Abstract
In the last 20 years specific methods of dialysis, different from those usually provided in chronic uremic patients has been developed for CRRT in ICUs patients. In the last 20 years, clinical experiences have demonstrated that continuous and intermittent techniques (prolonged intermittent renal replacement therapy, PIRRT) are overlapping for efficacy and patients survival. In addition, in this brief review we focus on recent data shedding light on two crucial points of high clinical impact on CRRT practice, such as dose of dialysis and anticoagulation of extracorporeal circuit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Continuous Renal Replacement Therapy
- Extracorporeal Circuit
- Regional Citrate Anticoagulation
- Dialysis Dose
- Blood Circuit
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Since preliminary experiences of Kramer with arteriovenous hemofiltration at low blood flow in 1977 [1], technological improvements led to develop a dedicated hardware (monitors, filters) for Continuous Renal Replacement Therapy (CRRT).
After some technical aspects of CRRT, this brief review will focus on two crucial points of high clinical impact on CRRT practice such as dose of dialysis and anticoagulation of extracorporeal circuit.
8.1 CRRT: An Overview of the Different Techniques
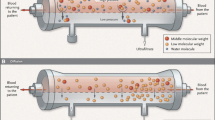
Dialysis circuit consists of : (1) the blood circuit; (2) the dialysate circuit. The core of dialysis process is the filter, where a semipermeable membrane separates the blood and dialysate compartments. Dialysis membrane rules the exchanges of water and solutes between blood and dialysate compartments driven by diffusive and convective fluxes (Fig. 8.1).
The different dialytic methodologies differ in a different assembly of the components, that is in a increasing degrees of technical complexity. They ranges from SCUF, in which there is a loss of water and solutes by exclusive convective motion without replacement liquid infusion, to continuous hemodiafiltration (CVVHDF) in which there are an exchange of solutes with both convective and diffusive motions, and a replacement liquid infusion.
8.1.1 Filters for CRRT
Filters for CRRT are hollow fiber filters with synthetic membrane, in polysulfone, polyethersulfon, polyamide, or polymethylmetacrilate.
Filters for CRRT are characterized by a membrane exchange area of 0.8–2 mq, and by an inlet blood flow of 100–300 ml/min. The membrane is highly permeable, with a cut-off for diffusible solutes of 20–40,000 Da. By these filters, convective motion of solutes throughout membranes allows a sustained clearance of middle and middle–high-molecular weight substances (1,000–30,000 Da). In addition, by these filters convective exchange can be coupled with a dialysate flow, allowing also a diffusive exchange of solutes.
The ability of a molecule to pass through a semipermeable membrane is expressed as sieving coefficient value (SC, see formula in Fig. 8.2).
Several hydraulic factors such as blood flow, transmembrane pressure, and filtration fraction (FF) affect SC during dialysis. In particular, SC varies inversely proportional to the FF. In order to avoid a marked reduction of SC due to an excessive concentration of blood and plasma proteins along fibers, FF is optimal for values of 15–20 % of blood flow. At these FF values, phenomena of fouling of membrane are limited, and the membrane can maintain a constant SC for whole treatment duration. As a matter of fact, membranes such as polymethylmethacrylate also have an adsorption capacity. Therefore, with polymethylmethacrylate molecules trapped in the membrane are not found in ultrafiltrate.
8.1.2 SCUF-Slow Continuous Ultrafiltration
-
SCUF needs a pump in blood circuit and a pump in ultrafiltrate circuit (optional)(two- or one-pump circuit).
-
SCUF do not use infusion replacement fluid or dialysate.
-
Ultrafiltrate rate is modulated by patient’s hemodynamic tolerance.
-
Indicative flow rates: blood pump flow rate: 80–150 ml/min; ultrafiltrate flow rate 10–30 ml/min.
-
Indications:. Los of excess fluid in overloaded patients not responsive to diuretic therapy (edemigene syndromes and congestive heart failure).
-
Comments: Depurative capacity is very poor, and it is proportional to amount of ultrafiltrate.
