Abstract
The light use efficiency of photosynthesis dynamically adapts to environmental factors and is one major factor determining crop yield. Optical remote sensing techniques have the potential to detect physiological and biochemical changes in plant ecosystems, and non-invasive detection of changes in photosynthetic energy conversion may be of great potential for managing agricultural production in a future bio-based economy. Here we give an overview on the principles of optical remote sensing in crop systems with a special emphasis on investigating hyperspectral reflectance data and the sun-induced fluorescence signal. Especially sun-induced fluorescence as a parameter, which becomes important in remote sensing research may have great potential quantifying the physiological status of the photosynthetic apparatus. Both remote sensing principles were applied during the CEFLES2 campaign in Southern France, where the structural and functional status of several crops was measured on the ground and using state-of-the-art optical remote sensing techniques. Sun-induced fluorescence measurements over a variety of crops showed that additional information can be retrieved also over dense canopies, where classical remote sensing signals often saturate. With a view to the future, we discuss how hyperspectral reflectance and sun-induced fluorescence can quantitatively be related to photosynthetic efficiency and help to measure and manage productivity of natural and agricultural ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Photosynthetic Efficiency
- Pigment Composition
- Saturate Light Pulse
- Normalize Difference Vegetation IndexNormalized
- Natural Canopy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Background on Optical Spectroscopy of Plant Canopies
Solar radiation that interacts with plant tissues or plant canopies is either reflected, absorbed or transmitted (Fig. 6.1, left). The spectral characteristics of the three components at leaf or canopy scale are a function of (I) leaf level absorption and scattering, (II) the optical properties of other canopy components and the canopy architecture itself, and (III) external effects (illumination, observation geometry) (Goel 1988, 1989, Chen et al. 2000). Optical spectroscopy mainly focuses on the reflected part of radiation as a measure to derive information about the biochemical and structural properties of plants at leaf and canopy level (Fourty and Baret 1998, Liang 2004), whereas the optical properties of leaf tissues significantly determine the canopy optical parameters (Asner 1998, Otterman et al. 1995). For instance, the low intensity reflectance of plant leaves in the visible (400–700 nm) part of the light spectrum results from strong absorbance by the photosynthetic foliar pigments, while the high reflectance in the near infrared (700–1,100 nm) is due to low absorption of light by the internal leaf mesophyll tissues, and the reflectance intensity in the shortwave infrared (1,100–2,500 nm) is strongly affected by the amount of water in plant tissues (Curran 1989) (Fig. 6.1, right).
During the phenological cycle, in response to an adaption of plants to environmental conditions, or between different species, leaf biochemical components vary and cause variations in the interaction of solar radiation with leaf tissues (Gausman and Allen 1973, Grant 1987). This, in turn, results in changing optical properties of leaf tissues. Figure 6.2 exemplarily shows the variation of reflected radiation in response to varying chlorophyll and water content.
2 Remote Sensing of Photosynthesis
Sensing the state of photosynthetic activity, however, is much more complex and the sole information about pigment content is insufficient to predict the current photosynthetic rate. In fact, plant photosynthesis is a dynamically regulated process that quickly adapts to environmental conditions and is affected by the ecological plasticity of each species (Turner et al. 2003b, Rascher and Nedbal 2006). Consequently, photosynthetic rates may greatly vary between different species with similar pigment composition and, additionally, both photosynthesis and pigment composition is dynamically adjusted in diurnal and seasonal cycles (Schurr et al. 2006). Most of the time, natural canopy photosynthesis is not operating at its maximum potential rate and may be largely reduced under prevailing environmental conditions. For example, Bergh et al. (1998) estimated that the CO2 uptake by a frost stressed boreal forest over a growing season reached only 44% from its potential rate or, Rascher et al. (2004) observed a 30% decrease in photosynthesis as response of drought stress in a tropical ecosystem. These reductions of photosynthetic rates cannot be tracked by the pigment content and the pigment composition itself.
