Abstract
This book chapter deals with the production of biogas coupled with the use of waste effluents for microalgae biomass growth. Biogas production through anaerobic digestion of microalgae uses the whole organic content of microalgae to produce energy. Furthermore, biogas generation seems to be the least complex of the different energy conversion routes since anaerobic digestion avoids energy-intensive steps such as biomass drying and extraction. Biogas can be produced as the main product from microalgae (direct anaerobic digestion of the whole biomass) or can be a coproduct of an industry culturing microalgae for different purposes. The integration of different technologies in a biorefinery aims at maximizing benefits while reducing the environmental impact. The future of algae biorefineries would include the extraction of several components from microalgae. Waste biomass can be treated by anaerobic digestion, reducing the pollutant load while producing energy. Additionally, there is a synergy between anaerobic digestion and microalgae growth. Biogas contains a high percentage of CO2, and if it is combusted in CHP units, CH4 is converted to CO2. The digestate produced after anaerobic digestion is a liquid medium where most of the nutrients of the organic substrate are mineralized. Therefore, the two main products of anaerobic digestion could serve as sources of nutrients for microalgae growth. If the nutrient loop is closed, profitable processes can be achieved. Consequently, biofuels and high-value products would be obtained at the same time from microalgal biomass, reducing environmental impact and increasing profits.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaerobic Digestion
- Switch Grass
- Organic Matter Solubilization
- Hydraulic Retention Time
- Methane Production
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Algae are aquatic organisms which obtain energy via photosynthesis. Algae capture CO2 and transform it into organic carbon. Algae comprises of two major groups – macroalgae and microalgae.
Microalgae are a diverse group of unicellular organisms with more than 30,000 known species. Although microalgae, in their strictest sense, are eukaryotes, this chapter will also consider cyanobacteria (blue-green algae), which are prokaryotes with photosynthetic capacity. In spite of the great number of species that belong to this group of living forms, only a handful are currently of commercial significance (Bruton et al. 2009).
Microalgae are attracting great interest in recent years due to their high photosynthetic efficiency, which can be as high as 5 % (Acién et al. 2012a). As a consequence, they grow faster than terrestrial plants and have a high potential for bioenergy production (Brennan and Owende 2010). Moreover, microalgae show other advantages compared to traditional energy crops such as their high CO2 consumption during their growth (Chisti 2007), their ability to grow on marginal lands, and use of marine or wastewater (Park et al. 2011). This biomass can be used for biofuel production or extraction of a wide range of value-added products. Pigments, ω-3 fatty acids, feed, food, fertilizers, and natural food colorants can be obtained from microalgal biomass (Spolaore et al. 2006; Romero García et al. 2012). The economical revenue obtained from these value-added compounds makes biofuel production from algae even more attractive. However, the current market is not yet open to high value-added products obtained from waste streams, and thus, as of today, the only recovery from this biomass can be achieved by its conversion into an energy form.
In this context, this book chapter is devoted to the production of biogas coupled with the use of waste effluents for microalgae biomass growth. Considering all available technologies producing biofuels, anaerobic digestion along with thermal liquefaction is the only conversion route that uses the whole organic content of microalgae to produce energy. Furthermore, biogas generation seems to be the least complex since anaerobic digestion avoids energy-intensive steps such as biomass drying and extraction. Therefore, AD has a higher energy efficiency compared to the other options (Sialve et al. 2009). Biogas can be produced as the main product from microalgae (direct anaerobic digestion of the whole biomass) or can be a coproduct of an industry culturing microalgae for different purposes (organic waste treatment in biorefineries) (Ramos-Suárez and Carreras 2014). Additionally, there is a synergy between anaerobic digestion and microalgae growth: (1) biogas contains a high percentage of CH4 and CO2, and if it is combusted in CHP units, CH4 is converted to CO2; (2) the digestate produced after anaerobic digestion is a liquid medium where most of the nutrients of the organic substrate are mineralized. Therefore, the two main products of anaerobic digestion (CO2 and nutrients) could serve as sources of growth enhancer for microalgae culture (Sialve et al. 2009; Uggetti et al. 2014).
2 The Anaerobic Digestion Process
Anaerobic digestion is a suitable technology for the treatment of almost all types of organic residues generated in agro-industrial processes. This technology has been shown to be environmentally friendly and cost competitive with more than 9232.7 ktoe of primary energy produced from decentralized biogas plants only in Europe during 2012–2013 (EurObserv’ER 2014).
The anaerobic digestion or biomethanation is a biological process whereby organic matter is degraded into a number of gaseous products, known as biogas, and a by-product known as digestate. This bioprocess is conducted in the absence of oxygen where degradation of organic matter is performed through a complex series of biochemical reactions that are carried out by different groups of anaerobic microorganisms.
2.1 Anaerobic Process Stages
Biochemical and microbiological studies conducted so far divide the anaerobic decomposition process into four phases or stages: (1) hydrolysis, (2) acidogenesis, (3) acetogenesis, and (4) methanogenesis. Any of these four stages can be the limiting step in terms of the overall reaction rate. Hydrolysis is usually the limiting step when dealing with complex substrates, as it is the case for some microalgal strains (Mussgnug et al. 2010). Figure 5.1 shows a diagram of the various steps involved in the anaerobic digestion process, the microorganisms involved, and intermediate products generated. The numbers indicate the bacterial population responsible for the process.
Degradation steps of anaerobic digestion process and microorganisms involved (1 fermentative bacteria, 2 hydrogen-producing acetogenic bacteria, 3 homoacetogenic bacteria, 4 hydrogenotrophic methanogenic bacteria, 5 acetoclastic methanogenic bacteria) (Source: adapted from Gujer and Zehnder (1983))
2.1.1 Hydrolysis
Out of the four stages, hydrolysis is the initial step in the anaerobic degradation of complex organic substrates. Anaerobic microorganisms can only use soluble organic matter that can pass through the cell wall, and thus, hydrolysis of organic matter is a must. The organic material mainly comprises of three basic types of macromolecules: carbohydrates, proteins, and lipids. During hydrolysis, the bacteria transform the complex organic substrates into simple soluble compounds, i.e., proteins, carbohydrates, and fats are converted to amino acids, monosaccharides, and fatty acids, respectively.
The hydrolysis of these complex molecules is carried out through the action of extracellular enzymes produced by hydrolytic microorganisms.
The rate of hydrolytic degradation of lignocellulose materials composed mainly of lignin, cellulose, and hemicellulose is so slow that it is often the limiting step of the AD process. This is because lignin is highly resistant to degradation by the anaerobic microorganisms and also affects the biodegradability of the cellulose, hemicellulose, and other carbohydrates. It is noteworthy to mention that in contrast to lignocellulosic material, microalgal biomass does not contain lignin, and therefore, their hydrolysis and overall methane production is favored.
2.1.2 Acidogenic Phase
During acidogenesis, the fermentation of soluble organic molecules takes place via facultative bacteria. The end product of this reaction includes alcohol, hydrogen, carbon dioxide, and several fatty acids like acetic acid, formic acid, propionic acid, valeric acid, lactic acid, etc. Some of the end products of this reaction like acetic acid, formic acid, and hydrogen can directly be used by the methanogenic bacteria.
The formation of one or another acid depends on the concentration of H2 produced during the digestion. When the H2 concentration in the gas produced is very low (5–50 ppm), acetic acid is preferably formed. When the H2 concentration increases, acetic acid decreases, and the fraction of long-chain acids (e.g., propionic, butyric, etc.) increases.
In this phase, also alcohols are produced. The kinetics of the process is relatively fast; the acid-producing bacteria are fast growing with minimum doubling times of 30 min.
2.1.3 Acetogenic Phase
In the third stage, known as acetogenesis, the other products of the acidogenic phase, namely, propionic acid, butyric acid, and alcohols, are transformed by acetogenic bacteria in hydrogen, carbon dioxide, and acetic acid. The small organic molecules, especially VFA (volatile fatty acids), are converted into acetic acid. The bacteria involved in this process are facultative, live in close collaboration with methanogenic bacteria, and can only survive in symbiosis with the genre that consumes hydrogen, since they are inhibited by high hydrogen concentrations (Anderson et al. 2003). These bacteria have slower growth rates than acidogenic bacteria, with minimum doubling times from 1.5 to 4 days.
2.1.4 Methanogenic Phase
This phase constitutes the final stage in which compounds such as acetic acid, hydrogen, and carbon dioxide are transformed into CH4 and CO2. Bacteria involved constitute a single group composed of several species of different shapes and cell structures. There are two main types of strictly anaerobic microorganisms, which degrade acetic acid (acetoclastic methanogenic bacteria) and those that consume hydrogen (hydrogenotrophic methanogens). The main route for methane formation is the first one, with about 70 % of the methane produced.
Acetoclastic methanogenic bacteria produce methane from acetate. They have a slow growth (minimum doubling time of 2–3 days) and are not affected by the concentration of hydrogen in the biogas. The hydrogen-consuming methanogenic bacteria produce methane from hydrogen and CO2. This reaction has a dual function in the anaerobic digestion process; on the one hand, methane is produced and, on the other, gaseous hydrogen is removed.
With high ammonia concentrations, acetate-utilizing methanogens have limited activity in methanogenesis (Hansen et al. 1998). Symptoms of limitation of acetoclastic methanogens have also been observed in anaerobic digestion of microalgae due to high ammonia concentrations (Ramos-Suárez et al. 2014b).
2.1.5 Formation of Hydrogen Sulfide
In addition to the bacteria described above, there is a group called sulfate-reducing bacteria (SRB), which are particularly important if sulfates are also present (Espinosa-Chávez et al. 2007). SRB are able to reduce sulfate to sulfide. This reaction is crucial in anaerobic digestion, since SRB can compete with methanogens and decrease the production of methane. Moreover, SRB are also able to reduce the sulfates using the hydrogen produced by the acid-forming bacteria. In this case, the hydrogen cannot be used by the methanogenic bacteria. Sulfur content of microalgae cells varies normally between 1.5 and 1.6 μg mg−1 dry weight, whereas the concentration range of sulfur (in the form of salts) in the growth media is usually in the range of grams per liter (Grobelaar 2004). Remains of growth media will probably be introduced in the digester together with microalgal biomass, increasing sulfur content and, therefore, hydrogen sulfide production. Moreover, chemicals with sulfur could be used in some extraction process, which could remain in spent biomass under digestion (Romero García et al. 2012). It is therefore important to monitor the presence of sulfates during the process, since besides affecting the methanogenesis, hydrogen sulfide is corrosive and can affect several processes and structures (Hidalgo and García 2001). In microalgal biorefineries, if biogas is supplied as carbon source, hydrogen sulfide can be inhibitory for the growth of microalgae (Kao et al. 2012a, b).
2.2 Process Parameters
Anaerobic digestion is affected by various parameters which influence the kinetics of the different reactions and the production of biogas. Those parameters affecting the digestion process include: control parameters (nutrients, temperature, pH, redox potential) and operational parameters (agitation, hydraulic retention time, organic load). Moreover, many of these parameters are used to monitor the digestion course in industrial plants.
2.2.1 pH and Alkalinity
The different groups of bacteria involved in the process have optimum activity levels around neutral pH:
-
Fermentative bacteria: 7.2–7.4
-
Acetogenic bacteria: 6.0–6.2
-
Methanogenic bacteria: 6.5–7.5
Generally, the pH of the digester should not be lower than 6.0 or higher than 8.3 (Bazara et al. 2003). If the pH of the medium is below 6.5, the activity of methanogenic acetoclastic bacteria diminishes, while at pH below 4.5, the activity ceases completely (Lema and Méndez 1997).
Organic overloading can mediate instability of the digestion process due to organic acid accumulation. A high concentration of organic acids decreases the pH, decreases methane production, and can cause reactor souring or reactor failure (Rittmann and McCarty 2001).
Alkalinity is defined as the capacity to neutralize acids (Rittmann and McCarty 2001). Bicarbonate alkalinity of at least 500–900 mg/l CaCO3 is required for a pH greater than 6.5 (Rowse 2011). In the anaerobic digestion of microalgae besides the alkalinity caused by the carbonate-bicarbonate equilibrium, ammonia produced from protein degradation plays an important role increasing the buffer capacity.
2.2.2 Redox Potential
Methanogenic bacteria are strict anaerobes; therefore, their tolerance to changes in the redox potential is lower than other microorganisms involved in the digestion process. In pure cultures, methanogens require a redox potential of at least −350 mV to ensure the strongly reduced environment that these bacteria need for optimal activity (Anderson et al. 2003).
