In decreasing order of importance, cladocerans showed biomass values between a minimum of 0.3 (LP1) and a maximum of 1.6 (SJSR), being absent at NCH. They were followed by rotifers, with comparatively lower values of biomass (0.01 at LP2 and 0.2 at NCH). Absolute biomass varied in the order SJSR>MSR>LP1>SCH>NCH>LP2 with 11.1, 4.9, 2.7, 1.5, 1.2, and 1.1 μg L–1, respectively.
10.3.2.3 Species Richness and Species Diversity
A total of 74 species were recorded, from which 13.5% corresponded to copepods, 22.9% to cladocerans, and 63.5% to rotifers. At MSR a total of 59 species were recorded, while 56 species were recorded at SJSR, 38 at LP1, 17 at SCH, 16 at NCH, and 13 at LP2. Therefore, species richness decreased among the sampling sites in the following order: MSR>SJSR>LP1>SCH>NCH>LP2. In function of richness, two environmental groups can be formed: the tributaries, with lower species richness (between 13 and 36 species), and the main river course at MSR and SJSR, with almost twice the number of species (between 56 and 59 species). The dominant group was rotifers, which were present at all sampling sites. In the river (MSR and SJSR), 99% of all rotifer species were represented. At LP1, 50% of species were represented; at LP2, 22%; and only 24% at NCH and SCH, with some species being exclusive from these environments. Such is the case of Anuraeopsis fissa and Euchlanis dilatata. The second group was copepods, with low species richness [one to two species in the tributaries and somewhat higher (six to seven species) in the river], while cladocerans contributed significantly to the community only at the reference site (RSSJ), where they showed a more uniform abundance. Figure 10.1 shows the relative richness of Rotifera, Copepoda, and Cladocera when considering the 20 most frequent species recorded at each sampling site. In the direction of the basin current, i.e., from NCH to MSR and in relation to RSSJ, the absence of cladocerans was observed at NCH, with absolute dominance of rotifers and scarce copepods. This situation was maintained at the other contaminated sites, but the presence of cladocerans increased progressively toward the river at Manucho (MSR). A similar proportion (that means higher equity) for the three groups was found in the river at San Justo (SJSR). Species diversity showed low values (0.35–1.56) in the tributaries and higher values in the Salado River at Manucho (3.0) and San Justo (3.16) (Fig. 10.1).
10.4 Discussion
There were differences between the concentration of metals in water in the more polluted sites and the control site. Heavy metals, especially chromium, copper, and cadmium, appear to be an important problem to the studied freshwater environment. When the effects of euthrophication and heavy metal contamination were assessed on the zooplanktonic community, we found that total density, by-group density (Copepoda, Cladocera, and Rotifera), micro and mesozooplankton density, biomass, species richness (S), and species diversity (H) were all good indicatos of water pollution: total density of zooplankton was significantly higher in the river than in the channels and streams (p < 0.001), with dominance of rotifers but a higher copepod biomass. Calanoida dominated over Cyclopoidea and Harpacticoida. Total species richness was 74, the highest values (59 and 56) being shown at the points corresponding to the Salado River at localities Manucho and San Justo (MSR, SJSR) and the lowest ones in North and South channels (NCH and SCH with 16 and 17 species, respectively) and in the two sampling stations of Las Prusianas stream (LP1, LP2) with 13–38 species. The species diversity showed low values (1.8–2.3) in channels and streams but higher values (3.0) in the Salado River at Manucho and San Justo. Absolute biomass varied in the order SJSR>MSR>LP1>NCH>SCH>LP2, similar to absolute density, which varied in the order SJSR>MSR>LP1>NCH>SCH>LP2. The comparison of the content of heavy metals in water between the control site (SJSR) and the most contaminated sites showed significant differences with the North Channel and Las Prusianas 1 and 2 streams (ANOVA; p=0.001, 0.012, and 0.011, respectively) and non-significant differences, although close to the significance level, with the South Channel and Manucho (p=0.08, 0.059, respectively). The following positive correlations were found: depth with mesozooplankton density, H, and S (p < 0.001); temperature with microzooplankton density, H, and S (p < 0.004); and a negative correlation between dissolved oxygen with mesozooplankton density, H, and S (p < 0.01) but not with microzooplankton, indicating a higher tolerance of the organisms belonging to this zooplankton fraction. A negative correlation was found between biomass of copepods and concentration of Pb and Cu (p < 0.05 and p=0.01, respectively). Rotifers were the most tolerant to heavy metal contamination, followed by copepods and cladocerans. Species diversity values (H) allowed differentiating between pollution levels. We conclude that S and H are good indicators of stress in polluted systems. Species richness (S) allowed separating studied environments into two groups: the tributaries, with lower species richness, and the river, with higher species richness. The decrease in specific richness and diversity observed at stations closer to the effluent source was related to the increase in chromium and sulfide concentrations. This result suggests that both substances and not only chromium are highly toxic to this community, which is generally not considered when the effects of tannery effluents on biota are discussed. Another result found in this study was the decrease in zooplankton biomass at a higher concentration of heavy metals. This result indicates that this parameter is also a good indicator of polluted aquatic systems.
