Abstract
Dynamics of hydrophytic and helophytic vegetation is analyzed in relation to environmental changes from 1956 until the present day. These changes are mainly related to hydrological characteristics and water quality. In the case of submerged vegetation, community changes are related to alterations of the salinity and eutrophication regime, manifested in a decreased coverage and the extinction of some species. In the case of the helophytic vegetation, changes are associated with the fragmentation of the original vegetation patches, originally dominated by Cladium mariscus. Nowadays emergent vegetation is dominated by Typha domingensis, Phragmites australis, and, in the last years, by annual vegetation, nitrophilous taxa and woody species (Tamarix canariensis, T. gallica). This vegetation type indicates wet and saline conditions in soils. Despite these changes at the structural level, there is also evidence of a considerable increase in the biomass that accumulates every year in the wetland and which accelerates wetland siltation in the long term.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Macrophytes in wetlands are important biotic elements that usually function as ecosystem engineers. Some of them, mostly helophytes, provide the characteristics of the wetland (Keddy 2000), i.e. they contribute to shape the wetland landscape. They also play other environmental roles, without which wetland performance would be quite different. Among them we can point out the following:
-
1.
Helophytes greatly increase local transpiration (Sánchez-Carrillo et al. 2004).
-
2.
Helophytes limit water evaporation because their patches reduce wind fetch and, hence, wind effects on the water surface (Anderson and Idso 1987).
-
3.
Surface water motion is limited by helophyte patch distribution, focusing it through preferential paths. The distribution and flow of particulate and dissolved material in the wetland is, therefore, somewhat related to helophyte distribution (Kadlec and Knight 1996).
-
4.
Helophytes modify the sedimentary chemical environment through their root activity. Submerged vegetation also takes up dissolved nutrients and increases dissolved oxygen in water tables (Duarte et al. 1994; Mitsch and Gosselink 2001; Kufel and Kufel 2002).
-
5.
Macrophyte decomposition increases nutrient contents when decaying (Mason and Bryant 1974).
-
6.
Macrophyte decay increases organic matter accumulation if there is no material export downstream, contributing to wetland siltation (Cronk and Fennessy 2001).
-
7.
They provide refuge for many invertebrate and vertebrate animals, including mainly waterfowl (Dykyjová and Kvet 1978, www.ramsar.org).
All these ecological services can be observed in Las Tablas de Daimiel wetland. The structural and dynamic changes of flora and aquatic vegetation in the last 40 years are related to the same impacts that affect all wetlands in Mediterranean Europe: diminishing water availability as a result of agricultural practices, drying, changing morphometry of their basin, water pollution, salinization, invasive species and fires (Cirujano et al. 2002). Similar changes have also been found in tropical wetlands, such as in the Everglades of Florida (Ogden 2005). In this chapter we will describe the changes that submerged and emergent vegetation of this wetland has experienced since 1956.
The first studies relating water quality to macrophytes were carried out after the area was declared a National Park in 1970s (Pascual 1976; Sáez-Royuela 1977). These studies clearly state that aquifer overexploitation and surface water pollution were the main problems threatening the aquatic flora of this wetland, a finding which was also sustained by more recent research. The aim of this chapter is to review this previous research, building especially on the papers of Pascual (1976), Álvarez-Cobelas and Cirujano (1996, 2007), Cirujano et al. (1996, 2002), Camargo and Cirujano (1996), Álvarez-Cobelas et al. (2001, 2008), Ribeiro (2005) and Ribeiro et al. (2004).
2 Materials and Methods
2.1 Surveys and Reconstructions
To survey the flora we used common botanical methods, with seasonal surveys over most years since 1974. The long-term reconstructions were based on emergent vegetation maps of cut-sedge and reed and were drawn from aerial photographs taken in 1956, 1977, 1984, 1993, 1997, 2001 and 2007. In 1993 one member of the team (Cirujano) carried out field surveys which allowed to ascribe photographic patterns to either cut-sedge or reed vegetation in all maps. In 2001, along with Typha increase, a special effort was made to separate Phragmites and Typha covers in aerial photographs, through intensive field work, but this proved to be very difficult (see also Maheu-Giroux and de Blois 2005), and, hence, both plant species had to be considered together for the 2001 plant cover quantification. All maps were rasterized using the ARCVIEW-GIS software package (ESRI 2006). Maximum flooded area of the wetland (1,675 ha) was divided into a 1,675 (100 × 100 m) cells grid. The aerial cover of the two major emergent species was recorded in each cell, using the ARCVIEW planimetric tool, and results were reported as percentage cover.
2.2 Estimation of Growth and Decay
To measure the individual growth of helophytes, ten plants of each species were tagged in four stands distributed in the wetland to cover environmental variability, and their main leaf (Cladium and Typha) or stem (Phragmites) growth was recorded monthly. Growth rates of these elongating shoots were calculated assuming an exponential growth mode for all plant species, applying the commonly used relative growth rate equation (e.g. Hutchings 1997).
The decay of helophyte biomass (Cladium and Phragmites) was studied during 18 months in triplicate bags, using Polunin’s (1982) methods to assess nutrient leaching and overall plant decomposition.
