Summary
Plants identified as having C4 photosynthesis have a C4 metabolic cycle with phosphoenolpyruvate carboxylase as the initial catalyst for fixation of atmospheric CO2, and a C4 acid decarboxylase (NADP-malic enzyme, NAD-malic enzyme, or phosphoenolpyruvate carboxykinase), which releases CO2 for fixation by the C3 cycle. Effective donation of CO2 to Rubisco minimizes competition by O2 and photorespiration, and thus increases photosynthesis under conditions where CO2 is limiting. To achieve this, fixation of atmospheric CO2 in the cytosol by phosphoenolpyruvate carboxylase must be separated from the donation of CO2 to Rubisco by the decarboxylation of C4 acids. In most documented C4 plants, this is accomplished through evolution of various forms of Kranz anatomy, with fixation of atmospheric CO2 in mesophyll cells and donation of CO2 from C4 acids to Rubisco in bundle sheath cells. In the family Chenopodiaceae, two alternative means of accomplishing this spatial separation evolved within individual photosynthetic cells, whereby one cytoplasmic compartment specializes in fixation of atmospheric CO2 in the carboxylation phase of the C4 cycle, and the other cytoplasmic compartment specializes in donating CO2 from C4 acids to Rubisco. In this chapter, biochemical and structural variations of Kranz anatomy in three major C4-containing families, Poaceae, Cyperaceae, and Chenopodiaceae, as well as other known forms for dicots, are summarized. Then, the phylogeny, biogeography, development, and structure-function relationships of the single-cell C4 systems are discussed in comparison to Kranz type C4 plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 I. Introduction
1.1 A. What Does It Take to Be C4?
This question was posed in a short commentary on the history of C4 by (Edwards et al., 2001) who noted that the minimum requirements for the CO2 concentrating mechanism in C4 photosynthesis are “(a) cell-specific amplification of enzymes of C4 photosynthesis; i.e. phosphoenolpyruvate carboxylase (PEPC) in mesophyll, and C4 acid decarboxylases and Rubisco in bundle sheath cells, with complementary adjustments of photosystem and electron transport activities; (b) novel cell-specific organelle metabolite translocators; (c) symplastic connections of the spatially separated sources and sinks of 4C-dicarboxylic acid transport metabolites; and (d) barriers to CO2 diffusion between the site of CO2 fixation by PEPC in mesophyll cells and sites of CO2 release and refixation by Rubisco in bundle-sheath cells”. These requirements have been met through the multiple, independent evolution of C4 photosynthesis in different groups of terrestrial plants. Until the recent discovery of two alternative means of performing C4 photosynthesis within individual chlorenchyma cells (single-cell C4), all terrestrial C4 plants were presumed to have Kranz anatomy.
1.2 B. Occurrence of C4 Among Terrestrial Plants
The earliest studies which led to the identification of C4 plants were on maize and sugarcane, members of family Poaceae (see review by Hatch, 1999). Since then, C4 plants have been found in 19 families with the largest number of species appearing in families Poaceae, Cyperaceae and Chenopodiaceae. C4 is estimated to have evolved independently over 50 times (Muhaidat et al., 2007), resulting in three biochemical subtypes (see Chapter 14 by Drincovich et al.) based on the mechanism of C4 acid decarboxylation: NADP-malic enzyme (NADP-ME), NAD-malic enzyme (NAD-ME), and phosphoenolpyruvate carboxykinase (PEP-CK).
2 II. Structural and Biochemical Diversity in Kranz Type Anatomy
The occurrence of Kranz anatomy (Kranz means wreath in German) has been known since its initial characterization by Haberlandt (1884). In a broad sense, Kranz anatomy can now be functionally defined to accommodate all known structural variants of Kranz type C4 plants. A double concentric layer of chlorenchyma cells together form the Kranz tissue with the outer layer capturing atmospheric CO2 in the C4 cycle, and the inner layer donating CO2 from C4 acids to Rubisco in the C3 cycle. The outer layer is commonly referred to as mesophyll (M) cells (usually consisting of palisade parenchyma) and the inner layer as specialized bundle sheath (BS) cells or Kranz cells. The M cells are always closer to the atmosphere than the BS cells, and the BS cells, as a rule, have limited contact with intercellular air space. The cells of chlorenchymatous M and BS layers are usually adjacent to one another, but in some cases they are separated by an additional layer of cells. The ratio of M/BS cells is lower than in C3 plants, and most of the M cells are in direct contact with BS cells. There are two common structural forms, a double concentric layer of chlorenchyma around individual veins, and a double concentric layer which surrounds all the vascular tissue in the leaf. Since C4 evolved multiple times from different C3 leaf anatomies, there are various structural types. Specific types are described below which represent striking examples of evolutionary convergence on a common suite of anatomical features.
2.1 A. Structural Diversity
Among C4 plants, there is considerable variation in features relevant to the C4 mechanism (Carolin et al., 1973, 1975, 1977, 1978; Laetsch, 1974; Brown, 1975, 1977; Ellis, 1977; Edwards and Walker, 1983; Dengler et al., 1985; Voznesenskaya and Gamaley, 1986; Prendergast and Hattersley, 1987; Prendergast et al., 1987; Ueno et al., 1988a; Hattersley and Watson, 1992; Dengler and Nelson, 1999; Sage, 2004; Muhaidat, et al., 2007). While distinct biochemical and anatomical types have been catalogued since C4 photosynthesis was first described more than three decades ago (see Hatch, 1971), new structural subgroups continue to be discovered, providing further insight into the evolution of the syndrome. Usually, several characteristics are taken into account to distinguish between different structural and biochemical subtypes. The most important among them are: (1) number of BS layers; (2) presence or absence of a mestome sheath (MS) and its positioning in relation to other parenchyma sheaths (notably in grasses); (3) presence or absence of a suberin lamella (SL) in BS cell walls (in grasses); (4) position of BS organelles (mainly chloroplasts); and (5) chloroplast differentiation between M and BS: BS cells in NADP-ME species have grana-deficient chloroplasts with a few, small mitochondria, while NAD-ME species have chloroplasts with well-developed grana and numerous, large mitochondria with specialized cristae. In both subtypes, M chloroplasts have a reversed pattern of grana development to that expressed in the BS, with abundant, large grana in NADP-ME species, and a deficiency of grana in chloroplasts of M cells in NAD-ME species. Some other features, such as the shape (outline) of the outer parenchyma BS, have been mentioned as being useful in characterizing certain subtypes, for example an uneven outline of the outer BS in PEP-CK type grasses; however, this does not seem to be especially important phylogenetically in relation to C4 photosynthetic subtypes, as there are many exceptions (Prendergast et al., 1987). Only features of organelle differentiation provide an easy means to predict biochemical subtypes, while other structural characters only give additional information for considering evolutionary development of Kranz anatomy. The main structural forms of Kranz that are known to occur among C4 species are illustrated in Figs. 1, 2 and 3. Historically, different forms of Kranz anatomy have been referred to either by taxonomic names, or by names descriptive of their structure. We have used taxonomic names to be consistent and concise, recognizing that they are used descriptively and do not always imply phylogenetic identity. An exception is description of the three classical forms of Kranz anatomy associated with the three biochemical subtypes in family Poaceae, which accounts for many C4 grasses.
Illustrations of the forms of Kranz anatomy in family Poaceae. Sketch of vascular bundles in maize for classical NADP-ME, of a large vein in Eragrostis sp. for classical NAD-ME, and of species representing PEP-CK type. Sketches of leaf structure in Arundinella hirta for Arundinelloid type, of large veins in Aristida adscensionis for Aristidoid type, of Stipagrostis pennata for Stipagrostoid type, of Eriachne aristidea for Eriachneoid type, of Alloteropsis semialata ssp. semialata for Neurachneoid type, and of Triodia scariosa for Triodioid type (Pictures are adapted from Voznesenskaya and Gamaley, 1986; Prendergast and Hattersley, 1987; Dengler and Nelson, 1999). B, biochemical subtype; BS, bundle sheath; Chl, chloroplast; M, mesophyll; Mito, mitochondria; MS, mestome sheath; OP BS, outer parenchymatous BS; SL, suberin lamella; VB, vascular bundle.
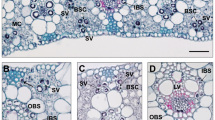
Illustrations of the forms of Kranz anatomy in family Cyperaceae. Sketches of vascular bundles in Fimbristylis sp. for Fimbristyloid type, in Cyperus sp. for Chlorocyperoid type, in Rhynchospora sp. for Rhynchosporoid type and in Eleocharis retroflexa for Eleocharoid type (Drawings to illustrate the anatomy were made from light micrographs, Dengler and Nelson, 1999). For abbreviations, see Fig. 1.
Illustrations of the forms of Kranz anatomy in family Chenopodiaceae. Sketches of leaf structure in Atriplex sp. for Atriplicoid type (tribe Atripliceae), Bassia hyssopifolia for Kochioid type (tribe Camphorosmeae), Salsola collina for Salsoloid type (tribe Salsoleae), Suaeda taxifolia for Salsinoid type (tribe Suaedeae), Suaeda eltonica for Schoberioid type (tribe Suaedeae), and Tecticornia (=Halosarcia) indica for Kranz-Tecticornioid type (tribe Salicornieae) (Some pictures are adapted from Voznesenskaya and Gamaley, 1986). For abbreviations, see Fig. 1.
2.1.1 1. Poaceae
For C4 grasses, important distinguishing characteristics include the presence or absence of a MS, and, when present, the size of MS cells and thickness of their cell walls, the positioning of chloroplasts in BS cells, the presence or absence of a SL and, when present, its distribution in the Kranz cell walls (Carolin et al., 1973; Brown, 1975, 1977; Ellis, 1977; Hattersley and Browning, 1981; Hattersley, 1992; Hattersley and Watson, 1992). At least nine structural subtypes have been distinguished on the basis of these features, and most of the known C4 grasses fit into these subtypes (described below and illustrated in Fig. 1 and Table 1). It is suggested from phylogenetic analyses that C4 evolved from C3 a minimum of 17 times in family Poaceae (Christin et al., 2008, 2009). Certain forms of Kranz anatomy evolved multiple times in the family (Table 1).
In the Poaceae, C4 species occur in subfamilies Panicoideae, Chloridoideae, Aristidoideae, and Micrairoideae (Sanchez-Ken et al., 2007; Vicentini et al., 2008; Christin et al., 2008, 2009). While most C4 species in subfamily Panicoideae are NADP-ME type, NAD-ME and PEP-CK type species also occur in the subfamily as discussed below. In subfamily Chloridoideae, most C4 species are NAD-ME type, while a few genera have PEP-CK type species. C4 species identified in subfamilies Aristidoideae and Micrairoideae are NADP-ME type (Table 1). Structural forms of Kranz anatomy among these biochemical subtypes are discussed below.
2.1.1.1 Classical NADP-ME Type Anatomy
This type of anatomy was originally the so-called Panicoid (Carolin et al., 1973). Among the three major biochemical C4 subgroups first identified in Poaceae (Gutierrez et al., 1974; Hatch et al., 1975; Brown, 1977), classical NADP-ME type species have a single parenchyma BS (the Kranz BS which is derived from provascular tissue and, thus, lacks a MS), with BS chloroplasts in a centrifugal/peripheral position and with a deficiency in their grana development, whereas, M chloroplasts have well-developed grana (Fig. 1) (Voznesenskaya and Gamaley, 1986; Hattersley, 1992; Yoshimura et al., 2004). C4 species with classical NADP-ME type anatomy in subfamily Panicoideae occur in tribes Paniceae, Arundinelleae, and Andropogoneae (Hattersley and Watson, 1992; Sage et al., 1999; GPWG, 2001; Vicentini et al., 2008). The degree of grana reduction in chloroplasts in the BS cells varies, from having nearly agranal BS chloroplasts (representatives of tribe Andropogoneae), to having numerous, small grana (tribe Paniceae), to having a few rather large grana (Panicum obseptum or Rhynchelytrum repens). This subtype has fewer mitochondria in BS cells than in the NAD-ME and PEP-CK subtype (Yoshimura et al., 2004). The SL is present in the outer tangential wall, and partly in the radial cell wall of the BS cells (Hattersley and Browning, 1981).
2.1.1.2 Classical NAD-ME Type Anatomy
This structural type of C4 grasses has a double sheath: a MS with thick cell walls and a few plastids, surrounded by a Kranz type chlorenchyma sheath (derived from ground tissue). Bundle sheath chloroplasts are in a centripetal position and have well-developed grana, while M chloroplasts show different degrees of grana reduction according to species (Fig. 1). A SL is usually absent in BS cells; but if present, is only in BS cell walls adjacent to sclerenchyma cells which do not contain chloroplasts (Hattersley and Browning, 1981). This subgroup also has abundant, large specialized mitochondria in BS cells, the site of the C4 acid decarboxylase (Gutierrez et al., 1974; Hatch et al., 1975; Brown, 1977; Hattersley and Watson, 1992; Yoshimura et al., 2004). This form of anatomy was originally named Eragrostoid (Carolin et al., 1973). It occurs particularly in subfamily Chloridoideae (the core Chloridoideae and Centropodia lineages); but, also in subfamily Panicoideae, tribe Paniceae, evolving once in the Panicum, Urochloa, Setaria clade, Table 1 (Sage et al., 1999; GPWG, 2001; Aliscioni et al., 2003; Christin et al., 2008, 2009).
2.1.1.3 Classical PEP-CK Type Anatomy
The classical PEP-CK type has a double chlorenchyma sheath similar to the NAD-ME type, with an inner MS and outer Kranz chlorenchyma sheath with grana-containing BS chloroplasts in a centrifugal position, or scattered peripherally around the cell, see Fig. 1 (Gutierrez et al., 1974; Brown, 1977; Dengler and Nelson, 1999; Yoshimura et al., 2004). The level of grana development is very similar in BS and M chloroplasts, and the BS mitochondria are quite small (generally comparable in size to M mitochondria) and are usually more numerous than in NADP-ME species, but less abundant than in NAD-ME species (Yoshimura et al., 2004; Voznesenskaya et al., 2006). Suberin lamella is present in the outer tangential BS cell walls and extends approximately to the middle of the radial cell walls (Hattersley and Browning, 1981). This subtype has been found in subfamily Chloridoideae in Bouteloua, Eleusine, Muhlenbergia, Spartina, Sporobolus, and Zoysia, and in subfamily Panicoideae, evolving once in the Panicum/Urochloa/Setaria clade, e.g. in Brachiaria, Chaetium, Eriochloa, Melinis, and Urochloa, see Table 1 (Sage et al., 1999; Guissani et al., 2001; Aliscioni et al., 2003; Christin et al., 2008).
