Abstract
The purpose of radiation therapy planning is to maximize dose delivery to the target while simultaneously decreasing radiation dose to the surrounding normal tissues. In the era of image-guided radiation therapy (IGRT), the greatest challenge is target delineation. Over the last two decades, technological advances in radiographic imaging, biochemistry, and molecular biology have played an increasing role in radiation treatment planning, delivery, and evaluation of response. In earlier times, fluoroscopy was the basis of radiation treatment planning. In the late 1980s, computed tomography (CT) became the basis for modern radiation treatment planning and delivery. Also multimodality anatomic imaging was found to be the solution to augment delineation of tumors and surrounding structures on CT-based treatment planning. Although these imaging modalities provide the customary anatomic details necessary for radiation treatment planning, they have limitations, including difficulty with identification of tumor extension, and distinction from scar tissues. To overcome these limitations, PET and, more recently, PET-CT have been innovative regarding the extent of disease appraisal, target delineation in the treatment planning, and assessment of therapy response. The use of multi-modality imaging fusion and the introduction of more sensitive and specific PET-CT tracers may further assist target definition. Novel markers of tumor hypoxia or proliferation have the potential to modify the delineation of target volumes, allowing for “dose painting” in selected subvolumes. Furthermore, the potential to predict early outcome or even detect early recurrence of tumor may allow for the tailoring of intervention in cancer patients. The implementation of three-dimensional radiotherapy and IMRT requires adequate selection and delineation of target volumes on the basis of anatomic or molecular imaging modalities, appropriate dose prescription and (dose) specification with regard to dose volume constraints, and quality control for both the clinical and the physical aspects of the entire procedure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Target Volume
- Intensity Modulate Radiation Therapy
- Radiation Therapy Planning
- Radiation Treatment Planning
- Surrounding Normal Tissue
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The purpose of radiation therapy planning is to maximize dose delivery to the target while simultaneously decreasing radiation dose to the surrounding normal tissues. In the era of image-guided radiation therapy (IGRT), the greatest challenge is target delineation. Over the last two decades, technological advances in radiographic imaging, biochemistry, and molecular biology have played an increasing role in radiation treatment planning, delivery, and evaluation of response. In earlier times, fluoroscopy was the basis of radiation treatment planning. In the late 1980s, computed tomography (CT) became the basis for modern radiation treatment planning and delivery. Also multimodality anatomic imaging was found to be the solution to augment delineation of tumors and surrounding structures on CT-based treatment planning. Although these imaging modalities provide the customary anatomic details necessary for radiation treatment planning, they have limitations, including difficulty with identification of tumor extension, and distinction from scar tissues. To overcome these limitations, PET and, more recently, PET-CT have been innovative regarding the extent of disease appraisal, target delineation in the treatment planning, and assessment of therapy response. The use of multi-modality imaging fusion and the introduction of more sensitive and specific PET-CT tracers may further assist target definition. Novel markers of tumor hypoxia or proliferation have the potential to modify the delineation of target volumes, allowing for “dose painting” in selected subvolumes. Furthermore, the potential to predict early outcome or even detect early recurrence of tumor may allow for the tailoring of intervention in cancer patients. The implementation of three-dimensional radiotherapy and IMRT requires adequate selection and delineation of target volumes on the basis of anatomic or molecular imaging modalities, appropriate dose prescription and (dose) specification with regard to dose volume constraints, and quality control for both the clinical and the physical aspects of the entire procedure.
For target volume selection and delineation, anatomic imaging modalities, such as CT and, to a lesser extent, MRI, remain the most widely used modalities. CT is widely available, does not have geometric distortion, and provides intrinsic information on the electronic densities of various tissues—information that is used in dose calculation algorithms. As a limitation, CT lacks contrast resolution for normal soft-tissue structures and tumor extent. This limitation has led to significant inter- and intra-observer variations in delineation of the gross tumor volume (GTV) in head and neck, lung, esophageal, prostate, breast, cervical, and brain tumors.
