Abstract
Environmental implications and climate change due to greenhouse gas emissions have raised concerns about the sequestration of CO2. Photosynthetic microalgae have shown excellent potential as a precursor for renewable biofuels, commercial bioproducts, and animal or aquaculture feed. Utilization of CO2 for cultivation of microalgae is a sustainable and environmentally friendly approach for biological CO2 sequestration. There are engineering constraints and challenges to make the overall process economically feasible which needs to be addressed. Integrating this biological CO2 sequestration approach in a microalgal biorefinery with utilization of wastewater is a green approach for clean energy and environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Global warming is currently at a disturbing level owing to the rise in anthropogenic greenhouse gases of which CO2 contributes up to 68 % of total emissions. Power plants are responsible for more than 22 % of CO2 emissions worldwide as CO2 is the primary emission of flue gases (Radmann et al. 2012). Flue gas emitted from fossil fuels usually contains N2, CO2, O2, and water vapor as well as minor quantities of CO, NOx, SOx, and particulate matters (Kumar et al. 2011). Carbon dioxide mitigation strategies employed globally thus far can broadly be grouped into physical and biological methods (Kumar et al. 2010, 2011; Ho et al. 2011). However, due to various problems associated with physical techniques, there is a demand to develop other appropriate technologies (Kumar et al. 2010, 2011).
Biological carbon sequestration could offer advantages as an intermediate solution for reduced carbon emissions. Carbon dioxide, which is a necessary compound in the formation of complex sugars by green plants and microalgae through photosynthesis, has also shown much potential in significantly increasing the growth rates of most microalgal species (Kumar et al. 2010, 2011; Ho et al. 2011). This application could therefore prove useful for closed systems, using specific microalgal strains to maximize CO2 conversion to biomass thus absorbing this greenhouse gas. Microalgal biomass could thus represent a natural sink for carbon. Furthermore, such systems could minimize capital and operating costs, complexity, and energy required to transport CO2 to other places. However, further research is required on various fronts, such as: separation of flue gas or direct utilization, CO2 capture, introduction of CO2 to the photosynthetic system and the physiological response this would have on cells, supply and distribution of light, as well as temperature requirements (Kumar et al. 2010, 2011).
2 Environmental Implications of Carbon Dioxide Gas
CO2 is the major contributor of the greenhouse gases (GHG). Greenhouse gas emission is primarily responsible for the global warming. The rise in the temperature is associated with many environmental implications and disturbance in the climate. Global warming is directly related to glacial melting and rise in the ocean level. Climate changes are also associated with the reduced agricultural productivities. Irregular rainfall due to climate change causes the water shortage. Environmental and climate irregularities are also associated with the species extinctions. The United Nations has established the Kyoto Protocol setting the objective of decreasing the GHG emissions by 5.2 % based on 1990 emissions (Pires et al. 2012). Thus, it becomes imperative to reduce the CO2 levels in the atmosphere for sustainable future of the planet.
3 Existing Technologies for CO2 Sequestration and Their Limitations
There are numerous techniques that are currently been employed globally to limit the amount of CO2 escaping into the atmosphere. However, over the years there has been much debate on the selection of the most appropriate technology. Carbon dioxide alleviation strategies that are currently been applied worldwide can broadly be grouped into physical or biological techniques. Physical-based methods essentially comprise three important steps: capture, transportation, and storage. Physical methods begin with the collection of CO2 from a fixed source. Examples of fixed sources that release large amounts of CO2 daily into the atmosphere include power plants and cement manufacturing facilities. After capture of a sufficient amount of CO2, the gas is then converted into a supercritical fluid. Conversion to a supercritical fluid enables simple transportation by ship or pipeline to a safe place of storage. Common storage methods utilized include injection into deep geological or oceanic trenches and mineralization (Khoo et al. 2011; Pires et al. 2012). These disposal techniques are energy intensive and expensive, require large amounts of land, and eventually lead to CO2 leakage over time. Hence, these methods are often considered unsustainable (Stewart and Hessami 2005). This technology, however, still remains a popular choice, even with the abovementioned drawbacks. This could be attributed to the fact that this technique allows communities to sustain their current carbon-based infrastructure while aiming to lessen the outcomes of CO2 on global warming (Pires et al. 2012). Communities at large are also often nervous and skeptical to venture out and try new techniques.
Another popular alternative would be to make use of the accumulated CO2. For instance, precipitated calcium carbonate (PCC) can be formed by managing the reaction of CO2 with lime. This can then be utilized as a replacement for titanium dioxide or kaolin in the production of paper products. Carbon dioxide can also be used in the production of paint, plastic, solvent, and packaging. However, this would use relatively minute amounts of CO2, when compared to the large quantities released into the atmosphere annually.