2.1.1.4 Arundinelloid: Biochemical Subtype NADP-ME
This type of anatomy was studied in detail in genus Arundinella (tribe Arundinelleae) subfamily Panicoideae. Like the classical NADP-ME type, species in this genus have NADP-ME type biochemistry, with BS chloroplasts having reduced grana and in the centrifugal position; MS is absent in all vascular bundles. However, the Kranz anatomy in veins is widely spaced, and there is the unusual occurrence of a row, or rows, of Kranz assemblies between the veins, which sometimes are referred to as distinctive cells because these bundle sheath-like cells are not associated with vascular tissue (Tateoka, 1958), see Fig. 1. A SL is usually present and continuous in the distinctive cells, or interrupted in the radial cell walls in BS cells surrounding the vascular tissue (Hattersley and Browning, 1981). Distinctive cells have structural and biochemical characteristics similar to the BS cells (Crookston and Moss, 1973; Dengler et al., 1990, 1996; Dengler and Dengler, 1990; Wakayama et al., 2002, 2003, 2006). Distinctive cells have also been found in genera Arthraxon and Microstegium (tribe Andropogoneae), where they have ultrastructural characteristics similar to those shown for Arundinella (Ueno, 1995), and in genus Garnotia; but, there is no additional biochemical or ultrastructural data for species of this genus (Tateoka, 1958).
2.1.1.5 Aristidoid: Biochemical Subtype NADP-ME
The Kranz anatomy of species in genus Aristida, tribe Aristideae in subfamily Aristidoideae, is unusual in having three distinct layers of chlorenchyma cells surrounding the vascular tissue: an inner BS, an outer BS, and the M cells (Brown, 1958; Johnson, 1964; Bisalputra et al., 1969), see Fig. 1. Aristida species have NADP-ME type biochemistry, based on analyses of several species in the genus (Gutierrez et al., 1974; Hattersley, 1987; Prendergast et al., 1987; Voznesenskaya, et al., 2005b). Mestome sheath is absent; both the inner and outer sheaths are chlorenchymatous. However, in the inner sheath, chloroplasts are nearly agranal, while the outer BS contains chloroplasts with well-developed grana similar to the M chloroplasts. In this type, only the inner sheath functions as Kranz cells, while the outer BS functions mainly for storage of starch and, possibly, for refixation of photorespired CO2 (Voznesenskaya et al., 2005b). In the inner BS, chloroplasts are scattered around the cell or tend to be centrifugal, while in the outer parenchyma BS, chloroplasts are located in a centripetal position. The SL is absent in cell walls of both types of BS (Hattersley and Browning, 1981).
2.1.1.6 Stipagrostoid: Biochemical Subtype NADP-ME
The genus Stipagrostis belongs to tribe Aristideae in subfamily Aristidoideae. Like Aristida, Stipagrostis species also evolved NADP-ME type photosynthesis, but they have a different type of Kranz anatomy, named Stipagrostoid (Voznesenskaya et al., 2005a). This subtype has an inner MS consisting of enlarged cells with thinner cell walls and few chloroplasts, and an outer layer of Kranz cells with chloroplasts in the centripetal position (Brown, 1975). In the Kranz cells of Stipagrostis, mitochondria are few and small, and the chloroplasts are deficient in grana compared to M chloroplasts, which have well-developed grana. In contrast, the classical NADP-ME subtype grasses lack a MS and they have Kranz cells with chloroplasts in a centrifugal position. Also, the Kranz cells of Stipagrostis lack a SL in the walls, whereas the classical NADP-ME type grasses have SL in the Kranz cells, which are thought to have originated from the MS.
2.1.1.7 Eriachneoid: Biochemical Subtype NADP-ME
This subtype, which occurs in genera Pheidochloa and Eriachne (subfamily Micrairoideae, Christin et al., 2008, 2009), has NADP-ME type biochemistry (Prendergast et al., 1987) and an inner MS with an outer Kranz sheath, like the Stipagrostoid type. However, unlike Stipagrostis, which has chloroplasts in a centripetal position in Kranz cells, in Pheidochloa and in most Eriachne species (18 of 21) the chloroplasts in Kranz cells are in a centrifugal position (Hattersley, 1987; Prendergast and Hattersley, 1987; Prendergast et al., 1987; Taniguchi et al., 2003). The BS chloroplasts have well-developed grana with numerous, long intergranal thylakoids; in Eriachne aristidea, the degree of grana stacking is lower compared to the M chloroplasts (Taniguchi et al., 2003). For other species, the situation is not very clear. Bundle sheath chloroplasts of E. glabrata, E. obtusa and P. gracilis have an unexpectedly large number of grana (up to 21 thylakoids in a stack) for an NADP-ME subtype (Prendergast et al., 1987); however, there is no data about the degree of grana differentiation in M chloroplasts of these species. The SL is absent in cell walls of Kranz cells (Prendergast et al., 1987).
2.1.1.8 Neurachneoid: Biochemical Subtypes NADP-ME and PEP-CK
Species with this type of anatomy have a double parenchymatous sheath and the MS is absent; however, in this case the inner sheath is Kranz and the outer sheath is a non-specialized parenchyma BS containing only a small number of chloroplasts (see Dengler et al., 1985; Hattersley et al., 1986; Prendergast et al., 1987; Ueno and Sentoku, 2006). It was suggested that the inner Kranz BS in all species having this type of anatomy originated from the MS of C3 grasses (Brown, 1975, 1977; Dengler et al., 1985). Species with this type of anatomy which perform NADP-ME type photosynthesis are Neurachne munroi, Paraneurachne muelleri in the Neurachne clade, and also Panicum petersonii and P. prionitis in section Prionita; all belong to the subfamily Panicoideae. In both N. munroi and P. muelleri, thick cell walls of the Kranz inner sheath have a SL, which is only continuous in the outer tangential walls and outer parts of radial walls. The outer parenchyma sheath has relatively thin cell walls without SL. Chloroplasts in Kranz cells are distributed evenly in N. munroi, but are in a centrifugal position in P. muelleri. Kranz cell chloroplasts have granal stacks which are less pronounced than in the M chloroplasts (Hattersley et al., 1986).
Alloteropsis semialata, subfamily Panicoideae, represents a very unique case, where diversity in the form of photosynthesis occurs among subspecies, with ssp. semialata being C4 and ssp. eckloniana being C3 (Frean et al., 1983; Prendergast et al., 1987; Ueno and Sentoku, 2006; Ibrahim et al., 2009). An Australian accession of spp. semialata biochemically is PEP-CK type with Neurachneoid type anatomy (Prendergast et al., 1987). The Kranz sheath, unlike the classical PEP-CK type species, is considered to be derived from the MS sheath of C3 plants. Most anatomical characteristics are similar to those cited above: cell walls of Kranz cells are thicker than in the parenchymatous BS and they have a SL. There are abundant chloroplasts and mitochondria in Kranz cells which do not have a special orientation, and chloroplasts have well-developed grana like those in the M cells (Ueno and Sentoku, 2006). The ssp. semialata has high PEP-CK activity and variable amounts of NADP-ME which may be influenced by growth conditions (Prendergast et al., 1987; Ueno and Sentoku, 2006).
2.1.1.9 Other Forms of NAD-ME Type Anatomy
There are several NAD-ME C4 species in family Poaceae having some anatomical features that are not characteristic of the classical NAD-ME type C4 species. Like the classical NAD-ME and PEP-CK types, these species have a double parenchyma sheath, with an inner MS and outer Kranz chlorenchyma sheath with grana-containing BS chloroplasts. Also, the BS cells have abundant mitochondria characteristic of NAD-ME type species. However, the BS chloroplasts are not arranged in the centripetal position, but are located in a centrifugal or peripheral position like the PEP-CK species. Also, the BS cell walls generally have a SL which is usually absent in the classical NAD-ME type species. This includes some species in genera Eragrostis, Enneapogon, Triraphis, and some Panicum species of the section Dichotomiflora (Ohsugi and Murata, 1980; Ohsugi et al., 1982; Prendergast et al., 1986). Interestingly, different cultivars of one NAD-ME type species, P. coloratum, were found to have different positions of chloroplasts in the Kranz cells, centripetal versus centrifugal (Ohsugi et al., 1982), which further shows that this feature cannot be taken as a criterion for distinguishing between different biochemical types.
A more extreme structural variant of NAD-ME type species is the Triodioid type anatomy. Species with Triodioid anatomy have two BS: an inner, thin-walled MS and an outer, chlorenchymatous Kranz BS which lacks SL in the cell walls. There are two variants of Kranz anatomy in this genus: Kranz BS form (“drape”) extensions between adjacent vascular bundles, as in Triodia pungens (Hattersley and Watson, 1992), or BS extensions towards patches of M cells on both the abaxial and adaxial sides of the leaf, which are not associated with vascular bundles, for example T. irritans and T. scariosa, as illustrated in Fig. 1 (Dengler and Nelson, 1999). The species which have been studied have NAD-ME type biochemistry; the appearance of mitochondria in Kranz cells is typical for NAD-ME species (Craig and Goodchild, 1977). The chloroplasts in Kranz cells have well-developed grana; but, unlike classical NAD-ME species, they, are in a centrifugal position or peripherally scattered around the cytoplasm, as in PEP-CK type species (Craig and Goodchild, 1977; Prendergast et al., 1987).
2.1.2 2. Family Cyperaceae
In family Cyperaceae, there are four types of Kranz anatomy (Fig. 2). As in family Poaceae, C3 Cyperaceae species have an inner MS and an outer parenchyma sheath around the vascular tissue. In C4 Cyperaceae species, the Kranz cells are considered to have evolved either internal to the MS (Fimbristyloid, Chlorocyperoid and Eleocharoid) or from the MS (Rhynchosporoid) (Brown, 1975; Carolin et al., 1977; Gilliland and Gordon-Gray, 1978; Bruhl et al., 1987; Bruhl and Perry, 1995; Soros and Dengler, 1998, 2001; Dengler and Nelson, 1999). In the first case, the MS, which is situated between M and Kranz cells, is nonphotosynthetic, thick-walled and generally suberized (Carolin et al., 1977; Ueno et al., 1988b; Ueno and Samejima, 1989; Bruhl and Perry, 1995; Soros and Dengler, 2001). This sheath may contribute to diffusional resistance of gases and help to minimize leakage of CO2 generated from decarboxylation of C4 acids in the Kranz cells. Most C4 representatives in the family are NADP-ME type; NAD-ME species have only been found in genus Eleocharis (Bruhl et al., 1987; Ueno et al., 1988a; Murphy et al., 2007).
2.1.2.1 Fimbristyloid: Biochemical Subtypes NADP-ME and NAD-ME
This type of anatomy was found in C4 species of the tribe Fimbristylideae, for example in Bulbostylis and Fimbristylis (see Carolin et al., 1977; Ueno et al., 1988a) and more recently in genus Eleocharis (Bruhl et al., 1987; Ueno, 1998a; Murphy et al., 2007). The Kranz cells originated internal to the MS and do not form a continuous wreath; rather, they are interrupted by metaxylem elements. In this type, there are three BS layers around all vascular bundles, even small ones: Kranz BS surrounded by the MS, and parenchymatous BS (external to the MS) having fewer chloroplasts than in M cells. Both NADP-ME biochemical subtype in genera Fimbristylis and Bulbostylis (Ueno et al., 1986; Ueno, 1998a), and NAD-ME subtype in some species of Eleocharis (Ueno, 1998b; Murphy et al., 2007), have been reported to have Fimbristyloid anatomy. The species of Fimbristylis have Kranz cells with chloroplasts that are centrifugally located and nearly agranal having numerous small, short grana; mitochondria are small and few, consistent with NADP-ME type photosynthesis (Carolin et al., 1977; Gilliland and Gordon-Gray, 1978). One population of Eleocharis vivipara (type 1) and E. retroflexa ssp. chaetaria are NAD-ME type C4 species with Fimbristyloid-like anatomy; the latter has Kranz cell chloroplasts with well-developed grana and large mitochondria, typical of NAD-ME type C4 species (Ueno and Samejima, 1989; Ueno et al., 1989; Ueno, 1996a). Immunolocalization studies show M and parenchymatous BS cells of E. vivipara type I (Ueno, 1996b) and Fimbristylis dichotoma (Ueno, 1998a) have PEPC and pyruvate, Pi dikinase (PPDK), indicating both cell types function to capture CO2 by PEPC, with delivery of C4 acids to the Kranz cells, where Rubisco is located.
2.1.2.2 Chlorocyperoid: Biochemical Subtype NADP-ME
The Chlorocyperoid type, as a rule, has two layers of BS, with the Kranz cells internal to the MS. Chlorenchyma cells external to the MS include a partial parenchymatous chlorenchyma sheath (occurring in large vascular bundles, it is less developed than in the Fimbristyloid, and may be completely absent in some species) and palisade-like M cells, both of which are considered to function in the carboxylation phase of the C4 cycle. Kranz cells contain few large centrifugally-arranged chloroplasts having mostly single stroma thylakoids, convoluted in loops. The degree of grana reduction varies in different species. The SL is usually discontinuous in the radial cell wall of the MS and absent from Kranz BS (Ueno et al., 1988a, b; Bruhl and Perry, 1995). In some species (as with the Fimbristyloid), both M and parenchymatous BS cells may function to capture CO2 by PEPC, with delivery of C4 acids to the Kranz cells, where Rubisco is exclusively localized (Ueno, 1998a). This type of anatomy has been found in genera Cyperus, Kyllinga, Pycreus and Torulinium of the tribe Cypereae and Lipocarpha in the Lipocarpheae (Carolin et al., 1977; Gilliland and Gordon-Gray, 1978; Ueno et al., 1986, 1988a). Representative species having Chlorocyperoid type anatomy have NADP-ME type C4 photosynthesis (Ueno et al., 1986; Bruhl et al., 1987). Eleocharis baldwinii, which has NAD-ME biochemistry and ultrastructure, has an intermediate type of anatomy called sub-Chlorocyperiod (Ueno and Samejima, 1989; Ueno, 2004).