8.1.3 CVVHF-Continuous Veno-Venous Hemofiltration
-
CVVH needs a pump on blood circuit, a pump for ultrafiltration, and a pump for replacement fluid in dialysate circuit (three-pumps circuits). The method is exclusively convective, and it optimally works with a FF of 15–20 % of blood flow.
-
In CVVHF replacement fluid can take place after dialysis filter (post dilution CVVHF), before the dialysis filter (pre dilution CVVHF), or before and after the filter (pre/postdilution CVVHF).
-
In post dilution CVVHF an important hemoconcentration of blood along dialysis fibers occurs. Circuit clotting risks are greater, and in general post dilution CVVHF needs higher amount of anticoagulant than predilution CVVHF.
-
In predilution CVVHF solute dilution occurs before filter exchanges. However, solute clearance is lower than that predicted by theory, probably for the diffusion of solutes across the membrane of erythrocytes in circuit line after predilution site. For example, in the case of urea with Qb of 150 mL/min and a sample predilution of 75 ml/min, measured clearance reduction is equal to 30 %, compared with a theoretical reduction of 40 % (an increase about 10 % of urea clearance has been demonstrated).
-
Indicative flow rates:
Postdilution CVVHF: blood pump 150–200 ml/min; ultrafiltrate 20–40 ml/min.
Predilution CVVHF: blood pump 100–150 ml/min; ultrafiltrate 30–60 ml/min.
8.1.4 CVVHD: Continuous Veno-Venous Hemodialysis
-
Method is exclusively diffusive, and requires a pump for blood circuit, a pump for inlet dialysate and a pump for outlet dialysate (three-pumps circuits).
-
Dialysate is usually set at low flow rate, not exceeding 30 % of blood flow value. As a result, saturation of small- to medium-sized molecules in the effluent is equal to 100 %.
8.1.5 CVVHDF: Continuous Veno-Venous Hemodiafiltration
-
CVVHDF is mixed method combining diffusion and convection. In addition to dialysate, CVVHDF uses an infusion fluid solution in pre and/or postfilter. Predilution CVVHDF (infusion fluid in prefilter) provides same hemorheology benefits described for predilution CVVHF.
-
CVVHDF is the best compromise to getting high clearance of both small and medium-sized molecules. In fact, CVVHDF by adding a diffusive component is more efficient than CVVHF (pure convection) in removing low-molecular weight substances. On the other side, in comparison to CVVHD CVVHDF shows a good clearance for substances with a molecular weight close to membrane cut-off, i.e., between 5,000–30,000 Da,
-
CVVHDF is the currently most used method in CRRT.
8.1.6 HV-HF: High Volume Hemofiltration
-
HV-HF uses high exchange volumes, generally reaching 50–70 L/day.
-
To ensure these volumes of ultrafiltrate, in HV-HF blood flow rate are usually 200–250 mL/min and are coupled with high permeability and high surface filters (>2 sqm).
-
Infusion is usually done simultaneously in both pre and postdilution to get an excellent convective clearance of high-molecular weight substances, and to take advantage of the predilution effects on filter duration.
8.2 Dose of Dialysis
The dose of dialysis, like every other therapy, is intended as a dosage of a drug, which in turn depends on specific indications, on therapeutic efficacy and on side effects presence. In this context, first the dose of dialysis in ICUs is conditioned by catabolic state and patient’s muscle mass, by presence of sepsis, of comorbidities, of electrolytic disorders and hydration state.
RRT lasting 4 h and 3 times a week at high dialytic efficiency usually allows survival in chronic uremic patients. However, when these patterns of short dialysis protocol were applied in ICUs patients they suffered from poor dialysis tolerance and insufficient dialysis dose. In these patients, mortality was significantly increased in comparison to patients treated with intermittent daily long treatments [2]. In fact, short treatments were abandoned for prolonged and continuous intermittent treatments [3].