Monoethanolamine (MEA) scrubbing is a popular method whereby CO2 is removed from flue gases during the combustion process. This method involves a chemical absorption process together with the use of a MEA solvent. During this procedure, the MEA solution makes contact with the flue gases and mixes in the absorber. The MEA solution that is now rich in CO2 is then transported to a stripper. It is then reheated to discharge almost pure CO2. This captured pure CO2 can then be utilized for various industrial processes. The MEA solution can be recycled to the absorber. This process requires large equipment sizes and high regeneration energy requirements; therefore, it is considered to be uneconomic. Other technologies that have been investigated (such as membrane separation, cryogenic fractionation, and adsorption using molecular sieves) are even less energy efficient for them to be considered economically viable (Stewart and Hessami 2005).
A carbon fiber composite molecular sieve was developed by the Oak Ridge National Laboratory. This carbon monolith was capable of separating CO2 from CH4, CO2 from air, and CO2, CO, H2S, and H2O from a mixture of gases. This separation technology displayed some potential as it proved to be cost effective, produced minimal waste, and could be adapted to numerous carbon sequestration strategies (Stewart and Hessami 2005).
Desiccant adsorption is another process that can be employed. This is often termed a pressure and temperature swing adsorption (PTSA) that could possibly be applied to electric power plant flue gas. Carbon dioxide can be adsorbed at near normal pressure using zeolite or alumina as the desiccant. Target removal efficiencies of 90–99 % purity of CO2 removed were achieved using this technology. A major problem with this method, however, is the reaction of the desiccant with SOX present in the flue gas (Stewart and Hessami 2005).
Biological CO2 fixation, which can be accomplished during the photosynthesis of terrestrial plants and photosynthetic microorganisms, seems to be the only economical and environmentally viable technique of the future without the aforementioned shortcomings (Kumar et al. 2010, 2011; Ho et al. 2011). Even though terrestrial plants fix around 500 billion tons of CO2 per annum, they are anticipated to play a minor role (3–6 %) in the overall reduction of atmospheric CO2 (Skjanes et al. 2007). Microalgae and cyanobacteria have rapid growth rates as opposed to terrestrial plants; their CO2-fixation efficiency is about 10–50 times better, and they are also known to have higher tolerance to extreme environments (Costa et al. 2000; Ho et al. 2011). These microorganisms have therefore come to the forefront of studies as they offer greater possibility in the long run. The biological mitigation of CO2 using autotrophic microalgae offers numerous advantages: no additional CO2 is created, while nutrient utilization can be accomplished in a continuous manner leading to the production of biofuels and other secondary metabolites (Kumar et al. 2010, 2011). In 2010, Sydney confirmed that carbon uptake by microalgae is essentially dependent on the metabolic activity of the particular microalgal strain. However, research has shown that microalgae supplied with higher levels of CO2 (>5 %) respond much better (on a biomass basis), as opposed to microalgae exposed to ambient air only (Kumar et al. 2010, 2011; Ho et al. 2011). Microalgae have the ability to generate approximately 280 tons of dry biomass per ha per year by utilizing only 9 % of the freely available solar energy. During this process, roughly 513 tons of CO2 can be sequestered (Sydney 2010). A study by Borkenstein and Knoblechner in 2011 investigated the growth of C. emersonii for 30 days with both pure CO2 and flue gas. Results revealed that when the Chlorella strain was supplied with CO2 containing flue gas, it produced 2.06 g L−1 biomass.
4 Bio-mitigation of CO2 by Microalgae
4.1 CO2 Capture
Microalgae are capable of accumulating large amounts of inorganic carbon in their cytoplasm. Most often, these carbon concentrations are much higher when compared to concentrations on the outside. This process is referred to as a CO2-concentrating mechanism (CCM). An important factor in photosynthesis is the CO2 concentration. If there is a very high concentration of CO2, this would increase the mass transfer mechanism from the gas mixture to the medium. This would eventually lead to a drastic drop in pH. The pH reductions often adversely affect the growth and productivity of most microalgal species. Oxygen, produced during photosynthesis, is another important factor that can hinder microalgal growth. Therefore, it is imperative that gas is regularly removed from a microalgal system and not allowed to accumulate (Pires et al. 2012).
4.2 Fate of CO2 in Microalgal Physiology
Photosynthesis occurs in two stages within microalgae cells. The first stage only occurs when cells are exposed to light and involve light-dependent reactions. This step utilizes light energy to produce the energy-storage molecules adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH). Dark reactions (carbon-fixation reactions) transpire during the second stage of photosynthesis. These reactions can occur both in the presence and in the absence of light. It is at this stage that energy-storage products produced during light reactions are employed to capture and reduce CO2 (Calvin 1989). The two major photoactive complexes (photosystem I (PSI) and photosystem II (PSII)) transfer sunlight into the electron transport chain using the excited chlorophyll dimer (Calvin 1989; Iverson 2006; Ho et al. 2011). Photosynthesis starts in the PSII complex. An electron is then transferred to the primary electron acceptor molecule once a chlorophyll molecule at the center of the PSII complex attains ample excitation energy. This process is referred to as photoinduced charge separation. An electron transport chain is then responsible for transporting electrons across the membrane. PSI then takes up the electrons from PSII and moves them via the P700 dimer of chlorophyll, which is oxidized from light-excited antenna chlorophyll to strongly reducing ferredoxin and NADPH (Cerveny et al. 2009; Ho et al. 2011). During photophosphorylation, energy harvested during the light reactions can be stored by the formation of ATP (Yang et al. 2000). Research has found that 1.3 ATP molecules are created per pair of electrons moving through the photosynthetic electron transport chain (Yang et al. 2000).