2.1.2.3 Rhynchosporoid: Biochemical Subtype NADP-ME
Unlike the other forms of Kranz anatomy in Cyperaceae, in Rhynchosporoid type the Kranz cells evolved from the MS (Takeda et al., 1980). This thick-walled sheath is surrounded by an incomplete chlorenchymatous parenchyma sheath and palisade-like M cells which are considered to function in fixation of atmospheric CO2 into C4 acids. Both M and outer parenchyma sheath chloroplasts have similar thylakoid structure with large grana. Kranz cells have numerous, centrifugally-arranged agranal chloroplasts but, unlike the convoluted thylakoids in the previous types, here the thylakoid membranes usually have a parallel arrangement (Gilliland and Gordon-Gray, 1978; Ueno et al., 1988a; Bruhl and Perry, 1995). Mitochondria are comparable in size and number in M and BS cells. The SL is mostly continuous around the cell but can be discontinuous in the radial cell walls. Biochemical analysis indicates NADP-ME type photosynthesis (Ueno et al., 1986; Bruhl et al., 1987).
2.1.2.4 Eleocharoid: Biochemical Subtype NAD-ME
The Eleocharoid type anatomy was named after C4 species of Eleocharis which have three types of BS, with the innermost Kranz cells forming a continuous wreath. As in Chlorocyperoid and Fimbristyloid types, the Kranz cells originate internal to the MS. The outer parenchyma BS contain some chloroplasts, while the middle MS lacks, or contains only a few, chloroplasts filled with starch; Kranz cells contain numerous organelles typical of NAD-ME species, with no strict orientation in the cell (scattered around the periphery) or tending slightly towards centrifugal. Chloroplasts of Kranz cells have well-developed grana and store starch; chloroplasts of parenchyma BS are smaller than those in M cells, but in both types of cells there are well-developed grana. Usually, a SL is present on both the inner and outer tangential cell walls in the MS, but sometimes it is absent on the radial cell walls (Ueno and Samejima, 1989; Bruhl and Perry, 1995). The Kranz cells have abundant and large mitochondria, typical of NAD-ME type C4 grasses and dicots (Ueno and Samejima, 1989; Bruhl and Perry, 1995; Ueno, 2004; Ueno and Wakayama, 2004). The genus Eleocharis is very diverse in forms of photosynthesis between species (C3, C3–C4, C4-like). It includes amphibious species which change their mode of photosynthesis between submerged and terrestrial growth; and both Eleocharoid and Fimbristyloid type anatomy with NAD-ME type photosynthesis has been found among its C4 species (Bruhl et al., 1987; Bruhl and Perry, 1995; Ueno, 2004; Ueno and Wakayama, 2004; Murphy et al., 2007).
2.1.3 3. Dicotyledons
Among dicot families, it is well-established that family Chenopodiaceae has the largest number of C4 species and also the greatest diversity in leaf anatomy, including C3, C4 Kranz and C4 single-cell types (Carolin et al., 1975; Pyankov et al., 1992; Sage et al., 1999; Edwards et al., 2004; Voznesenskaya et al., 2007). This family has been studied most extensively, resulting in classification of six types of Kranz anatomy (Fig. 3) which has been extended to several other families (Carolin et al., 1975, 1982; Jacobs, 2001). The C4 types of leaves vary in the structure and arrangement of chlorenchyma tissue, in arrangement of water storage and vascular tissue, and by the presence, or absence, of various specialized hypodermal cells. Within these six main types of Kranz anatomy, additional anatomical differences have been recognized, indicating potential for further subdivision of structural types of Kranz in the family (Kadereit et al., 2003). The C4 structural types in family Chenopodiaceae are named after the corresponding taxonomic names, as indicated below. These main structural forms were also given descriptive names (Vasilevskaya and Butnik, 1981; Voznesenskaya and Gamaley, 1986) which are also referred to in the descriptions below. In addition to the six Kranz types in the Chenopodiaceae, other forms have been recognized in dicot lineages found in Cleome (Cleomaceae), Isostigma and Glossocardia (Asteraceae) and Portulaca (Portulacaceae).
2.1.3.1 Atriplicoid: Biochemical Subtypes NAD-ME and NADP-ME
In C3 dicots, all the vascular bundles are surrounded by a parenchyma sheath which is more or less distinguishable from M tissue; this sheath becomes a specialized Kranz BS in C4 species. In Atriplicoid type of anatomy which occurs in some dicot species having laminate leaves, the Kranz tissue forms a classical wreath-like structure with concentric layers of chlorenchyma around each vascular bundle. The Kranz BS encloses vascular bundles; although it can become disrupted on the phloem side in larger bundles. There is structural diversity and potential for recognition of additional subtypes where Kranz encloses individual veins in C4 dicots having flattened leaves. In this type, hypodermal tissue when present usually fulfills the role of water storage tissue. For example, Portulaca oleracea has extensively developed water storage hypoderm with variable positioning of the veins between the abaxial and adaxial sides of the leaf. In Atriplicoid type, palisade M cells are usually arranged radially; but this can vary in different species (Rathnam et al., 1976; Dengler and Nelson, 1999; McKown et al., 2005; Muhaidat et al., 2007). For species of families Chenopodiaceae and Amarathaceae, Kadereit et al. (2003) distinguished four different types of anatomy in laminate leaves within the Atriplicoid type, which differ in the presence or absence of hypoderm, the occurrence of parenchyma cells between M cells, or the occurrence of additional layers of spongy parenchyma on the abaxial side of the leaf. Nevertheless, similar features may be found in other taxons. Two biochemical subtypes, NADP-ME and NAD-ME, have been found in species having this leaf structure, each having differences in chloroplast ultrastructure (Laetsch, 1968; Kennedy and Laetsch, 1974; Carolin et al., 1975, 1978; Rathnam et al., 1976; Gamaley and Voznesenskaya, 1986; Voznesenskaya and Gamaley, 1986; Sage et al., 1999; Marshall et al., 2007; Muhaidat et al., 2007; Akhani et al., 2008). In the NADP-ME subtype, BS chloroplasts have reduced grana, as is typical for this subtype. The NADP-ME subtype is present in Acanthaceae, Aizoaceae, Amaranthaceae, Asteraceae, Boraginaceae, Cariophyllaceae, Chenopodiaceae, Euphorbiaceae, Nyctaginaceae, Portulacaceae and Zygophyllaceae. The opposite variant, NAD-ME type, which has well-developed grana in BS chloroplasts and reduced grana in M chloroplasts, is found in Acanthaceae, Aizoaceae, Amaranthaceae, Chenopodiaceae, Cleomaceae, Euphorbiaceae, Gisekiaceae, Molluginaceae and Portulacaceae. As a rule, Kranz BS have thickened cell walls, but the thickness varies; they are usually thinner in NAD-ME species. Organelles are usually arranged centripetally in BS cells, except for Trianthema triquetra (Aizoaceae), which has centrifugal positioning of organelles (Carolin et al., 1975).
2.1.3.2 Kochioid: Biochemical Subtypes NAD-ME and NADP-ME
Kochioid type species, also referred to as Semi-Wreath type, have laminate or semi-terete to terete succulent leaves with water-storage tissue underneath the chlorenchyma. The main vascular bundle is in the center, and the remaining vascular bundles are located in two paradermal planes on the leaf periphery or around the periphery in terete leaves. The chlorenchyma tissue is distributed along the peripheral veins; BS and M cells form arcs above the vascular bundles. Bundle sheath cells have relatively thick cell walls, and organelles are located in the centripetal position. Kadereit et al. (2003) recognized three different types of anatomy with such distribution of chlorenchyma tissues, differing in the presence or absence of hypoderm in two Kochia species, while in Kirilowia species, vascular bundles with arcs of chlorenchyma are distributed in the lateral plane only on the adaxial side of the leaf, with spongy parenchyma on the abaxial side. Species with this type of anatomy have been found to have NADP-ME type biochemistry and chloroplast ultrastructure (reduction in grana in BS cells is highly pronounced, up to having totally agranal chloroplasts) in genera Bassia and Kochia of family Chenopodiaceae (Gutierrez et al., 1974; Carolin et al., 1975; Gamaley, 1985; Voznesenskaya and Gamaley, 1986; Pyankov et al., 2000a; Jacobs, 2001), and NAD-ME type biochemistry in C4 species of the genus Zygophyllum with respective granal chloroplasts and numerous specialized mitochondria in BS cells (Crookston and Moss, 1972; Muhaidat et al., 2007). In the latter case, the leaf is cylindrical with the main vascular bundle in the center of water storage tissue. Two layers of Kranz tissue form arcs outside of the small peripheral veins.
2.1.3.3 Salsoloid: Biochemical Subtypes NAD-ME and NADP-ME
Species with Salsoloid type anatomy, also referred to as Kranz-Centrical type, have cylindrical or terete leaves (or stems in aphyllous species) with two concentric layers of chlorenchyma, typical of C4 Kranz anatomy, located around the periphery of assimilating organs. The central part is occupied by water storage tissue with the main vein in the middle. The net of secondary vascular bundles penetrates into the water storage tissue; and the small peripheral veins contacting with BS cells are facing toward the chlorenchyma by their xylem. In some desert species, a scleromorphous variant of this type has been found which has a high volume of sclerenchymatous tissue in the center around the main vein and/or in the peripheral bundles, with only a small amount of water storage tissue, for example, in reduced leaves of Nanophyton erinaceum, in the leaves and stems of Arthrophytum lehmannianum from family Chenopodiaceae, or in some Calligonum species of family Polygonaceae (see Butnik et al., 2001). Kadereit et al. (2003) distinguished five different types of anatomy within this type: Salsola type with or without hypoderm, Nanophyton type with sclerenchyma, Climacoptera type having no contact of peripheral veins with chlorenchyma and Halothamnus auriculus type with flattened leaves and several secondary veins distributed in the water storage parenchyma in lateral plane and the net of small peripheral veins adjacent to BS cells. Two biochemical subtypes, NADP-ME (in genera Salsola, Halothamnus, Haloxylon, Horaninovia and some others in family Chenopodiaceae) and NAD-ME (for example in genera Salsola, Climacoptera, Halocharis in Chenopodiaceae and Calligonum in Polygonaceae), with their respective ultrastructural chloroplast subtypes, have been found in species with this anatomy (Winter et al., 1977; Voznesenskaya and Gamaley, 1986; Pyankov and Vakhrusheva, 1989; Sage et al., 1999; Pyankov et al., 2000c; Muhaidat et al., 2007). Variants occur with or without hypodermal tissue, which, if present, plays the role of additional water storage tissue. Usually BS chloroplasts are in the centripetal position, but species of the genus Halothamnus (previously named Aellenia) have centrifugally-arranged chloroplasts, see Edwards et al. (2004).
2.1.3.4 Salsinoid: Biochemical Subtype NAD-ME
Species with Salsinoid type anatomy (also referred to as Kranz-Isopalisade Circular type) occur in genus Suaeda, section Salsina (Kadereit et al., 2003; Schütze et al., 2003). They have terete leaves with two concentric layers of chlorenchyma, palisade M and Kranz cells, around the leaf periphery, and water storage tissue in the center of the leaf. The vascular tissue forms a network in the lateral longitudinal plane; there are no peripheral vascular bundles and only the lateral veins may have contact with chlorenchyma. Only one biochemical subtype has been found, NAD-ME, and structural characteristics are typical for this subtype: numerous specialized mitochondria and chloroplasts with well-developed grana in BS cells, and reduced grana in M chloroplasts. Unlike other C4 subtypes, the Kranz cells have a large vacuole with less abundant organelles which occur in a centripetal position in a relatively thin layer of cytoplasm. This type was originally called Kranz-Suaedoid (Carolin et al., 1975; Jacobs, 2001). However, subsequently this form of Kranz was recognized as Salsina type after the section of Suaeda in which it occurs (Schütze et al., 2003), and is called Salsinoid here for consistency in nomenclature. For a description of the structural and functional features, see (Shomer-Ilan et al., 1975, 1979, 1981; Fisher et al., 1997; Voznesenskaya et al., 2007).
2.1.3.5 Schoberioid: Biochemical Subtype NAD-ME
This is another form of Kranz anatomy in genus Suaeda which recently was called Schoberia after the section in which it occurs (Kadereit et al., 2003; Schütze et al., 2003); it is called Schoberioid type here for consistency in nomenclature (also referred to as Kranz-Isopalisade type). Before more recent phylogenetic analyses of the Suaedoideae subfamily, it was referred to as Conospermoid type anatomy by Freitag and Stichler (2000). It is found in semi-terete leaves with positioning of vascular bundles in a lateral plane. This subtype is unique in having the vascular bundles enclosed by two layers of Kranz type chlorenchyma in the central part of the leaf, with continuous BS extensions between the veins. Large hypodermal cells, which are located between the chlorenchyma and epidermis, function as water storage tissue. These are NAD-ME type species with typical ultrastructural features for this subtype: BS cells have granal chloroplasts and specialized mitochondria, and M cells have reduced chloroplasts with less grana development. Unlike the Salsinoid type, Schoberioid type species have BS chloroplasts located in the centrifugal position. A variant of this type of anatomy has been found inSuaeda cochlearifolia, which has only one layer of BS cells between the vascular bundles (Voznesenskaya et al., 2007).