2.2.3 Temperature
Methanogenic microorganisms are extremely sensitive to temperature changes. There are three temperature ranges: (1) psychrophilic (5–20 °C), (2) mesophilic (25–45 °C, with the optimum of 30–37 °C; above 40 °C can cause denaturation of the enzymes), and (3) thermophilic (45–65 °C, being the optimum 55–60 °C) (Anderson et al. 2003). At the industrial level, it is common to find mesophilic (35–40 °C) and thermophilic digesters (55–60 °C). Stability decreases with increasing temperature, as a consequence of higher accumulation of VFA, greater toxicity of ammonium, an increased sensitivity to temperature changes, and foam and odor problems (Parkin and Owen 1986).
Specific methane production rates are 50–100 % higher for thermophilic anaerobic digestion than for mesophilic anaerobic digestion (Rittmann and McCarty 2001). In the anaerobic degradation of microalgae, the use of higher temperatures sometimes leads to higher methane yields (Golueke et al. 1957). Other studies showed a decrease in the methane yield (Samson and LeDuy 1986). Whereas Golueke et al. (1957) suggested a higher rate of degradation of algal cells due to the increased temperature, the latter study pointed to an increase of ammonia sensitivity with increasing temperatures due to a shift to the unionized form of ammonium.
2.2.4 Nutrients
The main nutrients required for growth of microorganisms are carbon, nitrogen, and phosphorus. Also, a series of mineral elements such as sulfur, potassium, sodium, calcium, magnesium, and iron should be present at trace levels. Carbon is the main power source for bacteria and the main component of biogas. This carbon comes mainly from carbohydrates contained in the biomass degraded.
On the other hand, nitrogen is a major source for the synthesis of proteins. Nitrogen deficiency disables bacteria to metabolize the carbon, which would lead to a reduced efficacy in the degradation. Conversely, if there is an excess of nitrogen, its accumulation in the form of NH3 (ammonia) is toxic to anaerobic microorganisms.
Therefore, the C/N ratio of the substrate is a key indicator of digestibility and potential methane yield of the biomass (substrate) to be degraded. This value is specific to each substrate, and in the case of microalgae, it depends on the species and the cultivation process (Sialve et al. 2009). Optimal C/N ratio for anaerobic digestion is between 10 and 30, and it depends on operational conditions, substrate composition, and microorganism acclimation to a particular substrate (Chen et al. 2008; Pagés Díaz et al. 2011). Microalgae, due to their high protein content, show normally a low C/N ratio which could be detrimental for the anaerobic digestion process (Sialve et al. 2009).
2.2.5 Inhibitors
The anaerobic digestion process can be inhibited by the presence of toxics in the system which affects the development of bacterial activity. Furthermore, the threshold toxicity concentration of specific compounds (ammonium, sulfide, volatile fatty acids, etc.) depends also on other parameters such as temperature or pH. Out of the anaerobic microorganisms, methanogenic bacteria are generally the most sensitive although generally all groups of microorganisms involved in the process are affected.
2.2.6 Mixing
Mixing aims at facilitating mass transfer processes and preventing dead zones and insufficient contact between organic matter and microorganisms. Mixing increases the kinetics of the anaerobic digestion process by accelerating the biological conversion. Additionally, mixing allows uniform heating of the reactor (Tchobanoglous et al. 2003).
Mixing can be provided mechanically through conventional impellers rotating immersed within the digester at low speed, pneumatical recirculation by injecting biogas via spargers at the bottom of the digester (Tchobanoglous et al. 2003), or by recycling the effluent at the bottom of the digester.
2.2.7 Retention Time
The retention time defines the time that the substrate is in contact with the active biomass within the digester. In digesters without biomass retention mechanisms (e.g., CSTR), the retention time is equivalent to the hydraulic retention time (HRT). HRT is defined as the average amount of time one reactor volume of actively digesting sludge stays within the reactor. HRT can be calculated with the following equation:
where:
-
HRT = hydraulic retention time (d)
-
V = volume of reactor (m3)
-
Q = influent flow rate (m3/d)
Anaerobic reactors should be designed with sufficient retention time to allow an effective volatile solid conversion to biogas and the development of methanogenic populations (Vesilind 1998; Parkin and Owen 1986). In this context, there are digesters (e.g., fluidized bed reactors, membrane reactors) where the solid retention time (SRT) is increased by means of biomass retention mechanisms which separate the liquid and the solid streams. These digesters are normally used for the treatment of high volumes of wastewater with low solid content. Although not applied yet, these types of digesters could be used for degrading highly diluted microalgal biomass.
2.2.8 Organic Loading Rate (OLR)
The organic loading rate (OLR) is defined as the mass of volatile solids added each day per reactor volume (Vesilind 1998) or the amount of BOD or COD applied to the reactor volume per day (Tchobanoglous et al. 2003). Organic loading rate is related to the hydraulic retention time by the following equation:
where:
-
OLR = organic loading rate (kg VS m−3 d−1)
-
Q = volumetric flow rate (m3 d−1)
-
CVS = concentration of volatile solids in the substrate (kg VS m−3)
-
V digester = reactor volume (m3)
-
HRT = hydraulic retention time (days)
OLR thus depends on the waste composition and the retention time. It is one of the parameters commonly used to characterize the treating capacity of anaerobic digesters.
According to Rittmann and McCarty (2001), the recommended rate of organic loading for high-rate anaerobic digestion is 1.6–4.8 kg VSS m−3 d−1, and the recommended organic loading rate for low-rate anaerobic digestion (digestion with no heat and no mixing) is 0.5–1.6 kg VSS m−3 d−1. Vesilind (1998) recommended that the peak organic loading rate for high-rate anaerobic digestion should be 1.9–2.5 kgVS m−3 d−1. These values can vary for different substrates and digesters. If the loading rate is too high for the system conditions, methanogenesis can become inhibited by organic overloading. Therefore, organic loading rate should be set in a conservative way.
2.3 Biogas Recovery and Use
Biogas is mainly composed of methane and carbon dioxide. The energetic value of biogas (between 20 and 25 MJ m−3) is determined by the concentration of methane in the biogas (Werner et al. 1989). Besides methane and carbon dioxide, biogas contains other minor constituents such as water vapor (H2O), hydrogen sulfide (H2S), ammonia (NH3), hydrogen (H2), and nitrogen (N2). Additionally, some traces of volatile organic compounds (e.g., siloxanes, mercaptans, terpenes) can be present in biogas produced in landfills but are rarely produced in agro-industrial applications (Rasi 2009). Biogas composition affects the possibilities for its use, since the methane concentration determines the LHV of the fuel. Moreover, high concentrations of some of these trace components may impede the use of biogas for certain energetic purposes.
Biogas can be used practically for the same energy applications developed for natural gas. Nowadays, the applications of greatest interest (see Fig. 5.2) are (1) heat by direct combustion, (2) power generation in engines, (3) power generation in engines with heat recovery (cogeneration), (4) integration in natural gas grid, and (5) fuel for vehicles. Nevertheless, the most common ones are direct combustion for heat and power generation with cogeneration engines. However, there is a growing interest in other alternatives such as its application as motor fuel and its integration into the natural gas grid (AEBIOM 2009).
Therefore, depending on the application in which biogas is used, a different degree of cleaning is required. Biogas purification systems are based on different techniques (chemical, physical, biological) and are normally designed for the removal of a single component. In agro-industrial biogas plants, the minor constituents that require removal include water vapor, carbon dioxide, and hydrogen sulfide, depending on their concentration and the intended application. Commercial techniques for hydrogen sulfide removal include iron sponges, activated carbon, micro-aeration (inside digester), water scrubbing, chemical absorption, biological filters, and membranes. Whereas for CO2 removal, the following techniques are available in the market: physical and chemical absorption, water scrubbing, pressure swing adsorption (PSA), vacuum swing adsorption (VSA), membrane separation, and cryogenic separation.
Microalgae cultures can be used for biogas purification by fixing CO2 and sulfur in their biomass. Although this option is still under study and no commercial systems based on this technology exists, biogas purification with microalgae could show some benefits compared to the other techniques, such as no use of chemicals, production of a valuable product (microalgal biomass) or additional energy (if biomass is digested), and simultaneous removal of CO2, H2S, and other compounds. This option will be further discussed in this chapter.
2.4 The Digestate
The anaerobic digestion process produces a by-product commonly known as digestate. It is a mixture of stabilized influent and microbial biomass. For a certain substrate, the type of digester and the operation parameters determine the properties of the digestate. As already mentioned, during the anaerobic process, part of the organic matter is converted into methane; therefore, the organic content of the digestate is lower than in the influent. The reduction of the C/N is beneficial when the end product is intended for agricultural purposes.
The agricultural valorization of digestates focuses mainly on two aspects: the direct use of the digestate as fertilizer and solid–liquid separation and further usage of the solid fraction for the preparation of high value-added fertilizers through composting and the use the liquid fraction as a liquid fertilizer.
The fertilizer value of the digestate depends mainly on the nutrient concentration of the degraded substrate. During the digestion process, organic carbon is converted to methane and carbon dioxide, whereas most of the nutrients that were associated to organic molecules are mineralized and remain in the digestate. For instance, organic nitrogen is converted to ammonia nitrogen, whereas total nitrogen remains virtually unchanged (some ammonia will be found in the gas phase). Therefore, the digestate exhibits a great fertilizer value and could be used for the growth of terrestrial crops and also for microalgae cultures, as it will be further discussed in this chapter.
3 Biogas Production from Microalgae
3.1 Introduction
The initial studies on anaerobic digestion of microalgae are from the end of the 1950s (Golueke et al. 1957; Golueke and Oswald 1959). In their very first work published, Golueke et al. (1957) observed the difficulties that the anaerobic degradation of microalgal biomass (Scenedesmus and Chlorella) could entail, both in mesophilic and thermophilic range. Authors inferred three possible reasons: (1) low C/N ratio, (2) ability of algae to survive in the digester, and (3) resistance of algae cell wall to bacterial attack.
Gunnison and Alexander (1975a) worked to understand the resistance of microalgae to bacterial attack under natural conditions. The main conclusion of their study was that the composition of microalgal cell wall is a decisive factor for this resistance. Afterwards, the same authors analyzed the constituents of the cell wall of some microalgal species that showed an effective resistance to bacterial degradation (Gunnison and Alexander 1975b). In many species (e.g., Staurastrum sp., Pediastrum duplex, and Fischerella muscicola), complex carbohydrates supported high resistance to bacterial attack and decomposition. Considering these results, research conducted in the recent years has pursued different goals:
-
Coupling anaerobic digestion to microalgal growth units for clean energy production
-
Application of pretreatments to break microalgal cell walls in order to enhance biodegradation and consequently biogas production potential
-
Co-digestion of microalgal biomass with high C/N substrates in order to balance nutrients and to enhance microbial activity
-
Anaerobic digestion of microalgal residues produced after the extraction of high-value products, as waste treatment process in a biorefinery
3.2 Microalgae as Energy Crop
The literature on the direct use of microalgal biomass for biogas production is to some extent scarce. However, in the recent years, a new approach is emerging: the use of microalgae grown in wastewater for direct biogas production. In this case, the energy savings generated by replacing the traditional wastewater treatment processes by microalgae-based treatments make the cultivation process profitable, and the use of mixed algal-bacterial populations for biogas production adds to even higher energy gains. There are important R&D projects trying to implement this concept at medium and industrial scale with encouraging results to date (e.g., All-Gas project, led by Aqualia: http://www.all-gas.eu/).
Different microalgae species have been assessed for methane production. Microalgae assessed in the different studies include chlorophytes or green microalgae (Chlorella sp., Scenedesmus sp. Chlamydomonas reinhardtii, Dunaliella salina, Monoraphidium sp.), cyanobacteriae (Arthrospira platensis, Oscillatoria sp., Spirulina maxima), euglenoids (Euglena graciliis), and haptophytes (Isochrysis galbana) (Golueke et al. 1957; Golueke and Oswald 1959; Samson and LeDuy 1986; Varel et al. 1988; Mussgnug et al. 2010; Ras et al. 2011; Hernández et al. 2013; Prajapati et al.; 2014a; Tran et al. 2014; Mottet et al. 2014; Santos et al. 2014). The results are reported to be influenced by operational parameters and experimental conditions of each study (e.g., batch or continuous tests, temperature, hydraulic retention time, organic loading rate), but they provide an insight into intra- and interspecies variability in methane potential of microalgae (see Fig. 5.3 for a comparative evaluation of some of these results).