Water level and the content of nitrogen and phosphorus in sediments and the water column were related with instantaneous growth rates of each species. The duration of flooding was correlated with yearly-averaged growth rates of each species, using non-parametric Spearman correlations (Conover 1999). The STATISTICA6.0 statistical package was used for most tests.
2.3 Biomass and Primary Production Estimations
Biomass and plant cover were recorded in smaller patches less than 1 ha. During late summer in 2000, 2001, 2002 and 2008, maximum aboveground biomass was cut in 1-m2 plots distributed throughout the wetland in 5–20 well developed patches. For charophytes, the biomass in 0.25-m2 plots were gathered in 1996, 1997, 2001 and 2008. After counting all plants in each plot to estimate species density, fresh biomass was dried in an oven at 65°C up to constant weight to obtain dry weight. For patch biomass, it was assumed that 1-m2 plots were roughly representative of biomass in the 1-ha (or smaller) patches; preliminary replicates of biomass measurements within 1-ha plots showed coefficients of variation lower than 30% for Cladium mariscus, Phragmites australis, and Thypha domingensis involved (Cirujano et al. 2003). Belowground biomass was measured only in 2002 and 2008; using a retrodigger, square plots of 1 m2 were excavated down to the deeper layer of roots (usually 1.0–1.5 m deep). All roots, rhizomes and stems were gathered, sorted, sent to the laboratory, dried and weighted separately for each species. Drawings of underground organs, reported elsewhere (Conway 1936; Fiala 1978; Rodewald-Rudescu 1974), enabled us to separate rhizomes from roots of each species prior to belowground biomass quantification. Primary production of Cladium and Phragmites in 2008 was determined using the methods reported in Cronk and Fennessy (2001).
2.4 Spatial Distribution of Helophytes
Spatial patterns were estimated by means of spatial correlograms (Legendre and Fortin 1989). Correlograms have been calculated on plant cover using Moran’s I autocorrelation statistic (Moran 1950). Because we dealt with sites on a surface (i.e. a grid of 1,675 cells), we expected a high number of values that could have complicated computations. Therefore, we reduced distance values by grouping and recoding them into distance classes by means of the R-Package (Casgrain and Legendre 2001), considering distance classes of equal length (141 m) (equidistant classes). Critical values for the autocorrelation values at each distance class were determined by means of a randomization method, followed by a Bonferroni correction (Legendre and Legendre 1998), whereas the overall significance of correlograms was tested following the Oden’s (1984) method, which also used the Bonferroni correction.
Cluster analysis, using a UPGMA algorithm (Legendre and Legendre 1998), was used to search for similarity of spatial patterns among correlograms of both emergent species. Euclidean distance was the metric used to calculate each correlation matrix for cluster analyses, because this metric more readily detects differences among correlograms and, hence, facilitated the identification of spatial structure. The overall correlogram was interpreted as a surrogate of spatial structure of combined environmental and biological traits.
Water depth and quality appear to be the main variables responsible for controlling cover of emergent plants (Cronk and Fennessy 2001). We reconstructed earlier data of flooding using gauge data in the catchment at the inlets of the wetland, recorded by the Guadiana Water Authority since 1940. Using both flooding data and a digital terrain model (Sánchez-Carrillo 2000), we calculated average water depth in 1-ha cells in the same years of aerial photographs (see above).
Surface water always entered the wetland through the NE Gigüela stream, and the main outlet was located in the SW part of the wetland; hence that NE–SW direction comprised the main wetland gradient. Since no data on water quality prior to 1993 were available, we used the coordinates of each cell in the NE–SW gradient as a surrogate of water quality, because most pollution entered the wetland through the Gigüela stream. The rationale for such an approach was that water pollution always impacted the wetland through the Gigüela river, and that this pollution effect decreased with distance to the Gigüela inlet (Sánchez-Carrillo and Álvarez-Cobelas 2001). Therefore, the closer a given site was from that inlet, the more impaired was its water quality.
To outline the main environmental factors explaining the spatial scale of plant cover, we regressed the principal coordinates selected in the principal coordinates of neighbour matrices with either water depth or water quality. Furthermore, to relate spatial patterns and controlling factors, four variables were examined, namely, reed- and cut-sedge cover in 1-ha plots, space as the Euclidean distance of the given 1-ha cell from the main water input to the wetland, and average water level in the preceding year of plant cover assessment in that 1-ha cell. Water depth and plant cover were measured in cm and percentage of the 1-ha cell, respectively.
Total phosphorus is usually considered as the key factor controlling primary productivity in many freshwater environments (Vollenweider 1968) and, more recently, in wetlands (Keddy 2000). Because most water pollution entered the wetland through the NE stream, water quality effects were more important the closer a given wetland area was to the NE stream. We calculated that effect as the Euclidean distance to the input site. Total available phosphorus data showed a high correlation with those distances, and they were considered as a proxy for phosphorus distribution in the wetland.