The remote observation of photosynthetic rates can principally be grouped into two approaches: methods that indirectly relate photosynthesis to environmental stresses and approaches that estimate photosynthesis directly from remote sensing data. Recent research effort has focused on estimating the physiological status of photosynthesis directly from remotely sensed data because remote sensing provides the only practical approach to characterize photosynthesis and productivity of vast crop and natural ecosystems. The efficiency of photosynthesis is controlled on various levels involving biophysical and biochemical mechanisms (see Schulze and Caldwell 1995 for a summary on the ecophysiology of photosynthesis). Light absorbed by chlorophyll can be used to (I) drive photosynthesis, and (II) excess energy can be dissipated by a variety of non-photochemical processes usually as heat or, (III) it can be reemitted as fluorescence at longer wavelengths. These three processes compete with each other and an increase in the efficiency of one will result in the decrease of yield in the other two. The major component of the Non-Photochemical Quenching (NPQ) is related to a transthylakoid pH gradient, which activates enzymes altering the epoxidation state of xanthophyll molecules associated with the light harvesting complex and this affects the energy dissipation as heat (Demmig-Adams and Adams 1996, Müller et al. 2001, Baker 2008).
In the following two state-of-the-art remote sensing approaches (based on hyperspectral reflectance data and on the emitted sun-induced fluorescence signal) are described for the potential to directly measure the functional status of photosynthesis .
2.1 Photochemical Reflectance Index (PRI)
The Photochemical Reflectance Index (PRI) is related to NPQ and was developed to serve as an estimate of photosynthetic light use efficiency . This normalized difference reflectance index uses two wavebands (Eq. 1): 531 nm, which is correlated with the xanthophyll pigment composition during the NPQ energy dissipation, and 570 nm, which serves as a reference waveband (Gamon et al. 1992).
PRI has been used in a variety of case studies and positively correlates with photosynthetic efficiency. It has been successfully used to detect changes in photosynthetic efficiency at the leaf level (see Rascher et al. 2007, for an overview of the literature).
However, PRI values vary greatly between species with the same photosynthetic capacity (Guo and Trotter 2004). Additionally, the PRI is greatly affected by the geometry of the sun, the leaf, and the detector (Barton and North 2001). As natural canopies are an assembly of differently oriented leaves that additionally change their orientation during development of the plant and as a response to environmental conditions, canopy measurements of PRI often were greatly affected by seasonal changes in canopy structure (Filella et al. 2004). Thus, challenges remain to transfer the very promising results from the laboratory to the canopy and field scale. One of the first successful demonstrations of field measurements was performed in a Siberian forest (Nichol et al. 2002). Since then several groups have further evaluated the potential of PRI and are currently identifying procedures to scale and use the PRI on natural canopies (Hall et al. 2008).
2.2 Fluorescence
Light energy that is absorbed in photosynthetic pigments is partly re-emitted as fluorescence light with well defined wavelength characteristics. Chlorophyll fluorescence is emitted in two broad, overlapping bands with peaks at 685 nm and around 740 nm (Fig. 6.3a; Lichtenthaler and Rinderle 1988). However, the total amount of the emitted fluorescence signal is small in comparison the reflected light (Fig. 6.3b). The intensity of the emitted fluorescence signal is reversely correlated to the energy used for photosynthesis and thus can serve as an indicator for photosynthetic light conversion (see Baker 2008 for a recent review). Fluorescence approaches for analysis of photosynthesis have been developed over the past couple of decades. The most commonly used technique is the Pulse Amplitude Modulation (PAM) fluorometry, which uses the saturating light pulse method (Schreiber and Bilger 1993, Schreiber et al. 1995, Genty et al. 1989, Maxwell and Johnson 2000). PAM data can be analyzed to determine the efficiency with which absorbed photons are being used for photosynthesis, the rates of electron transport, and the degree of non-photochemical protection. This approach requires measurements very close to the leaf as a saturating light pulse has to be applied and is therefore not practical for measurements of plant canopies. However, processes within canopy with millions of leaves – each in its unique environment and all contributing to the overall performance of the canopy – provide many challenges and cannot be derived from single leaf measurements. Therefore, techniques and instruments for measurements on canopy scale are required and several approaches are currently being developed for application from a distance for the remote quantification of plant canopies and fields (Osmond et al. 2004, Rascher et al. 2009).