8.2.1 Prescribed and Delivered Dialysis Dose
Delivered dialysis dose is different from prescribed dose, because the latter always overestimates the dose actually delivered. As recently confirmed by the International Collaborative Multicentre DOse REsponse Initiative (DoReMi) [4], the difference is the “down-time” from therapy that is dialysis time window in which CRRT machine does not work [4]. The down-time during dialysis can be due to bag and/or circuit exchanges (for early coagulation, interruption for diagnostic/therapeutic procedures and other), during periods of inactivity or low efficiency dialysis for vascular access problems (real dialysis flow rate lower than prescribed), or even in the dialysis prescription errors. In quantitative terms, the difference between the prescribed and delivered dose is usually significant, reaching the 30–40 % of the total daily prescribed dose [4, 5].
8.2.2 Calculation of Dialysis Dose
The exact calculation of delivered dialysis dose needs the direct measurement of solutes in effluent, in order to calculate solutes mass transfer and clearance. Since these measurements are not feasible in clinical practice, taking into account SC of urea is equal to 1 the urea effluent concentration is considered equal to blood concentration. Therefore, volume of effluent is clearance of urea, the low-molecular weight nitrogen catabolite. However, the concept of a direct relationship between effluent volume and urea clearance has some important limitations: (1) there is no proof that toxicity in acute uremic patients is due to urea, or that urea is a good marker of uremic toxicity; (2) where urea was a good marker, measurement of dialysis dose should be done in patients with a stable urea generation and predictable urea distribution volume, conditions not appropriate for critically ill ICU patients; (3) if it is correct that effluent volume is equal to clearance in postdilution CVVHF, this is not the case in predilution CVVHF, or in HV-HF (at high flow rate exchanges); (4) clearance of metabolites in medium–high-molecular weight range (1,000–10,000 Da) is meaningful only in hemofiltration/hemodialfiltration, and concerned only with low protein binding solutes.
Keeping in mind all these limitations, in clinical large trials effluent dose is the usual way to calculate the dialysis dose. Dialysis dose is usually expressed as L/h or ml/Kg/hour, or better with ml/Kg/day (in the latter case, also PIRRT could be compared with continuous treatments) [6].
8.2.3 Dialysis Dose and Survival
A relationship between dialysis dose and survival was first reported in 1996 by Paganini et al. [7]. By stratifying clinical severity of disease and dialysis dose in 842 patients treated with intermittent hemodialysis or CRRT, Paganini et al. demonstrated that survival of the extreme less serious cases and more serious ones were not affected by the dialysis dose, and that in these patients mortality was related to severity of illness score regardless of the dose of dialysis. In contrast, in patients with intermediate illness score, survival improved proportionally to the increased dialysis dose [7]. In 2000 a randomized monocentric trial involving 425 patients Ronco et al. confirmed a significant increase in survival with increasing dialysis dose, and established a dialysis dose of 35 ml/Kg/h as optimal survival target [8].
However, more recently two large polycentric randomized studies involving more than 2,000 patients in United States and Australia/New Zealand (ATN Study and RENAL Study) [9, 10] compared a dialysis dose “normal” (20–25 ml/Kg/day) to a “more intensive” dialysis dose (35–40 ml/Kg/day). Both 2 trials failed to demonstrat any advantage with increasing dialysis dose [9, 10]. In details:
-
ATN study: comparison in CVVHDF between a prescribed “normal” dose of 20 ml/Kg/hour (hemodynamic unstable patients) or intermittent 3 times a week HDF (hemodynamically stable patients) versus an “intensive” dose of 35 ml/Kg/h in CVVHDF (hemodynamically unstable patients) or daily intermittent HDF (hemodynamically stable patients) [9]. Intensive dose did not demonstrate any added benefit to normal dose (cumulative mortality rate in intensive and normal dose groups: 53.5 vs. 51.5 %, p 0.10), including analysis done in subgroups of septic patients and of those treated with vasopressor amines [9].
-
RENAL Study: comparison in CVVHDF between a prescribed “normal” dose of 25 ml/Kg/h versus an “intensive” dose of 40 ml/Kg/h [10]. Mortality at 90 days was 44.7 % in “normal” dose versus 44.7 % in “intensive” dose (p 0.99).