Dark reactions or carbon-fixation reactions involve the Calvin cycle (Calvin 1989; Iverson 2006; Yang et al. 2000). It is during this cycle that CO2 is converted into the enzyme RuBisCO (Ribulose 1,5-bisphosphate carboxylase/oxygenase). Ribulose 1,5-bisphosphate carboxylase/oxygenase is also involved in oxygenase activity and forms glycolate 2-phosphate as an end product. This product and its synthesis consume considerable amounts of cellular energy, yet it is of no use to the cell. The oxygenase activity of RuBisCO is known to hinder around 50 % of biomass formation (Giordano et al. 2005; Kumar et al. 2011).
Photosynthesis in microalgae is often measured as rates of carbon accumulation or O2 evolution. Either measurement can be converted into the other using the photosynthetic quotient (Pires et al. 2012). In 2010, Jacob-Lopes et al. studied the influence of photoperiods on the rates of CO2 sequestration using a cyanobacterial strain in both BGN medium and refinery wastewater. They observed a linear decrease in biomass productivity during a longer dark period when cultivated in BGN medium. When the strain was grown in refinery wastewater, a photosynthetic quotient of 0.74 was recorded. This reading basically means that 1 g of CO2 utilized corresponds to the release of 0.74 g of O2. This study showed that the gas-exchange pattern within a system is greatly influenced by the intermittent light cycle. During the dark phase, the microalgal cells were able to consume organic carbon through heterotrophic metabolism and release CO2 in the process.
Chlorococcum littorale is an extremophile that has the ability to grow well at CO2 levels up to 60 % (Miyachi et al. 2003). Research conducted on this microalga demonstrated that when exposed to high levels of CO2, there is a rapid state transition of the photosynthetic apparatus (Demidov et al. 2000; Miyachi et al. 2003; Solovchenko and Khozin-Goldberg 2013). A state transition is often caused due to a reduction in the plastoquinone pool owing to accumulation of NADPH. This shift in the photosynthetic apparatus from state I to state II causes an increase in the cyclic electron transport over PSI. The additional ATP generated during this change is used to sustain pH homeostasis in the microalgal cell (Miyachi et al. 2003). In 2004, Muradyan et al. stated that CO2-intolerant species often exhibit signs of PSI damage when exposed to high CO2 conditions as they lack the state transition response (Solovchenko and Khozin-Goldberg 2013).
4.3 Microalgal Strains for CO2 Sequestration
Microalgae and cyanobacterial species that are often employed for CO2 sequestration include Anabaena sp., Botryococcus braunii, Chlamydomonas reinhardtii, Chlorella sp., Chlorococcum littorale, Scenedesmus sp., and Spirulina sp. (de Morais and Costa 2007; Ota et al. 2009; Packer 2009; Chen et al. 2010; Chiang et al. 2011; Ho et al. 2011). Green microalgae that are efficient carbon sequesters usually belong to the genera Chlorococcum, Chlorella, Scenedesmus, and Euglena. In 1970, Seckbach and Libby isolated microalgal species from the abovementioned genera. These strains were even able to tolerate pure (100 %) CO2. Research on the Scenedesmus culture showed that this strain was capable of flourishing under a 100 % CO2 level cell concentration increased for up to 30 days, achieving 3.65 g L−1. This proved to be a considerable increase in cell concentration, when compared to the 1.19 g L−1 recorded during exposure to atmospheric CO2 (0.036 %).
In another study, A Chlorella TX 71105 strain was supplemented with pure CO2 at a rate of 3.3 mL min−1 over a 28-day period. It was observed that during the first 6 days, the effluent gas collected contained more than 96 % O2. On the 12th day of cultivation, an increase in temperature from 37 to 39 °C was recorded. A 32 % CO2 concentration was noted in the effluent gas on the 13th day. The gas flow was then turned off, and the culture was not supplied with CO2 for 3.5 h. There were no adjustments to the experiment during the last 6 days. For the duration of this period, it was observed that the effluent gas contained 18 % CO2. When this strain was grown under 41, 71, and 100 % CO2, the mean biomass concentration noted was 3.15, 2.71, and 2.49 g L day−1, respectively. These cell concentrations achieved are reasonably similar to those obtained with other Chlorella species (Geckler et al. 1962).