Compared to less polluted systems of the region, zooplankton density in this system was similar but zooplankton biomass was much lower. This indicates the settlement and proliferation of smaller size species (rotifers). Rotifers were the most tolerant species; copepods followed rotifers, while cladocerans only contributed significantly to the community at San Justo, where a higher equity was also registered. Cladocerans showed very low tolerance to the toxic action of heavy metals. The clustering of biological and physicochemical variables and the concentration of heavy metals in water resume the picture of the effect on zooplankton assemblage and show three groups of environments (Fig. 10.5): the first one was the main course of the river, with lower contamination by heavy metals and higher density, biomass, H, and S, which separated clearly from the other two groups of the tributaries composed by channels (SCH, NCH) and streams (LP2, LP1). In the tributaries, r-strategists and a few tolerant species, such as E. neumani, proliferated. In general, the river offered better conditions for the development of the community: a higher flow and degree of dissolved oxygen than in the tributaries. This allowed the settlement of significant populations at Manucho, one of the polluted sites, and at San Justo, the initial reference site. Due to the high tolerance to tannery effluents and ubiquity of E. neumani, it is proposed as a water quality indicator species. In synthesis, the pollution gradient of the studied sites was Las Prusianas>Manucho>South Channel>North Channel>San Justo. The results of this study show that zooplankton responds as a good descriptor of water quality, constituting an efficient tool to assess eutrophication and heavy metal contamination. Data analysis shows the urgency to perform biological studies and to carry out remediation actions in the lower Salado River basin.
Hierarchical cluster analysis (Euclidean measures, UPGMA method) based on biological zooplankton parameters and concentrations of heavy metals in water at the sampling sites (Modified from Gagneten and Paggi 2009)
10.4.1 Integrating Possible Effects of Eutrophication and Heavy Metal Contamination on the Trophic Webs of Freshwater Ecosystems
When contamination by heavy metals is added to an eutrophication process it can turn out to be a very complex situation. As Clements and Newman (2002) pointed out, the studies of community-level impacts are a very useful tool for understanding pollution effects on the ecosystems. In this sense, responses of zooplanktonic species assemblage are a possible and reliable approach. This community, as it is constituted by organisms of different sizes and trophic habits and complex life cycles (parthenogenesis, sexual reproduction with larval and juvenile stages), is a valuable tool to characterize the environment biologically in areas with different degrees of anthropogenic impact (Fig. 10.5). Figure 10.6 summarizes the complex interrelations that can occur. Through biomonitoring, it is possible to study the attributes of the zooplanktonic community with a great potential as indicators of environmental stress: species richness (S), species diversity (H), equity (E), and biomass (B). These attributes allowed us to detect structural and functional changes. Structural changes: alterations in the community size structures are produced. Macrozooplankton reduces its number or disappears and rotifers, which would be the most tolerant species, increase markedly their density. Therefore, the composition changes, diversity decreases, and the community remains constituted by small size species, mainly smaller than 500 μm, i.e., rotifers, nauplii, and lower size cladocerans. The structure and size ranges in plankton are the first indicators of stress situations at the community level (Moore and Folt 1993). There is an inverse relationship between stress situations and zooplankton body size, with a proliferation of r-strategist species (rotifers) and opportunistic species (nauplii larvae), a dominance of tolerant species (E. neumani, in this example), and a decrease in the most sensitive ones, such as larger size crustaceans (copepods and cladocerans). Similarly, Takamura et al. (1999) registered a shift of zooplankton community structure from a Daphnia–Acanthodiaptomus community to a Bosmina–rotifer community, which probably led to a decrease of secchi disc transparency. The tolerance of rotifers would be determined by their lower sensitivity to toxics, their more rapid growth without molts, and their higher resilience (José de Paggi 1997). Functional changes: alterations in the intrazooplanktonic competition are produced. Macrozooplankton is substituted by microzooplankton. The selective elimination of larger size herbivore crustaceans (cladocerans and calanoid copepods) affects another trophic level, i.e., fish populations. Thus, changes in the trophic web are generated by the effects of the decrease in the available resource for larvae and juveniles