Pearson correlation among the four variables (helophyte cover of both species, hydrolevel, total phosphorus) was calculated to relate spatial pattern and their controlling factors. Its significance test was corrected using the CRH procedure to account for underlying spatial autocorrelation (Clifford et al. 1989), by means of the PASSAGE package (Rosenberg 2002). Briefly, this test adjusts degrees of freedom depending upon autocorrelation of spatial variables, based on the amount of autocorrelation in the data. Moran’s I statistic estimates the spatial autocorrelation in the plant cover and environmental data sets. Space (as a surrogate for water quality), water depth and plant cover of the other species (as an index of competition between emergent species) could be considered the main environmental controlling factors other than the biological traits of a given species. Therefore, the unexplained variability of a plant species cover by those factors in a given year can be considered as a proxy for biological traits of that species (Seabloom et al. 2001). This approach was used to reveal controlling factors of the spatial structure of both emergent plants at the whole wetland scale.
3 Aquatic Flora
As highlighted in previous chapters, Las Tablas de Daimiel wetland was flooded by water from different sources, having also specific chemical features, which resulted in two different habitats: one having permanent, fresh (860 ± 125 μS cm−1) and deeper (1–6 m) waters, and another having seasonal, oligohaline (2,778 ± 1,073 μS cm−1) and shallower (0–1 m) waters. While the former was due to the upwelling of the Llanura Manchega groundwater (see Chapter 1) and entered the wetland through the Guadiana River, the latter habitat was the result of flooding by the temporary Gigüela River (Fig. 7.1). The aquatic flora of these habitats also showed somewhat different composition and structure.
Large hydrophytes grew (Chara hispida var. major, Nuphar luteum, Nymphaea alba, Potamogeton lucens, Hydrocharis morsus-ranae, Fig. 7.1, Table 7.1) in the permanent habitat on peat substrate arising from the accumulation of decaying wetland vegetation lying on calcareous rock. In the shallow areas, and growing on sulfate-carbonate-rich limestone that were transported by the Gigüela river, there were compact submerged meadows comprising several charophyte species (Chara canescens, C. hispida, C. aspera, C. hispida var. major, etc.), locally termed “ovas” (Morillo and González 1996; Álvarez-Cobelas et al. 2001; Cirujano et al. 2002; Fig. 7.1, Table 7.1).
Helophytic vegetation was very homogenous and included large formations of cut-sedge (or giant sword grass, Cladium mariscus), locally called “masegones”. Littoral areas were covered by the remaining helophytes, mostly Phragmites australis, Typha domingensis, T. latifolia, Carex riparia and C. hispida (Table 7.2).
The pristine landscape structure was based upon the equilibrium between helophyte patches and helophyte-free areas, the latter being more frequent and extensive in the eastern, shallower area of the wetland, whose bottom areas were mostly covered by charophytes (Fig. 7.1, Zone 2). During the last 40 years, such a structure gradually changed, as a result of many anthropogenic impacts that started with partial desiccation and changing morphometry of the basin in the late 1960s, followed by corn and sunflower crops of the western part (25.5% out of the whole wetland). Crop cultivation within the wetland was discontinued in the 1980s, but fires that burnt the central area in 1987 (20% out of the total) caused new ecosystem-level alterations. Diminishing water inputs and the ongoing water pollution that has taken place since the 1970s to the present day have contributed to disrupt the natural hydrological regime in the wetland.
The main change was towards a chemically more homogeneous environment, but more fluctuating and somewhat stochastic as well, as a result of strong changes in water availability and ongoing eutrophication (see Chapter 6). This influenced plant species richness in a variety of ways. While the absence of fresh and permanent waters was the cause for the disappearance of large macrophytes (33.3% of species lost), water pollution, lower hydrolevels and hydroperiods and salt contents increasingly enhanced the occurrence of plant species that were better adapted to these conditions (Lemna minor, L. gibba and Chara connivens; Ranunculus peltatus, R. trichophyllus; Ruppia maritima, Tolypella hispanica, respectively). The final outcome was that the numbers of species associated in the area with the temporary Gigüela river did not change, but species composition did (Table 7.1).
The wetland is gradually reducing its submerged flora in favour of helophytes, and is even characterized by the invasion of terrestrial vegetation (see below). This phenomenon has also been reported in other agriculturally-impacted wetlands, such as Clear Lake in Iowa (Egertson et al. 2004).
Submerged meadows of charophytes are the best index of ecological conservation for this wetland. Its occurrence and abundance are dependent upon hydroperiod, water quality and the pressure of two invasive species that strongly feed on them, the common carp (Cyprinus carpio) and, more specifically, the American red swamp crayfish (Procambarus clarkii). An assay on crayfish food has demonstrated that it feeds selectively on charophytes rather than on other aquatic plants (Ceratophyllum submersum), to that charophyte consumption enhances crayfish weight from 0.27 ± 0.06 to 0.78 ± 0.12 g DW (Cirujano et al. 2004; Fig. 7.2).