In 2011, Zhao et al. did a comparative study of the growth and CO2 biofixation of a Chlorella sp. under two different modes of cultivations. Results obtained suggest that closed cultivation significantly enhanced microalgal performance with regard to growth and carbon biofixation. During conditions of closed cultivation, specific growth rate and CO2-fixation rate were observed to be 1.78 and 5.39 times higher, respectively, when compared to that of open cultivation. Closed systems permit effective gas bubble motion, which plays an essential role in displacing dissolved O2 buildup. Under appropriate cultivation modes, Chlorella sp. display much potential as effective carbon sequesters. Kurano et al. (1995) demonstrated that at a 20 % CO2 concentration, C. littorale was able to attain a high cell concentration of 4.9 g L−1. A short lag phase prior to active photosynthesis was observed when this strain was exposed to CO2 concentrations of greater than 20 %. It is important to note that the performance of microalgal strains is not solely influenced on CO2 concentrations, but also on experimental and culture conditions (culture medium, temperature, light intensity, as well as reactor design). Variation in any of these conditions could have an adverse effect on the CO2-fixation efficiency of the strains (Ho et al. 2011).
5 Making CO2 Available for Microalgae
5.1 Cultivation Systems
Once an appropriate strain has been selected, the next step would be suitable cultivation of the microalgae. It is essential that artificial propagation of microalgae both mimics and enhances the optimum natural growth conditions (Brennan and Owende 2010; Vasumathi et al. 2012). Open ponds and closed photobioreactors (PBR) have been extensively exploited for the growth of microalgae (Molina et al. 2001; Suh and Lee 2003; Chisti 2008; Brennan and Owende 2010). However, with regard to utilizing microalgae for CO2 mitigation, there has been ongoing debate as to which would be a better cultivation system. Raceway ponds are the most popular growth systems as they are cost effective and relatively simple to maintain. These systems, however, utilize CO2 much less efficiently when compared to PBRs as there is a significant loss of CO2 to the atmosphere (Borowitzka 1999; Chisti 2007; Brennan and Owende 2010).
Over the years, much research into PBR technology has been conducted to overcome some of the important problems linked with open pond production systems. Photobioreactors are more advantageous than open systems as they allow for culture of single species of microalgae for long durations with lower risk of contamination (Brennan and Owende 2010). Due to higher cell mass productivities when using a PBR system, harvesting costs are also often reduced. Carbon dioxide is also utilized more effectively using these systems (Chisti 2007; Brennan and Owende 2010; Vasumathi et al. 2012). Even though a great deal of work has been done to design and produce PBRs for microalgal propagation, and effective CO2 consumption, more research is still needed to improve PBR technologies and know-how of microalgal cultures. For the efficient mass cultivation of microalgae for carbon sequestration, PBR design and development is possibly one of the first major steps that should be undertaken (Ugwu et al. 2008).
Photobioreactors equipped with unique designed light systems have been examined for effective CO2 sequestration and greater biomass productivities (Lee 2001). In 2003, Suh and Lee designed and operated an internally illuminated airlift PBR. This reactor was constructed to study the light distribution and to ultimately maximize the photosynthetic efficiency to promote greater carbon uptake by a Synechococcus sp. Another important feature when developing a closed reactor is the volumetric gas transfer coefficient. By increasing the gas transfer coefficient, cell growth rate can be enhanced (Kumar et al. 2011). A series of trials was conducted by Zhang et al. in 2002 to comparatively investigate gas transfer in different PBRs at varying CO2 percentages. From the results obtained, they were able to conclude that a decrease in the CO2 concentration from the inlet gas stream leads to an increase in the gas transfer coefficient. Furthermore, it is imperative that the CO2 solubility within the cultivation media is established as this will give the researcher an idea on the amount of CO2 available for growth.
Borkenstein and Knoblechner (2011) investigated the growth of C. emersonii in 5.5 L airlift PBRs using both flue gas and pure CO2. The experiment lasted 30 days. At the end of experimentation, it was noted that there was no significant difference in biomass yields obtained. When the Chlorella sp. was supplied with CO2 containing flue gas, it produced 2.00 g L−1 biomass, and when cultivated with pure CO2, a biomass yield of 2.06 g L−1 was recorded. However, an important conclusion to this study was that when supplied with flue gas, C. emersonii was able to grow as successfully as when it was purged with pure CO2.
5.2 Parameters Affecting CO2 Uptake
Carbon dioxide can be an effective supplement to promote microalgal growth. However, high levels of CO2 (5 %) can often hinder growth of certain microalgal strains. High percentages of CO2 can cause acidification of the cellular content, which eventually hampers growth and productivity (Lee and Lee 2003; Solovchenko and Khozin-Goldberg 2013). In 2000, Watanabe et al. demonstrated that microalgal cultures that grew well at CO2 levels between 5 and 10 % had significant reductions in their growth rates at CO2 percentages above 20 %. Elevated levels of CO2 often lead to drastic drops in pH, due to the formation of large amounts of bicarbonate. pH can drop to 5 or even lower in some cases. Extreme decreases in pH cause an environmental stress that leads to a biological reduction in the ability of microalgal cells to sequester CO2. Microalgal growth is only slightly affected when there is a small drop in pH, but extreme pH changes could possibly inhibit all growth (Kumar et al. 2011; Solovchenko and Khozin-Goldberg 2013). Screening studies have identified microalgae that are capable of tolerating and flourishing under CO2 concentrations between 30 and 70 % (Hanagata et al. 1992; Iwasaki et al. 1996; Sung et al. 1999). In 2003, Olaizola demonstrated that microalgal growth could even be sustained at a 100 % CO2 level. This can only be achieved if changes in pH were monitored strictly and CO2 was only supplied to the strain on demand.