(a) Fluorescence and reflectance spectra of a sugar beet leaf. (a) Fluorescence emission spectrum in the region between 650 and 820 nm. (b) Reflectance with and without fluorescence , i.e. real and apparent reflectance. Measurements were performed on a sugar beet leaf under solar illumination with an ASD FieldSpec Pro coupled with the FluoWAT leaf clip (Alonso et al. 2007), which enables the extraction of the fluorescence spectrum by selectively filtering the incoming light. For details see Meroni et al. (2009)
One way of remote quantification of photosynthesis by fluorescence relies on making it possible to measure and analyze fluorescence transients at a distance from the target leaf. A newly developed Laser Induced Fluorescence Transient (LIFT) instrument makes use of a telescope to collect light from target leaves and a low power laser to manipulate the light regime of the target. Constraints on the power of lasers for use in open environments make it impossible to use the same protocols that have been used with PAM fluorometers. New approaches make use of the laser to make much smaller but highly replicated modification of the light regime to analyze the efficiency of photosynthesis (Kolber et al. 2005). The LIFT instrument is required to make measurements that are at the noise limit and computer assisted fitting of the data to a theoretical model are substituted for brute force and simple analysis used in PAM fluorometery (Ananyev et al. 2005, Kolber et al. 1998). The LIFT approach was successfully used to monitor spatial and temporal dynamics of the photosynthetic properties of leaves in the inaccessible outer canopy of trees (Osmond et al. 2004, Rascher and Pieruschka 2008). However, it is limited to measurements at a distance of 5–50 m from the canopy.
2.3 Retrieval of Remotely Measured Sun-Induced Chlorophyll Fluorescence
Fluorescence measurement of large ecosystems relies on passive measurements of solar induced fluorescence (Fs). The approach uses Fraunhofer lines with reduced incoming solar radiation reaching the Earth surface in three main absorption bands in the red and near infrared spectral domain: the Hα line at 656.3 nm is due to the hydrogen absorption by the solar atmosphere whereas two bands at 687 nm (O2-B) and 760 nm (O2-A) are due to the molecular oxygen absorption by the terrestrial atmosphere. Fluorescence originated from the canopy occurs in these otherwise ‘black’ absorption bands and, therefore, can be selectively quantified. Especially the O2-A and O2-B bands overlap with the chlorophyll fluorescence emission spectrum and are wide enough to allow quantifying fluorescence from air- and space-borne platforms. The Fraunhofer Line Discrimination method (FLD) has been proposed for this purpose (Plascyk and Gabriel 1975) and was used with success in different works (Carter et al. 1990, Moya et al. 2004, Meroni et al. 2009).
Retrieval of sun-induced fluorescence takes advantage of the great difference in incoming radiation in a small spectral window around the atmospheric oxygen absorption lines. Incoming and outgoing radiance is measured on the shoulders outside of the absorption lines and inside the absorption lines. Fluorescence is a spectrally comparably broad signal which is added to the reflected signal and by comparing values inside and outside of the oxygen absorption feature sun-induced fluorescence can be quantified (Fig. 6.4).
Principle of retrieval of sun-induced fluorescence in the atmospheric absorption lines, where I is incoming radiance and L is reflected, i.e. outgoing radiance, the subscripts ‘in’ and ‘out’ refer to radiance inside and outside of the atmospheric absorption lines (courtesy Jose Moreno, University of Valencia)
Approaches to quantify Fs can be divided in two major categories: radiance- and reflectance-based approaches. Radiance-based approaches exploit the narrow absorption feature of a Fraunhofer line and so make use of high spectral resolution data (from few nanometres up to 0.03 nm Full Width at Half Maximum (FWHM)). The main methods proposed in the literature require 2–3 spectral channels near the investigated absorption line, while other three methods require a set of contiguous channels covering the whole spectral range of interest. Reflectance-based approaches on the contrary compute optical indices related to Fs but cannot provide direct Fs estimates, neither in physical nor in auxiliary units. In fact, these methods exploit the effect of Fs on the apparent reflectance spectrum in the red-edge region (from 650 to 800 nm) and several indices have been proposed for this purpose (Meroni et al. 2009).
Taking the advantages and disadvantages of the different approaches into account and considering several sensitivity studies, the method proposed by Maier et al. (2003) may be a good compromise between low complexity and stability among a wide range of applications. According to the common measurement procedure of field spectroscopy , this method needs the hyperspectral reflectance measurement of a white (non-fluorescing) reflectance standard (e.g. a calibrated Spectralon™ panel) that is mounted on the same height as the vegetation or alternatively if applied on the larger scale non-fluorescent surfaces, such as fields of bare soil that are large enough to be not-influences by diffuse fluorescence from adjacent fields. Radiance measurements from the non-reflecting surface are compared with radiance measurements of the fluorescing canopy. Fluorescence can then be calculated according to Eq. (2)
with I being the incoming radiance, L is the radiance of vegetation, and the subscripts ‘in’ and ‘out’ indicate the wavelengths within and outside of the absorption line, respectively (see also Fig. 6.4).