Mean values (n = 5) of dry- and wet weight (g) of daily consumption of Chara hispida var. major by crayfish specimens of different size (small: 15.3 ± 1.25 g; medium: 25.9 ± 2.8 g and large: 40.8 ± 5.72 g) from Las Tablas de Daimiel in a mesocosm experiment (Cirujano et al. 2004)
Water pollution impaired growth of charophyte meadows, total phosphorus being a measure of such impairment (Blindow 1988, 1992; Kufel and Kufel 2002). In this wetland, charophyte meadows normally grow better at 0.17 ± 0.11 mg P l−1, starting to decline when total phosphorus concentration reaches 1.77 ± 0.41 P l−1. Charophyte cover has fluctuated as a result of both impaired water quality and reduced flooding; nowadays it is lower than 1 ha, caused by a recent drought (Fig. 7.3), but it is likely that increased flooding might promote recolonization of charophytes from propagule banks in former areas of growth.
4 Helophyte Cover and Growth
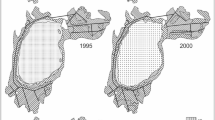
From a spatial perspective, changes in emergent vegetation resulted in strong fragmentation and cover loss of cut-sedge patches (Cladium mariscus; see below, Álvarez-Cobelas et al. 2008), with a decrease of 89% of the original cover, and a variable increase of reed (Phragmites australis) and cattail (Typha domingensis) cover, up to 24% and 237%, respectively. Such a substitution was caused by the elimination of 405 ha of cut-sedge cover in the period 1969–1977, the subsequent burning of 110 ha in 1987, and the stochastic flooding occurring later, that enhanced reed growth (Figs. 7.4 and 7.5). A key factor for maintaining biological richness of this wetland was the balance between helophyte-free and helophyte covered areas. Thus, helophyte-free area in 1956, when the wetland was in pristine condition, amounted to 22.6% of the whole wetland and since then it fluctuated between 14.5% and 38% without any clear time trend. This did not imply that those areas were colonized by charophyte meadows, since either water scarcity or pollution prevented or limited submerged plant growth (Fig. 7.6).
In recent years, water scarcity changed vegetation dynamics into terrestrial plant communities in which helophyte-free areas were colonized by invasive nitrophilous species (Erygeron canadensis, E. bonariensis, Aster squamatus), that occupied 79% and 95% of total helophyte-free areas in 2007 and 2008, respectively, with a growing contribution of woody species (Tamarix canariensis, T. gallica) that comprised the potential forest vegetation of wet and saline soils (Fig. 7.7).
Growth rates of cut-sedge were higher at NE sites, ranging from 0.001 to 0.022 d-1. Reed growth was similar at all sites, its overall range being 0.001–0.045 d-1. In 2001, cattail growth rates were markedly higher at SW sites than at NE sites, its range being 0–0.040 d-1 (Álvarez-Cobelas and Cirujano 2007).
Sedimentary nutrients displayed significant relationships with plant growth rates. While Cladium growth rate was positively related with nitrogen, Phragmites growth rate was negatively related with the N:P ratio, with the critical value around 4. Typha growth rate was negatively related with sedimentary nitrogen and phosphorus (Álvarez-Cobelas and Cirujano 2007). Water level was weakly but positively related with growth rates of Cladium and Typha (Álvarez-Cobelas and Cirujano 2007).
Only leaf elongation rates of Typha came closer to the highest reported rates (Dykyjová and Kvet 1978). Cladium and Phragmites never reached the highest reported rates (Conway 1938; Dykyjová and Kvet 1978; Lorenzen et al. 2001). Furthermore, these species presented strong inter-site variability. Therefore, helophyte growth in our studied wetland was suboptimal at the single plant level, as also judged by linear relationships between growth and environmental controls. For shoot elongation, controlling factors were found to differ among species. For Cladium it was nitrogen, as in other studies (Urban et al. 1993; Miao and Sklar 1998). Sedimentary N:P ratio was important for Phragmites; this has also been reported by Güsewell et al. (2003) for many wetland species, thus providing an explanation of conflicting evidence about abiotic controls of reed growth (Clevering 1998; Kohl et al. 1998). In the case of Typha, however, growth rate was negatively related to water N and P, despite positive relationships in other wetlands (Grace 1988; Miao et al. 2000).
Cut-sedge and reed cover were related with average flooding in the preceding year, albeit in an opposite way: as flooding increased, Cladium increased and Phragmites decreased (Fig. 7.8). These lagged responses to flooding are common in other wetlands (Armentano et al. 2006; Egertson et al. 2004).
Average water level appeared to control Cladium biomass up to 20–40 cm, above which water level increase did not enhance biomass increase (Álvarez-Cobelas and Cirujano 2007). We also observed a statistically significant, negative relationship between sedimentary phosphorus and cut-sedge biomass and a 1-year delayed, unimodal response of biomass to average water level, suggesting inhibition of plant growth at deeper sites (Álvarez-Cobelas and Cirujano 2007).
Reed biomass could be controlled by water level variability (positive relationship) as well as by averaged water level of the preceding year (negative relationship, Álvarez-Cobelas and Cirujano 2007). Cattail biomass appeared to be related with sedimentary phosphorus, also showing a positive and nonlinear relationship with average duration of flooding in the preceding year (Álvarez-Cobelas and Cirujano 2007).