Light is a necessary requirement for photosynthesis. The photosynthesis-irradiance response (P-I) curve accurately depicts the relationship between light and photosynthesis. This curve has three distinct regions: light-limited photosynthesis, light-saturated photosynthesis, and photoinhibition (Ralph and Gademann 2003). Optimum light intensity is essential for effective CO2 fixation and biomass production. Light intensity below the optimum becomes the limiting factor for microalgal growth, whereas too high light intensity causes photoinhibition to microalgal cells. Photoinhibition occurs when there is damage to the PSII repair mechanism – this also causes inactivation of other systems (electron carriers, oxygen-evolving systems, and the related D1/D2 proteins). Light intensity is dependent on numerous factors (wavelength, cell concentration, and the penetrating distance of light as well as then geometry of the system) (Kumar et al. 2011).
5.3 Novel Techniques for Facilitating Supply
The CO2-fixation rate can be improved if research was conducted on the Calvin cycle, PEP carboxylase, and/or through synthetic pathways (Rosgaard et al. 2012; Gimpel et al. 2013). Previous research efforts have met with varying degrees of success. Studies have focused on the following aspects: engineering RuBisCO to promote higher catalysis rates of carboxylation and decreases in the oxygenation reactions, improving the activation state of RuBisCO, accelerating the regeneration phase of the Calvin cycle, and enriching CO2 around RuBisCO in an attempt to inhibit the oxygenase reaction. From these studies it was concluded that the challenge lies in the activity of RuBisCO for carbon flux through the carbon flux when the media is not enriched with CO2 or during extreme temperature/light conditions. Chlamydomonas reinhardtii are ideal candidates for RuBisCO engineering as they are RuBisCO deficient and therefore able to complete their life cycle heterotrophically. Chlamydomonas sp. has been engineered for the efficient exploitation of energy, carbon and nitrogen. This has been accomplished using the nuclear genome of an MRL1-deficient strain and expressing the rbcl mRNA maturation factor MRL1 at varying levels. As opposed to the wild type, results for the deficient strain illustrated that RuBisCO could sustain phototrophic growth even when it was lowered up to 15 %. Based on these findings, it can be concluded that depending on the culture conditions (light intensity or CO2 concentration), an inducible promoter for MRL1 could successfully be applied to modify RuBisCO accumulation (Rosgaard et al. 2012; Gimpel et al. 2013).
As discussed above (section on photosynthesis), an increase in the PSI/II ratio suggests that microalgal species require an increase in their PSI light-harvesting antenna in order to grow at high CO2 concentrations. However, it must be noted that these changes can often be reversible. A reduction in the ATP demand caused by the overall acclimation of the microalgal cell to high levels of CO2/or a drop in the CCM activity often leads to the ratio returning to its original level (Miyachi et al. 2003; Solovchenko and Khozin-Goldberg 2013).
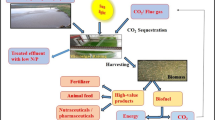
6 Integrated Biorefinery: From Waste to Value Addition
An integrated biorefinery has been considered as the sustainable and economical approach for the cultivation of microalgae for generation of biomass. In this approach maximum outputs and goals can be achieved in the single integrated cultivation system (Singh et al. 2015). Wastewater can be utilized as the growth medium for microalgae; flue gases can be used to provide the CO2. The microalgal biomass thus generated can be utilized for a number of purposes such as biofuels (biodiesel, bioethanol, biomethane, etc.), pigments (carotenes), therapeutic biomolecules, animal or fish feed, etc.
6.1 Waste Utilization: Flue Gas and Wastewater
The ability to fix atmospheric CO2 via photosynthesis by microalgae is 10 times more efficient than the terrestrial plants (Pires et al. 2012). Carbon is key component which constitutes 36–56 % of dry matter of microalgal cell. For per kg of dry biomass generation, 1.3–2.4 kg CO2 is fixed by microalgae (Van Den Hende et al. 2012). Microalgae can be cultivated by supplying CO2 from flue gases. Direct flue gases or CO2 separated from flue gases can be applied for cultivation of microalgae. Direct use of flue gases is energy- and cost-saving approach. Microalgal strain should be resistant toward the high percentage of CO2 (15 %) and presence of SOX and NOX for application of flue gases in the cultivation (Maeda et al. 1995). Maeda et al. (1995) investigated several microalgal species for flue gas application and observed a Chlorella sp. strain with high growth rate at temperature of 35 °C and 15 % CO2 concentration. Table 12.1 depicts the microalgal species cultivated using flue gases. Flue gases composed of several compounds like CO2, SOX, NOX, CO, CxHy, halogen acids, and particulate matter. Some of these contents could be toxic for the microalgal growth. Microalgae can be grown in open or closed system. Delivery of the flue gases to the cultivation system and its proper mixing have several challenges which need to be addressed for successful implementation of this technology. This biorefinery approach of utilization of flue gases for CO2 can minimize environmental concerns as well as earn carbon credits.