Keeping in mind that in patients with AKI in ICU uremia per se is an independent risk factor of mortality [11]; nowadays, suggested prescription of dialysis dose for patients in ICU with AKI (in CRRT or in PIRRT) is 20–25 ml/kg/h, or better expressed as 480–600 ml/Kg/day [5, 12].
8.3 Anticoagulation of Extracorporeal Circuit
In critically ill patients, the main limit of application of CRRT remains the need of an efficient anticoagulation of the extracorporeal circuit to prevent its premature clotting. Even if in most cases systemic anticoagulation is an invasive procedure, there are many good reasons to obtain a clinical effective anticoagulation of extracorporeal circuit (Table 8.1).
However, bleeding is really of clinical significance as its incidence is high (varies from 4 to 23 %). A bleeding from cruel or surgical wounds, or from tracheostomy tube, gastrointestinal tract or upper respiratory tract mucosa during suction maneuvers are serious complications. When bleeding occurs it will always affect heparin anticoagulation protocol in a restrictive diagram [13].
During the development of extracorporeal circulation hirudin extracted from leeches was the first applied anticoagulant. Then, at the end of 1920s heparin extracted from pig intestinal mucosa was available in pure enough quantity.
After more than 90 years from its appearance unfractionated heparin (UFH) and low-molecular weight heparin (LMWH) are still the most systemic anticoagulant used in the world in maintaining patency of extracorporeal circuit. However, alternative anticoagulation methods designed to avoid bleeding as heparin main side adverse effect has been proposed and applied with mixed success. These alternative methods include systemic anticoagulation methods (heparinoids, thrombin inhibitors, nafamostat, prostacyclin) and regional methods (saline flushes, protamine-heparin, citrate) (Table 8.2).
Undoubtedly regional anticoagulation methods are the most interesting, because in this case anticoagulation is virtually restricted to extracorporeal circuit. Among these methods, citrate is now emerging as an effective, safe, and feasible technique. In recent KDIGO guidelines citrate is suggested as choice anticoagulant during RRT in ICUs [14].
8.3.1 Unfractionated Heparin and Low-Molecular Weight Heparin
UFH is a mixture of branched glycosaminoglycans ranging from 3,000 to 30,000 Da with a mean molecular weight of 15,000 Da (about 45 monosaccharide unit). On the contrary, LMWH is a more homogeneous glycosaminoglycans composition with a mean molecular weight of 5,000–6,000 Da (less than 18 monosaccharide units) (Fig. 8.3).
Anticoagulant activity of UFH is mainly due to its interaction with antithrombin III (ATIII) and subsequent formation of heparin–antithrombin complex (Heparin-AT) with ATIII consumption. Heparin-AT complex changes ATIII inhibitory activity on thrombin, physiologically slow and progressive, in a rapid and irreversible inhibition on thrombin. Heparin binds ATIII by a well defined pentasaccharide sequence, which has been recently synthesized and constitutes the new heparinoid anticoagulant fondaparinux.
As to differentiate between UHF and LMWH, it should be highlighted that: (1) inhibitory activity to thrombin needs the formation of a trimolecular complex given by heparin-ATIII complex (via the pentasaccharide sequence specific binding) and thrombin (via non-specific charge binding between heparin and thrombin); (2) thrombin is about 10 times more sensitive to inhibition of heparin-ATIII complex compared to factor Xa, thus thrombin is the main target of anticoagulation activity of UHF; (3) inhibitory activity to factor Xa requires only the specific binding between the heparin-ATIII complex by pentasaccharide sequence; (4) by administering a bolus dose of UFH, only one-third of the dose can bind to ATIII to form the complex heparin-ATIII.
Therefore, LMWH (less than 18 monosaccharide molecules) loses the ability to inhibit thrombin (LMWH is not able to tie together thrombin and heparin), but keeps the ability to inhibit factor Xa by pentasaccharide sequence.
As to elimination of UFH, heparin high-molecular weight molecules have two mechanisms of clearance: (1) a cellular clearance, due to the binding of heparin to endothelium and macrophage receptors, which in turn promote the removal of heparin from bloodstream by a fast and saturable mechanism; (2) a renal clearance, characterized by a slow elimination kinetics and not saturable mechanism. Based on this elimination kinetics, LMWH (and the fraction of UFH of lower molecular weight) has only the renal clearance. In addition during CRRT LMWH clearance by filter is negligible even with highly permeable membranes [15].
