Abstract
Optimal cardiovascular function requires appropriate coupling of the left ventricle and the arterial system and good interaction of its components. To appreciate the pathophysiology of cardiovascular function, we need a diagnostic and monitoring modality that can represent left ventricular–arterial coupling or the matching of ventricular elastance (E ES) with arterial elastance (E A). E ES (ventricular function) indicates the variations of left ventricular end-systolic volume (ESV) (and indirectly the variations of stroke volume, SV) in response to the variations of end-systolic pressure (ESP) (arterial pressure) (ESP/ESV), and E A (arterial mechanical properties) indicates the variations of arterial pressure (ESP) in response to the variations of SV (ESP/SV). It has been suggested that the E A/E ES ratio is the left ventricular–arterial coupling parameter and that when E A/E ES = 1, that is, when the E A slope is equal to the E ES slope, the external work (mechanical work) is optimized, whereas for E A/E ES = 0.5, that is, the E A slope is lower than the E ES slope, cardiac efficiency is maximal. There are multiple potential sources of error in the noninvasive assessment of left ventricular–arterial coupling (E A/E ES ratio). However, this integrated monitoring approach (ESP, SV, ESV) gives appropriate attention to the concept of left ventricular–arterial coupling and it demonstrates the feasibility of monitoring the E A/E ES ratio throughout the noninvasive echocardiographic assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Left ventricular–arterial coupling
- Ventricular elastance
- Arterial elastance
- EA/EES ratio
- Left ventricular end-systolic pressure–volume relation
- Ejection fraction
- External work
- Mechanical work
- Cardiac efficiency
- Echocardiography
- Noninvasive hemodynamic monitoring
- Pulse contour methods
1 Left Ventricular–Arterial Coupling
The primary role of the cardiovascular system is to deliver energy sources and oxygen to the tissues. The periphery itself has the ability to autoregulate blood flow to the metabolic activity of any individual organ, but it needs an adequate perfusion pressure and a constant flow in the capillaries. Since the left ventricle functions as a volume phasic pump, the proximal arterial vascular system (the large arteries) acts as a large capacitor with a high output resistance (peripheral arterioles) to convert the pulsatile flow to a constant one, like a hydraulic filter (Fig. 41.1). Thus, optimal cardiovascular function requires appropriate coupling of the left ventricle and the arterial system and good interaction of its components. To fully appreciate the pathophysiology of cardiovascular function, we need a diagnostic and monitoring modality that explains the concept of a pump that generates flow (cardiac output), ejecting phasically in a pressurized compartment. In other words, we need an integrated monitoring modality that represents left ventricular–arterial coupling or the matching of ventricular elastance with arterial elastance. The clinical dilemma in a hypotensive patient with low cardiac output is whether the pump or the circuit is working out of the normal range or whether a combination of both exists.
Functional analysis of this interaction requires that the left ventricle and the arterial system be described in similar terms.
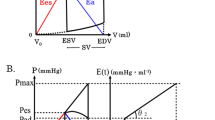
The left ventricle is evaluated best by plotting left ventricular pressure versus volume throughout the cardiac cycle (Fig. 41.2). With every heart beat a full loop is described. Starting at end-diastole, the first part of the loop is the isovolumic contraction. When the aortic valve opens, ejection begins and during the ejection period, the volume decreases and the pressure changes relatively little. After closure of the aortic valve, the isovolumic relaxation follows. When the mitral valve opens, filling starts and the volume increases with a very small increase in left ventricular pressure until the end-diastolic volume (EDV) is reached. The lower right-hand corner of the left ventricular pressure–volume loop is end-diastole, with EDV and end-diastolic pressure; the upper left-hand corner of the left ventricular pressure–volume loop is end-systole, with end-systolic pressure (ESP) and end-systolic volume (ESV). This is the point at which the left ventricle reaches maximal stiffness. The difference between EDV and ESV is the stroke volume (SV), and the ejection fraction (EF) is the relationship between SV and EDV. The area inside the loop is the ventricular effective stroke work (SW), which is the product of volume and pressure. Different loading conditions in different beats determine different SVs, so different ESVs come across different ESPs (Fig. 41.3).