Industrial and domestic wastewater discharges to the environment are adding organic and inorganic nutrients, pathogens, heavy metals, suspended solids, and oxygen-demanding material to the water bodies. Freshwater resource is limited to cater industrialization and socioeconomic developments. Cultivation of microalgae in open ponds needs around 11–13 million L ha−1 year−1 freshwater (Chinnasamy et al. 2010). In biorefinery concept, utilization of wastewater for cultivation of microalgae serves dual purpose of biomass generation as well as nutrient removal from the final effluent (Rawat et al. 2011; Singh et al. 2014). The microalgal biomass can be utilized for several purposes, viz., energy, value-added products, and feed production. Utilization of wastewater for microalgal cultivation reduces the freshwater footprint and also provides treated water for other uses. Microalgal growth needs inorganic nutrients like nitrogen and phosphorous. Nutrient supplementation is the contributor to the cultivation cost. This cost is reduced if the wastewater is utilized as the source. Various microalgal strains have been investigated using wastewater nutrient medium (Ramanna et al. 2014).
6.2 Utilization of Microalgal Biomass: Energy, Bioproducts, and Feed
Microalgal biomass can be utilized for several applications like renewable energy generation, bioproducts such as pigments and therapeutic biomolecules, and also as fish or animal feed. In biorefinery approach, focus is on deriving as many products as possible from the microalgal biomass. Multiproduct approach is favorable for economics, as microalgal biomass produces lipids, carbohydrates, and proteins which can be used for various purposes. Biodiesel is most studied biofuel from the microalgae. Microalgae have capabilities of fast growth rates and high lipid accumulation (Guldhe et al. 2015). Microalgal lipids are used as feedstock for biodiesel production. Anaerobic digestion of microalgae for biomethane generation is also another approach of biofuels generation. Either whole microalgal biomass or the lipid-extracted residue can be used for biomethane generation from microalgae. Lipid-extracted algae are nitrogen rich as protein content is high; thus, it can be co-digested with other carbon-rich substrates such as primary sewage sludge to maintain the desired C/N ratio (Sahu et al. 2013). Carbohydrate constituent of microalgae can be used for bioethanol production. Several microalgae can store energy in the form of starch, which can be used as a substrate for bioethanol generation. Biohydrogen is considered as the cleaner renewable fuel. Microalgae can be utilized for biohydrogen generation. Microalgae can photosynthetically produce the biohydrogen or microalgal biomass can be used as substrate for dark fermentation using bacteria for biohydrogen production (Batista et al. 2014).
Microalgae are diverse group of organisms which produces numerous secondary metabolites. Primary metabolites like carbohydrates, lipids, and proteins from microalgae can be used to produce numeral commercial products. The secondary metabolites from microalgae like fatty acids, sterols, carotenoids, phycocolloids, lectins, mycosporine-like amino acids, halogenated compounds, polyketides, and toxins have also numerous commercial applications (Singh et al. 2015). In an integrated biorefinery approach, microalgal biomass can be utilized for generation of various products of therapeutic and cosmetic significance, health food, coloring agents, and aquaculture and animal feed. The biomolecules from microalgae having therapeutic and cosmetic significance are high-value products. A biorefinery based on CO2 sequestration, wastewater utilization for growth of microalgal biomass for energy, bioproducts, and feed generation is a sustainable and economical approach.
7 Challenges and Future Prospects for CO2 Sequestration by Microalgae
The CO2 sequestration by microalgae is an environmentally friendly method, as the CO2 captured is converted to valuable biomass while the oceanic or geological storage of CO2 only delays its release in the environment. However, cultivation of microalgae and CO2 supply to cultivation system have several challenges which need to be addressed. The mass transfer coefficient of CO2 is low, and thus mass transfer from gaseous phase to liquid phase is a major bottleneck in application of this technology. Microalgae can be cultivated in closed or open system. In closed system maintaining the high flow rate could alleviate this mass transfer problem. Efficient mixing and aeration in open pond system facilitate the CO2 mass transfer (Pires et al. 2012). Delivery of the CO2 to the microalgal cultivation system is also cost incurring and thus needs economical and efficient technologies to overcome this barrier. When the flue gases are used for CO2 supply, it composed of several other components which could be toxic to the microalgal growth. Thus, further investigation is important assessing the effect of each component from the flue gases on the microalgal growth physiology. The CO2 sequestration technology by microalgae is still in its early stages. Engineering of the photosynthetic mechanism of microalgae could also improve the CO2 capture efficiency. Designing efficient closed or open cultivation system at the site of CO2 generation such as power plants and cement factories could reduce the transportation cost. Biorefinery concept where waste refusals from industry are used to cultivate microalgae and subsequent biomass utilized for maximum applications could make CO2 sequestration by microalgae economically feasible.