2.1.3.6 Kranz-Tecticornioid: Biochemical Subtype NAD-ME
This unique structural subtype of Kranz anatomy is found in the genus Halosarcia (H. indica, Salicornieae, family Chenopodiaceae) (Carolin et al., 1982; Jacobs, 2001), which is now included in the broadly circumscribed genus Tecticornia (Shepherd and Wilson, 2007). In general appearance, it is similar to the Kranz-Centrical (Salsoloid) type of leaf anatomy, with peripheral distribution of two chlorenchyma layers in cylindrical assimilating stems and with a net of small peripheral vascular bundles adjoining BS cells. However, in the Kranz-Tecticornioid type, these small veins are oriented with the phloem side facing towards the chlorenchyma. A striking feature of this type is the presence of bands of thick-walled colorless parenchyma cells between groups of chlorenchymatous M cells. Also, in the Kranz cells granal chloroplasts tend to be located centrifugally, or occasionally scattered around the periphery of the cell. Western blot analysis for C4 acid decarboxylases and immunolocalization studies indicate NAD-ME type C4 photosynthesis. There are numerous mitochondria in the Kranz cells, which compared to other NAD-ME species in the family are smaller, but they have a similar specialized structure (Voznesenskaya et al., 2008). Mesophyll chloroplasts have reduced grana, characteristic of this biochemical subtype.
2.1.3.7 Pilosoid: Biochemical Subtype NADP-ME
An interesting variant of Kranz anatomy occurs in species of the clade Pilosa, genus Portulaca, family Portulaceae (for example, in P. grandiflora, P. pilosa, P. villosa, P. sclerocarpa) which have terete cylindrical leaves with a circular arrangement of the small vascular bundles around the leaf periphery, with main vein and water storage cells in the center. Each peripheral vein is surrounded by BS cells (sometimes less developed on the inner side), with M cells forming a wreath-like structure only on the outer and lateral sides of the vascular bundles as illustrated in Nishioka et al. (1996) and Kim and Fisher (1990). Thus, the structure of the mesophyll-bundle sheath-vascular bundle complex of each vein is similar to one of the variants of Atriplicoid type anatomy, but differs in having a peripheral arrangement of veins around the leaf (Fig. 4). Flattened leaves of P. amilis have similar arrangement of VB but only about four layers of water storage tissue in the middle part of the leaf. The whole leaf anatomy can be considered to represent an intermediate stage of evolution from laminate Atriplicoid anatomy to Kochioid or directly to Salsoloid. NADP-ME type of biochemistry is well known for P. grandiflora (Gutierrez et al., 1974; Guralnick et al., 2002). It was recently also shown for two other species of this clade, P. pilosa and P. amilis (Voznesenskaya et al., 2010); and, all other studied species with this type of anatomy have similar ultrastructural features of BS and M chloroplastscharacteristic of this biochemical subtype. A similar distribution of vascular bundles was found in Zygophyllum simplex; but, with Kranz tissue forming open arcs typical for Kochioid type of anatomy, showing similar evolutionary trends in different families.
Illustration of other forms of Kranz anatomy among Dicotyledonae. Sketches of vascular bundles in Portulaca grandiflora for Pilosoid type, Portulaca cf. bicolor for Portulacelloid type, Cleome angustifolia cotyledon for Angustifolioid type, Glossocardia bosvallia and/or Cleome angustifolia leaf for Glossocardioid type, Isostigma simplicifolium for Simplicifolioid type, and Isostigmoid type (adapted from Fig. 2, Peter and Katinas, 2003). SP, spongy parenchyma tissue; for other abbreviations, see Fig. 1.
2.1.3.8 Portulacelloid: Biochemical Subtype NADP-ME
Species of the clade/section Portulacella, genus Portulaca (Portulacaceae) have vascular bundles surrounded by two concentric layers of Kranz anatomy distributed only on the adaxial side of the leaf; there are 4–5 layers of water storage tissue on the abaxial side (Voznesenskaya et al., 2010). As in Pilosoid type, palisade M cells are better developed on the upper and lateral sides of vascular bundles. It is NADP-ME biochemical type with centripetal position of grana-deficient chloroplasts in bundle sheath cells.
2.1.3.9 Glossocardioid: Biochemical Subtype NAD-ME and NADP-ME
Kranz anatomy similar to Salsoloid type was reported for representatives of family Asteraceae Glossocardia bosvallia (Das and Raghavendra, 1976) and some Isostigma species (Peter and Katinas, 2003), and also for Cleome angustifolia leaf (Cleomaceae); all these species have semi-terete to terete leaves with concentric layers of Kranz type chlorenchyma surrounding the leaf on the periphery. Leaf venation consists of the central main vein with or without lateral secondary veins embedded in the water storage parenchyma, with sclerenchyma tissue being either present around veins or absent depending on the species. Small peripheral bundles are in contact with BS cells with their xylem side, characteristic of Salsoloid type anatomy. The main difference from the classic Salsoloid anatomy is the absence of even distribution of small veins around the leaf periphery. The biochemical subtype of G. bosvallia based on western blot analysis is NADP-ME, while C. angustifolia is NAD-ME, the biochemical subtype for Isostigma peucedanifolium is NADP-ME based on deficiency of grana in BS chloroplasts (Voznesenskaya, Koteyeva and Edwards, unpublished). This type of structure was designated as Eryngiophyllum (= Chrysanthellum) in Peter and Katinas (2003); but it has only been observed in Isostigma and Glossocardia in family Asteraceae. Therefore, according to the genus where it was first described (Das and Raghavendra, 1976), we define this as Glossocardioid type anatomy.
2.1.3.10 Simplicifolioid: Biochemical Subtype NADP-ME
Isostigma simplicifolium (family Asteraceae) has a form of Kranz anatomy in which the chlorenchyma tissue forms a continuous layer around flattened leaves (Fig. 4). The major veins, which run in parallel with rare anastomoses, are enclosed in the well-developed sclerenchyma sheaths, with only very small bundles lacking sclerenchyma tissue. There is some water storage parenchyma between major veins. The biochemical subtype is NADP-ME based on deficiency of grana in BS chloroplasts (E. Voznesenskaya, N. Koteyeva and G. Edwards, unpublished).
2.1.3.11 Isostigmoid: Biochemical Subtype unknown
An unusual form of Kranz anatomy, called Isostigmoid, was reported for several species of the genus Isostigma in family Asteraceae having flattened leaves (Peter and Katinas, 2003). In Isostigmoid type, instead of Kranz anatomy encircling individual veins, as occurs in Atriplicoid, the two chlorenchyma layers surround several veins together (illustrated in Fig. 4). It is considered an intermediate form between Atriplicoid type and anatomical forms having a continuous ring of Kranz anatomy around the leaf. The biochemical subtype is unknown.
2.1.3.12 Angustifolioid: Biochemical Subtype NAD-ME
This form of Kranz anatomy occurs in cotyledons of the C4 species Cleome angustifolia (family Cleomaceae). Kranz tissue continuously surrounds the central part of the blade with vascular bundles distributed in the lateral longitudinal plane and with the main vein in the center of the leaf. Vascular bundles are separated by water storage parenchyma. Chlorenchyma layers, consisting of palisade M and BS cells, are located under the epiderm on the adaxial side; whereas, on the abaxial side the M cells adjacent to BS are small and rounded, and they are separated from the epiderm by two layers of spongy M cells (Fig. 4). Bundle sheath and M cells have ultrastructural features characteristic of NAD-ME biochemistry with granal BS chloroplasts and numerous mitochondria in BS cells; but, there is no difference in the granality between BS and M chloroplasts. This type of anatomy differs from all other known C4 types. The concentric layer of Kranz tissue around veins has some features of Salsinoid type, but this type is unusual in having spongy parenchyma on the abaxial side of the leaf. The biochemical subtype of this species is NAD-ME (Voznesenskaya, Koteyeva and Edwards, unpublished).
2.2 B. Biochemical Diversity: C4 Cycles and Energy Requirements for C4 Subtypes
2.2.1 1. Chloroplasts and Mitochondria
Despite C4 photosynthesis having evolved multiple times in different families and subfamilies with structural variations on Kranz anatomy, species belonging to each biochemical subtype tend to have features in common in terms of the structure of chloroplasts in M and BS cells, and the occurrence of mitochondria in BS cells. In NADP-ME type C4 species, BS chloroplasts are deficient in grana compared to M chloroplasts, whereas in NAD-ME type species, the M chloroplasts tend to be more deficient in grana development (Gamaley, 1985; Voznesenskaya and Gamaley, 1986; Fisher et al., 1997; Voznesenskaya et al., 1999). In PEP-CK type C4 species, the granal development is similar for M and BS chloroplasts (Yoshimura et al., 2004; Voznesenskaya et al., 2006). This has been quantified in a number of studies on different photosynthetic types by determining the granal index (the length of all appressed thylakoid membranes as a percentage of the total length of all thylakoid membranes in a chloroplast). Early studies indicate that the degree of grana development in BS chloroplasts correlates to the capacity for photosystem II (PSII) activity, linear electron flow and capacity for generation of NADPH, with low grana-containing chloroplasts being richer in photosystem I (PSI)-mediated cyclic electron flow producing ATP (Edwards and Walker, 1983; Anderson, 1999). Thus, differences in grana development are associated with differences between M and BS cells in the need for NADPH relative to ATP to support C4 photosynthesis.
Mitochondria are most abundant, and often larger, in BS cells of NAD-ME type species (where the decarboxylase is located in mitochondria), and least abundant in BS cells of NADP-ME type species (where the decarboxylase is located in chloroplasts). Bundle sheath mitochondria in PEP-CK species also function to provide ATP to support decarboxylation via PEP-CK in the cytosol (Burnell and Hatch, 1988), and they are generally intermediate in size and number compared to NAD-ME or NADP-ME type species (Yoshimura et al., 2004; Voznesenskaya et al., 2006).
The basal energy required per CO2 assimilation in C4 photosynthesis is the sum of energy to support the C4 and C3 cycles. Analyses to date on biochemical subtyping (by Western blots, enzyme assays) indicate each species has a major form of delivery of CO2 to Rubisco through one type of C4 acid decarboxylase. For each biochemical subtype, the amount of energy required to support the C4 cycle for delivery of CO2 and the C3 cycle for fixation of CO2 can be calculated. This is shown in Fig. 5, with illustrations of how the provision of energy can be met cooperatively by M and BS chloroplasts. This demonstrates how the photochemical demands for energy can be shared between the cell types, and the differences between the three types of C4 cycles. However, the exact photochemical demands for energy within each subgroup may vary between species. This is most evident in NADP-ME type C4 species, as discussed below.
Illustrations of the three types of C4 cycles and their bioenergetics: a, NADP-ME, b, NAD-ME and c, PEP-CK subtypes. αkg, α-ketoglutarate; Ala, alanine; Asp, aspartate; Atm, atmospheric; Glu, glutamate; Mal, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; PEPC, PEP carboxylase; PGA, 3-phosphoglyceric acid; Pi, inorganic phosphate; pyr, pyruvate; RuBP, ribulose 1,5-bisphosphate; triose-P, triose phosphate. Panels a and b, adapted from Voznesenskaya et al., 1999, Oxford University Press.
2.2.2 2. Illustration of Energetics for NADP-ME Type Species
Summary
-
1.
5 ATP, 2 NADPH required per CO2 assimilated (2 ATP for the C4 cycle, 3 ATP, 2 NADPH for the C3 cycle).
-
2.
The C4 cycle delivers primarily malate to BS cells (NADP-ME species are mainly malate formers).
-
3.
In NADP-ME type C4 species, BS chloroplasts have fewer grana than do M chloroplasts, but there is variation in the degree of deficiency of grana in the BS chloroplasts. The extremes range from the BS chloroplasts being agranal (in sugarcane, sorghum), to BS chloroplasts having granal indices about half that of M chloroplasts, observed in members of family Chenopodiaceae (see Voznesenskaya et al., 1999). The illustration in Fig. 5a is based on the granal index and linear electron flow to generate NADPH being two fold higher in M than in BS chloroplasts, as observed in some NADP-ME type chenopods (Voznesenskaya et al., 1999). The two fold higher use of reductive power in BS cells could vary due to the amount of 3-phosphoglyceric acid (PGA) shuttled from BS to M chloroplasts for reduction (in the current scheme, one sixth of the PGA). Alternatively, this balance in reductive power could be modified in NADP-ME dicots by a partial shuttle of aspartate from M to BS cells through NADP-ME, see Moore et al. (1984) and Meister et al. (1996). Also, the degree of grana development in BS chloroplasts of NADP-ME type species appears to correlate with the development of a secondary aspartate PEP-CK shuttle (Gutierrez et al., 1974; Wingler et al., 1999; Voznesenskaya et al., 2006).
The deficiency in PSII in BS chloroplasts in this subtype is thought to reduce production of O2 in BS cells and help maintain a high CO2/O2 ratio, which is favorable for limiting ribulose 1,5-bisphosphate (RuBP) oxygenase activity and photorespiration. This results in an increased need for photochemically-produced NADPH in M chloroplasts, see (Edwards and Walker, 1983).
2.2.3 3. Illustration of Energetics for NAD-ME Type Species
Summary
-
1.
5 ATP, 2 NADPH required per CO2 assimilated (2 ATP for the C4 cycle, 3 ATP, 2 NADPH for the C3 cycle).
-
2.
The C4 cycle delivers primarily aspartate to BS cells (NAD-ME species are primarily aspartate formers). The aspartate cycle requires only production of ATP (but not reductive power) to drive PEP regeneration from alanine by the M chloroplasts (Edwards and Walker, 1983; Voznesenskaya et al., 1999). This ATP may be provided via PSI cyclic electron flow in the M chloroplasts. Extensive studies of NAD-ME-type species in family Chenopodiaceae have shown that M chloroplasts are deficient in grana compared to BS chloroplasts (Gamaley, 1985; Gamaley and Voznesenskaya, 1986; Voznesenskaya and Gamaley, 1986; Glagoleva et al., 1991).
-
3.
In the illustration of Fig. 5b, the use and generation of reductive power in BS chloroplasts is twofold higher than in M chloroplasts. This occurs with the granal index and linear electron flow to generate NADPH being two fold higher in BS than M chloroplasts, as observed in some NAD-ME type chenopods (Voznesenskaya et al., 1999). Again, this partitioning of reductive power can be regulated by the amount of PGA shuttled from BS to M chloroplasts for reduction to triose-P (in the current scheme, one third of the PGA is shuttled to M cells).
2.2.4 4. Illustration of Energetics for PEP-CK Type Species
Summary
-
1.