MRI with various sequences (e.g., unenhanced T1-weighted, contrast-enhanced T1-weighted, and T2-weighted sequences with or without the fat suppression option) is another anatomic imaging modality that can complement or sometimes replace CT. MRI has been shown to be more accurate than CT for evaluating the soft tissue or bone extent of nasopharynx, prostate, and brain tumors. However, for pharyngeal–laryngeal tumors, the advantage of MRI over CT has not been confirmed, either in terms of interobserver variability or in terms of target volume delineation.
Over the last few years, the use of molecular imaging, particularly the use of positron-labeled 18F-FDG, has become increasingly popular in oncology. Given that adequate tracers are used, molecular imaging with PET enables visualization of the various molecular pathways of tumors, including metabolism, proliferation, oxygen delivery and consumption, and receptor or gene expression. Applied in the clinic, PET can be useful for tumor staging, for prediction of the tumor response, for selection or delineation of radiotherapy target volumes, for assessment of the tumor response to treatment, for the detection of early recurrence, or as a tool to evaluate modifications in organ function after treatment. The use of PET in general and of PET with FDG in particular for radiotherapy planning purposes has taken on increasing importance, so that more and more radiation oncologists believe that target volume selection and delineation cannot be adequately performed without the use of PET with FDG. It is important to discuss why should metabolic information be considered more important than the anatomic information provided by CT or MRI? What is the evidence supporting the use of FDG in the treatment planning process?
The ultimate goal of the planning process is to select and delineate target volumes (and organs at risk) on the basis of all of the available diagnostic information and on the knowledge of the physiology of the disease, that is, the probability of local and nodal infiltration. This goal is achieved in part through the use of various imaging modalities, which depict more or less accurately the true tumor extent. The difficulty with imaging modalities is that none of them has a sensitivity of 100 % (no false-negative examinations) or a specificity of 100 % (no false-positive examinations). Thus, false-negative and false-positive results for depicting neoplastic processes occur.
How the sensitivity and specificity of a particular imaging modality influence the radiation planning process depends on the underlying objective of the treatment. If, for a particular disease, the objective is to avoid missing a tumor at any expense, a highly sensitive approach needs to be selected. Such a selection will, of course, result in a lower specificity and in the inclusion of nonneoplastic tissue in the target volume. However, this approach reduces the likelihood of missing neoplastic cells. If, on the other hand, the aim is to avoid inclusion of nonneoplastic cells in the target volume to protect normal tissue, a highly specific approach needs to be adopted. However, such an approach reduces sensitivity and increases the risk for missing tumor cells.
When a novel imaging modality (e.g., PET with the tracer FDG) is introduced, its sensitivity and specificity need to be compared with those of the standard test which is for radiotherapy planning CT. Furthermore, its potential impact on treatment planning needs to be determined. For example, if an additional lymph node is visualized with a new imaging modality known to be more specific than the standard modality, it may be legitimate to increase the target volume(s) beyond what would have been used with a standard procedure; conversely, if fewer nodes are visualized with a new imaging modality known to be more sensitive than the standard modality, it may be legitimate to decrease the target volume(s) below what would have been used with a standard procedure.
Comparative analysis of using FDG-PET and CT to determine target volume has yielded different results in different cancers and locations. For example, compared with anatomic imaging modalities such as CT and MRI, FDG PET is not likely to be superior for the selection of the target volume in neck lymph nodes. In contrast, the sensitivity for the staging of lymph node involvement in lung cancer is significantly higher for FDG-PET than for CT. For esophageal cancer, the sensitivity of FDG-PET is similar to that of CT. However, FDG PET is more specific for the staging of lymph node involvement outside the mediastinum, like supraclavicular or celiac lymph node . For paraaortic lymph nodes in patients with cervical carcinoma, FDG-PET has been reported to be more specific than CT or MRI.
All these considerations have become redundant to some extent as more and more centers are using dual PET-CT systems. Selection of target volume using PET-CT systems has become more accurate than previous treatment planning using conventional imaging modalities.
The advent of dual-modality integrated PET-CT systems offers a unique opportunity of improving target localization and facilitating treatment planning for radiation therapy in contemporary oncologic practice.
