Abstract
In order to restore environmental balance, the utility of phytoremediation to remediate environmental contamination has received much attention in the last few years. Considerable effort has been devoted to making the transition from the laboratory to commercialization. Although plants have the inherent ability to detoxify contaminants, they generally lack the catabolic pathway for the complete degradation of these compounds as compared to microorganisms. There are also concerns over the potential for the introduction of contaminants into the food chain and how to dispose of plants that accumulate them in high quantities is also a serious concern. Hence, the utility of phytoremediation to remediate environmental contamination is still somewhat in question. For these reasons, researchers have endeavored to engineer plants with genes that can bestow superior degradation abilities. Genes from microbes, plants, and animals are being used successfully to enhance the ability of plants to tolerate, remove, and degrade pollutants. Although improvement of plants by genetic engineering opens up new possibilities for phytoremediation, it is still in its research and development phase, with many technical issues needing to be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Transgenic Plant
- Heavy Metal Tolerance
- Transgenic Poplar
- Carbon Preference Index
- Pentaerythritol Tetranitrate
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
13.1 Introduction
Elemental pollutants such as arsenic (As), copper (Cu), cadmium (Cd), mercury (Hg), zinc (Zn), and lead (Pb) are difficult to remediate from air, water, and soil because these toxic elements are immutable by all biochemical reactions, hence remain in the ecosystem. They seep into surface water, groundwater, or soil and may channel into the food chain through crops growing on such soil and water. Apart from these metals, small organic contaminants and volatile organic compounds are other prevalent contaminants of the environment. These include petroleum hydrocarbons (PHC), halogenated hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and pesticides, and solvents like trichloroethylene (TCE), carbon tetrachloride, and salts. PAHs, in general, are ubiquitous environmental pollutants and are formed from both natural and anthropogenic sources. Anthropogenic sources are by far the major contributors and include the burning of coal refuse banks, coke production, automobiles, commercial incinerators, and wood gasifers. PAHs are an environmental concern because they are not only toxic to aquatic life but also several are suspected human carcinogens
The term phytoremediation (“phyto” meaning plant, and the Latin suffix “remedium” meaning to restore) refers to a diverse collection of plant-based technologies that use plants (either naturally occurring or genetically engineered) to clean contaminated environments. Phytoremediation is popular because of its cost-effectiveness, environmentally friendly aesthetic advantages, long-term applicability, and good influence on the physical, chemical, and biological aspects of the environment. The technology can be used at hazardous waste sites where other methods of treatment are too expensive or impractical; it may also be used at low-level contaminated sites where only “polishing treatment” is required over a long period of time. Compared to the use of other organisms, the use of plants costs less as they capture solar energy to synthesize the proteins and other structures that are necessary for the remediation. Phytoremediation can be used in conjunction with other technologies as a final cap. Phytoextraction, a type of phytoremediation, is an effective means of remediating a site because it reduces the overall mass to be treated from tons of widespread contaminated soil to plant tissue that can be dried to a small volume. Unlike other engineering methods that might remove the fertile topsoil, phytoremediation does not reduce the fertility of the site (Chaney et al. 1997).
To be effective, the concentration of the metal in the harvestable part of the plants must be higher than its concentration in the soil so that the volume of the hazardous plant waste is less than the volume of the contaminated soil. Plants that are especially good at concentrating the pollutants are termed as hyperaccumulators. The ‘bioconcentration factor’ (BCF) refers to the metal concentration in plant tissues relative to the metal concentration in the substrate, and a value >1 indicates that the plant actively concentrates the metal. A metal hyperaccumulator is defined as a plant that can concentrate the metals to a level of 0.1 % for nickel (Ni), cobalt (Co), Cu, and Pb, 1 % for Zn and 0.01 % for Cd (Baker et al. 2000). For example, Pteris vittata (Chinese brake fern) efficiently hyperaccumulates arsenic in its fronds. Another hyperaccumulator is Thlaspi caerulescens, which can concentrate cadmium, a highly toxic and probably carcinogenic metal, in the above-ground tissues at concentrations 1,000 times higher than the normal toxic concentration of only 1 ppm.
Although plants have innate capabilities of remedying hazardous contaminants from the environment, the rate is directly proportional to plant growth rate and the total amount of remediation is correlated with the plant total biomass, making the process very slow. Further, phytoremediation using natural plants has other limitations that include the potential for introducing the contaminant or its metabolites into the food chain, long cleanup times required to achieve regulatory action levels, and toxicity encountered in establishing and maintaining vegetation at waste sites. The plants may also suffer toxicity symptoms.