3.6 ATP, 2.3 NADPH required per CO2 assimilated (0.6 ATP and 0.3 NADPH per CO2 for the C4 cycles; 3 ATP, 2 NADPH for the C3 cycle). The PEP-CK type requires less ATP, but more NADPH per CO2 fixed than the malic enzyme type species.
-
2.
There are two C4 cycles, one shuttling aspartate, the other shuttling malate (Fig. 5c). Aspartate is utilized in the cytosol to generate oxaloacetate for the PEP-CK reaction. Malate is used by the mitochondria via NAD-ME, which generates CO2 and NADH; the NADH is then utilized by the mitochondria to generate ATP to support the PEP-CK decarboxylase reaction (Burnell and Hatch, 1988; Walker and Chen, 2002; Voznesenskaya et al., 2006). In this scheme, the aspartate cycle generates about 70%, and the malate cycle about 30%, of the total CO2 delivered to the BS cells.
-
3.
In this illustration (Fig. 5c), an equal amount of reductive power is required by M and BS cells. This is consistent with M and BS chloroplasts of PEP-CK species having a similar granal index, suggestive of equivalent capacity for PSII activity for generating NADPH (Voznesenskaya et al., 2006). Again, this partitioning of reductive power can to some extent be regulated by the amount of PGA shuttled from BS to M chloroplasts for reduction (in the scheme, about 40%).
2.2.5 5. Additional Energy Requirements in C4 Photosynthesis
Besides the basic requirements for energy to support the C3 and C4 cycles, additional energy will be consumed by over-cycling of the C4 cycle (CO2 leakage from BS cells, that is 2 ATP per CO2 lost from BS cells), and due to the occurrence of limited photorespiration because of some O2 reacting with RuBP, see Kanai and Edwards (1999). The cost of photorespiration is illustrated in Fig. 6. The requirement for reductive power per O2 reacting with RuBP (eq. to 2 NADPH) is the same as for CO2 reacting with RuBP, and the scheme illustrates how this can be shared equally between M and BS chloroplasts.
3 III. Single-Cell C4 Photosynthesis in Terrestrial Plants
For decades following the discovery of C4 photosynthesis in the 1960s, it was considered that the requirements for C4, as summarized in the Introduction, could only be met in terrestrial plants by the presence of Kranz type anatomy. Thus, it was surprising to find species in family Chenopodiaceae that undergo traditional C4 photosynthesis, but have a unique anatomy that does not consist of the Kranz dual-cell system. Instead, the single-cell C4 system functions in individual chlorenchyma cells by means of intracellular biochemical and organelle compartmentation. Two very novel means of accomplishing this evolved in subfamily Suaedoideae. These systems function by spatial development of two cytoplasmic domains in chlorenchyma cells, which contain dimorphic chloroplasts. The arrangement of M and BS cells that so long has defined terrestrial forms of C4 plants has now been joined by single-cell C4 systems as functional anatomical alternatives (Voznesenskaya et al., 2001, 2002; Sage, 2002; Edwards et al., 2004; Akhani et al., 2005; Park et al., 2010).
3.1 A. Occurrence (Family and Phylogeny)
In family Chenopodiaceae, which has C3 and C4 species, all C4 genera except for subfamily Chenopodioideae (including genus Atriplex) occur in a succulent clade made up of subfamilies Salicornioideae/Suaedoideae/Salsoloideae. The single-cell C4 type species occur in subfamily Suaedoideae (Fig. 7), in two species in genus Bienertia (B. cycloptera Bunge ex Boiss., B. sinuspersici Akhani sp. nov.) and in one species in genus Suaeda, S. aralocaspica (Bunge) Freitag & Schütze (Kadereit et al., 2003; Schütze et al., 2003; Akhani et al., 2005; Kapralov et al., 2006). Suaeda aralocaspica, originally named Borszczowia aralocaspica Bunge, was subsequently classified in the monotypic Suaeda section Borszczowia, with a leaf type called Borszczowoid (Freitag and Stichler, 2000; Schütze et al., 2003; Kapralov et al., 2006).
Phylogenetic position of “single-cell” C4 in Chenopodiaceae. The single maximum likelihood phylogram based on combined complete sequence information on nuclear ITS and five chloroplast DNA regions. Numbers above branches refer to bootstrap percentages and those below branches refer to Bayesian inference posterior probabilities (adapted from Kapralov et al., 2006).
There are four independent origins of C4 photosynthesis in subfamily Suaedoideae: two parallel origins of Kranz C4 anatomy (Salsinoid and Schoberioid in genus Suaeda) (see Section II A 3), and two independent origins of single-cell C4. The single-cell C4 plant Suaeda aralocaspica is in tribe Suaedeae. In this tribe, the veins are invariably located in one plane, with the primary vein in the center of the leaf and all deviating bundles in lateral positions. However, unlike other Suaeda species, S. aralocaspica has a primary vein in the center with peripheral veins adjacent to the chlorenchyma tissue (Freitag and Stichler, 2000; Schütze et al., 2003). This species is positioned between the C3 section Schanginia and a C3 shrubby section Suaeda, which suggests this type of photosynthesis evolved from C3 ancestors rather than from a C4 ancestor with Kranz anatomy (Kapralov et al., 2006). The two Bienertia species occur in an isolated tribe, Bienertieae, with a leaf type called Bienertioid, and the species have no known close relatives. We will use the common names Bienertia and Borszczowia to refer to these two types of single-cell C4 taxa which are classified as C4 structural forms called Bienertioid and Borszczowoid, respectively.
3.2 B. Biogeography of Single-Cell C4 Species
The single-cell C4 species grow in desert conditions. Borszczowia grows in central Asia from northeast of the Caspian lowlands east to Mongolia and western China (Fig. 8). It is a hygro-halophyte that grows in temperate salt deserts with low night temperatures. The habitat consists of a high water table in salt marshes, which can support continuous leaf development and growth (Freitag and Stichler, 2000; Boyd et al., 2007).
Map showing the wide-spread distribution of single-cell C4 species, Suaeda (=Borszczowia) aralocaspica, Bienertia cycloptera, and B. sinuspersici. The lines on the figure show the range for each species. Further research is needed to determine whether there is more diversity among these populations (subtypes or new species).
Bienertia cycloptera grows from east Anatolia eastward to Turkmenistan and Pakistani Baluchestan. Its leaves are very succulent and sensitive to extended drought, which can cause wilting and leaf drop. It grows on dryer soils and is confronted with drought stress during the summer (Akhani et al., 2003).
Bienertia sinuspersici occurs in hot climates, and at lower latitudes and elevations than B. cycloptera. It is in a natural biogeographic range occurring from the westernmost coasts of Pakistan and extending westward along the coastal areas in southern Iran and countries surrounding the Persian Gulf. It shows an arc-like, latitudinal range that is separated from the range of B. cycloptera populations by the Zagros and Makran Mountains (Fig. 8). Bienertia sinuspersici differs anatomically by having mostly one to two layers of chlorenchyma cells, versus two to three layers in B. cycloptera. Furthermore, B. sinuspersici is distinguished from B. cycloptera in having longer cotyledon leaves and leaves proper, larger seeds, larger flowers, and larger chromosomes, together with a set of micro-morphological features (Akhani et al., 2005).
3.3 C. Overview of Two Types of Single-CellC4 Photosynthesis in Terrestrial Plants
Two means of partitioning the function of C4 photosynthesis between two cytoplasmic compartments evolved in family Chenopodiaceae. Borszczowia produces elongated palisade chlorenchyma cells with dimorphic chloroplasts polarized towards opposite ends of the cell. This is somewhat analogous to having Kranz anatomy, with the M and BS arrangement, without the intervening cell walls. Surprisingly, a completely different solution to performing C4 photosynthesis in a single cell is found in Bienertia. The chlorenchyma cells of the two Bienertia species have a peripheral, chloroplast-containing, thin layer of cytoplasm (peripheral compartment) and a very unusual chloroplast-containing central cytoplasmic compartment, which are proposed to function like M and BS cells, respectively, in Kranz type C4. In both systems, the partitioning of biochemically distinct organelles into discrete compartments is considered to result in concentration of CO2 around the Rubisco-containing chloroplasts, causing inhibition of Rubisco oxygenase activity and photorespiration, as occurs in the typical Kranz system. Models of how these systems operate C4 photosynthesis have been proposed (Fig. 9, Edwards et al., 2004).
General models of proposed function of C4 photosynthesis in the two types of single-cell C4 systems. The left panel for Bienertia illustrates atmospheric CO2 entering the peripheral cytoplasm where it is fixed by PEPC, and a scheme which shows the path of carbon through the NAD-ME type C4 cycle. The C4 cycle delivers CO2 to Rubisco (this immunogold-treated section for Rubisco shows label appearing as light deposits in the central cytoplasmic compartment). The right panel for Borszczowia illustrates atmospheric CO2 entering the proximal end of the cell where it is fixed by PEPC; CO2 is donated to Rubisco in the proximal end of the cell (immunolabeling for Rubisco shows light deposits in chloroplasts in the proximal end) via an NAD-ME C4 cycle (as in Bienertia). PC, peripheral chloroplast; CCC, central cytoplasmic compartment; Channel, cytoplasmic channel connecting the PC and CCC; PPDK, pyruvate, Pi dikinase; PEPC, PEP carboxylase; NAD-ME, NAD-malic enzyme.
3.4 D. Biochemical Evidence for Function of C4 Photosynthesis in Single-Cell C4 Plants
3.4.1 1. General Features Characteristic of C4
3.4.1.1 Western Blots and Analysis of C4 Enzymes
Analyses of photosynthetic enzymes by Western blots and enzyme assays show that the single-cell C4 species have high levels of C4 cycle enzymes PEPC and PPDK, similar to Kranz type Suaeda, and in contrast to very low levels in the C3 type Suaeda species (Fig. 10, also see Voznesenskaya et al., 2002). Assays for C4 acid decarboxylases show that these single-cell C4 species are NAD-ME type (Fig. 10), as are all Kranz type C4 species which have been examined in subfamily Suaedoideae, see Voznesenskaya et al., 2007. Also, the single-cell C4 species have a C4 type PEPC similar to that in Kranz type species in subfamily Suaedoideae (Lara et al., 2006). This includes having high specific activities, a serine residue near the amino-terminus which undergoes phosphorylation/dephosphorylation, and light/dark regulation by phosphorylation with differential sensitivity to malate (Lara et al., 2006).
Western blots of Rubisco and three C4 cycle enzymes, PPDK, PEPC, and NAD-ME in the single-cell C4 species Suaeda (=Borszczowia) aralocaspica, Bienertia cycloptera and B. sinuspersici, Kranz type S. eltonica and the C3 S. heterophylla (see Chuong et al., 2006). Copyright American Society Plant Biologists, www.plantcell.org
3.4.1.2 C4 Type Carbon Isotope Composition
Reports on carbon isotope values in Bienertia (Winter, 1981; Akhani et al., 1997, 2005; Freitag and Stichler, 2002; Voznesenskaya et al., 2002) and Borszczowia (Freitag and Stichler, 2000; Voznesenskaya et al., 2001) indicated that they have C4/CAM (Crassulacean acid metabolism) type carbon isotope composition. Although succulent, subsequent studies showed no evidence for performance of CAM (Voznesenskaya et al., 2001, 2002, 2003). Various collections of the two Bienertia species and Borszczowia, from natural habitats and from plants grown under controlled conditions in high light, show that they have C4 type carbon isotope composition. Analysis of the carbon isotope composition during a growing season in Iran showed B. cycloptera performs C4 photosynthesis during its life cycle in nature similar to Kranz type C4 species (Akhani et al., 2009). In Bienertia species, more negative values (−16% to −19%) have been observed in young leaves and during growth under low light (100–200 photosynthetic photon flux density) (Freitag and Stichler, 2002; Voznesenskaya et al., 2002, 2005c). In young leaves, C4 type chlorenchyma have not fully developed (Voznesenskaya et al., 2005c); growth under low light may limit the developmental transition from C3 to a fully functional C4 system (possibly due to incomplete development of dimorphic chloroplasts, or ability to concentrate CO2 around Rubisco).
3.4.1.3 Physiological Response
The single-cell C4 species and the Kranz type Suaeda species have low sensitivity of photosynthesis to O2 under atmospheric levels of CO2, and low CO2 compensation points, typical of C4 plants (Voznesenskaya et al., 2001, 2002, 2007; Edwards et al., 2007). Also, the water use efficiency (μmol CO2 mmol–1 water) is about two fold higher in the single-cell C4 species and the Kranz type C4 species than in representative C3 species in subfamily Suaedoideae (Edwards et al., 2007).
3.4.1.4 Resistance to CO2 Loss
For C4 photosynthesis to function, high efficiency in trapping of the CO2 generated by the C4 pump, and in refixation of photorespired CO2, are required. This means that the diffusive resistance in C4 plants for CO2 from Rubisco to the intercellular air space must be substantially higher than that in C3 plants. Analyses of the diffusive resistance in the single-cell C4 species show it is about 50-fold higher than that in C3 species, and that it is of the same order of magnitude of Kranz type C4 plants (Edwards et al., 2007).
3.4.2 2. Spatial Compartmentation Enabling Function of NAD-ME Type C4 Photosynthesis
Critical experiments showing how C4 photosynthesis functions in these single-cell C4 species were performed by studying the structural organization by microscopy, by immunolocalization of several photosynthetic enzymes, and by localization of starch. In each single-cell C4 system, the mechanism of photosynthesis and spatial compartmentation of function are analogous to those of NAD-ME type Kranz species (Fig. 9, Voznesenskaya et al., 2001, 2002; Edwards et al., 2004). In the Bienertia species, the peripheral cytoplasm functions analogous to Kranz type C4 M cells in fixation of atmospheric CO2 into C4 acids, while the central cytoplasmic compartment functions analogous to BS cells in donation of CO2 from C4 acids to Rubisco (Voznesenskaya et al., 2001, 2002; Edwards et al., 2004). As illustrated in the model in Fig. 9, CO2 is fixed by PEPC in the peripheral cytoplasm leading to formation of C4 acids (aspartate and malate) and their transport via cytoplasmic channels to the central compartment and decarboxylation in mitochondria via NAD-ME, with CO2 donation to Rubisco. C3 acids formed from decarboxylation are transported to the peripheral compartment and used for regeneration of PEP from pyruvate in the peripheral chloroplasts. In Borszczowia, the carboxylation phase of the C4 pathway, with fixation of atmospheric CO2, functions in the distal part of the cell (analogous to M cells in Kranz type), while donation of CO2 to Rubisco from decarboxylation of C4 acids occurs in the proximal part of the cell (analogous to BS cells in the Kranz type NAD-ME species).