Mesophilic methane yield of the different species ranged from 139.7 ± 0.9 LCH4 kgVS−1 for Scenedesmus sp. (Tran et al. 2014; Ramos-Suárez et al. 2014a) to 387 LCH4 kgVS−1 for Chlamydomonas reinhardtii (Mussgnug et al. 2010) or 446.8 LCH4 kgVS−1 for an algal sludge mainly composed of Scenedesmus, Chlorella, Euglena, and Oscillatoria (Golueke and Oswald 1959), the latter at thermophilic digestion (45 °C).
From all these studies, the main conclusion that can be drawn is that biodegradability, and therefore methane potential production, is species and strain specific (Mussgnug et al. 2010; Prajapati et al. 2014a). Moreover, within the same species, this potential could change depending on the culture growing method, or more specifically, on the nutrients supplied to the culture during its growth which determine its biochemical composition and, therefore, its methane production potential (Hernández et al. 2013).
It has been concluded, that biodegradability is mainly conditioned by the presence or absence of a cell wall composed of complex polymers, hardly biodegradable, that impede the degradation of intracellular organic molecules by microorganisms (Mussgnug et al. 2010).
Another drawback for an optimum anaerobic degradation of microalgae is the high content of nitrogen and low C/N ratio of this biomass. The major fraction of microalgae cultured without nutrient limitation is protein (Rebolloso Fuentes et al. 2000), which could be up to 60 % (Mahdy et al. 2014a). During anaerobic digestion, proteins are degraded and ammonium is produced. If ammonium concentration reaches important levels, microorganisms could be inhibited reducing or even stopping biogas production (Samson and LeDuy 1986). In literature, a wide range of ammonia inhibition thresholds are shown (from 1.7 to 14 g L−1 being the necessary concentration to cause a 50 % reduction in methane production). The inhibiting concentration could change with differences in substrates, inocula, environmental conditions (temperature and pH), and acclimation period (Chen et al. 2008).
Additionally, for marine species, salinity could also play an important role, as high salinity levels can diminish anaerobic digestion performance. Mottet et al. (2014) showed that salinity could be counteracted by means of adapted or acclimated inoculum, which could withstand high salinity levels yielding similar methane as low-salinity-level microalgae.
3.2.1 Enhancement of Methane Yields
3.2.1.1 Pretreatments for Microalgal Biomass
Algae consist of complex organic matter which implies a difficult enzymatic hydrolysis and hence limits the efficiency of anaerobic digestion. The limited hydrolysis is due to the biomass features such as biochemical composition and cell wall characteristics of the different microalgae strains. One of the approaches followed for enhancing methane production is the pretreatment of microalgae biomass. This approach enhances the hydrolysis rate of microalgae during anaerobic digestion by facilitating cell wall disruption. In this sense, organic matter contained in microalgal biomass becomes readily available for anaerobic microorganisms, and therefore, methane production and productivity increase. Pretreatments to open up the cell wall structure have been widely studied in activated sludge and lignocellulosic biomass; however, there is not enough information regarding the effect of those pretreatments on microalgae. Even though microalgae does not contain lignin which renders this substrate easier to degrade than lignocellulosic substrates, microalgal biomass contains some other compounds, such as algaenans and sporopollenin, which confer the cell wall a high resistance to bacterial attack (Burczyk and Dworzanski 1988).
3.2.1.1.1 Pretreatment Features: Organic Matter Solubilization and Structural Changes
The pretreatments applied to microalgal biomass prior to anaerobic digestion results in different changes in biomass structure and organic matter solubilization. By these means, microalgae biomass suffers changes which ultimately lead to methane production enhancement.
Traditionally, the organic matter solubilized upon pretreatment of activated sludge has been directly correlated to methane yield enhancement (Bougrier et al. 2005). Opposite to activated sludge, a parameter that can be used as an indicator of methane yield enhancement after pretreatment is lacking. As mentioned above, pretreatments employed for activated sludge are being adapted for microalgae biomass, and therefore, the first indicator followed was soluble COD increase after pretreatments. Nevertheless, this attempt has shown that microalgae biomass behaves differently than other biomasses upon pretreatments and no direct linkage exists (González-Fernández et al. 2012a; Alzate et al. 2012). In the constant search of finding a key indicator, proteins and carbohydrates have been also studied. These two macromolecules have been pointed out as potential indicators since microalgae biomass is mainly composed of these components (González-Fernández et al. 2010; Mendez et al. 2014a). In this sense, these macromolecules are main components, but what is indeed causing the low hydrolysis of these substrates in anaerobic digestion is the composition of the cell walls. It is important to understand the chemical composition of the targeted microalgal cell wall. C. vulgaris is one of the most commonly studied microalgae, and as reported in historical literature, it has a cellulose-based cell wall (Loos and Meindl 1984), but the latest studies are showing that this may not be correct (Gerken et al. 2013; Kim et al. 2014). By taking a closer look into carbohydrates, Mendez et al. (2014a) were able to identify a close relationship between carbohydrate solubilization and methane production enhancement after thermal pretreatment. Nevertheless, when trying to reproduce this trend in different microalgae strains, the results indicated that the overall fraction of carbohydrates was not a proper indicator (Mendez et al. 2014b). It can be thus concluded that the carbohydrate fraction itself cannot be used for this purpose, but study on the different carbohydrates that constitute the microalgae cell wall deserves further investigation. More specifically, uronic acids, neutral sugars, and amino sugars have been identified in the microalgae cell wall (Cheng et al. 2015). Studies have always claimed that this microalga possesses a carbohydrate-based cell wall mainly composed of cellulose and hemicellulose (González-Fernández et al. 2012b). Nevertheless, lately, few investigations have pointed out that this might not be true. For instance, Kim et al. (2014) used cellulases to hydrolyze C. vulgaris cell wall and the results were not as expected. Their investigation revealed that cellulase and amylase did not have any effect on cell wall disruption. To verify which enzyme would be more efficient for cell wall degradation, they tested pectinase, cellulase, amylase, xylanase, β-glucosidase, chitinase, lysozyme, and sulfatase. Their results confirmed that only pectinase had a significant effect on the degradation of polysaccharides from the cell wall of C. vulgaris. Similarly, another recent publication reached the same conclusion. It seems likely that C. vulgaris does not have a cellulose rigid cell wall, but rather uronic acids and amino sugars are conferring this microalgae its hardness (Gerken et al. 2013). At this point, it seems of relevant importance to characterize the carbohydrate fractions released upon pretreatments in order to gain insights on the effect that this disruption method has on the microalgae cell wall.
Proteins have been studied to a minor extent than carbohydrates. One of the main reasons for this limited information on proteins is related to the fact that proteins are weaker macromolecules than carbohydrates, and thus, upon pretreatments, proteins are quite often converted into other polymers and therefore not quantified in the soluble phase (Mendez et al. 2013). The low solubilization of proteins has been attributed to the occurrence of Maillard reaction. In this context, the available reducing sugars and amino acids reacted leading to the formation of complex molecules. Maillard reaction course is strongly affected by factors such as temperature, heating duration, water content, pH, and amino acid to sugar ratio. In this study, the low solubilization of proteins recorded was attributed to this type of reaction taking place when proteins and carbohydrates are soluble at high temperatures. As proteins react with reducing sugars, the amount of carbohydrates and proteins solubilized was indeed higher than the determined value. Nevertheless, the polymerization of the solubilized macromolecules reduced their solubility.
Microalgal cell walls have been reported to contain 10–30 % protein (Burczyk et al. 1999; Blumreisinger et al. 1983). The presence of proteins in the cell wall cannot be neglected since this polymer may also be responsible of the low anaerobic digestibility of microalgae. This macromolecular fraction has been pointed out as the polymer limiting the anaerobic digestion of other biomasses such as activated and primary sludge (Mottet et al. 2010; Miron et al. 2000). Lately, studies focusing on enzymatic hydrolysis of microalgae cell wall have elucidated the relevance of cell wall proteins for biogas production purposes. In this context, Mahdy et al. (2014a) tested two enzymatic cocktails, namely, carbohydrase and protease, which resulted in high hydrolysis efficiencies rendering almost all the particulate carbohydrates and proteins available in soluble phase. When subjecting carbohydrase and protease hydrolyzed biomass to anaerobic digestion, methane production was greatly enhanced for biomass pretreated with proteases. The importance of proteins for biogas production has been evidenced in chlorophyta, namely, Chlorella sp. and Scenedesmus sp. (Mahdy et al. 2015). Proteins frequently reported on microalgae cell wall conferring rigidity belong to the glycoprotein family (Voigt et al. 2014). Glycoproteins themselves are not difficult to digest but their linkage can be of great importance. For instance, Chlamydomonas reinhardtii exhibiting glycoproteins is a substrate easy to digest (Mahdy et al. 2014a), while Scenedesmus is probably one of the most difficult microalgae to degrade. In this latter one, glycoproteins are cross-linked via carbohydrate side chains and not via transglutaminase-dependent reactions or peroxidase-catalyzed isodityrosine formation as it happens in C. reinhardtii (Voigt et al. 2014).
The other change that is observed during microalgae pretreatments is the formation of aggregates upon cell wall disruption. Particle size distribution shifts to higher particle diameters when applying temperatures greater than 80 °C or when subjecting the microalgae biomass to ultrasound pretreatment at high energy levels (Ometto et al. 2014; González-Fernández et al. 2012c). The reason for this increased diameter has been attributed to the release of intracellular material. Some other evidences of cell wall disruption during pretreatments have been provided microscopically. Change in structural integrity or breakdown of microalgal cells are not detected under normal microscopic observation. However, cell wall disruption has been proved by using dyes, such as Sytox green (González-Fernández et al. 2012a), or through transmission electron microscopy (Passos and Ferrer 2014). These two methods are useful to fully ascertain that organic matter released during the different pretreatment is intracellular and not due to the exopolymers attached to the cell wall. In this context, Sytox green dyes the cell when the wall is disrupted; on the other hand, if only extracellular organic matter is released, microalgal cells would not be stained. Transmission electron microscopy has been also confirmed as a helpful tool to identify structural changes occurring upon microalgae cell wall pretreatments. During thermal hydrolysis, the cell wall are expanded and partially disaggregated inside the cell boundaries, while cell turgidity is less evident after enzymatic hydrolysis (Ometto et al. 2014). Authors attributed this less distorted cell structure after enzymatic hydrolysis to the degradation of specific cell wall components. FT-IR spectra of raw and pretreated microalgae biomass has also been used widely for obtaining information of chemical and structural changes in different biomasses (Monlau et al. 2012; Salehian et al. 2013). When applied to microalgal biomass, the intensity of peaks related to the bond C–O–C of polysaccharides diminishes at increasing temperatures, and also changes in the fingerprint regions of amide band (proteins) were registered during thermal pretreatment (Mendez et al. 2014a). Therefore, infrared spectra can support qualitative changes of the biomass upon pretreatment but not quantitative because the changes in peak intensity are too low to determine structural changes.
As it can be seen, the need of finding an indicator to elucidate whether the cell wall was damaged or not during biomass pretreatment has been successfully achieved during this last decade of research. Nevertheless, no clear indicator that can be related to methane production enhancement after biomass pretreatment has been provided. Most likely, the reason for this is the different cell wall composition and matrix linkage of the microalgae studied. However, the last decade of intensive research on microalgae biomass has shown a wide range of efficient pretreatments for biogas production. Most of the pretreatments studied recently are done through thermal, physical, or chemical means.