Radiation Therapy Planning
Radiation therapy planning can be defined the process of image acquisition, volume delineation, dose-fractionation prescription, assigning of treatment fields and beam modifiers, evaluation of dose distribution, and quality assurance before final approval for treatment delivery. The standard imaging technique used in radiotherapy planning is CT as it provides both good anatomic detail for defining target volumes and the electron density data required for dose calculations. Over the last couple of decades, advances in radiation therapy planning and delivery have ushered in the era of high-precision conformal radiotherapy allowing generation of dose distributions that conform closely to the shape of the target volume while minimizing high-dose regions in the surrounding normal tissues. In general, anatomical cross-sectional CT images are used to delineate treatment volumes and design multiple uniform intensity fields that are shaped using multi-leaf collimators. Intensity modulated radiation therapy (IMRT) is an advanced form of conformal radiotherapy wherein the beam intensity is modulated to produce highly conformal dose distributions around irregular and complex-shaped target volumes. Modern radiotherapy departments are equipped with volumetric image-guidance for precise alignment of the patient with respect to the beam line. Rapid advances in technology allow highly sophisticated treatment planning coupled with extremely accurate localization and precise radiation dose delivery. However, the technology for target volume delineation, i.e., accurately defining what regions or tissues need to be targeted is still not very robust and continues to evolve. One distinct advantage of PET-CT in radiotherapy planning is its potential to improve tumor delineation, reducing intra-observer and inter-observer variability and making treatment volumes more standard across individuals and institutions.
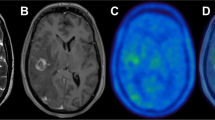
PET for radiation therapy planning can be used in several ways: visual aid for target delineation, fusion of PET and CT images acquired from separate scanners, or a planning PET-CT scan done on an integrated PET-CT unit with the patient in treatment position. Positioning tools should include a firm flat couch top, immobilization devices, laser beams for patient alignment, and a wide-bore scanner (>70 cm). The PET and CT images thus acquired are complementary as well as supplementary. PET images can identify areas of disease not readily visible on CT alone. CT images can provide improved spatial resolution helping to anatomically localize sites of involvement. Also, the low-noise CT data can be used to generate patient-specific map of attenuation coefficients for correcting PET emission data for errors from photon attenuation, scattered radiation, and other physical degrading factors such as partial volume effect. Thus dual-modality PET-CT can improve both the visual quality and the quantitative accuracy of the correlated radiotracer data. It is now widely accepted and acknowledged that PET-CT impacts significantly on planning in the modern radiation therapy clinic. PET-CT not only has a direct impact on target volume delineation in a wide variety of cancers, but can also lead to a significant change in the therapeutic approach in 10–30 % of patients as compared to other reference imaging modalities (Figs. 18.1, 18.2, and 18.3).
Limitations of PET-Guided Planning
One of the main difficulties is the delineation of the treatment volume from noisy PET data. Identification of lesion edges in general is not a trivial problem in PET imaging. Major problems encountered in functional volume quantitation are image segmentation and imperfect system response function. The difficulty in image segmentation is compounded by the low spatial resolution and high-noise characteristics of PET images. Manual delineation of target volumes using different window-level settings and look-up tables is the most common and widely used technique in the clinic. However, the method is highly operator-dependent with wide interobserver variability. Semi-automated or fully automated delineation techniques offer an advantage over manual techniques by improving reproducibility. A collaborative effort between nuclear medicine physicians, radiologists, and radiation oncologists is desirable to fully exploit the potential of PET-CT-guided radiation therapy planning.
Impact of PET-Guided Planning on Outcome
There have been recent reports of improved outcomes with PET-guided planning. The largest prospective dataset documenting improved outcomes comes out of a study consisting a total of 317 patients treated with a combination of whole-pelvis and split-field irradiation using an institutional step-wedge technique. Another 135 patients were treated with PET-CT-guided IMRT, using pseudo-step-wedge intensity modulation to match the target dose distribution of the conventional technique. Both groups had similar stage distribution, histology, brachytherapy, and concurrent chemotherapy. With a mean follow-up of 52 months for living patients, 178 patients (39IMRT, 139non-IMRT) had recurred. Patients in the PET-CT-guided IMRT group showed better overall and cause-specific survival (P < 0.0001). Only eight patients in the PET-CT-guided IMRT group developed Grade 3 or worse large bowel or bladder complications which was significantly lesser compared to 54 patients in the non-IMRT group (P = 0.0351).