Connecting the end-systolic points of variable loaded beats determines the left ventricular ESP–ESV relation (ESPVR) (Fig. 41.4). The points along this line indicate how much the left ventricular ESV will increase (and SV will decrease) in response to an increase of ESP. The slope of this relation expresses the left ventricular end-systolic elastance (E ES). This is a property of the heart linked to the contractility itself, and is very little affected by the loading conditions. The original results suggest a linear ESPVR with an intercept with the volume axis (V d). The linear relation implies that the slope of the ESPVR, the E ES, with the dimension of pressure over volume (mmHg/ml), can be determined. An increase in contractility, as obtained with epinephrine, shifts the relation to the left and increases E ES, but leaves the intercept volume (V d) unchanged. ESPVR can be calculated as the ratio between ESP and (ESV − V d).
On the other side of the aortic valve, the arterial system can be evaluated in an analogous manner [1]. Different SVs determine different expansions of the arterial system with different ESPs (Fig. 41.5): the higher the SV, the greater the arterial ESP, depending on compliance and resistance of the arterial tree (arterial mechanical properties). In this analysis, the arterial system is described by the relation between the SV and ESP. The slope of this relation expresses the effective arterial end-systolic elastance (E A); then E A can be calculated as the ratio of ESP to SV.
EES (ventricular function) indicates the variations of left ventricular ESV (and indirectly the variations of SV) in response to the variation of ESP (arterial pressure) [ESP/(ESV − Vd)], and EA (arterial mechanical properties) indicates the variation of arterial pressure (ESP) in response to the variation of SV (ESP/SV).
The intersection of the E ES slope (which represents ventricular function) and the E A slope (which represents arterial mechanical properties) is the “coupling point” (Fig. 41.6) and expresses the concept of the left ventricular–arterial coupling (E A/E ES ratio): this coupling point is, from a hemodynamic point of view, the ESP (arterial pressure) when the aortic valve has just closed and the total amount of the corresponding SV at the end of ejection phase has been ejected in the arterial tree.
Thus, the SV, the SW, and the ESP (arterial pressure) are dependent beat-to-beat on the balance between E ES (describing the left ventricle) and E A (describing the arterial system) and their corresponding slopes: different E A/E ES and different slopes determine different ESP (arterial pressure), different SV, and different SW (Figs. 41.7, 41.8, 41.9).
It has been suggested that this E A/E ES ratio is the left ventricular–arterial coupling parameter and that when E A/E ES = 1, that is, when the E A slope is equal to the E ES slope, the external work (mechanical work) is optimized (Fig. 41.10), whereas for E A/E ES = 0.5, that is, the E A slope is lower than the E ES slope, cardiac efficiency is maximal (Fig. 41.11).
To determine these two parameters, several monitoring simplifications have been used in clinical practice.
The arterial elastance (E A) can be approximated as follows. ESP is close to the mean arterial pressure (Fig. 41.12), and SV can be obtained through echocardiography (2D, 3D) or through continuous cardiac output with noninvasive hemodynamic monitoring systems (pulse contour methods). We can calculate E A = ESP/SV ≈ (beat-to-beat) P mean/cardiac output/heart rate.
The left ventricular end-systolic elastance (E ES) is calculated from E ES = ESP/(ESV − V d). V d (the intercept volume) is the volume of the heart at which the left ventricular ESPVR intercepts the x-axis (ideally the volume of the heart at zero ESP) (Fig. 41.13). ESV can normally be measured noninvasively by echocardiography (2D, 3D), but V d is hard to estimate. To derive this intercept volume, at least one other point of the ESPVR should be obtained. This would require changes in diastolic filling that are often not feasible in very sick patients. In a number of studies, it has simply been assumed that V d = 0.
This assumption leads to a very interesting simplification of the analysis (Fig. 41.14). With V d = 0, E ES = ESP/ESV = ESP/(EDV − SV). The E A/E ES ratio then becomes E A/E ES = (ESP/SV)/[ESP/(EDV − SV)] = (EDV − SV)/SV = ESV/SV = (1/EF) − 1.
It is important to underline that the ESP disappears altogether, leaving only the determination of two volumes, the ESV “inside the heart” and the SV, or the EF. This implies that the mechanical work is maximal when E A/E ES = 1 and EF = 0.5, or when ESV and SV are equivalent. Similarly, the cardiac efficiency is maximal when E A/E ES = 0.5 and EF = 0.67, or when SV is greater than ESV. In contrast, when E A exceeds E ES, that is, when E A/E ES > 1, EF falls, and ESV is greater than SV.