3.2.1.1.2 Thermal Pretreatments
Thermal pretreatments affect weak hydrogen bonds when mild temperatures (below 100 °C) are applied while complex carbohydrates solubilizes when higher temperatures are employed (González-Fernández et al. 2012a; Garrote et al. 1999). Mild temperatures of 50 °C when applied to microalgal biomass resulted in organic matter solubilization (Mahdy et al. 2014b). Carbohydrate solubilization recorded for Chlorella sp. was 15 and 32 % for Scenedesmus sp. Despite the organic matter release, methane yield of these biomasses did not increase significantly (Mahdy et al. 2014b; Passos et al. 2013). The almost negligible increase in methane yield was attributed to the fact that the organic matter solubilization was probably mediated by exopolymer released during the pretreatment rather than by cell wall breakage. At higher temperatures of around 90 °C, Scenedesmus biomass is disrupted after 30 min, and methane production is doubled (González-Fernández et al. 2012a). At this point, it should be stressed out that Scenedesmus is probably one of the most difficult microalgae to digest (Mussgnug et al. 2010). Similarly, out of the three mild temperatures tested on Nannochloropsis oculata, at 30, 60, and 90 °C for 4 h, an enhancement in methane yield of 41 % was recorded at 90 °C compared to the raw biomass (Marsolek et al. 2014). This was due to the organic matter solubilization registered after thermal treatment (40 %). In the same range, 32 % methane yield enhancement was registered after the thermal treatment at low temperature (60 °C for approximately 4 h) of a microalgal mixture of Pediastrum sp. and Micractinium sp. where 11 % organic matter was solubilized after the pretreatment (Kinnunen et al. 2014). Interestingly, these authors also reported the effect of freeze-thawing the biomass. This pretreatment resulted in 18 % organic matter solubilization but double methane yield. It can thus be confirmed that organic matter solubilization is a too general parameter, and the chemical composition of the different organic matter solubilized is crucial for methane production. This fact has also been confirmed by other authors. The different pretreatments and different pretreatment conditions differently affect microalgal biomass disruption, and thus, different disruption mechanisms lead to different chemical compositions of organic matter that ultimately determine the methane yield achievable by these pretreated substrates (González-Fernández et al. 2012a; Ometto et al. 2014). Overall, it may be hypothesized that this range of temperatures (around 100 °C) would be enough to open up all microalgae cell walls and make their organic material available for anaerobic microorganisms.
When evaluating higher temperatures, carbohydrate solubilization prevails over proteins (Mendez et al. 2013). This investigation dealt with Chlorella vulgaris subjected to 120 °C for 20 min, and 40 min resulted in 4- and 4.5-fold carbohydrate content in the soluble phase, respectively. Even though the differences attained were low, this had a major effect on the methane yield achieved. While biomass subjected to 120 °C for 20 min increased methane yield by 30 %, the biomass heated for 40 min doubled the methane production. Similarly, Cho et al. (2013b) reported a methane yield enhancement of 20 % together with 30 % organic matter solubilization when pretreating a microalgae mixture (70 % Chlorella sp. and 30 % Scenedesmus sp.) at 120 °C for 30 min with regard to 336 LCH4 kgVS−1 achieved by the raw biomass. These enhancements decreased to 14 % and 4 % when the mixture was pretreated at 80 and 50 °C, respectively. Nevertheless, as pointed out before, these enhancements are strain specific due to the differences in their cell wall and biochemical composition. In this manner, applying the same pretreatment to Scenedesmus sp. biomass supported an enhancement of 21–27 % (Mendez et al. 2014b). Even though carbohydrate profile for Scenedesmus biomass showed solubilization pattern similar to Chlorella biomass, the methane yield enhancement was lower for Scenedesmus than the observed value for Chlorella biomass. These results could be attributed to the high strength of Scenedesmus cell wall. In the case of saline biomass, thermal pretreatment at 120 °C for 2 h has been tested on Nannochloropsis salina (Schwede et al. 2013a). This investigation did not follow the organic matter solubilization, but authors observed a twofold increase in methane yield. The same enhancement was observed in semicontinuously operated reactors; however, the absolute methane yield values were diminished by half. In this sense, pretreated biomass digested in batch assay provided methane yield of 570 L kgVS−1, while the digestion in continuous mode mediated 270 L kgVS−1. The reason for such a decrease was attributed to the high ammonium and salt concentration in the feedstock which ultimately led to volatile fatty acid accumulation.
Moving upwards to higher temperatures in the range of 140–180 °C (applied for 10 min) increased the carbohydrate content in the soluble phase by four- to sixfold, while protein solubilization was enhanced by one- to twofold (Mendez et al. 2014a). Concomitantly with the enhanced carbohydrates solubilization, methane yield was improved by 1.4–1.6-fold in comparison to that of the raw Chlorella biomass. Ometto et al. (2014) also studied high temperatures in the range of 120–165 °C. Their results showed an organic matter solubilization of around 40 % when applying temperature at 165 °C for Scenedesmus obliquus and Chlorella sorokiniana. Despite the fact that the organic matter solubilization was similar, the methane yield in the case of Scenedesmus obliquus was enhanced by 200 % and for Chlorella sorokiniana by 100 % in comparison to the raw biomass (88 and 118 LCH4 kgVS−1, respectively). Within the high temperature range, high-pressure thermal hydrolysis (170 °C at 800 KPa) as pretreatment for Scenedesmus hydrolysis has been also tested (Keymer et al. 2013). These conditions of temperature and pressure were maintained for 30 min and resulted in 11-fold organic matter solubilization and 81 % increase in methane yield over that of raw algae (150 LCH4 kgVS−1).
3.2.1.1.3 Chemical Pretreatments
Chemical pretreatments have been studied to a lesser extent due to the need to readjust the pH changes prior to feeding into the reactor and associated chemical cost. The positive effect of acid catalysts in thermal pretreatment on other substrates, such as lignocellulose, has been confirmed. Thermo-acid pretreatment clearly affects the cell wall by solubilizing polymers, thus favoring anaerobic microbial degradation. This pretreatment, for instance, has been proven particularly efficient in solubilizing microalgae carbohydrates (Mendez et al. 2013). Treating Chlorella vulgaris at 120 °C for 40 min at pH 2 increased carbohydrates in the soluble phase by sevenfold, while the acid addition alone provided only 2.3-fold increase. Proteins were also solubilized; however, their conversion into other complex molecules made the quantification impossible. With regard to methane yield of this thermo-acid pretreatment, the higher solubilization of carbohydrates did not support an enhanced methane yield compared to only the thermally treated biomass. Thermally pretreated biomass yielded 267.7 LCH4 kg COD−1, while when this pretreatment was combined with pH reduction, methane yield reached 228.6 LCH4 kgCOD−1. Chemical supplementation combined with this temperature hindered the methane production, probably mediated by unidentified side product released during the pretreatment.
In case of lignocellulosic biomass, alkali addition disrupts the lignin structure and breaks the linkage between lignin and other cell wall carbohydrates. This feature does not affect microalgal biomass since these substrates are lignin-free. Nevertheless, alkali pretreatment enlarges the surface area of cellulose by biomass swelling and reduces cellulose crystallinity by cleavage of carbohydrate glycosidic bonds (Hsu 1996). Alkali pretreatment at pH 10 and temperature of 120 °C led to an increase of proteins in the soluble phase by 1.7- and 1.9-fold when pretreating Chlorella vulgaris biomass for 20 and 40 min, respectively (Mendez et al. 2013). Under this alkali scenario, carbohydrates were solubilized in a similar fashion like the thermal pretreatment which did not involve chemicals. Thermo-alkali pretreatment reported slightly higher values on methane yield than that observed for thermo-acidic pretreatment. Methane yield was reportedly enhanced by 1.73-fold when a combination of 120 °C for 40 min and pH 10 was used as pretreatment, while for the raw biomass, it was 139 LCH4 kgCOD−1 only.
Carbohydrate solubilization is also reported when pretreatment involves lower temperatures (50 °C), in addition to alkali (Mahdy et al. 2014b). The solubilization of polymer is strain specific. For C. vulgaris, it ranged from 1 to 18 %, while for Scenedesmus sp., it was 15–44 %. Once again, even though the organic matter solubilization was higher for Scenedesmus, methane yield enhancement was higher for C. vulgaris. The reason for that was attributed to the nature of carbohydrates solubilized. This study showed low methane production enhancement under this conditions (approximately 15 % when biomass was pretreated with 5 % w/w NaOH). This low enhancement was ascribed to the fact that the organic matter solubilization was probably mediated by exopolymers released during the pretreatment rather than those from within the cell. When alkali pretreatment was performed over a mixture of microalgae (70 % Chlorella sp. and 30 % Scenedesmus sp.) without heating, only 5 % organic matter solubilization was observed at pH 9 and 11, while this value increased up to 21 % at pH 13 (Cho et al. 2013b). Nevertheless, pH 11 and 13 negatively affected methane production and inhibited the process. Subjecting the biomass to pH 9 provided only a slight enhancement. It can be thus concluded that alkali pretreatment of microalgal biomass is not a promising method.
Overall, acidic pretreatments increased the carbohydrates released into the medium while under alkaline conditions; proteins were solubilized to a greater extent. The drawback of using concentrated chemicals includes material corrosion and formation of by-products that could result in inhibition of digestion (Monlau et al. 2014).
3.2.1.1.4 Physical Pretreatment
Physical pretreatment involves the reduction of particle size and increase in surface/volume ratio available for hydrolysis. Within this category, microwave and ultrasound are the most commonly used methodologies. Microwave pretreatment involves boiling of water using microwave radiation. This process resulted in cell hydrolysis and structural changes in proteins (Park et al. 2010). Microwave pretreatment (900 W for 3 min having specific energy of 70,000 kJ kg VS−1) has been tested on a mixture of microalgae, and the results on methane yield were not significant (Passos et al. 2014a). Minor increase in methane yield was attributed to the possible cell wall damage, but since lysis did not occur, any significant increase in yield was precluded. However, it remains to be seen if this pretreatment has strain specificity. For application of ultrasound to microalgal biomass, literature is more extensive. As a matter of fact, a detailed review on the effect of this pretreatment on different biomasses, including microalgae, is available (González-Fernández et al. 2014). Ultrasound consists of elastic waves with frequency range between 20 kHz and 1 GHz. Bubbles are formed in the liquid and filled with the liquid’s vapor and dissolved gases. Above a critical value of local pressure, the bubbles implode violently, producing powerful hydromechanical shear forces in the liquid medium surrounding them. On micro scale, the cavitation process produces temperature of around 5000 °C and pressure of 50 MPa for microseconds (Suslick 1990). Ultrasound pretreatment has also been proven as an effective pretreatment for two of the most robust microalgae, Chlorella vulgaris and Scenedesmus obliquus. When subjecting Scenedesmus obliquus to different levels of ultrasound energy, the results showed methane production at a twofold higher level on lower ultrasound levels (35.5 MJ kg−1 TS−1) and a fourfold level when applying higher energy levels (76.5–130 MJ kgTS−1) (González-Fernández et al. 2012b). The highest energy supplied (100–130 MJ kgTS−1) almost doubled the methane production for the untreated S. obliquus (51 LCH4 kgVS−1). This enhancement is similar to that observed for thermal pretreatment of this biomass, and therefore, due to lower energy requirements of thermal application, this latter one might be a preferable pretreatment (González-Fernández et al. 2012a). Ometto et al. (2014) compared the effect of ultrasound on three photosynthetic microorganisms, namely, C. vulgaris, S. obliquus, and A. maxima. This investigation showed that the microalgae C. vulgaris provided the highest methane yield enhancement (44 % in comparison to the raw biomass which attained 169 LCH4 kgVS−1) when the biomass was subjected to 35 MJ kgTS−1. In the case of A. maxima, 82 % organic matter solubilization and 33 % methane yield enhancement were observed, regardless of the increase in power output applied (from 0.35 up to 35 MJ kgTS−1). This was due to the fact that A. maxima has a weaker cell wall compared to other microalgae, and therefore, even the lowest energy level applied provided the very high solubilization and methane yield achievable out of this biomass. On the other hand, when applying a specific energy of 32 MJ kgTS−1 to a microalgal mixture composed of Monoraphidium sp., Stigeoclonium sp., and the diatoms Nitzschia sp. and Amphora sp., the methane yield enhancement was 11 % (raw biomass exhibited 148 LCH4 kgVS−1) (Passos et al. 2014b). This low enhancement was attributed to the presence of diatoms which have a silica-based cell wall which is only slightly degradable. Methane yield increased by 33 % after subjecting this biomass mixture to 67.2 MJ kgTS−1; nevertheless, the authors have also pointed out that the preliminary energy assessment indicated that the energy input was higher than the extra energy produced. The general conclusion that can be pointed out of the ultrasound pretreatment studies confirms that the thermal pretreatments can be more effective than ultrasound in enhancing microalgae digestibility (González-Fernández et al. 2012b; Cho et al. 2013b; Ometto et al. 2014).