of ichthyophagus fish and planktivorous adults. Similar results were obtained by Havens (1994) and Havens et al. (1993). Similar results were also recorded by Park and Marshall (2000) who investigated zooplankton and water quality parameters at the lower Chesapeake Bay and Elizabeth River to identify the changes of zooplankton community structure with increased eutrophication. The total micro- and mesozooplankton biomass decreased with the increase of eutrophication. However, the relative proportion of microzooplankton increased with increased eutrophication. Within highly eutrophied waters, the small oligotrichs (<30 μm) and rotifers dominated the total zooplankton biomass. However, tintinnids, copepod nauplii, and mesozooplankton significantly decreased with the increase of eutrophication. These patterns were consistent throughout the seasons and had statistically significant relationships. The authors also suggest that shifts in zooplankton community structure characterize an increasing eutrophication of an ecosystem. As shown in Fig. 10.6, cladoceran decrease generates changes in phytoplankton by decreasing the foraging pressure, which can increase the eutrophication process. This pattern was also addressed, among others, by Yang et al. (1998). A 12-year data analysis showed that since Daphnia feeds efficiently on phytoplankton, it could decrease concentration of Chl-a and enhance water transparency. Top-down control is an important type of interspecies interaction in food webs. Phytoplankton grazers contribute to the top-down control of phytoplankton populations, but chemical pollution may pose a threat to the natural top-down control of phytoplankton and water self-purification process (Ostroumov 2002, Bielmyer et al. 2006, Gama-Flores et al. 2006). On the one hand, a decrease in phytoplankton populations by direct toxic effect (Cu, for example, is a strong algaecide) can occur. This process can determine the decrease in the efficiency of carbon and energy transfer in the system. On the other hand, the decrease in phytoplankton affects negatively the filtering rate of cladocerans, which influences their growth rates. Water transparency and nutrient regeneration rate can also be affected (Somer 1998).
10.5 Summary
The contamination of the lower Salado River basin showed a very close relationship between the impact of heavy metals and the process of eutrophication on zooplanktonic assemblage. It is thus possible to conclude that zooplankters respond as good descriptors of water quality in complex situations, constituting an efficient tool together with other environmental parameters. Similarly, Beaver et al. (1998) suggested that abiotic factors which are known to directly affect phytoplankton may indirectly affect zooplankton composition in such a way as to use zooplankton assemblages as indicators of water quality. Moreover, pollutants finally reach the sea and can be found even in the traditionally less polluted environments. De Moreno et al. (1997) and Kahle and Zauke (2003) registered heavy metals in different groups of Antarctic invertebrates. The nutrient and metal removal from wastewaters through bioremediation using regional macrophytes such as Eicchornia crassipes, Salvinia herzogii, and Pistia stratiotes is proposed (Maine et al. 2004, 2005, 2006, 2007, Hadad et al. 2007). In other surveys, the growth response of Lemna minor and Spirodela polyrrhiza was studied for their possible application for remediating eutrophic waters (Ansari and Khan 2008, 2009). The biosorbent potential of algal cells for toxic metals also offers an effective and low-cost alternative to conventional methods for decontamination of industrial effluents containing metals (Rai et al. 2005, Baran et al. 2005, Beek et al. 2007, Regaldo et al. 2009, Gagneten et al. 2009). The most contaminant industries should be controlled in the tannery leather process, through the replacement of chromium salts by other less contaminant methods. In this sense, the United Nations Environmental Program (UNEP 1991) assessed that chromium salts should continue to be used because of their high affinity with carboxylic groups of the collagen fibers and of their price, which is comparatively lower compared to less contaminant methods. Finally, we should remember that the primary effects of contamination are exerted on the biota, including the human being. It is necessary to promote actions at different levels and sectors of the society, linked to the development of an adequate culture of water management, which will influence the improvement of life quality. In that sense, we can agree with the statement of Trevors and Saier (2009a, b): “We need to take care of the problems we currently recognize so that we are prepared to solve those about which we still have no inkling. The real rescue plan for the planet is environmental, not economic. Any rescue plan that does not aim to reduce the human population, the wasteful use of our resources and global pollution is doomed to failure.”