In patients with systolic heart failure, E ES is reduced and peripheral vascular resistance (and thus E A) is increased. In this situation, the left ventricle and the arterial system are suboptimally coupled (E A/E ES > 1). Any increase in heart rate will further increase E A, making the coupling worse. Vasodilator therapy, which lowers E A, will bring the E A/E ES ratio back down toward 1.0, improving the coupling. Alternatively, or contemporarily, inotropic therapy, which increases E ES, will also improve the E A/E ES ratio. Thus, in a patient with a failing heart, the left ventricle and the arterial system are inappropriately coupled, further reducing the heart’s mechanical work and efficiency. The coupling and performance in systolic heart failure can be improved by vasodilator and/or inotropic therapy and can be monitored by the E A/E ES ratio by an integrated monitoring, echocardiography and noninvasive hemodynamic monitoring (pulse contour methods).
The assumption of a negligible V d simplifies matters. However, negligible V d values are difficult to verify, mostly not correct, and certainly lead to possible large errors in the very sick patient (dilated cardiomyopathy).
There are multiple potential sources of error in the noninvasive assessment of left ventricular–arterial coupling. However, this integrated monitoring approach (ESP, SV, ESV) gives appropriate attention to the concept of left ventricular–arterial coupling and it demonstrates the feasibility of monitoring the E A/E ES ratio throughout the noninvasive echocardiographic assessment. Although not ready for routine use in the echocardiographic laboratory or in cardiac intensive care, this area certainly deserves further investigation and may ultimately improve our pathophysiologic understanding of cardiac disorders. Further studies may focus on assessing the effect of pharmacologic intervention on left ventricular–arterial coupling (improved E ES and/or reduced E A), on left ventricular function, on arterial compliance, and on left ventricular remodeling, thus leading to a correct prognostic evaluation.
Reference
Sunagawa K, Maughan WL, Sagawa K (1985) Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 56:586–595
Further Reading
Antonini-Canterin F, Enache R, Popescu BA, Popescu AC, Ginghina C, Leiballi E et al (2009) Prognostic value of ventricular–arterial coupling and B-type natriuretic peptide in patients after myocardial infarction. A five-year follow-up study. J Am Soc Echocardiogr 22:1239–1245
Binkley PF, Van Fossen DB, Nunziata E, Unverferth DV, Leier CV (1990) Influence of positive inotropic therapy on pulsatile hydraulic load and ventricular–vascular coupling in congestive heart failure. J Am Coll Cardiol 15:1127–1135
Borlaug BA, Kass DA (2008) Ventriculo-vascular interaction in heart failure. Heart Fail Clin 4:23–26
Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PYA et al (2001) Non-invasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38:2028–2034
Hettrick DA, Warltier DC (1995) Ventriculoarterial coupling. In: Warltier DC (ed) Ventricular function. Williams & Wilkins, Baltimore, pp 153–179
Little WC, Cheng CP (1991) Left ventricular–arterial coupling in conscious dogs. Am J Physiol 261:H70–H76
Ohte N, Cheng CP, Little WC (2003) Tachycardia exacerbates abnormal left ventricular–arterial coupling left ventricular–arterial coupling in heart failure. Heart Vessels 18:136–141
Saba PS, Roman MJ, Ganau A, Pini R, Jones EC, Pickering TG, Devereux RB (1995) Relationship of effective arterial elastance to demographic and arterial characteristics in normotensive and hypertensive adults. J Hypertension 13:971–977
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Italia
About this chapter
Cite this chapter
Sorbara, C., Salandin, V. (2013). ICU Echocardiography and Noninvasive Hemodynamic Monitoring: The Integrated Approach. In: Sarti, A., Lorini, F. (eds) Echocardiography for Intensivists. Springer, Milano. https://doi.org/10.1007/978-88-470-2583-7_41
Download citation
DOI: https://doi.org/10.1007/978-88-470-2583-7_41
Published:
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-2582-0
Online ISBN: 978-88-470-2583-7
eBook Packages: MedicineMedicine (R0)