3.2.1.1.5 Enzymatic Hydrolysis
For enzymatic hydrolysis, the correct choice of enzymes is crucial for a successful pretreatment. So far, very little work has been done on the effect of enzymatic hydrolysis of microalgae for methane production purposes (Miao et al. 2013; Ciudad et al. 2014). Cell wall enzymatic pretreatment may not be useful for biofuel production only but also for value-added products that can be extracted from this biomass. It is of high importance to understand the chemical composition of the targeted microalgae cell wall. Enzymatic pretreatment precludes the formation of inhibiting by-products. The overall process cost of enzymatic hydrolysis may be lower than thermochemical hydrolysis as it avoids corrosion of the container and requires mild temperature. Likewise, enzymes can be produced naturally by a wide range of bacteria and fungi.
As mentioned above, the cell wall composition is one of the key parameters. Chlorella vulgaris is probably one of the most studied microalgae, but the composition of its cell wall is still not clear. Some studies have claimed that this microalga possesses a carbohydrate-based cell wall mainly composed of cellulose and hemicellulose. Nevertheless, lately, few investigations have pointed out that this might not be true (Kim et al. 2014). It seems likely that C. vulgaris does not have a rigid cell wall because of cellulose, but uronic acids and amino sugars are conferring this microalgal cell wall its hardness (Gerken et al. 2013). Therefore, enzymatic hydrolysis may be extremely useful to gain insights on cell wall composition.
The addition of carbohydrase and protease mediated high carbohydrate and proteins solubilization (86–96 %) when applied on Chlamydomonas reinhardtii and Chlorella vulgaris (Mahdy et al. 2014a). Out of these two biocatalytic cocktails, protease addition was more beneficial for biogas production. In the case of C. reinhardtii, protease addition increased methane production by 1.17-fold in comparison to the raw biomass (263 LCH4 kgCOD−1). This enhancement was low due to the inherent high biodegradability of this biomass. On the other hand, hydrolyzed C. vulgaris mediated an enhancement of 51 % compared to the raw biomass (190 LCH4 kgCOD−1). In order to optimize the protease dosage, results were tested by decreasing dosages. This attempt resulted in diminished hydrolysis efficiency concomitantly with decreased methane yield enhancement (Mahdy et al. 2014c). Thus, the optimum protease dosage was set at 0.585 AU g DW−1. This dose was tested at increasing biomass loads to elucidate whether the viscosity of the broth was affecting the hydrolysis. The results indicated that this dose can be employed up to 65 g TS L−1 without markedly affecting the hydrolysis efficiency or the methane yield enhancement. At this point, it should be stressed out that proteases are released under biomass storage. Using some other biocatalyst cocktails, Ometto et al. (2014) tested a mixture of endoglucanase and cellulase and a mixture of esterase and protease in two microalgae and one cyanobacterium. Their results showed that the first mixture was better to hydrolyze Scenedesmus obliquus and increased methane yield by 5.6-fold, while the enhancement in the case of Chlorella vulgaris was decreased by 3.15-fold. The second mixture of enzymes provided an enhancement in methane yield of 3.9- and 3.2-fold for S. obliquus and C. vulgaris, respectively. In the case of the cyanobacterium Arthrospira maxima, no significant differences were attained between the two cocktails; thereby, both of them provided a biogas yield increase of approximately eightfold.
Only one study is available in the recent literature concerning the use of noncommercial enzymes for hydrolyzing microalgae biomass. In this context, marine bacteria with cellulolytic capacity (mainly exhibiting endoglucanase activity) were tested on Nannochloropsis gaditana (Muñoz et al. 2014). After 25 days of digestion, methane yield was enhanced by 2.5-fold compared to the raw biomass (109 LCH4 kgVSS−1). For commercial cocktails which are mostly active at 50 °C, these authors also pointed out that the activities of these enzymes were maximum at 30 °C. Thereby, it can be inferred that energy costs can be decreased due to moderate requirement of temperature. Once identified, the most appropriate enzyme (or a cocktail) which is able to disrupt a broad range of microalgae strain may be produced in situ.
3.2.1.2 Co-digestion
Due to the high nitrogen content, microalgal biomass is characterized by a low C/N ratio. Co-digestion aims to increase the C/N ratio of the substrate introduced into the digester in order to balance nutrients. Different substrates rich in carbon could be combined with microalgal biomass thereby increasing the C/N ratio up to levels close to the optimum for anaerobic digestion, which oscillates between 10 and 30 (Pagés Díaz et al. 2011). The reason for such wide optimum C/N ratio is that it depends on several factors such as the chemical composition of the digested substrate, temperature of the process, and adaptation of the microorganisms to high levels of potentially inhibitory compounds, such as nitrogen (Chen et al. 2008).
Algae could potentially be integrated in a wastewater treatment plant and combine the benefits of nutrient removal, energy production, and CO2 sequestration. Recently, a study was conducted to evaluate the potential of microalgae biomass as co-substrate for anaerobic digestion of primary and secondary sludge, thereby increasing the methane production and improving the energy balance of the whole process (Mahdy et al. 2014d).
Co-digestion with different substrates has been tested with different microalgae species such as Spirulina maxima (Samson and LeDuy 1983), Arthrospira platensis (El-Mashad 2013), Scenedesmus sp. (Yen and Brune 2007; González-Fernández et al. 2011; Ramos-Suárez et al. 2014a), Chlorella sp. (Yen and Brune 2007; Ehimen et al. 2009, 2011; González-Fernández et al. 2011; Wang et al. 2013; Park et al. 2013), Nannochloropsis salina (Park and Li 2012; Schwede et al. 2013b), Microcystis sp. (Zhong et al. 2012, 2013; Zhao and Ruan 2013), Isochrysis galbana, and Selenastrum capricornutum (Caporgno et al. 2015). In turn, co-substrates employed are diverse, mostly rich in carbon (sewage sludge, peat hydrolyzate, paper residues, glycerin, waste fat and oils, kitchen wastes, corn straw, switch grass, and prickley pear) and in once case a substrate rich in nitrogen: swine manure.
Most of these studies showed a positive effect when co-digesting microalgal biomass. Yen and Brune (2007) observed an increase in methane production and an improvement in the kinetics of degradation when co-digesting Scenedesmus and Chlorella with paper residues. Besides the beneficial effects of the balance of nutrients and alkalinity, they found an increase in the activity of cellulase when paper residues were added to the digesters together with microalgal sludge. The cellulase activity registered mediated a better degradation of Scenedesmus and Chlorella cell wall, and thus, the anaerobic biodegradability of these substrates was improved. Methane yield of microalgal sludge digested alone (C/N= 6.7) was 143.2 LCH4 kgVS−1, whereas a mixture composed of 40 % algal sludge and 60 % paper residues in VS basis (C/N= 22.6) increased methane yield by 124 %. On the other hand, Wang et al. (2013) suggested that the increase in the biodegradability of Chlorella when it was co-digested with activated sludge was consequence of the high quantity and diversity of microorganisms in the sludge that aided in the hydrolysis of microalgal cell wall.
However, not all the studies have shown positive results. González-Fernández et al. (2011) co-digested Chlorella vulgaris and Scenedesmus obliquus with swine manure expecting microalgae to act as a carbon source to improve the digestion of swine manure. Although the cell walls of both species are rich in carbon, their complexity impeded the degradation and the methane production decreased. A decrease in methane production was also observed when Spirulina platensis was co-digested with switch grass (El-Mashad 2013). According to the author, the high lignin content of switch grass was the cause of this reduction even though C/N ratio was higher.
Ammonia produced by nitrogen degradation increased the buffer capacity of the digestion system. Therefore, microalgae could be used as co-substrate of easily degradable energy crops, increasing the organic loading rates (OLR) achievable. Schwede et al. (2013b) observed that the addition of Nannochloropsis salina to maize silage in a continuous digestion process facilitated the increase in organic loading rates to higher levels than in the monodigestion of maize silage. Similarly, Ramos-Suárez et al. (2014a) reached OLR as high as 5.33 gVS L−1 d−1 (HRT of 15 days) in the co-digestion of Scenedesmus sp. and O. maxima with a methane yield of 307.8 L kgVS−1.
Considering the different studies concerning co-digestion of microalgal biomass, it seems that the addition of carbon-rich substrates enhanced the digestion process and an increase in the methane production along with an improvement in the kinetics of degradation. However, each co-substrate needs to be studied separately, since the increase in the C/N ratio cannot be used as a sole indicator of the process performance (Table 5.1).
3.3 Anaerobic Digestion in Microalgae Biorefineries
Definitely, there is a great potential in microalgae for biofuel production, but the truth is that nowadays microalgal biofuels are far from being economically viable. In this regard, the upper limit value of biomass production cost has been agreed at 0.5 US$ kg−1 (Acién et al. 2014), although Chisti (2012) suggested a production cost of 0.25 US$ kg−1 for algal fuels to be competitive with petroleum-derived fuel.
Production cost estimates vary widely from study to study due to the lack of industrial plants working at full capacity and a defined technology (Acién et al. 2014). Chisti (2007) estimated production costs of 2.95 US$ kg−1 and 3.80 US$ kg−1, respectively, for PBRs and raceways, assuming free carbon dioxide and a facility with an annual biomass production of 100 tons. Acién et al. (2012b) estimated production cost of 69 € kg−1 for a 3 m3 tubular PBR facility producing Scenedesmus almeriensis. Authors indicated that by a simplification of the production system and due to economics of scale, increasing annual biomass production up to 200 tons year−1 (1570 m3 of PBRs) could reduce production costs to 12.6 € kg−1, still far away from the upper limit mentioned above. Other authors (Norsker et al. 2011) estimated production costs of microalgae in closed tubular PBRs, flat panels, and raceways to be 4.15, 4.95, and 5.96 € kg−1, respectively, and stated that optimizing production conditions could reduce production costs to 0.68 € kg−1.
In any case, microalgae production costs need to be reduced to meet requirements of the energy market (Acién et al. 2012b). Microalgae industry is expected to keep growing in the near term for the nutraceutical, pharmaceutical, cosmetic, food, and feed industries, whereas fertilizers, bioremediation, and chemical demands are also future applications of interest. If several compounds and products are obtained from microalgae at the same time, the economics of production would improve substantially. It has to be taken into account that all these products have higher market prices than biofuels, and therefore, the production of the latter can be expected to be as a marginal case at the end of a production line. Whatever the compound/s to be extracted or product/s to be produced from microalgae, it is expected that in the large scale this activity would produce great amount of organic residues that would require appropriate treatment (Ramos-Suárez and Carreras 2014).
Anaerobic digestion can be used in a biorefinery concept, as the appropriate process with huge synergistic possibilities for energy generation with microalgae culture. The coupling of anaerobic digestion to the extraction of proteins from microalgae could improve the economics of the process by the generation of renewable energy, the recycling of the digestate as growth medium (Uggetti et al. 2014), and the use of raw biogas or combusted biogas as carbon source (Douškova et al. 2010).
3.3.1 Organic Waste Treatment and Clean Energy Production
The first goal of anaerobic digestion in any biorefinery is the treatment of the organic waste and the production of clean energy in form of biogas, and in the case of microalgal biorefineries, this is not different. In this regard, different options have been assessed up to now, although available literature is pretty scarce.
As already mentioned in this chapter, the major fraction of microalgae grown without nutrient limitation is protein (Rebolloso Fuentes et al. 2000). Therefore, if the goal of a biorefinery is to use most of the generated biomass, protein should be leveraged properly. After the extraction of proteins, the residual biomass could be converted into biogas. Furthermore, the digestion process is improved due to the disruption of the cell wall prior to protein extraction and the increase in the C/N ratio (Ramos-Suárez and Carreras 2014). In their study with Scenedesmus biomass digested in CSTR, Ramos-Suárez et al. (2014b) observed an increase in the biodegradability and biogas yield in amino acid-extracted residual biomass similar to that observed after thermal and thermochemical pretreatments by other authors. Biogas yield of Scenedesmus residual biomass increased up to 409 Lbiogas kgVS−1 (71 %CH4) with a digester efficiency of 1084 LCH4 m−3 digester d−1 at and OLR of 3.85 gVS L−1 d−1 and 20 days of HRT.
Several authors have pointed anaerobic digestion as a convenient supplemental process to biodiesel production from microalgae (Sialve et al. 2009; Chisti 2007), since the produced biogas could serve the electrical and thermal energy necessary to run the biodiesel production process. In this regard, different studies have assessed the combination of lipid extraction from microalgae and subsequent anaerobic digestion of lipid-extracted residual biomass.
