Abstract
Glutathione (γ-l-glutamyl-l-cysteinyl-glycine) is a well-known major antioxidant produced in living cells at the concentrations of 1–10 mM. It protects cells from nutritional, environmental, and oxidative stresses. Structurally, it is a nonprotein thiol compound made up of three amino acids – glutamate, cysteine, and glycine with an unusual γ-peptide bond. To meet the increasing commercial demand of GSH as a functional food supplement, various methods for its production methods have been tried. Forms of glutathione (GSH and GSSG) could be produced by enzymatic methods, which are expensive and unprofitable. The microbial biosynthesis could be achieved by fermentation method using natural or engineered microorganisms such as some strains of yeasts Saccharomyces cerevisiae and Candida utilis and some strains of bacteria Escherichia coli and Lactococcus lactis. However, the former approach is not commercially viable because of its high production cost as compared to the latter one, which is more practical and also cost-effective. Certain microorganisms such as Saccharomyces cerevisiae and Candida utilis have been studied as potential microorganisms for GSH production. For enhanced production of glutathione, using a bifunctional enzyme encoded by gshF from Streptococcus thermophilus has been expressed in Escherichia coli.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antioxidant
- Glutathione
- Fermentation
- Saccharomyces cerevisiae
- Oxidative stress
- Osmotic stress
- Esterification
1.1 Significance of Glutathione
Glutathione (GSH) possesses life-sustaining functions as it is an important antioxidant synthesized by mammals. The deficiency of GSH has been reported to cause several diseases (Ballatori et al. 2009; Lu 2009) due to the oxidative stress (Valencia et al. 2001). GSH a nonprotein thiol compound is found in cells at the concentration of 1–10 mM. GSH is found to be the most important antioxidant, because its anti-oxidizing capability is 100 times more than simple antioxidants. Therefore, there is a high demand and research on production of synthetic antioxidant (Nutracam 2007; Xiong et al. 2009; Das et al. 2011).
The antioxidants and their antioxidant activities are the subject of interest for the nutritionists (Nutrition Advisor 2007) and health professionals (World IPO 2007) and so its increasing demand in pharmaceutical and food industries. Antioxidants are the factors that protect cells against free radicals by preventing the oxidation of other molecules. The common examples of antioxidants are vitamin C, flavonoids, lipoic acid, vitamin E, and glutathione. Natural antioxidants including vitamin C, flavonoids, and coumarins are widely found in plants, many have been extracted from various plants using ultrasonic methods, and their antioxidant capacities have been studied in detail (Adam et al. 2009). Antioxidants are those compounds which prevent the natural formation of reactive oxygen species (free radicals) inside the living cells (Wu et al. 2004).
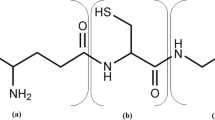
Glutathione (g-l-glutamyl-l-cysteinyl-glycine), a tripeptide, is a low molecular weight nonprotein thiol compound (Li et al. 2004). It is made up of three amino acids, namely, cysteine, glutamic acid, and glutamine. There is a γ-peptide bonding between glutamate and cysteine which makes this molecule very resistant toward its hydrolysis by most peptidases (Anderson 1998). GSH exists in two forms:
-
1.
Reduced form of glutathione with molecular weight 307.3 g/mol – referred as GSH
-
2.
Oxidized form of glutathione with molecular weight 612.6 g/mol – referred as GSSG
These forms of GSH can be measured using a practical HPLC method (Yilmaz et al. 2009).
1.2 Properties of Glutathione
GSH is associated with in various vital functions such as in the maintenance of cells, as radical scavenger, in detoxification and restoration of redox potential (Santos et al. 2007; Noctor et al. 1998). Glutathione was first identified in baker’s yeast cells extracted with ethanol as early as in 1888. Its molecular structure was established in 1921, and it was named as glutathione. Reduced form of glutathione is γ-glutamylcysteinylglycine, a nonprotein thiol compound, which has been reported (Meister and Tate 1976) to be present in most aerobic organisms.