Acute Phase Reactants proteins can affect in vivo UFH anticoagulant activity. As a matter of fact, UFH is a polyanion able to bind in an aspecific way many plasma proteins. This aspecific binding reduces UFH anticoagulant activity, and unpredictably contributes to so-called phenomenon of “UFH resistance”.
Apparent biological half-life of UFH is dose-dependent, and varies from 30 min after a bolus dose of 25 U/Kg up to 60 and 150 min observed after a bolus dose of 100 and 400 U/Kg, respectively. LMWH has a more predictable and much longer half-life, tending to accumulate over time. Curiously, after administration of UFH the part of low-molecular weight heparins present in UFH mixture tends to accumulate more and more. As a result, using UFH continuously over days anticoagulant activity of heparin will change with a decrease of antifactor-IIa/anti-Xa activity ratio.
From these physiological basis it comes out that in patients with AKI in CRRT without residual diuresis the use of UFH leads to anticoagulant effects not easily predictable.
In last 10 years in western countries LMWH is becoming the standard anticoagulant in chronic hemodialysis patients [15]. Conversely, LMWH in ICUs in patients treated with CRRT has failed to demonstrate any advantage (drug safety and filter life) in comparison to UFH [16–18]. In addition, daily costs of LMWH anticoagulant, including assays, are higher by about 10 % than of UHF anticoagulant [19].
Recommended dose of UFH in patients on CRRT is 1,000–2,500 IU as initial bolus, followed by 5–10 IU/Kg/h with a PTT target of 1–1.4 times.
Recommended dose of LMWH (nadroparin, enoxaparin, and dalteparin) in patients at bleeding risk should reach a anti-Xa activity target of 0.25–0.35 IU/ml.
8.3.2 Regional Citrate Anticoagulation
Citrate has been used in hemodialysis for the first time in 1960 by Morita et al. [20]. Thirty years later citrate, revised by Mehta et al. has been successfully applied as RCA in critically ill patients on CRRT [21]. Nowadays, 20 years after first experience in ICU impact of RCA on renal and intensivist practice is real and increasing. RCA is supposed to spread more and more because of increasing experience of staff, of availability of dedicated monitors and materials and of operational protocols simple and adaptable to the patient needs.
RCA is mainly popular in North America and Northern Europe. Since 1999 in Calgary (Canada) a standard system of anticoagulation with heparin alternative to RCA has been implemented for all CRRT [22]. Based on pediatric registry of CRRT in North America, 56 % of CRRT sessions are now done with RCA [23]. In the same way, in Italy RCA is in rapid development, although the available data are few. In a survey of dialysis practice of the year 2007 in Northwest Italy (4.5 million inhabitants with global data concerning all ICUs) anticoagulant heparin was by far the most widespread (5,296 on 7,842 days of dialysis, 67.5 % of cases) [24]. However, in patients at high risk of bleeding RCA was performed in 18 % of cases [24] in 2007, but it reached 25 % of all treatments in 2009 (unpublished data).
Based on these data an increase of RCA indications has been suggested by recent guidelines recommending RCA as standard anticoagulant for extracorporeal circuit in ICUs [14].
8.3.2.1 Extracorporeal Circuit During Regional Citrate Anticoagulation
Citrate is infused at the beginning of extracorporeal circuit. Citrate binds ionized calcium (iCa ++) and magnesium. In extracorporeal circuit citrate usually reaches a concentration of 3–5 mmol/L, whereas iCa ++ concentration decreases at 0.4–0.2 mmol/L. Since enzymes of coagulation cascade are iCa ++-dependent, blood clotting capacity inversely decreases. It should be noted that filter membranes are freely permeable to citrate (molecular weight 192 Da). Therefore, of whole citrate entering in filter a part (at about half as Ca ++-citrate complex) is lost in effluent (or dialysate), and the remaining part enters to patient by circuit venous line. During RCA inlet dialysate is Ca ++-free, and Ca ++ (iCa ++ or complexes Ca ++-citrate) is lost in effluent. Infusion of Ca ++ at the end of circuit line is only meant to replace the amount of Ca ++ lost in effluent, which is directly proportional to effluent volume (Fig. 8.4) [25].