Two studies assessed methane potential of Chlorella biomass both in batch and continuous mode and after different lipid extraction methods (Ehimen et al. 2009, 2011). A reduction in methane potential was observed when lipid-extracted biomass was digested, although kinetics of the process was improved. The decrease in the methane production was a consequence of the extraction of the lipid fraction, which yields higher methane than proteins and carbohydrates (Sialve et al. 2009). It is important to note the solvent used during the extraction method since it can remain the residual biomass and affect the microorganisms. In fact, it has been demonstrated that the extraction of lipids with a chloroform-methanol mixture in the conventional lipid extraction process can inhibit the process (Ehimen et al. 2009). Contrarily to the results observed by Ehimen et al. (2009), other studies have shown that the lipid extraction could benefit a subsequent digestion of the residues compared to raw biomass. An increase in methane yield for Scenedesmus and Nannochloropsis gaditana lipid-extracted biomass has been observed. For Scenedesmus biomass, increases of 33.3 and 51.3 % have been observed after lipid extraction (Keymer et al. 2013; Ramos-Suárez and Carreras 2014), whereas for Nannochloropsis gaditana, a slight increase of 10 % was observed (Alzate et al. 2014). Results obtained suggest that the increase or decrease of methane potential is dictated by the specie under digestion. In species with resistant cell walls, such as Scenedesmus, the rupture of the cell wall is enough to produce a significant increase in the methane production compared to that obtained from raw biomass, even though lipids are extracted from the biomass.
Although major part of the research on this field is focused on the use of lipid- or protein-extracted residual biomass, the ideal biorefinery would use all precious components in a multistep extraction before introducing residues in an anaerobic digester. Lipids and amino acids could be extracted in sequential processes; afterward, residues could be used for biogas production. Moreover, sugars or other high-value products depending on the species could also be extracted. However, the sequential application of different extraction processes is difficult due to the intensive processes used. Normally, the extraction of certain component causes the loss or degradation of the other components, preventing further use of generated residual biomass. Researchers in the University of Almería (Spain) are working currently in the development of multipurpose extraction process (see Fig. 5.4), minimizing residues but using them for anaerobic digestion as a final step to produce biogas and digestate which could be used as fertilizer (Fernández Sevilla 2014).
3.3.2 Closing the Nutrient Loop
As already said, the two main products of anaerobic digestion are biogas and the digestate. Biogas is formed mainly by methane and carbon dioxide, although it has typically other minor components such as water vapor, hydrogen sulfide, ammonia, hydrogen, and nitrogen. On the other hand, the digestate is an aqueous sludge where almost all nutrients remain in a mineralized form, therefore being used as biofertilizer and/or soil amendment. Both products could be used for microalgae culture, the first as carbon source for their autotrophic growth and the second as growth medium, supplying the necessary mineral nutrients for microalgae growth. Possibilities for their use are described in this section.
3.3.2.1 Biogas Upgrading
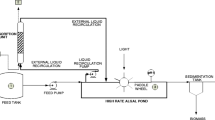
There are two possibilities for biogas to be used by microalgae (see Fig. 5.5): (1) biogas upgrading by microalgae to produce methane-enriched biogas and subsequent use of methane-enriched biogas in different applications which can provide additional CO2 for microalgae culture and (2) supplying the flue gases produced after the combustion of biogas in traditional CHP engines or boilers to microalgae cultures.
Based on these two basic combinations, different additional processes could be included. For instance, in Fig. 5.5a, instead of using the upgraded biogas in CHP units or boilers, biogas could be further upgraded to be used as vehicle fuels, in fuel cells, or to be injected in natural gas grids. Additionally, in both schemes, cultivated microalgae could be introduced in the anaerobic digester as co-substrate to increase biogas production. The use of flue gases as carbon source in microalgae cultures has been extensively studied and will not be covered in this chapter, where we will focus on biogas upgrading by microalgae.
The need of biogas purification depends on the application in which it is being used, as shown in Fig. 5.2. Hydrogen sulfide and carbon dioxide removal technologies are widely applied in biogas plants all over the world, with different existing options based on chemical, physical, or biological methods.
If microalgae are used for biogas upgrading, the technology needs to be competitive with the existing technologies in the market. Table 5.2 shows a comparison between the main technologies used nowadays for biogas purification. Microalgae show potential in biogas purification at farm scale although capital costs are high compared to other low-cost technologies such as iron sponge, activated carbon, iron chloride addition to substrate, and microareation. Moreover, it is the only technology that has the potential for simultaneous removal of H2S and CO2.
The main benefits of microalgal purification over the other technologies are:
-
Effective absorption of CO2 in the form of biomass.
-
Generation of microalgal biomass which can be further marketed.
-
Microalgal biomass could be used as co-substrate increasing biogas production.
-
No residues are produced and no chemicals are used.
-
The digestate could be also used as growth medium, removing nitrogen and phosphorous
Microalgal biogas purification is a new technology, poorly developed, that even has some uncertainties that will be important to determine the feasibility of the process. Besides the factors influencing microalgal growth in any conventional culture system (light, mineral nutrients, temperature, pH, mass transfer capacity), if biogas is used as carbon source, some additional factors come into play.
Biogas composition influences purification efficiency according to three factors: (1) CH4 concentration, (2) CO2 concentration, and (3) H2S concentration. Another important point that limits the efficiency of the system is the oxygen concentration in the upgraded biogas.
3.3.2.1.1 Methane Concentration
Methane concentration in raw biogas could reach levels of up to 70 % in some cases. In several studies where biogas has been upgraded by microalgae, the growth rate of these microorganisms has been negatively affected due to high CH4 concentrations (Kao et al. 2012a; Yan et al. 2014; Douškova et al. 2010). Although there was an evident decrease in the growth rate, in all cases, it was a slight decrease that would not suppose an impediment to the use of biogas as carbon source.
Despite this limitation, methane concentration in purified biogas has been above 90 % in most cases (Kao et al. 2012a; Yan et al. 2014; Travieso et al. 1993; Yan and Zheng 2013; Converti et al. 2009).
3.3.2.1.2 Carbon Dioxide Concentration
In studies where biogas has been used as carbon source of microalgae, no inhibition has been observed with CO2 concentrations up to 55 % (Yan et al. 2014). However, in other studies performed with flue gases, inhibition of microalgal growth has been observed with CO2 concentrations above 5 % (Chiu et al. 2009).
In any case, an excessive addition of CO2 to microalgal cultures could cause the acidification of the growth medium, a decrease in microalgae growth rate, and the subsequent reduction of purification efficiency (Acién et al. 2012a; Sumardiono et al. 2014; Yan et al. 2014). Therefore, addition of CO2, and consequently of biogas, must always be controlled as a function of pH, matching CO2 addition to the CO2 consumption by microalgae.
Several studies have explored the possibility of injecting biogas and air intermittently, observing an increase in microalgae growth rate (Sumardiono et al. 2014; Kao et al. 2012b). This kind of systems would entail at list two duplicate systems working in parallel to achieve a constant-in-time purification of biogas, avoiding at the same time the dilution of purified biogas with air. Also, it has to be taken into account that there are microalgae species more resistant to high concentrations of CO2, as it is the case of a mutant strain of Chlorella sp. (Kao et al. 2012b).
3.3.2.1.3 Hydrogen Sulfide Concentration
Hydrogen sulfide is often present in biogas in concentrations up to 1 % (10,000 ppm). This component is probably the one which brings more uncertainties to the efficiency of the microalgal biogas upgrading system. Moreover, there exists little information about what happens with this component during biogas supplying to microalgal cultures. Some authors have hypothesized about what is the mechanism of removal of H2S from biogas. Travieso et al. (1993) suggested that this removal would be caused mainly by absorption of hydrogen sulfide into the aqueous growth medium; once absorbed, sulfur would be consumed by microalgae cells for the production of amino acids but at a slower rate than the absorption rate (Travieso et al. 1993). Therefore, at first, we might consider that biogas cleaning by microalgae removes both CO2 and H2S, as has been shown in some studies (Travieso et al. 1993; Mann et al. 2009; Douškova et al. 2010). However, other studies showed that the presence of H2S in the system is inhibitory to the growth of microalgae (Kao et al. 2012a). Consequently, in other studies, H2S is removed before employing biogas as carbon source for microalgae (Yan and Zheng 2013). It is probably an excessive accumulation of H2S in growth medium which caused inhibition of microalgal growth in some cases.
Although this point may not seem important, economic viability compared to other methods of biogas upgrading could change dramatically if there is a need to install an additional system to remove H2S, as often happens in the conventional systems shown in Table 5.2.
3.3.2.1.4 Oxygen Concentration in Upgraded Biogas
In any system where microalgae grow, oxygen will be produced due to photosynthetic activity. This oxygen should be removed from the growth medium to impede photosynthesis inhibition due to oxygen saturation (Acién et al. 2013). Therefore, one of the technical challenges is how to remove this oxygen from the growth medium avoiding at the same time the accumulation of oxygen in the purified biogas. The maximum oxygen concentration would be determined by the application in which the biogas is to be used and the flammability risks of the gas mixture.
Normally, the minimum oxygen concentration (MOC) below which the combustion of a gas mixture is not possible oscillates between 5 and 15 %. For the case of methane, the MOC is 12 %. However, this relation changes with pressure and temperature. The higher the pressure and the temperature, the lower the MOC, i.e., gas mixture is more explosive. This is a very important factor which has to be taken into account due to safety reasons.
3.3.2.2 Digestate Recycling as Growth Medium
The digestate is an aqueous medium which is composed of stabilized organic matter (active biomass and hardly biodegradable organic matter) and mineralized nutrients. Digestate is an easy product to handle and can successfully substitute mineral fertilizers in agricultural applications (Lukehurst et al. 2010). Anaerobic digestion is a closed system where the only inputs are the substrate and energy. Therefore, the fertilizer value of the digestate depends on the substrate composition, i.e., the nutrients that are supplied to a digester via the feedstock are the same as those in the digestate. However, nutrients in the feedstock will change in form during the anaerobic digestion process due to biochemical reactions that take place inside the digester, enhancing their availability to crops (Lukehurst et al. 2010).
The use of digestate as growth medium could improve economics and environmental impact of microalgae production by reducing water needs and fertilizer costs. For the specific case of biofuel production from microalgae, the availability of these two inputs is crucial for its viability (Uggetti et al. 2014).
Use of digestate as a growth medium for microalgae culture implies to consider some important points. First of all, it is necessary to remove the solid fraction of the digestate. The removal of the solids from the digestate will improve light availability and ease final separation of higher-quality microalgal biomass. Digestate liquid fraction is characterized by high ammonium content (Lukehurst et al. 2010) and high turbidity (Noike et al. 2004), factors that could influence microalgal growth and reduce the suitability of digestate as growth medium (Uggetti et al. 2014). According to Collos and Harrison (2014), who studied the effect of different ammonium concentrations on several species of microalgae, there are significant differences between classes of unicellular algae. According to this study, chlorophytes are the most tolerant class to high ammonium, whereas dinoflagellates are the least tolerant. Furthermore, ammonia toxicity is associated with NH3 at pH > 9, whereas at pH < 8, toxicity is likely to be associated with the ammonium ion. The effects of ammonium on microalgae are evident on long-term growth rates (days), but also on short-term physiological processes such as uptake rates, photosynthetic rates, and enzyme activities (minutes to hours).
Moreover, the use of digestate as fertilizer is normally regulated in order to protect animal and human health as well as the quality of the crops (Lukehurst et al. 2010). Therefore, the use of digestate as growth medium for microalgae culture could limit the ultimate fate of the produced biomass.
In literature, relatively very few recent studies have investigated on the use of digestate as growth medium for microalgae culture (Cho et al. 2013a; Uggetti et al. 2014; Ficara et al. 2014; Prajapati et al. 2014b; Fouilland et al. 2014; Franchino et al. 2012; Sheets et al. 2014; Marcilhac et al. 2014). From most of these studies, it can be concluded that the ammonium concentration in digestate negatively affects microalgae growth, and therefore, it should be diluted before being used (Cho et al. 2013a; Uggetti et al. 2014; Prajapati et al. 2014b; Fouilland et al. 2014; Franchino et al. 2012; Sheets et al. 2014). This statement applies to several microalgae strains: Chlorella, Scenedesmus, Chroococcus, Botryococcus, Nannochloris, Dunaliella, Lyngbya aestuarii, Neochloris and Nannochloropsis. The impact of ammonia varied from strain to strain, as already concluded by Collos and Harrison (2014). Conversely, Ficara et al. (2014) observed no severe inhibition when using undiluted digestate to grow an algal suspension made of a mixture of Scenedesmus and Chlorella, although a decrease in nitrogen removal efficiency was evident. Dilution could be done with any available water, but preferably, wastewaters with low ammonium content should be used in order to reduce environmental impact.