Glutathione is one of the major antioxidant, which has been detected at the concentration of up to 10 mM in living organisms and mammalian cells. GSH reacts with toxic compounds to form GSH conjugates in enzymatically or nonenzymatically catalyzed reactions.
The significant functions of GSH at cellular level have been placed in three main categories:
-
(a)
Antioxidant: This is the most important function of GSH. It protects cells against oxidative damage caused by the reactive oxygen species. It is also found to have an antiaging effect on the body.
-
(b)
Detoxifier: It detoxifies the thiol group of foreign compounds by conjugating with the exogenous electrophiles and diverse xenobiotics.
-
(c)
Immunity booster: GSH plays an important role in white blood cells production.
GSH has its role in the regulation of cellular events like protein synthesis, gene expression, and protein glutathionylation and in the regeneration of vitamin C and vitamin E to its un-oxidized state, conversion of prostaglandin H2 into prostaglandins D2 and E2 by endoperoxide isomerase. GSH converts methylglyoxal to d-lactate in a glyoxalase microbial pathway, and it also actively contributes in spermatogenesis and sperm maturation. Commercially, GSH has established its application in several cosmetic products, where it is used as an emulsifier and moisturizing agent, to enhance the whitening of skin and as an antiaging agent in sun-protection products (Wu et al. 2004; Bachhawat et al. 2009). If the cells become deficient of GSH, this condition may lead to certain diseases such as Alzheimer’s, Parkinson’s, cystic fibrosis, sickle cell anemia, cancer, and HIV (Wu et al. 2004). Wang et al. (2008) have reported that GSH can be used in brewing industries to maintain the high cell viability and to enhance the flavor of beer.
1.3 Occurrence of Glutathione
Natural glutathione is found in several fruits and vegetables, like orange, peach, squash, asparagus, potato, spinach, tomato, grapefruit, etc. The consumption of some vegetables, such as broccoli, cabbage, Brussels sprouts, cauliflower, kale, and parsley, in human diets contributes in two ways – firstly, providing their naturally occurring GSH and secondly also stimulating the human body to self-produce this powerful antioxidant.
Though there are many natural sources to extract GSH, but economically, yeast cells are proved to be the most suitable resource to extract GSH. The yeast can be easily and practically grown on larger scale in shorter time period and have a low-cost maintenance and storage compared to vegetables and fruits. Yeasts have exceptional capability to accumulate GSH in its cells, and low operational costs are involved in its extraction (Vitamin Stuff 2013). Research work has been focused on some specific yeast strains, e.g., Saccharomyces, Candida, Kluyveromyces, Pichia, Rhodotorula, Hanserzula, Debaryomyces, Torulopsis, or the fission yeast genus Schizosaccharomyces, which produced glutathione in simple cultivation medium. For the large-scale production of GSH, two yeasts – Saccharomyces cerevisiae and Candida utilis – are widely used (Cha et al. 2004; Liang et al. 2008a, b).
1.4 Biosynthesis of Glutathione
Several researches were actively carried out on fermentative and enzymatic production of GSH (Yamada et al. 1984; Murata 1989). Thornalley (1991) studied the esterification of glutathione for its bioavailability. Anderson (1998) overviewed the biosynthesis and modulation of glutathione. The optimization of biosynthesis of GSH using yeast cells of Saccharomyces cerevisiae has been studied by many workers (Liu et al. 1999; Chalil et al. 2010; Nigam and Owusu-Apenten 2011).
Glutathione can be enzymatically produced in the presence of ATP and its precursor amino acids, namely, l-glutamic acid, l-cysteine, and glycine (Lu 2013). But the commercialization of GSH was obtained by fermentative production process and not by the method of enzymatic production, because of its high production cost (Li et al. 2004). Later on the large-scale production of GSH was carried out by fermentation methods using Candida utilis and Saccharomyces cerevisiae (Wei et al. 2003a, b).