5 Plant Biomass, Production and Decomposition
Charophyte biomass was promoted by flooding and good water quality. Available data indicated that it could reach more than 3 kg DW m−2 (Fig. 7.9), a value that was higher than most reported values for other wetlands (Kufel and Kufel 2002).
Cut-sedge biomass attained the higher values at NE sites, where its populations always grew better. In 2001 and 2002, more biomass was recorded than in 2000 (Álvarez-Cobelas and Cirujano 2007). In 2008, there appeared to be an increase in cut-sedge biomass, but it was the result of the higher variability (Fig. 7.10). Overall, biomass variability ranged between 1 and 11 kg DW m−2. In fact, there were few instances in the wetland where cut-sedge reached its optimal growth. Belowground biomass, only measured in 2002 and 2008, showed strong fluctuations between years, the ratio above: belowground biomass hence showing changing ratios interannually (Fig. 7.10).
Reed biomass also appeared to be higher at the NE entrance (the shallower sites of the wetland and river discharge sites), and it was higher in 2000 than in 2001, 2002 and 2008, reaching 4.02 ± 1.76, 2.74 ± 1.18, 3.26 ± 1.50, 2.03 ± 0.80 kg DW m−2 in 2000, 2001, 2002 and 2008, respectively (Álvarez-Cobelas and Cirujano 2007; Fig. 7.9). Belowground biomass was higher than aboveground biomass in 2002 and 2008, increasing the ratio from 1.24 to 2.33.
When the hydroperiod lasted throughout the year, Typha domingensis thrived and its biomass was similar over time, showing low spatial variability in the wetland (2.63 ± 1.48, 2.14 ± 0.84 and 4.03 ± 2.41 kg DW m−2 in 2000, 2001 and 2002, respectively). Belowground biomass was similar to aboveground biomass in 2002 (3.97 ± 1.82 vs 4.03 ± 2.41 kg DW m−2; Álvarez-Cobelas and Cirujano 2007). In 2008, Typha did not grow in this wetland. In Las Tablas de Daimiel aboveground biomass of these three helophytes was often higher than the values reported in other studies (for instance, Dykyjová and Kvet 1978; Miao and Sklar 1998; Vymazal 1995). The aboveground: belowground biomass ratio of these helophytes, however, was lower in TDNP than in most wetlands (Dykyjová and Kvet 1978; Fiala 1978; Lorenzen et al. 2001).
In this wetland, aerial biomass of annual plant populations was negligible before 2006. As a result of a heavy drought, they thrived later and peaked in 2007 and 2008, when they attained 3.56 ± 3.06 and 1.40 ± 1.21 kg DW m−2, respectively (Fig. 7.9; Rodríguez-Murillo, personal communication). The relationship between above and belowground biomass in annual plants decreased, changing from 7.3 in 2007 to 0.61 in 2008.
In 2008, aerial production of cut-sedge reached 1.67 ± 1.28 kg DW m−2 and 1.53 ± 0.3 kg DW m−2 for a flooded and a non-flooded site, respectively. In 2008, aerial production of reed has ranged 0.91–3.05 kg DW m−2 throughout the wetland (Rodríguez-Murillo, personal communication).
Helophyte decomposition was a slow process for Cladium and Phragmites in Las Tablas de Daimiel, with 50% of the initial biomass being decomposed after 1.5 years. There were no significant correlations between environmental variables and decomposition rates. An initial phase of leaching with the highest rates (p < 0.05) of mass loss and nutrients occurred for both plant species. Throughout the incubation period, no significant differences were observed between both species and experiments, and the average daily rates of mass loss were low: 0.0022 ± 0.001 day–1 for C. mariscus and 0.0029 ± 0.002 day–1 for P. australis in the overall decomposition experiment, and 0.0019 ± 0.001 day–1 and 0.0016 ± 0.001 day–1 due to chemical-microbiological decomposition, suggesting the negligible significance of invertebrates in the plant decomposition process. Decaying mass rates of reed in this wetland were similar to those of Andersen (1978) in Danish wetlands whereas those of Cladium were lower than those recorded by Newman et al. (2001) for C. jamaicensis in the Florida Everglades. To our knowledge, there are no data on C. mariscus decomposition rates. Furthermore, no significant difference was observed for nutrient release, except that Phragmites lost more phosphorus than Cladium, but differences were not significant (p < 0.1). Plant litter decaying rates were roughly an order of magnitude lower than nutrient release rates in both species. Our results suggest that slow decomposition rates of emergent plant biomass increases organic matter sedimentation and reduces water quality at Las Tablas de Daimiel.