The amount of citrate (as Ca ++-citrate complexes) entering patient through circuit venous line is rapidly metabolized to bicarbonates in liver, muscle, and kidney.
8.3.2.2 Efficacy and Safety of Citrate
In comparison to heparin citrate is capable of maintaining the circuit patency for equal time, if not longer [12, 22, 26, 27]. A comparison among different studies is not easy, because they are monocentric and evaluated different populations. Circuit life can be affected both by factors inherent to studied patient such as procoagulant patient capacity, platelet counts, levels of ATIII, sepsis, or by factors inherent to dialysis such as membrane type, convective flux, filtration fraction, predilution, vascular access efficiency, blood flow rate, and type of monitoring and alarm circuit intervention.
In addition, during RCA circuit survival is strongly affected by citratemia reached in extracorporeal circuit varying from 2 to 5 mmol/l (mean citratemia 4 mmol/L) [21–23, 25–27]. As a matter of fact, for increasing values of citratemia (from 2 to 6 mmol/L) values of iCa ++ inversely decrease (from 0.5 to 0.1 mmol/L). At level of 6 mmol/L of citratemia iCa ++ concentration is 0.1 mmol/L, and blood coagulation is inhibited.
8.3.2.3 Metabolism and Kinetics of Citrate
After infusion plasma citrate is rapidly uptaken by liver, kidney, and muscle. Citrate enters citric acid cycle, generates bicarbonate and consumes H+. In quantitative terms, liver is the most important metabolic site of citrate.
During RCA some metabolic alterations can arise including: (1) an excessive citrate metabolism with systemic alkalosis; (2) accumulation of citrate for impaired metabolism, with hypocalcemia/hypomagnesemia, or total Ca ++/iCa ++ ratio >2.5 (or better demonstrated by citratemia dosage) [25]. When citrate accumulates iCa ++ concentration decreases whereas total Ca ++ concentration is constant. Therefore, total Ca ++/iCa ++ ratio increase is more accurate marker of citrate accumulation than iCa ++ decrease; (3) hypernatremia, most often seen in the past with old protocols of RCA (Table 8.3).
In general, in all studies assessing metabolic alterations during RCA (cumulative total of 770 patients) metabolic tolerance was good, with no significant electrolyte and acid-base alterations [25–27]. Risk of hypernatremia is usually prevented by using dialysate solutions containing lower sodium concentration, such as 120–132 mEq/L, and by providing a dialysate flow rate as high as able to enhance diffusive clearance of Na+ [12, 25–27].
In addition, in populations at risk of citrate accumulation the optimization of diffusive clearance in order to increase citrate loss in effluent allowed an excellent acid-base control [12, 25]. Citrate losses in effluent can be usefully exploited to reduce its metabolic load to patient. As shown in Fig. 8.5, in CVVHDF at blood flow ranging from 100 to 150 ml/min and at effluent flow ranging from 1,200 to 5,000 ml/hour, losses of citrate in effluent were directly related to effluent volume, and can reach up to 70 % of citrate amount passing the filter [25].