It is synthesized from its constituent amino acids by two ATP-dependent reaction steps which are catalyzed by two enzymes γ-glutamylcysteine synthetase (GSH1) and glutathione synthetase (GSH2). In the first step, γ-glutamylcysteine is formed from glutamate and cysteine, catalyzed by the enzyme γ-glutamyl synthetase. In the second step, glycine is added to the C-terminal of γGC by the enzyme glutathione synthetase (Li et al. 2004). The strong electron-donating factor of glutathione is generally due to the presence of a sulfhydryl group (SH) on the cystenial portion. It is mainly present in the body in reduced form; GSH is oxidized to GSSG by reacting with free radicals catalyzed by enzymes glutathione peroxidase or glutathione S-transferase. Regeneration of GSH is an NADPH dependent and is catalyzed by the enzyme glutathione reductase (Grant 2001). GSH reacts enzymatically or nonenzymatically with toxic products to form GSH conjugates (Anderson 1998). In humans, the metabolism takes place in the liver by absorbing amino acids to increase GSH level for detoxifying chemicals (Valencia et al. 2001).
Due to its vast functions and significance, the bioavailability of GSH to humans was of great concern. Many techniques were undertaken to make glutathione available on large scale but at a lower cost. Chemical method was one of the earliest method in which modified version of benzyl carbonate method was used to make synthetic glutathione. But the GSH synthesized in this process was optically inactive mixture of d- and L-isomers; therefore, the optical resolution was required to separate the L-form from the D-isomer which hindered this method (Bachhawat et al. 2009).
Enzymatic method has tried to avoid the problems and cost involved in chemical synthesis of GSH. The immobilized microbial cells and enzymes have also been studied in the bioreactor systems. The enzymatic method required the participation of ATP and coenzymes; however, the regeneration of ATP and the low activity of GSH1 and GSH2 became the limiting factors in GSH biosynthesis. This method was also expensive and time consuming because of the usage of three precursor amino acids. The biosynthetic approach via fermentative production method is the most economical one, which involves the sugar material as the fermentation raw substrate, and microorganisms are employed to carry out the fermentative-biosynthetic process (Li et al. 2004). Yeast strains are the most suitable microorganism for this purpose, yeasts like Saccharomyces cerevisiae and Candida utilis have been found to possess higher intracellular content of GSH. The biggest advantage using yeasts is that these yeasts can be easily grown on cheaper sources of sugar substrates, and the other major advantages in using yeast are that they do not cause any endotoxic reactions in humans. In research to increase yield in biosynthesis of GSH, recombinant techniques have been used to overexpress gshp1 and gshp2 in S. cerevisiae and E. coli, but any increased level of glutathione yield could not be achieved (Bachhawat et al. 2009). Research has been conducted by Li et al. (2011) on the production of glutathione using a bifunctional enzyme encoded by gshF from Streptococcus thermophilus which was expressed in Escherichia coli. The gene coding for a novel bifunctional enzyme that is catalytically involved in the reaction for glutathione synthesis, gshF, was cloned from Streptococcus thermophilus SIIM B218 and expressed in Escherichia coli JM109. The induced E. coli JM109 (pTrc99A-gshF) showed the accumulation of 10.3 mM GSH in 5 h in the presence of the precursor amino acids and ATP. Addition of higher concentrations of the precursor amino acids and ATP induced E. coli JM109 (pTrc99A-gshF) to produce up to 36 mM GSH. The molar yield of GSH based on added cysteine was 0.9 mol/mol and based on added ATP yield was found 0.45 mol/mol. Li et al. (2011) found that when ATP was replaced with glucose, E. coli JM109 (pTrc99A-gshF) could produce 7 mM GSH in 3 h. ATP was generated by Saccharomyces cerevisiae for GSH production. E. coli JM109 (pTrc99A-gshF) produced 33.9 mM GSH in 12 h with a yield of 0.85 mol/mol based on added l-cysteine in the presence of glucose and the pmr1 mutant of S. cerevisiae BY4742.