References
Ansari AA, Khan FA (2008) Remediation of eutrophied water using Lemna minor in a controlled environment. Afric J Aquat Sci 33:275–278
Ansari AA, Khan FA (2009) Remediation of eutrophied water using Spirodela polyrrhiza L. Shleid in controlled environment. Pan-American J Aquat Sci 4:52–54
Baran A, Baysal SH, Sukatar A et al (2005) Removal of Cr6+ from aqueous solution by some algae. J Environ Biol 26:329–333
Beaver JR, MillerLemke AM, Acton JK et al (1998) Midsummer zooplankton assemblages in four types of wetlands in the Upper Midwest, USA. Hydrobiologia 380:209–220
Beek B, Böhling S, Bruckman U, Franke C, Jöhncke U, Studinger G et al (2007) The assessment of Bioaccumulation. In: Beek B (ed) The Handbook of Environmental Chemistry, vol 2. Bioaccumulation. Springer, Berlin, Heidelberg
Bielmyer GK, Grosell M, Brix KV et al (2006) Toxicity of Silver, Zinc, Copper, and Nickel to the Copepod Acartia tonsa Exposed via a Phytoplankton Diet. Environ Sci Technol 40:2063–2068
Canadian environmental quality guidelines (2002) Canadian environmental protection act. Canadian Water Guideliness, Ottawa, ON
Canadian environmental quality guidelines (2003) Summary of the Existing Canadian Environmental Quality Guidelines. Canadian Council of Ministers of the Environment
Clements WH, Newman MC (2002) Community ecotoxicology. Wiley, Chichester, 336 pp
de Moreno JEA, Gerpe MS, Moreno VJ, Vodopivez C et al (1997) Heavy metals in Antarctic organisms. Pol Biol 17:131–140
Gagneten AM, Ceresoli N (2004) Efectos del efluente de curtiembre sobre la abundancia y riqueza de especies del zooplancton en el Arroyo Las Prusianas (Santa Fe, Argentina). Interciencia 29:702–708
Gagneten AM, Gervasio S, Paggi JC et al (2007) Heavy metal pollution and eutrophication in the lower Salado River Basin (Argentina). Wat Air Soil Pollut 178:335–349
Gagneten AM, Paggi JC (2009) Effects of heavy metal contamination (Cr, Cu, Pb, Cd) and eutrophication on zooplankton in the lower basin of the Salado River (Argentina). Wat Air Soil Pollut 198:317–334
Gagneten AM, Regaldo L, Troiani H et al (2009) Uso del SEM para determinar efectos tóxicos del cromo en la morfología de Chlorella sp. y Daphnia magna. SETAC LA “Quimica y Toxicología Ambiental en América Latina”. In: J Herkovits (ed) Sociedad de Toxicología y Química Ambiental (SETAC), pp 130–132
Gama-Flores JL, Sarma SS, Nandini S et al (2006) Effect of cadmium level and exposure time on the competition between zooplankton species Moina macrocopa (Cladocera) and Brachionus calyciflorus (Rotifera). J Environ Sci Health 41:1057–1070
Hadad HR, Maine MA, Nataleb GS, Bonetto C et al (2007) The effect of nutrient addition on metal tolerance in Salvinia herzogii. Ecolog Eng 31:122–131
Hair JF, Anderson RE, Tarham RL, Black WC et al (1999) Análisis Multivariante, 5th ed. Prentice Hall Iberia, Madrid, 832 pp
Havens KE (1994) Experimental perturbation of a freshwater plankton community: a test of hyotheses regarding the effects of stress. Oikos 69:147–153
Havens KE, Hanazato T et al (1993) Zooplankton community responses to chemical stressors: a comparison of results from acidification and pesticide contamination research. Environ Poll 82:77–288
INFOSTAT (2007) Grupo Infostat, Facultad de Ciencias Agrarias. Universidad Nacional de Córdoba, Córdoba
José de Paggi S (1997) Efectos de los pesticidas sobre el zoopancton de las aguas continentales: análisis revisivo. FABICIB 1:103–114
Kahle J, Zauke GP (2003) Trace metals in Antarctic copepods from the Weddell Sea (Antarctica). Chemosphere 51:
Khan FA, Ansari AA (2005) Eutrophication: an ecological vision. Bot Rev 71:449–482