On the other hand, there is no consensus about the effect of turbidity and color of the digestate on microalgal growth. Some authors pointed out that biomass concentration limits light availability, and therefore microalgal growth rate, to a greater extent than turbidity (Uggetti et al. 2014). Contrarily, other studies suggested that digestate color, even when diluted up to 30 %, hindered light availability and microalgal growth (Prajapati et al. 2014b; Marcilhac et al. 2014).
It should be noted again that digestate composition and color depend on the substrate being fed into the digester and on the process that takes place in each digester; therefore, the successful use of different digestates as growth medium will vary from case to case and also with a high dependence on microalgae strain (Marcilhac et al. 2014). This can be seen in Table 5.3, where the optimal dilutions found by some of the studies conducted are shown.
The use of digestate as growth medium will reduce, or even eliminate, the use of additional nutrients. The level of self-sufficiency of nutrients will depend on the composition and availability of the digestate. For instance, Ramos-Suárez et al. (2014b) prospected a reduction of 30 % in nitrogen needs if digestate from amino acid-extracted microalgae degradation was recycled as growth medium in a closed biorefinery concept. In this specific case, the extraction of amino acids reduced nitrogen content of digested biomass and, therefore, nitrogen content in the digestate. In this kind of closed microalgae biorefineries, for different classes of spent biomass, savings in fertilizer could be higher.
4 Conclusion and Future Prospects
The integration of different technologies in a biorefinery aims at maximizing benefits while reducing the environmental impact. Considering what we have shown in this chapter, the future of algae biorefineries would include the extraction of several components from microalgae and simultaneous reduction of the waste biomass. Waste biomass would be treated by anaerobic digestion thus reducing the pollutant load while producing energy and recycling nutrients for microalgae culture. A simplified scheme of the prospected biorefinery is shown in Fig. 5.6.
According to authors, current bottlenecks for the development of this concept of biorefinery include:
-
The extraction of different components from biomass in a sequential process
-
Effective biogas upgrading by microalgae
-
Use of the digestate as growth medium without dilution
If the nutrient loop is closed, profitable processes will be achieved. Consequently, biofuels and high-value products would be obtained at the same time from microalgal biomass, reducing environmental impact and increasing profits.
References
Acién FFG, González López CV, Fernández Sevilla JM, Molina Grima E (2012a) Conversion of CO2 into biomass by microalgae: how realistic a contribution may it be to significant CO2 removal? Appl Microbiol Biotechnol 96:577–586
Acién FG, Fernández JM, Magán JJ, Molina E (2012b) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30:1344–1353
Acién FFG, Fernández Sevilla JM, Molina Grima E (2013) Photobioreactors for the production of microalgae. Rev Environ Sci Biotechnol 12:131–151
Acién FG, Fernández JM, Molina-Grima E (2014) Economics of microalgae biomass production. In: Pandey A, Lee D-J, Chisti Y, Socool CR (eds) Biofuels from algae. Elsevier, Burlington, pp 313–325
AEBIOM-European Biomass Association (2009) A biogas road map for Europe. European Biomass Association, Brussels
Alzate ME, Muñoz R, Rogalla F, Fdz-Polanco F, Perez-Elvira SI (2012) Biochemical methane potential of microalgae: influence of substrate to inoculum ratio, biomass concentration and pretreatment. Bioresour Technol 123:488–494
Alzate ME, Muñoz R, Rogalla F, Fdz-Polanco F, Perez-Elvira SI (2014) Biochemical methane potential of microalgae biomass after lipid extraction. Chem Eng J 243:405–410
Anderson K, Sallis P, Uyanik S (2003) Anaerobic treatment processes. In: Mara D, Horan N (eds) The handbook of water and wastewater microbiology. Academic Press, London, pp 391–426
Bazara X, Galimany F, Torres R (2003) Digestión anaerobia en el tratamiento de efluentes y lodos residuales. Tecnología del Agua 233:34–46
Blumreisinger M, Meindl D, Loos E (1983) Cell wall composition of chlorococcal algae. Phytochemistry 22:1903–1904
Bougrier C, Carrere H, Delgenes JP (2005) Solubilisation of waste activated sludge by ultrasonic treatment. Chem Eng J 106:163–169
Brennan L, Owende P (2010) Biofuels from microalgae – a review of technologies for production, processing and extractions of biofuels and co-products. Renew Sust Energy Rev 14:557–577
Bruton P, Lyons H, Lerat Y, Stanley M, Rasmusse MB (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Sustainable Energy Ireland, Dublin
Burczyk J, Dworzanski J (1988) Comparison of sporopollenin-like algal resistant polymer from cell wall of Botryococcus, Scenedesmus and Lycopodium clavatum by GC-pyrolysis. Phytochemistry 27:2151–2153
Burczyk J, Smietana B, Terminska-Pabis K, Zych M, Kowalowski P (1999) Comparison of nitrogen content amino acid composition and glucosamine content of cell walls of various chlorococcal algae. Phytochemistry 51:491–497
Caporgno MP, Trobajo R, Caiola N, Ibáñez C, Fabregat A, Bengoa C (2015) Biogas production from sewage sludge and microalgae co-digestion under mesophilic and thermophilic conditions. Renew Energy 75:374–380
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064
Cheng YS, Labavitch JM, Vander Gheynst JS (2015) Elevated CO2 concentration impacts cell wall polysaccharide composition of green microalgae of the genus Chlorella. Lett Appl Microbiol 60:1–7
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2012) Raceways-based production of algal crude oil. In: Posten C, Walter C (eds) Microalgal biotechnology: potential and production. De Gruyter, Berlin, pp 113–146
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Cho S, Lee N, Park S, Yu J, Luong TT, Ohc Y-K, Lee T (2013a) Microalgae cultivation for bioenergy production using wastewaters from a municipal WWTP as nutritional sources. Bioresour Technol 131:515–520
Cho S, Park S, Seon J, Yu J, Lee T (2013b) Evaluation of thermal, ultrasonic and alkali pretreatments on mixed-microalgal biomass to enhance anaerobic methane production. Bioresour Technol 143:330–336
Ciudad G, Rubilar O, Azócar L, Toro C, Cea M, Torres A (2014) Performance of an enzymatic extract in Botrycoccus braunii cell wall disruption. J Biosci Bioeng 117:75–80
Collos Y, Harrison P (2014) Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar Pollut Bull 80:8–23
Converti A, Oliveira RPS, Torres BR, Lodi A, Zilli M (2009) Biogas production and valorization by means of a two step biological process. Bioresour Technol 100:5771–5776
Deublein D, Steinhauser A (2008) Biogas from waste and renewable resources. An introduction. Wiley-VCH Verlag GMBH & Co., Weinheim
Douškova I, Kaštanek F, Maleterova Y, Kaštanek P, Doucha J, Zachleder V (2010) Utilization of distillery stillage for energy generation and concurrent production of valuable microalgal biomass in the sequence: biogas-cogeneration-microalgae-products. Energy Convers Manag 51:606–611
Ehimen EA, Connaughton S, Sun Z, Carrington GC (2009) Energy recovery from lipid extracted, transesterified and glycerol codigested microalgae biomass. Glob Change Biol Bioenergy 1:371–381
Ehimen EA, Sun ZF, Carrington CG, Birch EJ, Eaton-Rye JJ (2011) Anaerobic digestion of microalgae residues resulting from the biodiesel production process. Appl Energy 88:3454–3463
El-Mashad HM (2013) Kinetics of methane production from the codigestion of switchgrass and Spirulina platensis algae. Bioresour Technol 132:305–312
Espinosa-Chávez B, Cervantes FJ, Celis-García LB, Razo-Flores E (2007) Sulfate reducing activity in methanogenic granular sludge of different size. In: Proceedings 11th world congress on anaerobic digestion, Brisbane, Australia, pp 23–27
EurObserv’ER (2014) Biogas barometer. Available at: http://www.energies-renouvelables.org/observ-er/stat_baro/observ/baro224_Biogas_en.pdf. Accessed 26 Jan 2015
Fernández Sevilla JM (2014) Integral use of biomass: an overview. Development of multipurpose extraction process. In: EMBS 2014 – Euromediterranean Microalgal Biotechnology Seminar & Workshop. Summaries and presentations. University of Almería, Spain, 20–24 October, 2014
Ficara E, Uslenghi A, Basilico D, Mezzanotte V (2014) Growth of microalgal biomass on supernatant from biosolid dewatering. Water Sci Technol 69:896–902
Fouilland E, Vasseur C, Leboulanger C, Le Floc’h E, Carré C, Marty B, Steyer JP, Sialve B (2014) Coupling algal biomass production and anaerobic digestion: production assessment of some native temperate and tropical microalgae. Biomass Bioenergy 70:564–569
Franchino M, Comino E, Bona F, Riggio VA (2012) Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 92:738–744
Garrote G, Dominguez H, Parajo JC (1999) Hydrothermal processing of lignocellulosic materials. Holz Roh Werkst 57:191–202
Gerken HG, Donohoe B, Knoshaug EP (2013) Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 237:239–253
Golueke CG, Oswald WJ (1959) Biological conversion of light energy to the chemical energy of methane. Appl Microbiol 7:219–227
Golueke CG, Oswald WJ, Gotaas HB (1957) Anaerobic digestion of algae. Appl Microbiol 5:47–55
González-Fernández C, Molinuevo-Salces B, García-González MC (2010) Open and enclosed photobioreactors comparison in terms of organic matter utilization, biomass chemical profile and photosynthetic efficiency. Ecol Eng 36:1497–1501
González-Fernández C, Molinuevo-Salces B, García-González MC (2011) Evaluation of anaerobic codigestion of microalgal biomass and swine manure via response surface methodology. Appl Energy 88:3448–3453
González-Fernández C, Sialve B, Bernet N, Steyer JP (2012a) Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenergy 40:105–111
González-Fernández C, Sialve B, Bernet N, Steyer JP (2012b) Impact of microalgae characteristics on their conversion to biofuel. Part II: focus on biomethane production. Biofuel Bioprod Bioref 6:205–218
González-Fernández C, Sialve B, Bernet N, Steyer JP (2012c) Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour Technol 110:610–616
González-Fernández C, Timmers RA, Ruiz B, Molinuevo-Salces B (2014) Biofuels and biorefineries. In: Fang Z, Smith RL Jr, Qi X (eds) Production of biofuels and chemicals with ultrasound. Springer, Heidelberg, pp 209–242
Grobelaar JU (2004) Part II. Mass cultivation of microalgae: algal nutrition. Mineral nutrition. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Gunnison D, Alexander M (1975a) Basis for the resistance of several algae to microbial decomposition. Appl Microbiol 29:729–738
Gunnison D, Alexander M (1975b) Resistance and susceptibility of algae to decomposition by natural microbial communities. Limnol Oceanogr 20:64–70
Hansen KH, Angelidaki I, Ahring BK (1998) Anaerobic digestion of swine manure: inhibition by ammonia. Water Res 32:5–12
Hernández D, Riaño B, Coca M, García-González MC (2013) Treatment of agro-industrial wastewater using microalgae-bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour Technol 135:598–603
Hidalgo MD, García PA (2001) Influencia del sulfato en la degradación anaerobia de materia orgánica. Ing Quim 383:183–191
Hsu TA (1996) Pretreatment of biomass. In: Wyman CE (ed) Handbook on bioethanol, production and utilization: pretreatment of biomass. Taylor & Francis, Washington, DC, pp 179–212
Kao CY, Chiu SY, Huang TT, Dai L, Hsu LK, Lin CS (2012a) Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy 93(2012a):176–183
Kao CY, Chiu SY, Huang TT, Dai L, Wang GH, Tseng CP, Chen CH, Lin CS (2012b) A mutant strain of microalga Chlorella sp. for the carbon dioxide capture from biogas. Biomass Bioenergy 36:132–140
Keymer P, Ruffell I, Pratt S, Lant P (2013) High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Bioresour Technol 131:128–133
Kim KH, Choi IS, Kim HM, Wic SG, Bae H (2014) Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour Technol 153:47–54