1.5 Parameters Control in GSH Biosynthesis
A number of researches were carried out to study the cellular responses of different microorganisms in order to maintain metabolic viability and vitality of strains for use in industrial fermentation. Thus, a number of practical approaches were carried out to enhance the fermentative glutathione production, and this could be performed either by increasing the cell biomass by high cell density cultivation or by optimizing the fermentation process parameters (Liang et al. 2008a, b, c). The overall production of GSH has been focused in three approaches: (a) production methods, (b) extraction methods, and (c) detection methods.
Initially glutathione was produced extracellularly by Candida tropicalis pk233. In this method, the filamentous form of organism was grown in the presence of ethanol. Initially at higher concentration of ethanol, the production of extracellular glutathione was low, but it increased rapidly along growth time, and the maximum glutathione was obtained after 96 h of cultivation at 5 % (v/v) concentration of ethanol (Yamada et al. 1984). Optimization of medium was one of the basic approaches for higher GSH production. Liu et al. (1999) optimized the growth medium by using peptone, magnesium sulfate, and glucose for enhanced synthesis of GSH by S. cerevisiae and obtained a yield of 124 mg/L. Later on Cha et al. (2004) improved the yield up to 204 mg/L by using a different medium composition consisting of glucose, yeast extract, KH2PO4, and cysteine.
Amino acids like cysteine, glutamic acid, and glycine are required to form glutathione. Hence, the enhanced GSH production was studied using amino acid supplementation using S. cerevisiae (Wen et al. 2004) and by amino acid modulation (Wen et al. 2006). Cysteine had a major impact, so a two-step strategy was undertaken where cysteine was added at the beginning of culturing, and then other amino acids were added after sometime which resulted in 2.67 times increase in GSH yield. Single shot of cysteine addition was shown to give higher yield as compared to continuous addition, because more amount of cysteine ceased the cell growth. Later studies used a strategy of combining amino acids with other parameters, where a feedback control strategy was used and the GSH yield and productivity could be increased by 25 and 70 % from S. cerevisiae. Santos et al. (2007) optimized culture conditions of S. cerevisiae, and GSH yield achieved was 154.5 mg/L after 72 h incubation. Subsequent studies in 2008 were designed on control of dissolved oxygen (DO) along with cysteine addition in C. utilis. It was undertaken by adapting a two-stage strategy where the cysteine was added initially; DO was initially controlled at 5 % v/v for the first 3 h, and then it was gradually increased to 20 % in the following 12 h, such strategy resulted in 1767 mg/L GSH yield (Liang et al. 2008a). In the same year, again, C. utilis was used by adapting precursor amino acids with ATP which was added after 15 h of amino acid addition resulting in GSH yield of 2043 mg/L after 72 h of incubation (Liang et al. 2008b).
Since yeast cells consist of stress molecule that is synthesized and stored under high stress conditions, therefore, other parameters like salinity, high temperature, and osmotic pressure were undertaken to obtain maximum GSH without inhibiting the cell growth. Earlier in 2003, effect of temperature was studied on C. utilis, and a two-stage temperature control strategy was chosen for increasing the cell density as well as higher GSH production by growing cells at 30 °C for 8 h and glutathione production at 26 °C ranging 235–385 mg/L (Wei et al. 2003a). With regard to pH studies in C. utilis, a low pH strategy was applied initially in 2005 and then later on combined low pH with cysteine addition (Liang et al. 2008c). The pH less than 1.5 with single and double shift addition led to 673 and 558 mg/L GSH excretion into the medium (Liang et al. 2008b, c). Dong et al. (2007) studied the effect of high pressure on glutathione accumulation in S. cerevisiae, in this approach the yield of GSH obtained was 103 g/mg GSH at 1.0 MPa.
Glutathione in reduced form is electron donor for GPx reaction (Izawa et al. 1995). Having this knowledge that exposure of cells to osmotic and oxidative stress results in inhibition of cell growth, the strategy of multiple oxidative stress using H2O2 and osmotic stress using NaCl was applied conditions on C. utilis. The maximum GSH yield obtained was 218 mg/L and 238 mg/L, respectively, under these two conditions. Increased level of intracellular cysteine and γ-glutamyl synthetase and GSH reductase were noticed indicating the involvement in GSH accumulation against the condition of stress imposed (Liang et al. 2009a, b).