There are increasing numbers of long-term studies in wetlands at the landscape level (Leendertsee et al. 1997; Visser et al. 1999; Alvarez et al. 2005). Our study covering the period 1956–2008 showed a trend of reed cover increase and cut-sedge cover decrease over time. In most studies, water level increase and water pollution are the causes for the observed changes. In Las Tablas de Daimiel, water pollution certainly is the most important cause for reed cover dynamics, but water level is the main factor responsible for long-term cover changes of cut-sedge. Notwithstanding, when cover expansion rates are considered, no average flooding effects are observed, and only a weak water quality effect may be suggested for cut-sedge decline at the landscape scale. Saltmarsh et al. (2006) report that, as water stress increases, photosynthetic efficiency of reed is higher than that of cut-sedge. This mechanism may be important in natural habitats, but it only partly explains the patterns observed in TDNP, given that water quality impairment may also affect emergent species in this wetland.
6 Spatial Heterogeneity of Helophytes
The size of Cladium and Phragmites patches, defined as their longest axial dimension, varied from 1956 to 2001 and ranged between 2,300–4,100 m and 1,300–4,500 m for cut-sedge and reed, respectively. The size of cut-sedge patches decreased from 1956 onwards, whereas the trend of reed patch size was inconsistent until 1984. Throughout the study, cut-sedge patches were often bigger than those of reed (Álvarez-Cobelas et al. 2008). Two groups of distinct spatial heterogeneity were recorded for cut-sedge, an earlier natural one (1945–1977) and a later, anthropogenically-driven one, with 1984 as the turning year. Because reed expansion in this wetland occurred in the early 1990s, it was not surprising that there also were two spatially-different groups which then split. The spatial pattern of both helophytes changed more in recent periods, along with anthropogenic change (Álvarez-Cobelas et al. 2008).
Water quality was partly responsible for spatial patterns of Cladium and Phragmites at the very broad scale (>1,000 ha), while water depth explained variability in plant cover at the very broad and broad scales (500–1,000 ha). The overall variability explained by biotic (the cover of the other species) and abiotic (water depth and water quality) factors never explained more than 39% of overall variability (Álvarez-Cobelas et al. 2008).
These results suggested changing patterns of emergent plant cover in space and time. From the temporal perspective, cut-sedge and reed showed distinct responses between periods dominated by natural variability (1945–1977) and anthropogenic change (1977 onwards). During the period of natural variability, no fragmentation of patches of both emergent plants took place. Man-made change, however, resulted in fragmentation of cut-sedge patches, and the influence of biological traits on its spatial patterns increased. Reed patches showed increasing cover in recent years, but the trend of its spatial pattern was unclear. Patch fragmentation is frequent in species subject to man-made change (Turner 2005).
Based on results obtained from the cold-temperate Delta Marsh (Canada), Seabloom et al. (2001) hypothesized that plant cover is controlled by environmental gradients under stable conditions but biological traits become more important for spatial patterns when the wetland turns to unstable situations. Results from forests support this hypothesis (Camarero et al. 2000; Pu et al. 2005). Cladium cover can be controlled by the abiotic environment during stable conditions (i.e. in pristine years), whereas species biology partly governs spatial pattern when unstable conditions prevail (Álvarez-Cobelas et al. 2008). By contrast, Phragmites did not follow such trends, perhaps because of its faster growth (Ribeiro 2005) which made species cover more fluctuating and unpredictable.
References
Alvarez MG, Tron F, Mauchamp A (2005) Sexual versus asexual colonization by Phragmites australis: 25-year reed dynamics in a Mediterranean marsh, southern France. Wetlands 25:639–647
Álvarez-Cobelas M, Cirujano S (eds) (1996) Las Tablas de Daimiel: ecología acuática y sociedad. Ministerio de Medio Ambiente, Madrid
Álvarez-Cobelas M, Cirujano S (2007) Multilevel responses of emergent vegetation to environmental factors in a semiarid floodplain. Aquat Bot 87:49–60
Álvarez-Cobelas M, Cirujano S, Sánchez-Carrillo S (2001) Hydrological and botanical man-made changes in the Spanish wetland of Las Tablas de Daimiel. Biol Conserv 97:89–97
Álvarez-Cobelas M, Sánchez-Carrillo S, Cirujano S, Angeler DG (2008) Long-term changes in spatial patterns of emergent vegetation in a Mediterranean floodplain: natural versus anthropogenic constraints. Plant Ecol 194:257–271