In another study of 115 patients with locally advanced NSCLC treated with definitive PET-guided conformal radiation therapy were analyzed for survival, local regional recurrence, and distant metastases. With a median follow-up of 18 months (range 3–44 months) for all patients, the median overall survival, 2-year actuarial overall survival, and disease-free survival were 19 months, 38, and 28 %, respectively. Majority of the patients died from distant metastases (overall rate of 36 %).
In a recent case control study, 45 patients with stage IVA pharyngeal carcinoma treated with definitive chemoradiation with PET-CT-guided IMRT were compared with 86 patients treated without PET-CT and 3D-conformal radiotherapy after matching with respect to gender, age, stage, grade, and tumor location. Median follow-up was 18 months (range, 6–49 months) for the PET-CT-IMRT group and 28 months (range, 1–168 months) for controls. PET-CT and treatment with IMRT improved cure rates compared to patients without PET-CT and IMRT. Overall survival of patients with PET-CT and IMRT was 97 and 91 % at 1 and 2 years respectively, compared to 74 and 54 % for patients without PET-CT or IMRT (P = 0.002). The event-free survival rate of PET-CT-IMRT group was 90 and 80 % at 1 and 2 years, respectively, compared to 72 and 56 % in the control group (P = 0.005). Thus more and more data is coming up showing distinct advantage of using PET-CT in radiation therapy planning.
Therapy Planning with Integrated MRI/PET
The combination of MRI and PET in a single gantry for simultaneous acquisition has been developed which has helped to bridge the gap between systems and molecular diagnosis. Both PET and MRI offer richly complementary information about disease. Their integration into a combined system has produced hybrid technology that is significantly better than the sum of its parts. The possibility of using this highly sophisticated hybrid technology for therapy planning is under way and may improve the results further.
Concluding Remarks
Radiation therapy planning has traditionally relied very heavily on CT imaging. Increasingly, FDG-PET-CT is being incorporated into the treatment planning process and promises to improve target volume delineation in a wide variety of cancers. The use of PET-CT for target volume selection should be considered within the framework of its sensitivity and specificity for various tumor types and also mandates specific tuning of parameters, such as image acquisition, processing, and segmentation. There is accumulating evidence that PET-CT guidance has significant impact on radiotherapy planning in many types of cancer. The potential benefits of improved staging and more accurate target localization can promote integrated PET-CT to become the gold standard for radiotherapy simulation and planning.
For Further Reading
Ford EC, Herman J, Yorke E, Wahl RL. 18F-FDG PET-CT for image-guided and intensity-modulated radiotherapy. J Nucl Med. 2009;50:1655–65.
Jarritt PH, Carson KJ, Hounsell AR, Visvikis D. The role of PET-CT scanning in radiotherapy planning. Br J Radiol. 2006;79:S27–35.
Rothschild S, Studer G, Seifert B, Huguenin P, Glanzmann C, Davis JB, et al. PET-CT staging followed by Intensity-Modulated Radiation Therapy (IMRT) improves treatment outcome of locally advanced pharyngeal carcinoma: a matched-pair comparison. Radiat Oncol. 2007;2:22.
Schlemmer HP, Pichler BJ, Krieg R, Heiss WD. An integrated MR/PET system: prospective applications. Abdom Imaging. 2009;34:668–74.
Vernon MR, Maheshwari M, Schultz CJ, Michel MA, Wong SJ, Campbell BH, et al. Clinical outcomes of patients receiving integrated PET-CT-guided radiotherapy for head and neck carcinoma. Int J Radiat Oncol Biol Phys. 2008;70:768–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Das, B.K. (2015). Application of PET (PET-CT) in Radiation Therapy Planning. In: Das, B. (eds) Positron Emission Tomography. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2098-5_18
Download citation
DOI: https://doi.org/10.1007/978-81-322-2098-5_18
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2097-8
Online ISBN: 978-81-322-2098-5
eBook Packages: MedicineMedicine (R0)