The downstream processing for the extraction of GSH after the fermentation process is the most important step in GSH recovery. The main objective is the selective separation of compound from the fermented medium. Despite the fact that extraction of GSH is the most important step, only a little work has been carried out on the extraction methods, and hence the detailed information is not available. Earlier glutathione was extracted by using aqueous acetone in Pirie’s method which extracted around 60 % of the GSH. In 1988, glutathione extraction was carried out from S. cerevisiae by selenite. Selenite was added into the medium, and intracellular GSH was leaked out turning the yeast cell culture red due to the presence of elemental selenium. In this reaction glutathione reductase was involved in the reduction of glutathione selenotrisulfide to glutathione selenopersulfide or to GSH and elemental selenium (Iizuka et al. 1988). In one work copper precipitation method and ion exchange chromatography were used for GSH extraction (Bachhawat et al. 2009). In 2005, a comparison of various techniques was made based on factors like extraction time and temperature. The techniques used were ultrasonic methods, homogenizing method, and autolysis. The autolysis method was found to be an efficient technique among others (Salleh et al. 2005).
The solvent ethanol was used for the extraction in cells of S. cerevisiae without disrupting the yeast cells by Xiong et al. (2009), thus having advantage over other techniques as it can maintain cells under appropriate condition with intact plasma membrane. This method required less time, and the solvent ethanol used for the extraction could be recycled, which reduced the cost of the overall production (Xiong et al. 2009). Surfactants were used to optimize the extracellular accumulation of GSH in a fermentation production process using S. cerevisiae by Wei et al. (2003b). GSH production was found mainly affected by the factors like processing temperature, extraction time, the concentration of the yeast cells, and the solvent used (Salleh et al. 2005); however, the extraction using ethanol has proven beneficial to extract GSH for a higher yield. Another important parameter was glutathione detection in yeast cells which was determined by the popular method known as Tietze method. This method includes the use of DTNB also known as Ellman’s reagent which reacts with GSH and produces an oxidized form GSSG and 2-nitro-5-thiobenzoic acid, which is yellow in color (Biomax Co. Ltd 2007). The formation of color can be determined by OD measurement at 412 nm using a spectrophotometer. The advantage of using this reagent was its convenience and short detection time, and DTNB produced can be recycled.
Many conventional techniques have been used like encapsulating the glutathione in the liposome or in combination with other pharmacological products like vitamins or statin, a cholesterol-lowering product (World Intellectual Property Organization 2007). In some research work, glutathione precursor is made available which induces glutathione formation inside the body (Nutrition Advisor 2007). The pure form of GSH is presently supplemented in the form of oral pills or tablets. However, the major drawback of this supplementation is that glutathione is broken down into its three constituent amino acids in the digestive system by the enzymes γ-glutamyl transpeptidases, and thus the glutathione doesn’t reach the end target (Nutra Cam 2007).
The bioavailability of GSH can be improved by esterifying the GSH with alcohol and using HCl as a catalyst. The monoester form of GSH can be regarded as major bioavailable form of glutathione (Thornalley 1991), as it can be easily taken up by the cells.
1.6 Future Prospects
The biosynthesis of GSH using yeast cells, particularly S. cerevisiae, has been studied by several researchers, because of its capability of regenerating sufficient ATP for GSH biosynthesis and due to simplified glycolytic pathway. Yeast cells can be used in large-scale biosynthesis of GSH due to the fact that these compared to other microorganisms are more capable of thriving under adverse growth conditions (Murata 1989). The potential ability of organism to produce higher amount of glutathione under osmotic stress condition using NaCl, and under oxidative stress using H2O2, could be used by applying the strategy of varying the doses and periods of stress factors. The advantages of use of ethanol in extraction studies and the comparison of different alcohol solvents should be explored for the effective extraction of glutathione to obtain maximum yield under high cell density achieved in fermentation process.
A modified HPLC method may be used to determine the oxidized and reduced form of GSH in animal tissues which would be faster and more sensitive and would not require derivatization process unlike normal method (Yilmaz et al. 2009). The bioavailability on larger scale can be enhanced by esterification of glutathione using the background information from previous studies (Thornalley 1991).