References
Kramer P, Wigger W, Rieger J, Matthaei D, Scheler F (1977) Arteriovenous haemofiltration: a new and simple method for treatment of over-hydrated patients resistant to diuretics. Klin Wochenschr 55:1121–1122
Schiffl H, Lang SM, Fischer R (2002) Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346:305–310
Vinsonneau C, Camus C, Combes A et al (2006) Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 368:379–385
Vesconi S, Cruz DN, Fumagalli R et al (2009) Dose Response Multicentre International collaborative Initiative (DO-RE-MI Study Group). Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care 13(2):R57
Kellum JA, Ronco C (2010) Dialysis: results of renal: what is the optimal CRRT target dose? Nat Rev Nephrol 6:191–192
Granado RC, Macedo E, Chertow GM et al (2011) Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol 6:467–475
Paganini EP, Tapolyai M, Goormastic M (1996) Establishing a dialysis therapy/patient outcome link in intensive care unit acute dialysis for patients with acute renal failure. Am J Kidney Dis 28(3):S81–S89
Ronco C, Bellomo R, Homel P et al (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
VA/NIH Acute Renal Failure Trial Network (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
RENAL Replacement Therapy Study Investigators (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
Metnitz PG, Krenn CG, Steltzer H et al (2002) Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30:2051–2058
Mariano F, Tedeschi L, Morselli M, Stella M, Triolo G (2010) Normal citratemia and metabolic tolerance of citrate anticoagulation for hemodiafiltration in severe septic shock burn patients. Intensive Care Med 36:1735–1743
van de Wetering J, Westendorp RG, van der Hoeven JG et al (1996) Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol 7:145–150
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:179–184
Davenport A (2009) Review article: Low-molecular-weight heparin as an alternative anticoagulant to unfractionated heparin for routine outpatient haemodialysis treatments. Nephrology 14:455–461
Reeves JH, Cumming AR, Gallagher L, O’Brien JL, Santamaria JD (1999) A controlled trial of low-molecular-weight heparin (dalteparin) versus unfractionated heparin as anticoagulant during continuous venovenous hemodialysis with filtration. Crit Care Med 27:2224–2228
Jeffrey RF, Khan AA, Douglas JT, Will EJ, Davison AM (1993) Anticoagulation with low molecular weight heparin (Fragmin) during continuous hemodialysis in the intensive care unit. Artif Organs 17:717–720
de Pont AC, Oudemans-van Straaten HM, Roozendaal KJ, Zandstra DF (2000). Nadroparin versus dalteparin anticoagulation in high-volume, continuous venovenous hemofiltration: a double-blind, randomized, crossover study. Crit Care Med 28:421–425
Mariano F (2012) Il citrato: un diverso approccio mentale all’anticoagulazione del circuito extracorporeo. G Ital Nefrol 19:27–32
Morita Y, Johnson RW, Dorn RE, Hall DS (1961) Regional anticoagulation during hemodialysis using citrate. Am J Med Sci 242:32–43
Mehta RL, McDonald BR, Aguilar MM, Ward DM (1990) Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int 38:976–981
Bagshaw SM, Laupland KB, Boiteau PJ, Godinez-Luna T (2005) Is regional citrate superior to systemic heparin anticoagulation for continuous renal replacement therapy? A prospective observational study in an adult regional critical care system. J Crit Care 20:155–161
Symons JM, Chua AN, Somers MJ et al (2007) Demographic characteristics of pediatric continuous renal replacement therapy: a report of the Prospective Pediatric Continuous Renal Replacement Therapy Registry. Clin J Am Soc Nephrol 2:732–738
Mariano F, Pozzato M, Canepari G et al (2011) Renal replacement therapy in intensive care units: a survey of nephrological practice in northwest Italy. J Nephrol 24:165–176
Mariano F, Morselli M, Bergamo D et al (2011) Blood and ultrafiltrate dosage of citrate as a useful and routine tool during continuous venovenous haemodiafiltration in septic shock patients. Nephrol Dial Transplant 26:3882–3888
Morgera S, Schneider M, Slowinski T et al (2009) A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med 37:2018–2024
Oudemans-van Straaten HM, Bosman RJ, Koopmans M et al (2009) Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med 37:545–552
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Italia
About this chapter
Cite this chapter
Mariano, F. (2014). Continuous Renal Replacement Therapy (CRRT) in Intensive Care. In: Allaria, B. (eds) Practical Issues in Anesthesia and Intensive Care 2013. Springer, Milano. https://doi.org/10.1007/978-88-470-5529-2_8
Download citation
DOI: https://doi.org/10.1007/978-88-470-5529-2_8
Published:
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-5528-5
Online ISBN: 978-88-470-5529-2
eBook Packages: MedicineMedicine (R0)