The magnitude of fluorescence emission is primarily driven by the amount of absorbed light in the photosynthetic ‘machinery’ and secondly depends on the physiological properties of the photosynthesis . If one is interested in the physiological status, the fluorescence signal (F s ) has to normalize by incoming or absorbed light. This can be achieved by rationing the number of photons emitted (F s ) and the number of photons absorbed by the plants (APAR). The resulting signal is termed fluorescence quantum yield (Fs yield). Changes in Fs yield are independent of the light level and thus reflect the functional status of photosynthesis.
3 Case Studies
3.1 CEFLES-2 Campaign
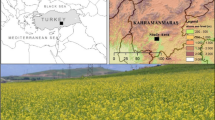
Field data were acquired as part of the European Space Agency (ESA) supported CEFLES-2 campaign in April, June and September 2007 (http://www.esa.int/esaLP/SEMQACHYX3F_index_0.html). The campaign was perfor-med in the Les Landes area, Southwest France. The main site is located in a plain of the Garonne valley and dominated by intensive agriculture (Fig. 6.5). CEFLES-2 was designed to provide extensive and spatially resolved validation of photosynthesis estimates based on remote sensing fluorescence measurements obtained by using airborne instrumentation. Remotely sensed fluorescence parameter were validated by extensive ground measurements of structural parameters (leaf area index (LAI)), canopy height or fractional cover (fcover), biochemical characterizations (chlorophyll, water and dry matter content), physiological parameters (PAM fluorometry, gas exchange) and standard field spectroscopy . These more traditional measurements were complemented with novel set-ups aimed to quantify fluorescence at the canopy level. Winter wheat and maize were chosen as species of major interest in April and September, respectively. Additionally, investigations were expanded to rapeseed , grassland , pine , maize, potato , sunflower , bean , kiwi , grapevine and oak forest . A detailed overview of the campaign is published in Rascher et al. (2009).
Aerial view of the main study site Les Landes in Southern France. The RGB false color image was derived from the Airborne Hyperspectral Scanner (AHS) airborne sensor (R: 855 nm, G: 652 nm, B: 539 nm) on September 15, 2007, around 11:44 local time, close to Marmande. The image dataset was acquired in a flight height of 2,840 m above ground with a spatial resolution of 6 m. Several corn fields (MC) and selected sunflower (SF), potato (MP), grass (MG, MBU), Kiwi (MK) and Rapeseed (MR) field that were intensively characterized for their structure and photosynthetic function are marked
3.2 Characterization of Spatial and Species Dependent Variability of Photosynthesis Using Fluorescence Estimates
Some basic information about the spatial and species dependent variability of canopy fluorescence was investigated covering 36 individual fields in 8 different crop types, including bare soil. Main focus of this analysis was to investigate the variability of sun-induced fluorescence within the same field, of the same crop, and in different canopies (Fig. 6.5). A comparison of the well established Normalized Difference Vegetation Index (NDVI) and Fs was performed to investigate the plausibility of the derived F s values. The NDVI typically shows the ‘greenness’ i.e. the index is a measure for green biomass or canopy chlorophyll content (Goetz and Prince 1999). As fluorescence is also a function of canopy chlorophyll content , we expect that low F s values correspond to low NDVI values and high F s values go along with high NDVI values respectively. Important is the dependency of F s to photosynthesis and F s covers complementary information about photosynthetic activity. Hence, we also expect a non linear relationship between both parameters.
A first relative evaluation of the data showed that the F s signal exponentially increases with increasing NDVI (Fig. 6.6). A clear difference in the inter- and intra-field variation was obvious for both parameters. This result is mirroring the heterogeneity of cultivation, nutrient availability, or simple species composition within one field being much lower compared to different fields. Principally, the results seem to describe the status of vegetation: the vital and dense winter wheat fields reach the highest F s values, slightly senescent corn field medium F s values and dry stressed grassland and senescent sunflowers have lowest F s values (Fig. 6.6). Moreover, the sensitivity of both parameters differs especially at the boundaries of the parameter range. On the one hand, the classical vegetation index saturated in dense canopies (e.g. when LAI is higher than 4) at a NDVI value of 0.9, where F s still provided a differentiation of values (e.g. Fig. 6.6, for winter wheat). On the other hand, NDVI showed a significant variability for non vegetated surface classes (e.g. bare soil or water). F s values of non-vegetated surfaces, such as bare soil and burned grass were close to zero, or greatly senescent sunflower field also showed F s slightly below zero (Fig. 6.6).