3.4.2.1 Dimorphic Chloroplasts (Structure, Enzymes and Starch)
In each structural type of single-cell C4, C4 photosynthesis is accomplished in part by the partitioning of two biochemically and ultrastructurally distinct chloroplast types into separate compartments within the cell (Voznesenskaya et al., 2001, 2002, 2005c). These chloroplasts are dimorphic in biochemistry of photosynthesis, in ability to store starch, and in ultrastructure. In addition to immunolocalization studies by confocal microscopy, immunolocalization in Bienertia and Borszczowia by transmission electron microscopy shows rather strong selective labeling of PPDK in one chloroplast type, and Rubisco in the other chloroplast type (E. Voznesenskaya, N. Koteyeva, G. Edwards, unpublished results). The outer chloroplasts supporting the carboxylation phase of the C4 cycle to fix atmospheric CO2 have PPDK, which generates the substrate for PEPC by converting pyruvate to PEP, they store little or no starch, and they have a deficiency in grana development. The inner chloroplasts, which fix CO2 generated from decarboxylation of C4 acids, have Rubisco, they store starch (and have ADPG pyrophosphorylase, the first committed step for starch biosynthesis), and they have well-developed grana. These features are the same as the respective M and BS chloroplasts in related Kranz type NAD-ME species. In C4 plants, BS chloroplasts usually store larger amounts of starch than M chloroplasts. In NAD-ME type C4 species, the grana-deficient chloroplasts are thought to be associated with a lower requirement for reductive power to support the C4 carboxylation phase in this subgroup (see Section B2).
3.4.2.2 Mitochondria and Peroxisomes
In the single-cell C4 species, the mitochondria are partitioned to the cytoplasmic compartment, where the Rubisco-containing chloroplasts are located (the proximal end of the cell in Borszczowia and in the central cytoplasmic compartment in Bienertia species). The mitochondria perform two important functions relative to C4 photosynthesis: generation of CO2 by decarboxylation of C4 acids via NAD-ME and decarboxylation of glycine as a result of any photorespiration. Also, peroxisomes are predominantly located in the cytoplasmic compartment with Rubisco-containing chloroplasts (based on transmission electron microscopy and immunolocalization of catalase), which are presumably associated with metabolism of glycolate to glycerate in the glycolate pathway (Voznesenskaya et al., 2001, 2002; Chuong et al., 2006). While the C4 cycle concentrates CO2 around Rubisco and suppresses photorespiration, some photorespiration does occur. The selective localization of glycine decarboxylase in BS mitochondria makes photorespired CO2 available for refixation by Rubisco.
3.5 E. Development of Spatial Compartmentation and Dimorphic Chloroplasts
An intriguing aspect of single-cell C4 photosynthesis is the development of spatial compartmentation of functions and dimorphic chloroplasts. There is evidence that very young chlorenchyma cells have a single type of chloroplast (monomorphic) which is in a C3 default mode, with all chloroplasts containing low levels of Rubisco without PPDK, and without spatial separation of organelles into two compartments (Voznesenskaya et al., 2005c). Since enzymes of the C4 cycle like PPDK and the small subunit of Rubisco are nuclear encoded, there must be posttranscriptional regulation for selective expression of certain proteins in chloroplasts (see chapter 12 by Berry et al. for selective expression in Kranz type C4). In mature chlorenchyma cells that have formed two cytoplasmic compartments and dimorphic chloroplasts, there is intricate development of the cytoskeleton, which consists of actin and microtubules. Cytoskeleton-disrupting drugs show that microtubules are important in maintaining the two cytoplasmic compartments (Chuong et al., 2006).
3.6 F. Form of Photosynthesis in Different Photosynthetic Organs in Single-Cell C4 Species
Plants are usually characterized by photosynthetic type according to the mechanism of carbon assimilation in the leaf, which is generally the main photosynthetic organ. Among chenopods having C4 photosynthesis in leaves, there is variation between species as to the type of photosynthesis in cotyledons (Butnik, 1979, 1984, 1991; Pyankov et al., 1999, 2000b, c; Voznesenskaya et al., 1999, 2004; Akhani and Ghasemkhani, 2007). There are C4 chenopods, for example Salsola richteri (Salsoloid type), which have the same type of Kranz anatomy in leaves and cotyledons. There are also C4 chenopods having C4 photosynthesis in leaves and cotyledons, but different types of Kranz anatomy, for example Salsola laricina, which has Salsoloid anatomy in leaves and Atriplicoid type anatomy in cotyledons. Finally, there are dicots which have C4 photosynthesis in leaves, but C3 type anatomy and photosynthesis in cotyledons; for example Salsola gemmascens has Salsoloid type anatomy in leaves and C3 type anatomy in cotyledons (Pyankov et al., 2000c).
Not all C4 plants have leaves as the primary photosynthetic organ during vegetative growth. For example, in family Chenopodiaceae, C4 species of Anabasis, Haloxylon, Halosarcia, Hammada and some species of Halothamnus have reduced leaves with stems as the primary photosynthetic organ (Ocallaghan, 1992; Akhani et al., 1997; Pyankov et al., 2000b).
With respect to single-cell C4 species, Borszczowia and both Bienertia species have green cotyledons, leaves, and flowers. Studies of the respective organs, including structure of chlorenchyma, immunolocalization of photosynthetic enzymes (Rubisco and PEPC), and Western blots of Rubisco and C4 cycle enzymes, show they all have unique chlorenchyma cells which are structurally and biochemically developed to perform single-celled C4 photosynthesis (Voznesenskaya et al., 2001, 2002, 2004; Edwards et al., 2004; Akhani et al., 2005; Boyd et al., 2007). Thus, these species perform single-cell C4 photosynthesis throughout their life cycle. Leaves make up the majority of the green tissue during vegetative growth of these species, although younger branches have green stems. However, the flowers have green tepals which become a major photosynthetic organ during the latter reproductive phase of growth. Photosynthesis in flowers may have adaptive value for survival of these desert plants. In Bienertia, under increasing temperatures as flowers develop, lower leaves wither and senesce and are replaced by smaller floral leaves consisting of green tepals (Boyd et al., 2007). Also, the stems of B. cycloptera have single-cell C4 type chlorenchyma cells beneath the epidermis. This, combined with the presence of stomata on the stems, starch in stem chlorenchyma, and light-dependent fixation of atmospheric CO2 by the stems, suggests they contribute to carbon assimilation in Bienertia. In contrast, the stems of Borszczowia have C3 type chlorenchyma cells scattered throughout the cortical tissue. They likely function to refix respired CO2 in stems, since light dependent fixation of external CO2 is only observed under high atmospheric levels (Boyd et al., 2007).
3.7 G. How Did Single-Cell C4 Evolve?
In considering how single-cell C4 plants might have evolved, an analogy can be made to the proposed evolutionary development of Kranz type C4 plants from C3 plants (see Chapter 6 by Bauwe). In C3 plants, M cells are the main photosynthetic tissue in the leaf, since the BS cells have few, or no, chloroplasts. It has been suggested that evolution of C4 has occurred multiple times by a stepwise progression of structural and biochemical changes which were induced by CO2-limiting conditions (Monson et al., 1984; Edwards and Ku, 1987; Monson and Moore, 1989; Rawsthorne and Bauwe, 1998; Sage, 2004). The occurrence of intermediates between C3 and C4 plants, particularly in the genus Flaveria (Asteraceae), has provided a basis for suggesting how C4 may have evolved, from C3, to intermediates which reduce photorespiration without a C4 cycle, to intermediates having a partially-functioning C4 cycle, to full development of C4. In the first type of intermediate, normal C3 type photosynthesis occurs in M cells; however, the release of CO2 in photorespiration occurs in mitochondria in BS cells (by selective localization of glycine decarboxylase in BS mitochondria), where a degree of refixation by chloroplasts occurs. This increases the efficiency of photosynthesis under limiting CO2 (von Caemmerer, 1989).
By analogy, under CO2-limiting conditions a single-cell C3-C4 intermediate species may develop by spatial separation of the fixation of atmospheric CO2 by the C3 cycle and the refixation of photorespired CO2 (see illustration in Fig. 11). This would generate a form of CO2 concentrating mechanism and reduce loss of CO2 by photorespiration. It could provide the initial spatial separation with chloroplasts in one cytoplasmic compartment fixing atmospheric CO2, and chloroplasts and mitochondria in another cytoplasmic compartment functioning to minimize CO2 loss by photorespiration. The spatial separation of chloroplasts and mitochondria in this hypothetical C3-C4 intermediate is like that in the single-cell C4 plants. This would provide the initial spatial separation of organelles, with subsequent differentiation of chloroplasts and expression of C4 cycle enzymes leading to development of a functional C4 system.
4 IV. Future Perspectives
The discovery of C4 plants among terrestrial species, approximately 40 years ago, and their association with Kranz anatomy, has led to numerous studies on the occurrence, photosynthetic mechanism, structural and biochemical diversity, molecular control of development of two photosynthetic cells, and evolution (Edwards and Walker, 1983; Hatch, 1987; Sage and Monson, 1999). With the paradigm that this occurred in land plants via development of Kranz anatomy, the finding that terrestrial species can conduct C4 photosynthesis within individual chlorenchyma cells provides a very different system to study C4. This includes stages in evolution of C4, the genetic control of development of the requisite spatial separation of functions, the mechanism of chloroplast differentiation, biochemical and biophysical requirements for the function of C4 photosynthesis, and development of strategies for engineering C4 photosynthesis into selected C3 crops.