Andersen OFO (1978) Effects of nutrient level on the decomposition of Phragmites communis Trin. Archiv Hydrobiol 84:42–54
Anderson MG, Idso SB (1987) Surface geometry and stomatal conductance effects on evaporation from aquatic macrophytes. Water Resour Res 23:1037–1042
Armentano TV, Sah JP, Ross MS, Jones DT, Cooley HC, Smith CS (2006) Rapid responses of vegetation to hydrological changes in Taylor Slough, Everglades National Park, Florida, USA. Hydrobiologia 569:293–309
Blindow I (1988) Phosphorus toxicity in Chara. Aquat Bot 32:393–395
Blindow I (1992) Decline of charophytes during eutrophication: comparison with angiosperms. Freshw Biol 28:9–14
Camarero JJ, Gutiérrez E, Fortin MJ (2000) Spatial pattern of subalpine forest-alpine grassland ecotones in the Spanish Central Pyrenees. For Ecol Manage 134:1–16
Camargo JA, Cirujano S (1996) Reduction in diversity of aquatic plants in a Spanish wetland: the effect of the size of inundated area. J Freshw Ecol 12:539–543
Casgrain P, Legendre P (2001) The R-Package for multivariate and spatial analysis, version 4.0 d3 – User’s manual. Departament de sciences biologiques, Université de Montreal, Montreal
Cirujano S, Casado C, Bernués M, Camargo JA (1996) Ecological study of Las Tablas de Daimiel National Park (Ciudad Real, Central Spain): differences in water physico-chemistry and vegetation between 1974 and 1989. Biol Conserv 75:211–215
Cirujano S, Medina L, Chirino M (2002) Plantas acuáticas de las lagunas y humedales de Castilla-La Mancha. Junta de Comunidades de Castilla-La Mancha – Real Jardín Botánico, Madrid
Cirujano S, Álvarez-Cobelas M, Riolobos P, Ribeiro MD, Sánchez-Carrillo S, Medina L, Moreno M, Angeler DG, Rojo C, Rodrigo MA, Armengol J, Ortega-Mayagoitia E (2003) Seguimiento y recuperación ambiental del P.N. Las Tablas de Daimiel. Informe 1999-2002 para el Organismo Público Parques Nacionales, Madrid
Cirujano S, Camargo JA, Gómez-Cordobés C (2004) Feeding preferences of the red swamp crayfish Procambarus clarkii (Girard) on living macrophytes in a Spanish wetland. J Freshw Ecol 19:219–226
Clevering OA (1998) An investigation into the effects of nitrogen on growth and morphology of stable and die-back populations of Phragmites australis. Aquat Bot 60:11–25
Clifford P, Richardson S, Hémon D (1989) Assessing the significance of the correlation between two spatial processes. Biometrics 45:123–145
Conover WJ (1999) Practical non-parametric statistics. Wiley, New York
Conway VM (1936) Studies in the autoecology of Cladium mariscus R.Br. I. Structure and development. New Phytol 35:177–205
Conway VM (1938) Studies in the autoecology of Cladium mariscus R.Br. III. Growth rates of the leaves. New Phytol 37:254–278
Cronk JK, Fennessy MS (2001) Wetland plants. Biology and ecology. Lewis, Boca Ratón, FL
Duarte CM, Planas D, Peñuelas J (1994) Macrophytes, taking control of an ancestral home. In: Margalef R (ed) Limnology now. Elsevier, Amsterdam
Dykyjová D, Kvet J (eds) (1978) Pond littoral ecosystems. Structure and function. Springer-Verlag, Berlin
Egertson CJ, Kopaska JA, Downing JA (2004) A century of change in macrophyte abundance and composition in response to agricultural eutrophication. Hydrobiologia 524:145–156
ESRI (2006) ArcGIS Version 9.2 and the Spatial Analyst Extension, Environmental Systems Research Institute, Redlands, California
Fiala K (1978) Underground organs of Typha angustifolia and Typha latifolia: their growth, propagation and production. Acta Sci Nat Acad Sci Bohemosl Brno 12:1–43
Grace JB (1988) The effects of nutrient additions on mixtures of Typha latifolia L. and Typha domingensis Pers. along a water-depth gradient. Aquat Bot 31:83–92
Güsewell S, Koerselman W, Verhoeven JTA (2003) Biomass N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol Appl 13:372–384
Hutchings MJ (1997) The structure of plant populations. Plant litter quality and decomposition: a historical overview. In: Crawley MJ (ed) Plant Ecol. Blackwell, Oxford
Kadlec RH, Knight RL (1996) Treatment wetlands. CRC Lewis, Boca Ratón, FL
Keddy PA (2000) Wetland ecology. Cambridge University Press, Cambridge
Kohl JG, Woitke P, Kühl H, Dewender M, König G (1998) Seasonal changes in dissolved amino acids and sugars in basal culm internodes as physiological indicators of the C/N-balance of Phragmites australis at littoral sites of different trophic status. Aquat Bot 60:221–240
Kufel L, Kufel I (2002) Chara beds acting as nutrient sinks in shallow lakes-a review. Aquat Bot 72:249–260
Leendertsee PC, Roozen AJM, Rozema J (1997) Long-term changes (1953-1990) in the salt marsh vegetation at the Boschplaat on Terschelling in relation to sedimentation and flooding. Plant Ecol 132:49–58
Legendre P, Fortin MJ (1989) Spatial pattern and ecological analysis. Vegetatio 80:107–138
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
Lorenzen B, Brix H, Mendelssohn IA, McKee KL, Miao SL (2001) Growth, biomass allocation and nutrient use efficiency in Cladium jamaicense and Typha domingensis as affected by phosphorus and oxygen availability. Aquat Bot 70:117–133