References
Batista AP, Ambrosano L, Graca S, Sousa C, Marques PA, Ribeiro B, Botrel EP, Castro Neto P, Gouveia L (2014) Combining urban wastewater treatment with biohydrogen production – an integrated microalgae-based approach. Bioresour Technol 184:230–235
Borkenstein CG, Knoblechner J (2011) Cultivation of Chlorella emersonii with flue gas derived from a cement plant. J Appl Phycol 23:131–135
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Brennan L, Owende P (2010) Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Calvin M (1989) 40 years of photosynthesis and related activities. Photosynth Res 21:3–16
Cerveny J, Setlik I, Trtilek M, Nedbal L (2009) Photobioreactor for cultivation and real-time, in situ measurement of O2 and CO2 exchange rates, growth dynamics, and of chlorophyll fluorescence emission of photoautotrophic microorganisms. Eng Life Sci 9:247–253
Chen CY, Yeh KL, Su HM, Lo YC, Chen WM, Chang JS (2010) Strategies to enhance cell growth and achieve high-level oil production of a Chlorella vulgaris isolate. Biotechnol Prog 26:679–686
Chiang CL, Lee CM, Chen PC (2011) Utilization of the cyanobacteria Anabaena sp. CH1 in biological carbon dioxide mitigation processes. Bioresour Technol 102:5400–5405
Chinnasamy S, Bhatnagar A, Hunt RW, Das KC (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101:3097–3105
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chiu SY, Kao CY, Huang TT, Lin CJ, Ong SC, Chen CD, Chang JS, Lin CS (2011) Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour Technol 102:9135–9142
Costa JAV, Linde GA, Atala DIP (2000) Modelling of growth conditions for cyanobacterium Spirulina platensis in microcosms. World J Microbiol Biotechnol 16:15–18
de Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
Demidov E, Iwasaki I, Satoh A, Kurano N, Miyachi S (2000) Short-term responses of photosynthetic reactions to extremely high-CO2 stress in a “High-CO2” tolerant green alga, Chlorococcum littorale and an intolerant green alga Stichococcus bacillaris. Russ J Plant Physiol 47:622–631
Doucha J, Straka F, Lívanský K (2005) Utilization of flue gas for cultivation of microalgae Chlorella sp.) in an outdoor open thin-layer photobioreactor. J Appl Phycol 17:403–412
Geckler RP, Sane JO, Tew RW (1962) Highly concentrated carbon dioxide as a carbon source for continuous algae cultures [Online]. http://contrails.iit.edu/DigitalCollection/1962/AMRLTDR62-116article06.pdf. [2013/03/06]
Gimpel JA, Specht EA, Georgianna DR, Mayfield SP (2013) Advances in microalgae engineering and synthetic biology applications for biofuel production. Curr Opin Chem Biol 17:1–7
Giordano M, Beardall J, Raven JA (2005) Mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Guldhe A, Singh B, Rawat I, Permaul K, Bux F (2015) Biocatalytic conversion of lipids from microalgae Scenedesmus obliquus to biodiesel using Pseudomonas fluorescens lipase. Fuel 147: 117–124
Hanagata N, Takeuchi T, Fukuju Y, Barnes DJ, Karube I (1992) Tolerance of microalgae to high CO2 and high-temperature. Phytochemistry 31:3345–3348
Ho SH, Chen CY, Lee DJ, Chang JS (2011) Perspectives on microalgal CO2-emission mitigation systems – a review. Biotechnol Adv 29:189–198
Iverson TM (2006) Evolution and unique bioenergetic mechanisms in oxygenic photosynthesis. Curr Opin Chem Biol 10:91–100
Iwasaki I, Kurano N, Miyachi S (1996) Effects of high-CO2 stress on photosystem II in a green alga, Chlorococcum littorale, which has a tolerance to high CO2. J Photochem Photobiol B Biol 36:327–332
Jacob-Lopes E, Scoparo CHG, Queiroz MI, Franco TT (2010) Biotransformations of carbon dioxide in photobioreactors. Energy Convers Manage 51:894–900
Khoo HH, Sharratt PN, Das P, Balasubramanian RK, Naraharisetti PK, Shaik S (2011) Life cycle energy and CO2 analysis of microalgae-to-biodiesel: preliminary results and comparisons. Bioresour Technol 102:5800–5807
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, Langenhove HV (2010) Enhanced CO2 fixation and biofuels production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Kumar K, Dasgupta CN, Nayak B, Lindblad P, Das D (2011) Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol 102:4945–4953
Kurano N, Ikemoto H, Miyashita H, Hasegawa T, Hata H, Miyachi S (1995) Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Convers Manag 36:689–692
Lee YK (2001) Microalgal mass culture systems and methods: their limitation and potential. J Appl Phycol 13:307–315
Lee JS, Lee JP (2003) Review of advances in biological CO2 mitigation technology. Biotechnol Bioprocess Eng 8:354–359
Maeda K, Owada M, KimurA N, Omata K, Karube I (1995) CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae. Energy Convers Manage 36:717–720