Maglianesi RE, Depetris PJ (1970) Características químicas del agua del Río Salado Inferior (Provincia de Santa Fe, República Argentina). PHYSIS 30:19–32
Maine MA, Suñe N, Hadad H, Sánchez G, Bonetto C et al (2005) Phosphate and metal retention in a small-scale constructed wetland for waste-water treatment. In: Golterman, HL,Serrano, L (eds) Phosphates in sediments. Proceedings 4th International Symposium on Phosphate in Sediments. Backhuys, Leiden, pp 21–32
Maine MA, Suñe N, Hadad H, Sánchez G, Bonetto C et al (2006) Nutrient and metal removal in a constructed wetland for waste-water treatment from a metallurgic industry. Ecolog Eng 26:341–347
Maine MA, Suñe N, Hadad H, Sánchez G, Bonetto C et al (2007) Removal efficiency of a constructed wetland for wastewater treatment according to vegetation dominance. Chemosphere 68:1105–1113
Maine MA, Suñé NL, Lagger SC et al (2004) Chromium bioaccumulation: comparison of the capacity of two floating aquatic macrophytes. Wat Res 38:1494–1501
Moore M, Folt C (1993) Zooplankton body size and community structure: effects of thermal and toxicant stress. Tree 8:178–183
Moss B (1999) Ecological challenges for lake management. Hydrobiologia 396:3–11
Ostroumov SA (2002) Inhibitory analysis of top-down control: new keys to studying eutrophication, algal blooms, and water self-purification. Hydrobiologia 469:117–129
Park GS, Marshall HG (2000) Estuarine relationships between zooplankton community structure and trophic gradients. J Plank Res 22:121–136
Rai UN, Dwivedi S, Tripathi RD, Shukla OP, Singh NK et al (2005) Algal biomass: an economical method for removal of chromium from tannery effluent. Bull Environ Contam Toxicol 75:297–303
Regaldo L, Gagneten AM, Troiani H et al (2009) Accumulation of chromium and interaction with other elements in Chlorella vulgaris (Cloroficeae) and Daphnia magna (Crustacea, Cladocera). J Environ Biol 30:213–216
Somer U (1998) Plankton ecology. Succession in plankton communities. Springer, New York, NY, 369 pp
Subsecretaria Recursos Hídricos de la Nación (2003) (http://www.hidricosargentina.gov.ar).
Takamura N, Mikami H, Mizutani H, Nagasaki K et al (1999) Did a drastic change in fish species from kokanee to pond smelt decrease the secchi disc transparency in the oligotrophic Lake Towada, Japan. Arch Fur Hydrobiol 144:283–304
Trevors JT, Saier MH Jr. (2009a) The Failures of Some Will Affect All. Wat Air Soil Pollut DOI 10.1007/s11270-009-0100–2
Trevors JT, Saier MH Jr. (2009b) Where is the global environmental bailout? Wat Air Soil Pollut 198:1–3
UNEP (1991) Tanneries and the Environment. A Technical Guide to Reducing the Environmental Impact of Tannery Operations. Technical Report Series No. 4. United Nations Environment Programme, Industry and Environment Office
Xu FL, Tao S, Dawson RW, Li PG, Cao J et al (2001) Lake ecosystem health assessment: indicators and methods. Wat Res 35:3157–3167
Yang YF, Huang XF, Liu JK (1998) Long-term changes in crustacean zooplankton and water quality in a shallow, eutrophic Chinese lake densely stocked with fish. Hydrobiologia 391:195–203
Zar JH (1984) Biostatistical analysis. Prentice-Hall, Englewood cliffs, NJ, N.718 pp
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Gagneten, A.M. (2010). Effects of Contamination by Heavy Metals and Eutrophication on Zooplankton, and Their Possible Effects on the Trophic Webs of Freshwater Aquatic Ecosystems. In: Ansari, A., Singh Gill, S., Lanza, G., Rast, W. (eds) Eutrophication: causes, consequences and control. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9625-8_10
Download citation
DOI: https://doi.org/10.1007/978-90-481-9625-8_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9624-1
Online ISBN: 978-90-481-9625-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)