Kinnunen V, Craggs R, Rintala J (2014) Influence of temperature and pretreatments on the anaerobic digestion of wastewater grown microalgae in a laboratory-scale accumulating-volume reactor. Water Res 57:247–257
Lema JM, Méndez RJ (1997) Tratamientos biológicos anaerobios. In: Bueno J, Sastre L, Lavín A (eds) Contaminación e ingeniería ambiental, vol 3. Contaminación de las aguas. F.I.C.Y.T, Oviedo
Loos D, Meindl D (1984) Cell wall-lytic activity in Chlorella fusca. Planta 160:357–362
Lukehurst CT, Frost P, Al Seadi T (2010) Utilisation of digestates from biogas plants as biofertilizer. IEA bioenergy task 37. Available at: http://www.iea-biogas.net/files/daten-redaktion/download/publi-task37/Digestate_Brochure_Revised_12-2010.pdf. Accessed 26 Jan 2014
Mahdy A, Mendez L, Ballesteros M, González-Fernández C (2014a) Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energy Convers Manag 85:551–557
Mahdy A, Mendez L, Ballesteros M, González-Fernández C (2014b) Autohydrolysis and alkaline pretreatment effect on Chlorella vulgaris and Scenedesmus sp. methane production. Energy 78:45–52
Mahdy A, Méndez L, Blanco S, Ballesteros M, González-Fernández C (2014c) Protease cell wall degradation of Chlorella vulgaris: effect on methane production. Bioresour Technol 171:421–427
Mahdy A, Mendez L, Ballesteros M, González-Fernández C (2014d) Algaculture integration in conventional wastewater treatment plants: anaerobic digestion comparison of primary and secondary sludge with microalgae biomass. Bioresour Technol (Article in press)
Mahdy A, Mendez L, Ballesteros M, González-Fernández C (2015) Enzyme-assisted methane production using Chlorella vulgaris and Scenedesmus sp. as substrates (Submitted)
Mann G, Schlegel M, Schumann R, Sakalauskas A (2009) Biogas-conditioning with microalgae. Agron Res 7:33–38
Marcilhac C, Sialve B, Pourcher AM, Ziebal C, Bernet N, Béline F (2014) Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res 64:278–287
Marsolek MD, Kendall E, Thompson PLS, Teodora R (2014) Thermal pretreatment of algae for anaerobic digestion. Bioresour Technol 151:373–377
McKinsey Zicari S (2003) Removal of hydrogen sulfide from biogas using cow manure compost. Dissertation, Cornell University
Mendez L, Mahdy A, Timmers RA, Ballesteros M, González-Fernández C (2013) Enhancing methane production of Chlorella vulgaris via thermochemical pretreatments. Bioresour Technol 149:136–141
Mendez L, Mahdy A, Demuez M, Ballesteros M, González-Fernández C (2014a) Effect of high pressure thermal pretreatment on Chlorella vulgaris biomass: organic matter solubilisation and biochemical methane potential. Fuel 117A:674–679
Mendez L, Mahdy A, Ballesteros M, González-Fernández C (2014b) Methane production of thermally pretreated Chlorella vulgaris and Scenedesmus sp. biomass at increasing biomass loads. Appl Energy 129:238–242
Miao H, Lu M, Zhao M, Huang Z, Ren H, Yan Q (2013) Enhancement of Taihu blue algae anaerobic digestion efficiency by natural storage. Bioresour Technol 149:359–366
Miron Y, Zeeman G, Van Lier JB, Lettinga G (2000) The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res 34:1705–1713
Monlau F, Barakat A, Steyer JP, Carrere H (2012) Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour Technol 120:241–247
Monlau F, Sambusiti C, Barakat A, Quéméneur M, Trably E, Steyer JP, Carrère H (2014) Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol Adv 32:934–951
Mottet A, Francois E, Latrille E, Steyer JP, Déléris S, Vedrenne F, Carrère H (2010) Estimating anaerobic biodegradability indicators for waste activated sludge. Chem Eng J 160:488–496
Mottet A, Habouzit F, Steyer JP (2014) Anaerobic digestion of marine microalgae in different salinity levels. Bioresour Technol 158:300–306
Muñoz C, Hidalgo C, Zapata M, Jeison D, Riquelme C, Rivas M (2014) Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl Environ Microbiol 80:4199–4206
Mussgnug JH, Klassen V, Schlüter A, Kruse O (2010) Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J Biotechnol 150:51–56
Noike T, Goo IS, Matsumoto H, Miyahara T (2004) Development of a new type of anaerobic digestion equipped with the function of nitrogen removal. Water Sci Technol 49:173–179
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production—a close look at the economics. Biotechnol Adv 29:24–27
Ometto F, Quiroga G, Pšeničkac P, Whitton R, Jefferson B, Villa R (2014) Impacts of microalgae pre-treatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res 65:350–361
Pagés Díaz J, Pereda Reyes I, Lundin M, Horváth IS (2011) Co-digestion of different waste mixtures from agro-industrial activities: kinetic evaluation and synergetic effects. Bioresour Technol 102:10834–10840
Park S, Li Y (2012) Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae biomass residue and lipid waste. Bioresour Technol 111:42–48
Park W-J, Ahn J-H, Hwang S, Lee C-K (2010) Effect of output power, target temperature, and solid concentration on the solubilization of waste activated sludge using microwave irradiation. Bioresour Technol 101:S13–S16
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42
Park K, Kweon J, Chantrasakdakul P, Lee K, Cha HY (2013) Anaerobic digestion of microalgal biomass with ultrasonic disintegration. Int Biodeter Biodegr 85:598–602
Parkin GF, Owen WF (1986) Fundamentals of anaerobic digestion of wastewater sludges. J Environ Eng 112:867–920
Passos F, Ferrer I (2014) Influence of hydrothermal pretreatment on microalgal biomass anaerobic digestion and bioenergy production. Water Res 68:364–373
Passos F, García J, Ferrer I (2013) Impact of low temperature pretreatment on the anaerobic digestion of microalgal biomass. Bioresour Technol 138:79–86
Passos F, Hernández-Mariné M, García J, Ferrer I (2014a) Long-term anaerobic digestion of microalgae grown in HRAP for wastewater treatment. Effect of microwave pretreatment. Water Res 49:351–359
Passos F, Astals S, Ferrer I (2014b) Anaerobic digestion of microalgal biomass after ultrasound pretreatment. Waste Manage 34:2098–2103
Prajapati SK, Malik A, Vijay VK (2014a) Comparative evaluation of biomass production and bioenergy generation potential of Chlorella spp. through anaerobic digestion. Appl Energy 114:790–797
Prajapati SK, Kumar P, Malik A, Vijay VK (2014b) Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: a closed loop bioenergy generation process. Bioresour Technol 158:174–180
Ramos-Suárez JL, Carreras N (2014) Use of microalgae residues for biogas production. Chem Eng J 242:86–95
Ramos-Suárez JL, Martínez A, Carreras N (2014a) Optimization of the digestion process of Scenedesmus sp. and Opuntia maxima for biogas production. Energy Convers Manag 88:1263–1270
Ramos-Suárez JL, García Cuadra F, Acién FG, Carreras N (2014b) Benefits of combining anaerobic digestion and amino acid extraction from microalgae. Chem Eng J 258:1–9
Ras M, Lardon L, Sialve B, Bernet N, Steyer JP (2011) Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour Technol 102:200–206
Rasi S (2009) Biogas composition and upgrading to biomethane. Dissertation. Jyväskylä University
Rebolloso Fuentes MM, Acién Fernández FG, Sánchez Pérez JA, Gil Guerrero JL (2000) Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem 70:345–353
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw Hill, New York
Romero García JM, Acién Fernández FG, Fernández Sevilla JM (2012) Development of a process for the production of L-aminoacids concentrates from microalgae by enzymatic hydrolysis. Bioresour Technol 112:164–170
Rowse LE (2011) Design of small scale anaerobic digesters for application in rural developing countries. Master’s thesis. University of South Florida
Salehian P, Karimi K, Zilouei H, Jeihanipour A (2013) Improvement of biogas production from pine wood by alkali pretreatment. Fuel 106:484–489
Samson R, LeDuy A (1983) Improved performance of anaerobic digestion of Spirulina maxima algal biomass by addition of carbon-rich wastes. Biotechnol Lett 5:677–682
Samson R, LeDuy A (1986) Detailed study of anaerobic digestion of Spirulina maxima algal biomass. Biotechnol Bioeng 28:1014–1023
Santos NO, Oliveira SM, Alves LC, Cammarota MC (2014) Methane production from marine microalgae Isochrysis galbana. Bioresour Technol 157:60–67
Schwede S, Rehman Z-U, Gerber M, Theiss C, Span R (2013a) Effects of thermal pretreatment on anaerobic digestion of Nannochloropsis salina biomass. Bioresour Technol 143:505–511
Schwede S, Kowalczyk A, Gerber M, Span R (2013b) Anaerobic co-digestion of the marine microalga Nannochloropsis salina with energy crops. Bioresour Technol 148:428–435
Sheets JP, Ge X, Park SY, Li Y (2014) Effect of outdoor conditions on Nannochloropsis salina cultivation in artificial seawater using nutrients from anaerobic digestion effluent. Bioresour Technol 152:154–161
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27:409–416
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Sumardiono BS, Syaizhurrozi I, Sasongko SB (2014) Utilization of biogas as CO2 provider for Spirulina platensis culture. Curr Res J Biol Sci 6:53–59
Suslick KS (1990) Sonochemistry. Science 247:1439–1445
Tchobanoglous G, Burton FL, Stensel HD (2003) Wastewater engineering: treatment and reuse. McGraw Hill, New York
Tran KC, Mendoza Martin JL, Heaven S, Banks CJ, Acien Fernandez FG, Molina Grima E (2014) Cultivation and anaerobic digestion of Scenedesmus spp. grown in a pilot-scale open raceway. Algal Res 5:95–102
Travieso L, Sánchez EP, Benttez F, Conde JL (1993) Arthrospira sp. intensive culture for food and biogas purification. Biotechnol Lett 15:1091–1094
Uggetti E, Sialve B, Latrille E, Steyer JP (2014) Anaerobic digestate as substrate for microalgae culture: the role of ammonium concentration on the microalgae productivity. Bioresour Technol 152:437–444
Varel VH, Chen TH, Hashimoto AG (1988) Thermophilic and mesophilic methane production from anaerobic degradation of the cyanobacterium Spirulina maxima. Resour Conserv Recycl 1:19–26
Vesilind PA (1998) Wastewater treatment plant design, 4th edn. IWA Publishing and the Water Environment Federation, London
Voigt J, Stolarczyk A, Zych M, Malec P, Burczyk J (2014) The cell-wall glycoproteins of the green alga Scenedesmus obliquus. The predominant cell-wall polypeptide of Scenedesmus obliquus is related to the cell-wall glycoprotein gp3 of Chlamydomonas reinhardtii. Plant Sci 215–216:39–47
Wang M, Sahu AK, Rusten B, Park C (2013) Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour Technol 142:585–590
Werner U, Stoehr U, Hees N (1989) Biogas plants in animal husbandry. Deutsche Gesellschaft fuer Technische Zusammenarbeit (GTZ) GmbH, Eschborn
Yan C, Zheng Z (2013) Performance of photoperiod and light intensity on biogas upgrade and biogas effluent nutrient reduction by Chlorella. Bioresour Technol 139:292–299
Yan C, Zhang L, Luo X, Cheng Z (2014) Influence of influent methane concentration on biogas upgrading and biogas slurry purification using Chlorella. Energy 69:419–426
Yen HW, Brune DE (2007) Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour Technol 98:130–134
Zhao MX, Ruan WQ (2013) Biogas performance from co-digestion of Taihu algae and kitchen wastes. Energy Convers Manag 75:21–24
Zhong W, Zhang Z, Luo Y, Qiao W, Xiao M, Zhang M (2012) Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour Technol 114:281–286
Zhong W, Chi L, Luo Y, Zhang Z, Zhang Z, Wu W-M (2013) Enhanced methane production from Taihu Lake blue algae by anaerobic co-digestion with corn straw in continuous feed digesters. Bioresour Technol 134:264–270
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Ramos-Suárez, J.L., Arroyo, N.C., González-Fernández, C. (2015). The Role of Anaerobic Digestion in Algal Biorefineries: Clean Energy Production, Organic Waste Treatment, and Nutrient Loop Closure. In: Singh, B., Bauddh, K., Bux, F. (eds) Algae and Environmental Sustainability. Developments in Applied Phycology, vol 7. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2641-3_5
Download citation
DOI: https://doi.org/10.1007/978-81-322-2641-3_5
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2639-0
Online ISBN: 978-81-322-2641-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)