Abbreviations
- BS:
-
Bundle sheath(s);
- Kranz cells:
-
An inner layer of chlorenchyma cells specialized for C4 photosynthesis, irrespective of whether there is contact with vascular bundles (sometimes referred to as BS cells in C4 plants);
- M:
-
Mesophyll;
- MS:
-
Mestome sheath(s);
- NAD-ME:
-
NAD-malic enzyme;
- NADP-ME:
-
NADP-malic enzyme;
- PEPC:
-
Phosphoenolpyruvate carboxylase;
- PEP-CK:
-
Phosphoenolpyruvate carboxykinase;
- PGA:
-
3-Phosphoglyceric acid;
- PPDK:
-
Pyruvate,Pi dikinase;
- PSI:
-
Photosystem I;
- PSII:
-
Photosystem II;
- RuBP:
-
Ribulose 1,5-bisphosphate;
- SL:
-
Suberin lamella
References
Akhani H and Ghasemkhani M (2007) Diversity of photosynthetic organs in Chenopodiaceae from Golestan National Park (NE Iran) based on carbon isotope composition and anatomy of leaves and cotyledons. Nova Hedwigia Suppl 131: 265–277
Akhani H, Trimborn P and Ziegler H (1997) Photosynthetic pathways in Chenopodiaceae from Africa, Asia and Europe with their ecological, phytogeographical and taxonomical importance. Plant Syst Evol 206: 187–221
Akhani H, Ghobadnejhad M and Hashemi SMH (2003) Ecology, biogeography and pollen morphology of Bienertia cycloptera Bunge ex Boiss. (Chenopodiaceae), an enigmatic C4 plant without Kranz anatomy. Plant Biol 5: 167–178
Akhani H, Barroca J, Koteeva N, Voznesenskaya E, Franceschi V, Edwards G, Ghaffari SM and Ziegler H (2005) Bienertia sinuspersici (Chenopodiaceae): a new species from Southwest Asia and discovery of a third terrestrial C4 plant without Kranz anatomy. Syst Bot 30: 290–301
Akhani H, Ghasemkhani M, Chuong SDX and Edwards GE (2008) Occurrence and forms of Kranz anatomy and characterization of NAD-ME subtype C4 photosynthesis in Blepharis ciliaris (L) B.L. Burtt (Acanthaceae). J Exp Bot 59: 1755–1765
Akhani H, Lara MV, Ghasemkhani M, Ziegler H and Edwards GE (2009) Does Bienertia cycloptera with the single-cell system of C4 photosynthesis exhibit a seasonal pattern of δ13C values in nature similar to co-existing C4 Chenopodiaceae having the dual-cell (Kranz) system? Photosyn Res 99: 23–36
Aliscioni SS, Giussani LM, Zuloaga FO and Kellogg EA (2003) A molecular phylogeny of Panicum (Poaceae: Paniceae): Tests of monophyly and phylogenetic placement within the Panicoideae. Am J Bot 90: 796–821
Anderson JM (1999) Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: a personal perspective. Aust J Plant Physiol 26: 625–639
Bisalputra T, Downton WJS and Tregunna EB (1969) The distribution and ultrastructure of chloroplasts in leaves differing in photosynthetic carbon metabolism. I. Wheat, Sorghum and Aristida (Gramineae). Can J Bot 47: 15–21
Boyd CN, Franceschi VR, Chuong SDX, Akhani H, Kiirats O, Smith M and Edwards GE (2007) Flowers of Bienertia cycloptera and Suaeda aralocaspica (Chenopodiaceae) complete the life cycle performing single-cell C4 photosynthesis. Funct Plant Biol 34: 268–281
Brown WV (1958) Leaf anatomy in grass systematics. Bot Gaz 119: 170–178
Brown WV (1975) Variations in anatomy, associations, and origin of Kranz tissue. Am J Bot 62: 395–402
Brown WV (1977) The Kranz syndrome and its subtypes in grass systematics. Mem Torrey Bot Club 23: 1–97
Bruhl JJ and Perry S (1995) Photosynthetic pathway-related ultrastructure of C3, C4 and C3-like C3-C4 intermediate sedges (Cyperaceae), with special reference to Eleocharis. Aust J Plant Physiol 22: 521–530
Bruhl JJ, Stone NE and Hattersley PW (1987) C4 acid decarboxylation enzymes and anatomy in sedges (Cyperaceae): first record of NAD-malic enzyme species. Aust J Plant Physiol 14: 719–728
Burnell JN and Hatch MD (1988) Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 plants: pathways of C4 acid decarboxylation in bundle sheath cells of Urochloa panicoides. Arch Biochem Biophys 260: 187–199
Butnik AA (1979) Types of seedling development of Chenopodiaceae Vent. Bot Zh 64: 834–842 (In Russian)
Butnik AA (1984) The adaptation of anatomical structure of the family Chenopodiaceae Vent. species to arid conditions. Summary of biological science doctor degree thesis. Academy of Sciences of Uzbek SSR, Tashkent (In Russian)
Butnik AA (1991) Family Chenopodiaceae. In: AL Tachtadjan (ed) Comparative seed anatomy. Dicotyledonous. Caryophyllidae-Dilleniidae, pp 77–82 (In Russian). Nauka, Leningrad
Butnik AA, Ashurmetov OA, Nigmatova RN and Paizieva SA (2001) Ecological anatomy of desert plants of Middle Asia. V. 2. Subshrubs, Subshrublets FAN, Tashkent (In Russian)
Carolin RC, Jacobs SWL and Vesk M (1973) The structure of the cells of the mesophyll and parenchymatous bundle sheath of the Gramineae. Bot J Linn Soc 66: 259–275
Carolin RC, Jacobs SWL and Vesk M (1975) Leaf structure in Chenopodiaceae. Bot Jahrbuch Syst Pflanzengesch Pflanzengeogr 95: 226–255
Carolin RC, Jacobs SWL and Vesk M (1977) The ultrastructure of Kranz cells in the family Cyperaceae. Bot Gaz 138: 413–419
Carolin RC, Jacobs SWL and Vesk M (1978) Kranz cells and mesophyll in the Chenopodiales. Aust J Bot 26: 683–698
Carolin RC, Jacobs SWL and Vesk M (1982) The chlorenchyma of some members of the Salicornieae (Chenopodiaceae). Aust J Bot 30: 387–392
Christin P-A, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V and Salamin N (2008) Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biol 18: 37–43
Christin P-A, Salamin N, Kellogg EA, Vicentini A and Besnard G (2009) Integrating phylogeny into studies of C4 variation in the grasses. Plant Physiol 149: 82–87
Chuong SDX, Franceschi VR and Edwards GE (2006) The cytoskeleton maintains organelle partitioning required for single-cell C4 photosynthesis in Chenopodiaceae species. Plant Cell 18: 2207–2223
Craig S and Goodchild DJ (1977) Leaf ultrastructure of Triodia irritans: a C4 grass possessing an unusual arrangement of photosynthetic tissues. Aust J Bot 25: 277–290
Crookston RK and Moss DN (1972) C-4 and C-3 carboxylation characteristics in the genus Zygophyllum (Zygophyllaceae). Ann MO Bot Gard 59: 465–470
Crookston RK and Moss DN (1973) A variation of C4 leaf anatomy in Arundinella hirta (Gramineae). Plant Physiol 52: 397–402
Das VSR and Raghavendra AS (1976) C4 photosynthesis and a unique type of Kranz anatomy in Glossocordia boswallaea (Asteraceae). Proc Indian Acad Sci 84B: 12–19
Dengler RE and Dengler NG (1990) Leaf vascular architecture in the atypical C4 NADP-malic enzyme grass Arundinella hirta. Can J Bot 68: 1208–1221
Dengler NG and Nelson T (1999) Leaf structure and development in C4 plants. In: Sage RF and Monson RK (eds) C4 Plant Biology. Physiological Ecology series, pp 133–172. Academic Press, New York
Dengler NG, Dengler RE and Hattersley PW (1985) Differing ontogenetic origins of PCR (“Kranz”) sheaths in leaf blades of C4 grasses (Poaceae). Am J Bot 72: 284–302
Dengler NG, Dengler RE and Drenville DJ (1990) Comparison of photosynthetic carbon reduction (Kranz) cells having different ontogenetic origins in the C4 NADP-malic enzyme grass Arundinella hirta. Can J Bot 68: 1222–1232
Dengler NG, Donnelly PM and Dengler RE (1996) Differentiation of bundle sheath, mesophyll, and distinctive cells in the C4 grass Arundinella hirta (Poaceae). Am J Bot 83: 1391–1405
Edwards GE and Ku MSB (1987) The biochemistry of C3-C4 intermediates. In: Hatch MD and Boardman NK (eds) The Biochemistry of Plants, pp 275–325. Academic Press, New York
Edwards GE and Walker DA (1983) C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis. Blackwell, Oxford
Edwards GE, Furbank RT, Hatch MD and Osmond CB (2001) What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiol 125: 46–49
Edwards GE, Franceschi VR and Voznesenskaya EV (2004) Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Ann Rev Plant Biol 55: 173–196
Edwards GE, Voznesenskaya EV, Smith M, Koteyeva N, Park Y-I, Park JH, Kiirats O, Okita TW and Chuong SDX (2007) Breaking the Kranz paradigm in terrestrial C4 plants: Does it hold promise for C4 rice? In: Sheehy JE, Mitchell PL and Hardy B (eds) Charting New Pathways to C4 rice, pp 249–273. International Rice Research Institute, World Scientific, Los Banos, Philippines
Ellis RP (1977) Distribution of the Kranz syndrome in the Southern African Eragrostoideae and Panicoideae according to bundle sheath anatomy and cytology. Agroplantae 9: 73–110
Fisher DD, Schenk HJ, Thorsch JA and Ferren WR, Jr. (1997) Leaf anatomy and subgeneric affiliation of C3 and C4 species of Suaeda (Chenopodiaceae) in North America. Am J Bot 84: 1198–1210
Frean ML, Ariovich D and Cresswell CF (1983) C3 and C4 photosynthetic and anatomical forms of Alloteropsis semialata (R. Br.) Hitchcock. II. A comparative investigation of leaf ultrastructure and distribution of chlorenchyma in the two forms. Ann Bot 51: 811–821
Freitag H and Stichler W (2000) A remarkable new leaf type with unusual photosynthetic tissue in a central Asiatic genus of Chenopodiaceae. Plant Biol 2: 154–160
Freitag H and Stichler W (2002) Bienertia cycloptera Bunge ex Boiss., Chenopodiaceae, another C4 plant without Kranz tissues. Plant Biol 4: 121–132
Gamaley YV (1985) The variations of the Kranz-anatomy in Gobi and Karakum plants. Bot Zh 70: 1302–1314 (In Russian)
Gamaley YV and Voznesenskaya EV (1986) Structural-biochemical types of C4 plants. Sov Plant Physiol 33: 616–630
Gilliland MG and Gordon-Gray KD (1978) Kranz and non-kranz cells in Cyperaceae. Proc Electron Microsc Soc Southern Africa 8: 85–86
Glagoleva TA, Voznesenskaya EV, Kol’chevskii KG, Kocharyan NI, Pakhomova MV, Chulanovskaya MV and Gamalei YV (1991) Structural-functional characteristics of halophytes of the Ararat valley. Sov Plant Physiol 37: 822
GPWG (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Ann MO Bot Gard 88: 373–457
Guissani LM, Cota-Sanches JH, Zuloaga FO and Kellogg EA (2001) A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. Am J Bot 88: 1993–2012
Guralnick LJ, Edwards GE, Ku MSB, Hockema B and Franceschi VR (2002) Photosynthetic and anatomical characteristics in the C4-Crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Funct Plant Biol 29: 763–773
Gutierrez M, Gracen VE and Edwards GE (1974) Biochemical and cytological relationships in C4 plants. Planta 119: 279–300
Haberlandt G (1884) Physiologische Pflanzenanatomie. Engelmann, Leipzig
Hatch MD (1971) Mechanism and function of C4 photosynthesis. In: Hatch MD, Osmond CB and Slatyer RO (eds) Photosynthesis and Photorespiration, pp 139–152. Wiley-Interscience, New York
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106
Hatch MD (1999) C4 photosynthesis: a historical overview. In: Sage RF and Monson RK (eds) C4 plant biology. Physiological Ecology series, pp 17–46. Academic Press, San Diego
Hatch MD, Kagawa T and Craig S (1975) Subdivision of C4-pathway species based on differing C4 acid decarboxylating systems and ultrastructural features. Aust J Plant Physiol 2: 111–128
Hattersley PW (1987) Variations in photosynthetic pathway. In: Soderstrom, TR Hilu KW, Campbell CS and Barkworth ME (eds) Grass systematics and evolution, pp 49–64. Smithsonian Institution Press, Washington, DC
Hattersley PW (1992) C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In: Chapman GP (ed) Desertified Grasslands: Their Biology and Management, pp 181–212. Academic Press, London
Hattersley PW and Browning AJ (1981) Occurrence of the suberized lamella in leaves of grasses of different photosynthetic types. I. In parenchymatous bundle sheaths and PCR (“Kranz”) sheaths. Protoplasma 109: 371–401
Hattersley PW and Watson L (1992) Diversification of photosynthesis. In: Chapman GP (ed) Grass evolution and domestication, pp 38–116. Cambridge University Press, Cambridge
Hattersley PW, Wong S-C, Perry S and Roksandic Z (1986) Comparative ultrastructure and gas exchange characteristics of the C3-C4 intermediate Neurachne minor S.T. Blake (Poaceae). Plant Cell Environ 9: 217–233
Ibrahim DG, Burke T, Ripley BS and Osborne CP (2009) A molecular phylogeny of the genus Alloteropsis (Panicoideae, Poaceae) suggests an evolutionary reversion from C4 to C3 photosynthesis. Ann Bot 103: 127–136
Jacobs SWL (2001) Review of leaf anatomy and ultrastructure in the Chenopodiaceae (Caryophyllales). J Torrey Bot Soc 128: 236–253
Johnson MSC (1964) An electron microscope study of the photosynthetic apparatus in plants, with special reference to the Gramineae. Ph.D. thesis, The University of Texas.