Maheu-Giroux M, de Blois S (2005) Mapping the invasive species Phragmites australis in linear wetland corridors. Aquat Bot 83:310–320
Mason CF, Bryant RJ (1974) Production, nutrient content and decomposition of Phragmites communis Trin. and Typha angustifolia L. J Ecol 63:71–95
Miao SL, Sklar FH (1998) Biomass and nutrient allocation of sawgrass and cattail along a nutrient gradient in the Florida Everglades. Wetlands Ecol Manage 5:245–263
Miao SL, Newman S, Sklar FH (2000) Effects of habitat nutrients and seed sources on growth and expansion of Typha domingensis. Aquat Bot 68:297–311
Mitsch WJ, Gosselink JG (2001) Wetlands, 3rd edn. Van Nostrand Reinhold, New York
Moran PAP (1950) Notes on continuous stochastic phenomena. Biometrika 37:17–23
Morillo C, González JL (1996) Management of Mediterranean Wetlands 2. Ministerio de Medio Ambiente, Madrid
Newman S, Kumpf H, Laing JA, Kennedy WC (2001) Decomposition responses to phosphorus enrichment in an Everglades (USA) slough. Biogeochemistry 54:229–250
Oden NL (1984) Assessing the significance of a spatial correlogram. Geogr Anal 16:1–16
Ogden JC (2005) Everglades ridge and slough conceptual ecological model. Wetlands 25:810–820
Pascual H (1976) Contribución al estudio ecológico de Las Tablas de Daimiel. I. La vegetación. Anales INIA 2:107–128
Polunin NVC (1982) Processes contributing to the decay of reed (Phragmites australis) litter in fresh water. Archiv Hydrobiol 94:182–209
Pu M, Jones RH, Guo D, Lister A (2005) Regeneration strategies, disturbance and plant interactions as organizers of vegetation spatial patterns in a pine forest. Landscape Ecol 20:971–987
Ribeiro MD (2005) Biología de los macrófitos emergentes en Las Tablas de Daimiel. Ph.D. thesis. Universidad Complutense, Madrid
Ribeiro MD, Alvarez-Cobelas M, Riolobos P, Cirujano S (2004) Descomposición de los helófitos en un humedal semiárido hipertrófico. Anal Jar Bot Madrid 61:53–61
Rodewald-Rudescu L (1974) Das Schilfrohr. Die Binnengewässer, 27. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart
Rosenberg MS (2002) PASSAGE. Pattern Analysis, Spatial Statistics, and Geographic Exegesis. Department of Biology, Arizona State University, Tempe, Version 1.0
Sáez-Royuela R (1977) Contributión al studio ecológico de las Tablas de Daimiell III, Las aguas (1974–1975). Anal INIA, Serie Recursos Naturales 3:101–149
Saltmarsh A, Mauchamp A, Rambal S (2006) Contrasted effects of water limitation on leaf functions and growth of two emergent co-occurring plant species, Cladium mariscus and Phragmites australis. Aquat Bot 84:191–198
Sánchez-Carrillo S (2000) Hidrología y sedimentación actual de Las Tablas de Daimiel. Ph.D. Thesis. Universidad Autónoma, Madrid
Sánchez-Carrillo S, Álvarez-Cobelas M (2001) Nutrient dynamics and eutrophication patterns in a semiarid wetland: the effects of fluctuating hydrology. Water Air Soil Pollut 131:97–118
Sánchez-Carrillo S, Angeler DG, Sánchez-Andrés R, Alvarez-Cobelas M, Garatuza-Payán J (2004) Evapotranspiration in semi-arid wetlands: relationships between inundation and the macrophyte cover:open water ratio. Adv Water Resour 27:643–655
Seabloom EW, Moloney KA, van der Valk AG (2001) Constraints on the establishment of plants along a fluctuating water-depth gradient. Ecology 82:2216–2232
Turner MG (2005) Landscape ecology: what is the state of the science? Ann Rev Ecol Evol Syst 36:319–344
Urban NH, Davis SM, Aumen NG (1993) Fluctuations in sawgrass and cattail densities in Everglades Water Conservation Area 2A under varying nutrient, hydrological and fire regimes. Aquat Bot 46:203–223
Visser JM, Sasser CE, Chabreck RH, Linscombe RG (1999) Long-term vegetation change in Louisiana tidal marshes, 1968–1992. Wetlands 19:168–175
Vollenweider RA (1968) Scientific Fundamentals on the Eutrophication of Lakes and Flowing Waters, with particular reference to Nitrogen and Phosphorus as Factors in Eutrophication. OECD Report, Paris
Vymazal J (1995) Algae and element cycling in wetlands. CRC Press, Boca Ratón, FL
Acknowledgments
We are very grateful to Juan Carlos Rodríguez-Murillo for his data on primary production and biomass of helophytes and terrestrial plants in 2008.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Netherlands
About this chapter
Cite this chapter
Cirujano, S., Álvarez-Cobelas, M., Sánchez-Andrés, R. (2010). Macrophyte Ecology and Its Long-term Dynamics. In: Sánchez-Carrillo, S., Angeler, D. (eds) Ecology of Threatened Semi-Arid Wetlands. Wetlands: Ecology, Conservation and Management, vol 2. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9181-9_7
Download citation
DOI: https://doi.org/10.1007/978-90-481-9181-9_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9180-2
Online ISBN: 978-90-481-9181-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)