The future prospects for the microbial biosynthesis of GSH should be focused on enhancing the yield of glutathione through the recovery of dense yeast cell mass and making it bioavailable using optimized method and suitable chemicals on a large scale. The large-scale fermentative production of glutathione can be done producing active yeast cell cultures in bioreactors under optimized conditions mainly DO and some stress agents. The formulation of GSH, according to its required applications, can be devised in the form of tablets, powder, or cream. Esterified GSH can be tested on animals for its bioavailability and functions and for the pharmaceutical applications.
References
Adam M, Dobias P, Eisner A, Ventura K (2009) Extraction of antioxidants from plants using ultrasonic methods and their antioxidant capacity. J Sep Sci 32:288–294
Anderson M (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 11:1–14
Bachhawat A, Ganguli D, Kaur J, Kasturia N, Thakur A, Kaur H, Kumar A, Yadav A (2009) Glutathione production in yeast. In: Satyanarayan T, Gunje G (eds) Yeast biotechnology diversity and applications. Springer, New York, pp 259–280
Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL (2009) Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 39:191–214
Biomax. Co. Ltd (2007) http://www.biomaxkorea.com/product/dojindo/dpo-appli/stress/stress.htm
Cha J, Park J, Jeon B, Lee Y, Cho Y (2004) Optimal fermentation conditions for enhanced glutathione production by Saccharomyces cerevisiae FF-8. J Microbiol 42:51–55
Chalil S, Das AJ, Nigam P, Owusu-Apenten R (2010) Novel approaches of production and downstream processing of glutathione from microbial resources, In: International Congress in Bioprocesses in the Food Industries (ICBF), University of Parana, Curitiba, Abstract book p 3
Das A, Chalil S, Nigam P, Magee P, Janneh O, Owusu-Apenten R (2011) Glutathione transferase-P1-1 binding with naturally occurring ligands: assessment by docking simulations. J Biophys Chem 2:401–407
Dong Y, Yang Q, Jia S, Qiao C (2007) Effects of high pressure on the accumulation of trehalose and glutathione in the Saccharomyces cerevisiae cells. Biochem Eng J 37:226–230
Grant CM (2001) Role of glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 39:533–541
Iizuka M, Murata K, Kimura A (1988) Induction of glutathione leakage from Saccharomyces cerevisiae cells by selenite. Agric Biol Chem 52:613–614
Izawa S, Yoshiharu I, Kimura A (1995) Oxidative stress response in yeast: effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett 368:73–76
Li Y, Wei G, Chen J (2004) Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol 66:233–242
Li W, Li Z, Yang J, Ye Q (2011) Production of glutathione using a bifunctional enzyme encoded by gshF from Streptococcus thermophilus expressed in Escherichia coli. J Biotechnol 154:261–268
Liang G, Cheng D, Chen J (2008a) A novel strategy of enhanced glutathione production in high cell density cultivation of Candida utilis – cysteine addition combined with dissolved oxygen controlling. Enzyme Microb Technol 42:284–289
Liang G, Liao X, Du G (2008b) Elevated glutathione production by adding precursor amino acids coupled with ATP in high cell density cultivation of Candida utilis. J Appl Microbiol 105:1432–1440
Liang G, Du G, Chen J (2008c) Enhanced glutathione production by using low-pH stress coupled with cysteine addition in the treatment of high cell density culture in Candida utilis. Lett Appl Microbiol 46:507–512
Liang G, Guocheng D, Chen J (2009a) Salt-induced osmotic stress for glutathione production in Candida utilis. Enzyme Microb Technol 45:324–329
Liang G, Liao X, Du G (2009b) A new strategy to enhance oxidative stresses in Candida utilis. Bioresour Technol 100:350–355
Liu C, Hwang C, Liao C (1999) Medium optimization for glutathione production by Saccharomyces cerevisiae. Process Biochem 34:17–23
Lu SC (2009) Regulation of glutathione synthesis. Mol Aspects Med 30:42–59