Kadereit G, Borsch T, Weising K and Freitag H (2003) Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Int J Plant Sci 164: 959–986
Kanai R and Edwards G (1999) The biochemistry of C4 photosynthesis. In: Sage RF and Monson RK (eds) C4 Plant Biology. Physiological Ecology series, pp 49–87. Academic Press, San Diego
Kapralov MV, Akhani H, Voznesenskaya E, Edwards G, Franceschi VR and Roalson EH (2006) Phylogenetic relationships in the Salicornioideae /Suaedoideae /Salsoloideae s.l. (Chenopodiaceae) clade and a clarification of the phylogenetic position of Bienertia and Alexandra using multiple DNA sequence datasets. Syst Bot 31: 571–585
Kennedy RA and Laetsch WM (1974) Plant species intermediate for C3, C4 photosynthesis. Science 184: 1087–1089
Kim I and Fisher DG (1990). Structural aspects of the leaves of seven species of Portulaca growing in Hawaii. Can J Bot 68: 1803–1811
Laetsch WM (1968) Chloroplast specialization in dicotyledons possessing the C4-dicarboxylic acid pathway of photosynthetic CO2 fixation. Am J Bot 55: 875–883
Laetsch WM (1974) The C4 syndrome: a structural analysis. Annu Rev Plant Physiol 25: 27–52
Lara MV, Chuong SDX, Akhani H, Andreo CS and Edwards GE (2006) Species having C4 single-cell-type photosynthesis in the Chenopodiaceae family evolved a photosynthetic phosphoenolpyruvate carboxylase like that of Kranz-type C4 species. Plant Physiol 142: 673–684
Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths H, Sage RF and Hibberd JM (2007) Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. Plant J 207: 886–896
McKown AD, Moncalvo J-M and Dengler NG (2005) Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. Am J Bot 92: 1911–1928
Meister M, Agostino A and Hatch MD (1996) The roles of malate and aspartate in C4 photosynthetic metabolism of Flaveria bidentis (L.). Planta 199: 262–269
Monson RK and Moore Bd (1989) On the significance of C3-C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant Cell Env 12: 689–699
Monson RK, Edwards GE and Ku MSB (1984) C3-C4 intermediate photosynthesis in plants. BioScience 34: 563–574
Moore Bd, Ku MSB and Edwards GE (1984) Isolation of leaf bundle sheath protoplasts from C4 dicot species and intracellular localization of selected enzymes. Plant Sci Lett 35: 127–138
Muhaidat RM, Sage RF and Dengler NG (2007) Diversity of Kranz anatomy and biochemistry in C4 eudicots. Am J Bot 94: 362–381
Murphy LR, Barroca J, Franceschi VR, Lee R, Roalson EH, Edwards GE and Ku MSB (2007) Diversity and plasticity of C4 photosynthesis in Eleocharis (Cyperaceae). Funct Plant Biol 34: 571–580
Nishioka D, Miyake H and Taniguchi T (1996) Suppression of granal development and accumulation of Rubisco in different bundle sheath chloroplasts of the C4 succulent plant Portulaca grandiflora. Ann Bot 77: 629
Ocallaghan M (1992) The ecology and identification of the southern African Salicornieae (Chenopodiaceae). South Afr J Bot 58: 430–439
Ohsugi R and Murata T (1980) Leaf anatomy, post-illumination CO2 burst and NAD-malic enzyme activity of Panicum dichotomiflorum. Plant Cell Physiol 21: 1329–1333
Ohsugi R, Murata T and Chonan N (1982) C4 syndrome of the species in the Dichotomiflora group of the genus Panicum (Gramineae). Bot Mag 95: 339–347
Park J, Okita TW and Edwards GE (2010) Expression profiling and proteomic analysis of isolated photosynthetic cells of the non-Kranz C4 species Bienertia sinuspersici. Funct Plant Biol 37: 1–13
Peter G and Katinas L (2003) A new type of Kranz anatomy in Asteraceae. Aust J Bot 51: 217–226
Prendergast HDV and Hattersley PW (1987) Australian C4 grasses (Poaceae): leaf blade anatomical features in relation to C4 acid decarboxylation types. Aust J Bot 35: 355–382
Prendergast HDV, Hattersley PW, Stone NE and Lazarides M (1986) C4 acid decarboxylation type in Eragrostis (Poaceae): patterns of variation in chloroplast position, ultrastructure, and geographical distribution. Plant Cell Env 9: 333–344
Prendergast HDV, Hattersley PW and Stone NE (1987) New structural/biochemical associations in leaf blades of C4 grasses (Poaceae). Aust J Plant Physiol 14: 403–420
Pyankov VI and Vakhrusheva DV (1989) Pathways of primary CO2 fixation in C4 plants of the family Chenopodiaceae from the arid zone of Central Asia. Sov Plant Physiol 36: 178–187
Pyankov VI, Kuzmin AN, Demidov ED and Maslov AI (1992) Diversity of biochemical pathways of CO2 fixation in plants of the families Poaceae and Chenopodiaceae from the arid zone of Central Asia. Sov Plant Physiol 39: 411–420
Pyankov VI, Artyusheva EG and Edwards G (1999) Formation of C4 syndrome in leaves and cotyledons of Kochia scoparia and Salsola collina (Chenopodiaceae). Russian J Plant Phys 46: 452–466
Pyankov VI, Gunin PD, Tsoog S and Black CC (2000a)C4 plants in the vegetation of Mongolia: their natural occurrence and geographical distribution in relation to climate. Oecologia 123: 15–31
Pyankov VI, Voznesenskaya EV, Kuzmin A, Ku MSB, Black CC and Edwards GE (2000b) Diversity of CO2 fixation pathways in leaves and cotyledons of Salsola (Chenopodiaceae) plants. Dokl Bot Sci 370: 1–5
Pyankov VI, Voznesenskaya EV, Kuzmin AN, Ku MSB, Ganko E, Franceschi VR, Black CC, Jr. and Edwards GE (2000c) Occurrence of C3 and C4 photosynthesis in cotyledons and leaves of Salsola species (Chenopodiaceae). Photosyn Res 63: 69–84
Rathnam CKM, Raghavendra AS and Das VSR (1976) Diversity in the arrangements of mesophyll cells among leaves of certain C4 dicotyledons in relation to C4 physiology. Z Pflanzenphysiol 77: 283–291
Rawsthorne S and Bauwe H (1998) C3-C4 intermediate photosynthesis. In: Raghavendra AS (ed) Photosynthesis. A comprehensive treatise, pp 150–162. Cambridge University Press, Cambridge
Sage RF (2002) C4 photosynthesis in terrestrial plants does not require Kranz anatomy. Trends Plant Sci 7: 283–285
Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161: 341–370
Sage RF and Monson RK (1999) C4 Plant Biology. Academic Press, San Diego
Sage RF, Li M and Monson RK (1999) The taxonomic distribution of C4 photosynthesis. In: RF Sage and RK Monson (eds) C4 Plant Biology, pp 551–584. Academic Press, New York
Sanchez-Ken JG, Clark LG, Kellogg EA and Kay EE (2007) Reinstatement and emendation of subfamily Micrairoideae (Poaceae). Syst Bot 32: 71–80
Schütze P, Freitag H and Weising K (2003) An integrated molecular and morphological study of the subfamily Suaedoideae Ulbr. (Chenopodiaceae). Plant Syst Evol 239: 257–286
Sede SM, Morrone O, Aliscioni SS, Giussani LM and Zuloaga FO (2009) Oncorachis and Sclerochlamys, two new segregated genera from Streptostachys (Poaceae, Panicoideae, Paniceae): a revision based on molecular, morphological and anatomical characters. Taxon 58: 365–374
Shepherd KA and Wilson PG (2007) Incorporation of the Australian genera Halosarcia, Pachycornia, Sclerostegia and Tegicornia into Tecticornia (Salicornioideae, Chenopodiaceae). Aust Syst Bot 20: 319–331
Shomer-Ilan A, Beer S and Waisel Y (1975) Suaeda monoica, a C4 plant without typical bundle sheaths. Plant Physiol 56: 676–679
Shomer-Ilan A, Neumann-Ganmore R and Waisel Y (1979) Biochemical specialization of photosynthetic cell layers and carbon flow paths in Suaeda monoica. Plant Physiol 64: 963–965
Shomer-Ilan AS, Nissenbaum A and Waisel Y (1981) Photosynthetic pathways and the ecological distribution of the Chenopodiaceae in Israel. Oecologia 48: 244–248
Soros CL and Dengler NG (1998) Quantitative leaf anatomy of C3 and C4 Cyperaceae and comparisons with the Poaceae. Int J Plant Sci 159: 480–491
Soros CL and Dengler NG (2001) Ontogenetic derivation and cell differentiation in photosynthetic tissues of C3 and C4 Cyperaceae. Am J Bot 88: 992–1005
Takeda T, Ueno O and Agata W (1980) The occurrence of C4 species in the genus Rhynchospora and its significance in Kranz anatomy of the Cyperaceae. Bot Mag 93: 55–65
Taniguchi Y, Taniguchi M, Kawasaki M and Miyake H (2003) Strictness of the centrifugal location of bundle sheath chloroplasts in different NADP-ME type C4 grasses. Plant Prod Sci 6: 274–280
Tateoka T (1958) Notes on some grasses. VIII. On leaf structure of Arundinella and Garnotia. Bot Gaz 120: 101–109
Ueno O (1995) Occurrence of distinctive cells in leaves of C4 species in Arthraxon and Microstegium (Andropogoneae-Poaceae) and the structural and immunocytochemical characterization of these cells. Int J Plant Sci 156: 270–289
Ueno O (1996a) Structural characterization of photosynthetic cells in an amphibious sedge, Eleocharis vivipara, in relation to C3 and C4 metabolism. Planta 199: 382–393
Ueno O (1996b) Immunocytochemical localization of enzymes involved in the C3 and C4 pathways in the photosynthetic cells of an amphibious sedge, Eleocharis vivipara. Planta 199: 394–403
Ueno O (1998a) Immunogold localization of photosynthetic enzymes in leaves of various C4 plants, with particular reference to pyruvate orthophosphate dikinase. J Exp Bot 49: 1637–1646
Ueno O (1998b) Induction of Kranz anatomy and C4-like biochemical characteristics in a submerged amphibious plant by abscisic acid. Plant Cell 10: 571–583
Ueno O (2004) Environmental regulation of photosynthetic metabolism in the amphibious sedge Eleocharis baldwinii and comparisons with related species. Plant Cell Env 27: 627–639
Ueno O and Samejima M (1989) Structural features of NAD-malic enzyme type C4 Eleocharis: an additional report of C4 acid decarboxylation types of the Cyperaceae. Bot Mag 102: 393–402
Ueno O and Sentoku N (2006) Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant Cell Env 29: 257–268
Ueno O and Wakayama M (2004) Cellular expression of C3 and C4 photosynthetic enzymes in the amphibious sedge Eleocharis retroflexa ssp. chaetaria. J Plant Res 117: 433–441
Ueno O, Takeda T and Murata T (1986) C4 acid decarboxylating enzyme activities of the C4 species possessing Kranz anatomical types in the Cyperaceae. Photosynthetica 20: 111–116
Ueno O, Takeda T and Maeda E (1988a) Leaf ultrastructure of C4 species possessing different Kranz anatomical types in the Cyperaceae. Bot Mag 101: 141–152
Ueno O, Samejima M and Koyama T (1989). Distribution and evolution of C4 syndrome in Eleocharis, a sedge group inhabiting wet and aquatic environments, based on culm anatomy and carbon isotope ratios. Ann Bot 64: 425–438
Ueno O, Samejima M, Muto S and Miyachi S (1988b) Photosynthetic characteristics of an amphibious plant, Eleocharis vivipara: expression of C4 and C3 modes in contrasting environments. Proc Natl Acad Sci USA 85: 6733–6737
Vasilevskaya VK and Butnik AA (1981) The types of the anatomical structure of the dicotyledon leaves (a contribution to the method of anatomical description). Bot Zh 66: 992–1001 (In Russian)
Vicentini A, Barber JC, Aliscioni SS, Giussani LM and Kellogg EA (2008) The age of the grasses and clusters of origins of C4 photosynthesis. Global Change Biology 14: 2963–2977
von Caemmerer S (1989) A model of photosynthetic CO2 assimilation and carbon-isotope discrimination in leaves of certain C3-C4 intermediates. Planta 178: 463–474
Voznesenskaya EV and Gamaley YV (1986) The ultrastructural characteristics of leaf types with Kranz-anatomy. Bot Zh 71: 1291–1307 (In Russian)
Voznesenskaya EV, Franceschi VR, Pyankov VI and Edwards GE (1999) Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae). J Exp Bot 50: 1779–1795
Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H and Edwards GE (2001) Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414: 543–546
Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H and Edwards GE (2002) Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J 31: 649–662
Voznesenskaya EV, Edwards GE, Kiirats O, Artyusheva EG and Franceschi VR (2003) Development of biochemical specialization and organelle partitioning in the single celled C4 system in leaves of Borszczowia aralocaspica (Chenopodiaceae). Am J Bot 90: 1669–1680
Voznesenskaya EV, Franceschi VR and Edwards GE (2004) Light-dependent development of single cell C4 photosynthesis in cotyledons of Borszczowia aralocaspica (Chenopodiaceae) during transformation from a storage to a photosynthetic organ. Ann Bot 93: 1–11
Voznesenskaya EV, Chuong SDX, Kirrats O, Franceschi VR and Edwards GE (2005a) Evidence that C4 species in genus Stipagrostis, family Poaceae, is NADP-malic enzyme subtype with nonclassical type of Kranz anatomy (Stipagrostoid). Plant Sci 168: 731–739
Voznesenskaya EV, Chuong SDX, Koteeva NK, Edwards GE and Franceschi VR (2005b) Functional compartmentation of C4 photosynthesis in the triple-layered chlorenchyma of Aristida (Poaceae). Funct Plant Biol 32: 67–77
Voznesenskaya EV, Koteyeva NK, Chuong SDX, Edwards GE, Akhani H and Franceschi VR (2005c) Differentiation of cellular and biochemical features of the single-cell C4 syndrome during leaf development in Bienertia cycloptera (Chenopodiaceae). Am J Bot 92: 1784–1795
Voznesenskaya EV, Franceschi VR, Chuong SDX and Edwards GE (2006) Functional characterization of phosphoenolpyruvate carboxykinase type C4 leaf anatomy: Immuno, cytochemical and ultrastructural analyses. Ann Bot 98: 77–91
Voznesenskaya EV, Chuong S, Koteyeva N, Franceschi VR, Freitag H and Edwards GE (2007) Structural, biochemical and physiological characterization of C4 photosynthesis in species having two vastly different types of Kranz anatomy in genus Suaeda (Chenopodiaceae). Plant Biol 9: 745–757
Voznesenskaya EV, Akhani H, Koteyeva NK, Chuong SDX, Roalson EH, Kiirats O, Franceschi VR and Edwards GE (2008) Structural, biochemical and physiological characterization of photosynthesis in two C4 subspecies of Tecticornia indica and the C3 species Tecticornia pergranulata (Chenopodiaceae). J Exp Bot 59: 1715–1734
Voznesenskaya EV, Koteyeva NK, Edwards GE and Ocampo G (2010) Anatomical and biochemical characterization of photosynthetic types in genus Portulaca L. (Portulacaceae). J Exp Bot 61:3647–3662
Wakayama M, Ueno O and Ohnishi J (2002) Cellular accumulation of photosynthetic enzymes during leaf development of Arundinella hirta, a C4 grass with unusual Kranz cells without contact with vascular tissues. Plant Cell Physiol 43: S173–S173
Wakayama M, Ueno O and Ohnishi J (2003) Photosynthetic enzyme accumulation during leaf development of Arundinella hirta, a C4 grass having Kranz cells not associated with vascular tissues. Plant Cell Physiol 44: 1330–1340
Wakayama M, Ohnishi J and Ueno O (2006) Structure and enzyme expression in photosynthetic organs of the atypical C4 grass Arundinella hirta. Planta 223: 1243–1255
Walker RP and Chen Z-H (2002) Phosphoenolpyruvate carboxykinase: Structure, function and regulation. In: Callow JA (ed) Advances in Botanical Research Incorporating Advances in Plant Pathology, pp 93–189. Academic Press, New York
Wingler A, Walker RP, Chen Z-H and Leegood RC (1999) Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle sheath of maize. Plant Physiol 120: 539–545
Winter K (1981) C4 plants of high biomass in arid regions of Asia. Occurrence of C4 photosynthesis in Chenopodiaceae and Polygonaceae from the middle east and USSR. Oecologia 48: 100–106
Winter K, Kramer D, Troughton JH and Card KA (1977) C4 pathway of photosynthesis in a member of the Polygonaceae: Calligonum persicum (Boiss. and Buhse) Boiss. Z Pflanzenphysiol 81: 341–346
Yoshimura Y, Kubota F and Ueno O (2004) Structural and biochemical bases of photorespiration in C4 plants: quantification of organelles and glycine decarboxylase. Planta 220: 307–317
Acknowledgments
This material is based upon work supported by the National Science Foundation under Grant Nos. IBN-0131098, IBN-0236959 and IBN-0641232, by Civilian Research and Development Foundation grants RB1-2502-ST-03 and RUB1-2829-ST-06, by Russian Foundation of Basic Research grant 05-04-49622 and 08-04-00936,and Bill and Melinda Gates Foundation to IRRI for C4 Rice Program. We also thank the Franceschi Microscopy and Imaging Center of Washington State University for use of their facilities and staff assistance, and C. Cody for plant growth management. The authors appreciate the helpful discussions with Dr. Nuria Koteyeva and the advice of two reviewers, especially in the sections devoted to grasses and sedges.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Netherlands
About this chapter
Cite this chapter
Edwards, G.E., Voznesenskaya, E.V. (2010). Chapter 4 C4 Photosynthesis: Kranz Forms and Single-Cell C4 in Terrestrial Plants. In: Raghavendra, A., Sage, R. (eds) C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Advances in Photosynthesis and Respiration, vol 32. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9407-0_4
Download citation
DOI: https://doi.org/10.1007/978-90-481-9407-0_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9406-3
Online ISBN: 978-90-481-9407-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)