Comparison between the NDVI and sun-induced fluorescence (F s ). Measurements were taken over a wide range of agricultural crops and surface classes. During the three campaigns in April, June, and September 2007, 8 different crops and bare soil were characterized. To cover the spatial heterogeneity of each field, four representative places were selected and three measurements per place were performed. Measurements were taken top-of-canopy using a FieldSpec Pro high resolution spectroradiometer (Analytical Spectral Devices, Boulder,USA), which measures reflected radiation within the spectral domain of 350–2,500 nm with a spectral resolution (FWHM) of 3.0 nm (350–1,050 nm) and a field-of-view (FOV) of 25°. A calibrated Spectralon™ panel (25 × 25 cm) served as white reference to estimate incident irradiance. The fluorescence signal was quantified using the FLD method according to Maier et al. (2003) in the O2-A band. At each place in the field, the instrument’s fibre optic was mounted on a tripod, approximately 1 m above the canopy. Three different spots with a circular area of 0.5 m diameter each were recorded moving the fibre optic manually over the canopy
The relationship between F s and NDVI indicates that both parameters are driven by canopy chlorophyll content , but also that the fluorescence signal is complementary driven by other parameters and may support the theory that F s is sensitive to photosynthetic activity. The results clearly show that the observed canopy F s signal is affected by various structural effects, e.g. canopy structure, fractional cover, or canopy height. A comparison of F s values from canopies with different structural characteristics and the linkage of derived F s values to photosynthesis necessitate a proper normalization for the mentioned structural effects.
4 Conclusions
Quantum yield of photosynthetic energy conversion can be related to photosynthetic light use efficiency (LUE), which represents photosynthetic processes, by the amount of fixed carbon per unit of absorbed solar radiation (Genty et al. 1989). Estimation of plant productivity is often based on the linear relationship between net primary productivity (NPP) and the fraction of absorbed PAR (fAPAR), with LUE as the slope of this relationship (Monteith 1972, 1977). However, LUE is often estimated from physiological models or look-up tables, and LUE can vary greatly among different vegetation types (Gower et al. 1999, Ruimy et al. 1995, Weis and Berry 1987). Monitoring of LUE by measurement of sun-induced fluorescence could greatly improve these models.
Crop productivity varies within fields and between fields due to various environmental factors, diseases, and management practices. Photosynthetic efficiency may be a promising parameter to detect limitations or down-regulation of photosynthesis regardless of its cause on thus may serve as an early indicator for reduced productivity. Air- and space-borne fluorescence or hyperspectral sensors may provide spatio-temporal information for better response and managing of crops.
Different studies have shown that fluorescence is somehow related to photosynthesis or LUE, respectively (Damm et al. 2009, van der Tol et al. 2009, Meroni et al. 2008). However, the existence of non-photochemical quenching mechanisms may influence the relationship of F s and LUE within a diurnal course and between different species. Understanding the relation of sun-induced fluorescence and photosynthetic efficiency in structurally complex and diverse canopies and ecosystems is extremely complex and challenging but it may provide a very useful tool to quantify productivity of natural and agricultural ecosystems.