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta (BBA) Gen Subj 1830:3143–3153
Meister A, Tate SS (1976) Glutathione and related γ-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem 45:559–604
Murata K (1989) Immobilization, chemical and industrial application and biosynthetic preparation of glutathione and related compounds. Coenzymes Cofactors 3:187–242
Nigam P, Owusu-Apenten R (2011) Production and downstream processing of glutathione from microbial sources. In: International conference on new horizons in biotechnology, Trivandrum, Abstract book p 52
Noctor G, Arisi MA, Jouanin L, Kunert K, Rennenberg H, Foyer C (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647
NutraCam (2007) Glutathione supplement, antioxidant glutathione chewing gum. http://www.thqueen.com/
Nutrition Advisor (2007) Glutathione, GSH and whey proteins. Information for physicians. http://www.nutritionadvisor.com/immunocal_balch.php
Salleh MB, Farouk AE, Jamal P (2005) Comparison studies among the methods used in isolating the GSH from baker yeast. J Appl Sci 5:151–154
Santos LO, Gonzales TA, Úbeda BT (2007) Influence of culture conditions on glutathione production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 77:763–769
Thornalley PK (1991) Esterification of reduced glutathione. Biochem J 275:535–539
Valencia E, Marin A, Hardy G (2001) Glutathione – nutritional and pharmacological viewpoints: part III. Nutrition 17:696–697
Vitamin Stuff (2013) Antioxidant benefits, information on supplements. http://www.vitaminstuff.com/glutathione.html
Wang Z, He X, Lium N, Zhang B (2008) Construction of self-cloning industrial brewing yeast with high- glutathione and low-diacetyl production. Int J Food Sci Technol 43:989–994
Wei G, Li Y, Du G, Chen J (2003a) Application of a two-stage temperature control strategy for enhanced glutathione production in the batch fermentation by Candida utilis. Biotechnol Lett 25:887–890
Wei G, Li Y, Dua G, Chen J (2003b) Effect of surfactants on extracellular accumulation of glutathione by Saccharomyces cerevisiae. Process Biochem 38:1133–1138
Wen S, Zhang T, Tan T (2004) Utilization of amino acids to enhance glutathione production in Saccharomyces cerevisiae. Enzyme Microb Technol 35:501–507
Wen S, Zhang T, Tan T (2006) Maximizing production of glutathione by amino acid modulation and high-cell- density fed-batch culture of Saccharomyces cerevisiae. Process Biochem 46:2424–2428
World Intellectual Property Organization (2007) Liposomally encapsulated reduced glutathione, including with other pharmacologic preparation, capable of administration as an oral, topical, intraoral or transmucosal preparation, for reversal and prevention of oxidation of cholesterol and of low density lipoprotein, vol. no. 053810
Wu G, Fang Y, Yang S, Lupton J (2004) Glutathione metabolism and its implications for health. J Nutr 43:489–492
Xiong Z, Guo M, Guo Y (2009) Efficient extraction of intracellular reduced glutathione from fermentation broth of Saccharomyces cerevisiae by ethanol. Bioresour Technol 100:1011–1014
Yamada Y, Tan Y, Kamihara T (1984) Production of extracellular glutathione by Candida tropicalis Pk 233. J Gen Microbiol 130:3275–3278
Yilmaz O, Keser S, Tuzcu M, Guvenc M, Cetintas B, Irtegun S, Tastan H, Sahin K (2009) A practical HPLC method to measure reduced (GSH) and oxidized (GSSG) glutathione concentrations in animal tissues. Anim Vet Adv 8:343–347
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Nigam, P.S., Owusu-Apenten, R. (2016). Studies on Biosynthetic Production of Antioxidant Glutathione Using Microbial Cultures. In: Shukla, P. (eds) Frontier Discoveries and Innovations in Interdisciplinary Microbiology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2610-9_1
Download citation
DOI: https://doi.org/10.1007/978-81-322-2610-9_1
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2609-3
Online ISBN: 978-81-322-2610-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)