Miyachi S, Iwasaki I, Shiraiwa Y, Yoshihiro S (2003) Historical perspective on microalgal and cyanobacterial acclimation to low- and extremely high-CO2 conditions. Photosynth Res 77:139–153
Molina GE, Belarbi EH, Fernandez FG, Medina AR, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Muradyan EA, Klyachko-Gurvich GL, Tsoglin LN, Sergeyenko TV, Pronina NA (2004) Changes in lipid metabolism during adaptation of the Dunaliella salina photosynthetic apparatus to high CO2 concentration. Russ J Plant Physiol 51:53–62
Olaizola M (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol Eng 20:459–466
Ota M, Kato Y, Watanabe H, Watanabe M, Sato Y, Smith RL Jr, Inomata H (2009) Effect of inorganic carbon on photoautotrophic growth of microalgae Chlorococcum littorale. Biotechnol Prog 25:492–498
Packer M (2009) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37:3428–3437
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simoes M (2012) Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew Sustain Energy Rev 16:3043–3053
Radmann EM, Camerini FV, Santos TD, Costa JAV (2012) Isolation and application of SOX and NOX resistant microalgae in biofixation of CO2 from thermoelectricity plants. Energy Convers Manage 52:3132–3136
Ralph PJ, Gademann R (2003) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Biol 82:222–237
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88:3411–3424
Rosgaard L, de Porcellinis AJ, Jacobsen JH, Frigaard NU, Sakuragi Y (2012) Bioengineering of carbon fixation, biofuels, and biochemicals in cyanobacteria and plants. J Biotechnol 162:134–147
Sahu AK, Siljudalen J, Trydal T, Rusten B (2013) Utilisation of wastewater nutrients for microalgae growth for anaerobic co-digestion. J Environ Manage 122:113–120
Seckbach J, Libby WF (1970) Vegetative life on Venus? Or investigations with algae which grow under pure CO2 in hot acid media at elevated pressures. Origins Life Evol Biospheres 2:121–143
Singh B, Guldhe A, Rawat I, Bux F (2014) Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew Sustain Energy Rev 29:216–245
Singh B, Guldhe A, Singh P, Singh A, Rawat I, Bux F (2015) Sustainable production of biofuels from microalgae using a biorefinary approach. In: Kaushik G (ed) Applied environmental biotechnology: present scenario and future trends. Springer, New Delhi
Skjanes K, Lindblad P, Muller J (2007) BioCO2 – a multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol Eng 24:405–413
Solovchenko A, Khozin-Goldberg I (2013) High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnol Lett 35:1745–1752
Stewart C, Hessami MA (2005) A study of methods of carbon dioxide capture and sequestration-the sustainability of a photosynthetic bioreactor approach. Energy Convers Manage 46:403–420
Suh IS, Lee CG (2003) Photobioreactor engineering: design and performance. Biotechnol Bioprocess Eng 8:313–321
Sung KD, Lee JS, Shin CS, Park SC, Choi MJ (1999) CO2 fixation by Chlorella sp. KR-1 and its cultural characteristics. Bioresour Technol 68:269–273
Sydney EB (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Van Den Hende S, Vervaeren H, Boon N (2012) Flue gas compounds and microalgae: (bio-)chemical interactions leading to biotechnological opportunities. Biotechnol Adv 30:1405–1424
Vasumathi KK, Premalatha M, Subramanian P (2012) Parameters influencing the design of photobioreactors for the growth of microalgae. Renew Sustain Energy Rev 16:5443–5450
Watanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES-collection list of strains, microalgae and protozoa. In: Microbial culture collections. National Institute for Environmental Studies, Tsukuba
Yang C, Hua Q, Shimizu K (2000) Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J 6:87–102
Zhang L, Happe T, Melis A (2002) Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga). Planta 214:552–561
Zhao B, Zhang Y, Xiong K, Zhang Z, Hao X, Liu T (2011) Effect of cultivation mode on microalgal growth and CO2 fixation. Chem Eng Res Design 9:1758–1762
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Guldhe, A., Bhola, V., Rawat, I., Bux, F. (2015). Carbon Dioxide Sequestration by Microalgae: Biorefinery Approach for Clean Energy and Environment. In: Singh, B., Bauddh, K., Bux, F. (eds) Algae and Environmental Sustainability. Developments in Applied Phycology, vol 7. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2641-3_12
Download citation
DOI: https://doi.org/10.1007/978-81-322-2641-3_12
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2639-0
Online ISBN: 978-81-322-2641-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)