References
Alonso L, Gómez-Chova L, Vila-Francés J et al (2007) Sensitivity analysis of the FLD method for the measurement of chlorophyll fluorescence using a field spectroradiometer. Proceedings of the 3rd International Workshop on Remote Sensing of Vegetation Fluorescence, Florence, Italy, 7–9 Feb 2007
Ananyev G, Kolber ZS, Klimov D et al (2005) Remote sensing of heterogeneity in photosynthetic efficiency, electron transport and dissipation of excess light in Populus deltoides stands under ambient and elevated CO2 concentrations, and in a tropical forest canopy, using a new laser-induced fluorescence transient device. Global Change Biol 11:1195–1206
Asner GP (1998) Biophysical and biochemical sources of variability in canopy reflectance. Rem Sens Environ 64:234–253
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Ann Rev Plant Biol 59:89–113
Barton CVM, North PRJ (2001) Remote sensing of canopy light use efficiency using the photochemical reflectance index; model and sensitivity analysis. Rem Sens Environ 78: 264–273
Bergh J, McMurtrie RE, Linder S (1998) Climatic factors controlling the productivity of Norway spruce: a model-based analysis. Forest Ecol Manage 110:127–139
Carter GA, Theisen AF, Mitchell RJ (1990) Chlorophyll fluorescence measured using the Fraunhofer line-depth principle and relationship to photosynthetic rate in the field. Plant Cell Environ 13:79–83
Chen JM, Li X, Nilson T et al (2000) Recent advances in geometrical optical modelling and its applications. Rem Sens Rev 18:227–262
Curran PJ (1989) Remote-sensing of foliar chemistry. Rem Sens Environ 30:271–278
Damm A, Elbers J, Erler E et al (2009) Remote sensing of sun induced fluorescence to improve modelling of diurnal courses of gross primary production (GPP). Global Change Biol DOI: 10.1111/j.1365-2486.2009.01908.x
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Filella I, Peñuelas J, Llorens L et al (2004) Reflectance assessment of seasonal and annual changes in biomass and CO2 uptake of a Mediterranean shrubland submitted to experimental warming and drought. Rem Sens Environ 90:308–318
Fourty T, Baret F (1998) On spectral estimates of fresh leaf biochemistry. Int J Rem Sens 19: 1283–1297
Gamon JA, Peñuelas J, Field CB (1992) A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Rem Sens Environm 41:35–44
Gausman HW, Allen WA (1973) Optical parameters of leaves of 30 plant species. Plant Physiol 52:57–62
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Goel NS (1988) Models of vegetation canopy reflectance and their use in estimation of biophysical parameters from reflectance data. Rem Sens Rev 4:1–122
Goel NS (1989) Inversion of canopy reflectance models for estimation of biophysical parameters from reflectance data. In: Asrar G (ed) Theory and applications of optical remote sensing. Wiley, New York, pp 205–251
Goetz SJ, Prince SD (1999) Modelling terrestrial carbon exchange and storage: evidence and implications of functional convergence in light-use efficiency. Adv Ecol Res 28:57–92
Gower ST, Kucharik CJ, Norman JM (1999) Direct and indirect estimation of leaf area index, f(APAR), and net primary production of terrestrial ecosystems. Rem Sens Environ 70:29–51
Grant L (1987) Diffuse and specular characteristics of leaf reflectance. Rem Sens Environ 22: 309–322
Guo JM, Trotter CM (2004) Estimating photosynthetic light-use efficiency using the photochemical reflectance index: variations among species. Funct Plant Biol 31:255–265
Hall FG, Hilker T, Coops NC et al (2008) Multi-angle remote sensing of forest light use efficiency by observing PRI variation with canopy shadow fraction. Rem Sens Environm 112:3201–3211
Jacquemoud S, Baret F (1990) PROSPECT – a Model of leaf optical-properties spectra. Rem Sens Environ 34:75–91
Kolber Z, Klimov D, Ananyev G et al (2005) Measuring photosynthetic parameters at a distance: laser induced fluorescence transient (LIFT) method for remote measurements of PSII in terrestrial vegetation. Photosyn Res 84:121–129
Kolber ZS, Prasil O, Falkowski PG (1998) Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochim Biophys Acta 1367:88–106
Liang SL (2004) Quantitative remote sensing of land surfaces. Wiley, Hoboken
Lichtenthaler HK, Rinderle U (1988) The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit Rev Anal Chem 19:S29–S85
Maier SW, Günther KP, Stellmes M (2003) Sun-induced fluorescence: a new tool for precision farming. In: McDonald M, Schepers J, Tartly L et al (eds) Digital imaging and spectral techniques: applications to precision agriculture and crop physiology, vol 66. ASA Special Publication, Madison, pp 209–222
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51: 659–668
Meroni M, Picchi V, Rossini M et al (2008) Leaf level early assessment of ozone injuries by passive fluorescence and photochemical reflectance index. Int J Rem Sens 29:5409–5422
