Abstract
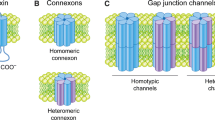
Similar to other transmembrane proteins, connexin translation occurs on the ribosomes attached to the endoplasmic reticulum (ER), followed by co-translational release of the protein in the lumen of ER. During the transport of connexins from ER to Golgi network, they are folded into the three-dimensional structure, followed by their oligomerization into hexameric hemichannels called connexons. Each connexon subunits can be either homomeric (made of similar connexins) or heteromeric (made of different connexins). The possibility of forming the heteromeric connexin channels is significant, as many cells express more than one type of connexin protein. Hence, more the expression of different connexins in a cell, the possibility of forming various permutation combinations of connexin channels increases significantly. Moreover, channels formed of heteromeric connexons have different properties from those of homomeric channels of the constituent connexins, thereby allowing for critical regulation of the permeability and conductance of gap junctions. However, it is regarded that there exists some selective compatibility between various connexins to form heterotypic channels, and this compatibility has been attributed to the N-terminal and C-terminal domains of each connexin. Furthermore, the transport of connexon hemichannels involves their packaging into vesicles, and then these vesicles deliver the connexon hemichannels to the plasma membrane. The transport of these vesicles to the plasma membrane has been demonstrated to be both microtubule and actin mediated. The insertion of connexon hemichannels to the plasma membrane is regarded to be random. However, some studies have shown that microtubules target the connexon hemichannels to a specific domains or regions of plasma membrane, which are rich in adherens junction proteins. The inserted connexon hemichannels interact with the apposing connexion hemichannels of adjacent cell, thus allowing the formation of complete intercellular gap junction channel. The interaction between the apposing connexon hemichannels is mediated by the external loop domains. The individual channels aggregate in the membrane to form plaques and hence are known as gap junction plaques. However, under certain conditions, the inserted connexon hemichannels remain uncoupled, thus connecting the intracellular milieu directly to the extracellular. The existence of these hemichannels has been well established and is known to play important roles in cell physiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Synthesis, Maturation, and Transport to the Cell Membrane
Similar to other transmembrane proteins, connexin translation occurs on the ribosomes attached to the endoplasmic reticulum (ER), followed by co-translational release of the protein in the lumen of ER. During the transport of connexins from ER to Golgi network, they are folded into the three-dimensional structure, followed by their oligomerization into hexameric hemichannels called connexons. Each connexon subunits can be either homomeric (made of similar connexins) or heteromeric (made of different connexins). The possibility of forming the heteromeric connexin channels is significant, as many cells express more than one type of connexin protein. Hence, more the expression of different connexins in a cell, the possibility of forming various permutation combinations of connexin channels increases significantly. Moreover, channels formed of heteromeric connexons have different properties from those of homomeric channels of the constituent connexins, thereby allowing for critical regulation of the permeability and conductance of gap junctions. However, it is regarded that there exists some selective compatibility between various connexins to form heterotypic channels, and this compatibility has been attributed to the N-terminal and C-terminal domains of each connexin. Furthermore, the transport of connexon hemichannels involves their packaging into vesicles, and then these vesicles deliver the connexon hemichannels to the plasma membrane. The transport of these vesicles to the plasma membrane has been demonstrated to be both microtubule and actin mediated. The insertion of connexon hemichannels to the plasma membrane is regarded to be random. However, some studies have shown that microtubules target the connexon hemichannels to a specific domains or regions of plasma membrane, which are rich in adherens junction proteins. The inserted connexon hemichannels interact with the apposing connexion hemichannels of adjacent cell, thus allowing the formation of complete intercellular gap junction channel. The interaction between the apposing connexon hemichannels is mediated by the external loop domains. The individual channels aggregate in the membrane to form plaques and hence are known as gap junction plaques. However, under certain conditions, the inserted connexon hemichannels remain uncoupled, thus connecting the intracellular milieu directly to the extracellular. The existence of these hemichannels has been well established and is known to play important roles in cell physiology.
4.2 Post-translational Modifications, Half-Life, and Degradation
Connexins are not regarded as mere intercellular channels that link two communicating cells, but connexins are now regarded to play important role in controlling various other cell functions. In part, these connexin functions are determined by various post-translational modifications, which include phosphorylation, hydroxylation, acetylation, nitrosylation, and palmitoylation. The most important of these connexin post-translational modifications is phosphorylation of various amino acid residues of connexins. Most of these phosphorylation events occur at the C-terminal domain of the connexins, thus modulating the function of each connexins and hence the gap junction channel. Most of the connexins, except Cx26, are known to be phosphorylated, which include Cx31, Cx32, Cx37, Cx40, Cx43, Cx45, Cx46, Cx50, and Cx57. Various kinases have been identified that target connexin proteins, which include Src, PKC, MAPKs, tyrosine kinases, etc. Phosphorylation serves as an important post-translational modification system that regulates various aspects of connexin in including the formation and modulation of gap junction channels. Phosphorylation events regulate the gating of connexin channels and thus control the opening and closing of these channels. The effect of phosphorylation on channel gating is very specific, as the phosphorylation of a connexin isoform such as Cx43 on different residues by the same kinase may lead to opposite effects with respect to enhancing or inhibiting gap junction function or gap junction intercellular communication (GJIC). Besides gating, connexin phosphorylation affects various other aspects of connexins such as trafficking, assembly and disassembly, and degradation. Moreover, connexin phosphorylation at specific residues controls the interaction of connexins with other proteins, and these events affect other cellular functions of connexins that are independent of gap junctions, including the control of growth and proliferation, cell migration, etc.
Just like other proteins in the cell, connexins are degraded in a controlled manner, and their turnover is very fast, with a half-life not exceeding few hours. The newly synthesized connexins are delivered to the membrane and incorporated at the existing gap junction plaques, while the older connexins are internalized from the existing gap junction plaques and finally degraded. Thus, the gap junction plaques are highly dynamic in nature, having newly delivered connexons localized to the periphery of existing gap junctional plaques, while those destined for degradation are present at the centre of the gap junctional plaque. The mechanism of gap junctional internalization is through the formation of annular junctions, which are large double-membrane vesicular structures that could contain the entire gap junction, or a fragment of it, and transport it from cell–cell boundaries into one of the two interacting cells. Following gap junction internalization, these complexes undergo preliminary degradation in the annular junctions, leading to disassembly of gap junctions and connexons into individual connexins. The connexin proteins undergo complete degradation either through proteasomes or through lysosomes, with each of the two degradation pathways having different roles.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Hussain, M.U. (2014). General Cell Biology of Connexins. In: Connexins: The Gap Junction Proteins. SpringerBriefs in Biochemistry and Molecular Biology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1919-4_4
Download citation
DOI: https://doi.org/10.1007/978-81-322-1919-4_4
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1918-7
Online ISBN: 978-81-322-1919-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)