Meroni M, Rossini M, Guanter L et al (2009) Remote sensing of solar induced chlorophyll fluorescence: review of methods and applications. Rem Sens Environ 113:2037–2051
Monteith JL (1972) Solar radiation and productivity in tropical ecosystems. J Appl Ecol 9:747–766
Monteith JL (1977) Climate and efficiency of crop production in Britain. Phil Trans Royal Soc London B Biol Sci 281:277–294
Moya I, Camenen L, Evain S et al (2004) A new instrument for passive remote sensing. – 1. Measurements of sunlight-induced chlorophyll fluorescence. Rem Sens Environ 91:186–197
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nichol CJ, Lloyd J, Shibistova O et al (2002) Remote sensing of photosynthetic-light-use efficiency of a Siberian boreal forest. Tellus B 54B:677–687
Osmond CB, Ananyev G, Berry J et al (2004) Changing the way we think about global change research: scaling up in experimental ecosystem science. Global Change Biol 10:393–407
Otterman J, Brakke T, Smith J (1995) Effects of leaf-transmittance versus leaf-reflectance on bidirectional scattering from canopy soil surface – an analytical study. Rem Sens Environ 54:49–60
Plascyk JA, Gabriel FC (1975) The Fraunhofer line discriminator MKII – an airborne instrument for precise and standardized ecological luminescence measurements. IEEE Trans Instrum Measure 24:306–313
Rascher U, Bobich EG, Lin GH et al (2004) Functional diversity of photosynthesis during drought in a model tropical rainforest – the contributions of leaf area, photosynthetic electron transport and stomatal conductance to reduction in net ecosystem carbon exchange. Plant Cell Environ 27:1239–1256
Rascher U, Agati G, Alonso L et al (2009) CEFLES2: the remote sensing component to quantify photosynthetic efficiency from the leaf to the region by measuring sun-induced fluorescence in the oxygen absorption bands. Biogeosciences 6:1181–1198
Rascher U, Nedbal L (2006) Dynamics of plant photosynthesis under fluctuating natural conditions. Curr Opin Plant Biol 9:671–678
Rascher U, Nichol CL, Small C et al (2007) Monitoring spatio-temporal dynamics of photosynthesis with a portable hyperspectral imaging system. Photogram Eng Rem Sens 73:45–56
Rascher U, Pieruschka R (2008) Spatio-temporal variations of photosynthesis: the potential of optical remote sensing to better understand and scale light use efficiency and stresses of plant ecosystems. Prec Agric 9:355–366
Ruimy A, Saugier B, Dedieu G (1995) Methodology for the estimation of terrestrial net primary production from remotely sensed data. J Geophys Res 99:5263–5283
Schreiber U, Bilger W (1993) Progress in chlorophyll fluorescence research: major developments during the past years in retrospect. Proc Bot 53:151–173
Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, Heidelberg, pp 49–70
Schulze ED, Caldwell MM (1995) Ecological studies. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, Heidelberg, p 100
Schurr U, Walter A, Rascher U (2006) Functional dynamics of plant growth and photosynthesis – from steady-state to dynamics – from homogeneity to heterogeneity. Plant Cell Environ 29:340–352
Turner DP, Urbanski S, Bremer D et al (2003) A cross-biome comparison of daily light use efficiency for gross primary production. Global Change Biol 9:383–395
Van der Tol C, Verhoef W, Rosema A (2009) A model for chlorophyll fluorescence and photosynthesis at leaf scale. Agric Forest Meteorol 149:96–105
Weis E, Berry JA (1987) Quantum efficiency of photosystem II in relation to `energy´-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta 894:198–208
Acknowledgements
This work has been made possible by the funding support of the ESA-projects (1) Technical Assistance for Airborne/Ground Measurements in support of Sentinel-2 mission during CEFLES2 Campaign (ESRIN/Contract No. 20801/07/I-LG) (2) Technical Assistance for Airborne/Ground Measurements in support of FLEX mission proposal during CEFLES2 Campaign (ESRIN/Contract No. 20802/07/I-LG) (3) FLEX Performance analysis and requirements consolidation study (ESTEC/Contract No. 21264/07/NL/FF). Additional financial and intellectual support was provided by the SFB/TR 32 ‘Patterns in Soil-Vegetation-Atmosphere Systems: Monitoring, Modelling, and Data Assimilation’ – project D2, funded by the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Rascher, U. et al. (2010). Sensing of Photosynthetic Activity of Crops. In: Oerke, EC., Gerhards, R., Menz, G., Sikora, R. (eds) Precision Crop Protection - the Challenge and Use of Heterogeneity. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9277-9_6
Download citation
DOI: https://doi.org/10.1007/978-90-481-9277-9_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9276-2
Online ISBN: 978-90-481-9277-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)