Keeping these limitations in view, it would be beneficial to enhance plants’ capacity for phytoremediation. This necessitates the identification of a fast growing (largest potential biomass and greatest nutrient responses) and more strongly metal accumulating genotype. Genetic engineering provides an excellent means to achieve this goal. It is possible to generate transgenic plants that can overexpress their genes or express exogenous genes, and thereby acquire new or improved phenotypes. One major advantage of genetically engineered plants is that the ability of specific enzymes for degradation of a contaminant could be transferred to a plant species that is either indigenous to an ecosystem or has other desirable remediation properties such as rapid growth, deep root structures, or high water uptake. Once an individual plant with suitable phenotype is produced, it is easy to multiply it by cloning methods, such as cuttings and somatic embryogenesis. Moreover, many trees continue to grow for many years as long as the conditions are suitable.
13.2 Genetic Engineering Strategies for Plant Modification to Enhance Phytoremediation
Many transgenic plants with new characteristics due to expression of genes from microorganisms, yeasts, mouse, or even human have been reported in scientific journals. Till date, all commercial and research activities have used naturally occurring plant species. Many of these are species that can be genetically engineered. In general, any dicotyledonous plant species can be genetically engineered using the Agrobacterium vector system, while most monocotyledonous plants can be transformed using particle gun or electroporation techniques.
Recent studies using transgenic aspen overexpressed with a nitroreductase, pseudomonas nitroreductase A (pnrA), isolated from the bacterium Pseudomonas putida have shown higher accumulation of TNT (2, 4, 6-trinitrotoluene) from liquid culture and soil, compared to nontransgenic plants. Furthermore, the tolerance limit toward TNT was also significantly higher than for nontransgenic plants.
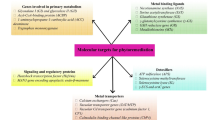
The genetic engineering approach has successfully facilitated to alter the biological functions of plants through modification of primary and secondary metabolism and by adding new phenotypic and genotypic characters to plants with the aim of understanding and improving their phytoremediation properties (Fig. 13.1). Transgenic plants produced for metabolizing herbicides and long-persisting pollutants can be used for phytoremediation of foreign chemicals in contaminated soil and water (Dowling and Doty 2009).
Metal-hyperaccumulating plants and microbes with unique abilities to tolerate, accumulate, and detoxify metals and metalloids represent an important reservoir of unique genes. These genes could be transferred to fast-growing plant species to improve phytoremediation (Fulekar et al. 2009). It has been established that the adaptive metal tolerance is governed by a small number of major genes and some minor modifier genes. Probably, it is this adaptive metal tolerance that gears a plant species for hyperaccumulation.
Use of tissue culture techniques to select genes having enhanced bio-degradative properties (for organics) or the enhanced ability to assimilate metals helps to select plants with the desired characters. Molecular techniques such as the analysis of molecular variance of the random amplified polymorphic DNA (RAPD) markers are useful to investigate the genetic diversity and heavy metal tolerance in plant populations, providing the opportunity to investigate the first steps in the differentiation of plant populations under severe selection pressure and to select plants for phytoremediation.
The genetic and biochemical basis is becoming an interesting target for genetic engineering. A fundamental understanding of both uptake and translocation processes in normal plants and metal hyperaccumulators, regulatory control of these activities, and the use of tissue specific promoters offers the ability to develop effective and economic phytoremediation plants for soil metals. Examples include genes controlling the synthesis of peptides that sequester metals, like phytochelatins, genes encoding transport proteins, or genes encoding enzymes that change the oxidation state of heavy metals. The genes involved in the metabolism of chemical compounds can be isolated from various organisms, including bacteria, fungi, plants, and animals, and these genes are then introduced into candidate plants. The desired characters for phytoremediation can be improved by identifying candidate protein, metal chelators, and transporter genes for transfer and/or overexpression of a particular gene.
13.2.1 Plant Metal Transporters
One of the promising approaches to enhance the ability of metal ions to enter plant cells is the identification of the metal transporter proteins and introducing genes encoding transporter molecules. These are generally proteins found in the cell membrane, that either have an affinity for metal ions, or that create favorable energetic conditions to allow metals to enter the cell. Till date, several plant metal transporters have been reported and more remain to be recognized. Some of the transporters identified so far include the Arabidopsis IRT1 gene that encodes a protein that regulates the uptake of iron and other metals (Eide et al. 1996) and the MRP1 gene encoding, an Mg-ATPase transporter, also from Arabidopsis (Lu et al. 1997).
Further, success in this approach is achieved by identifying proteins such as ZNT1, ZIP1-4, IRT1, COPT1, LCT1, and tVramp-1/3/4 on the plasma membrane-cytosol interface; ZAT, ABC type, HMT1, AtMRP, CAX2 seen in vacuoles; and RAN1 in Golgi bodies. Manipulations of these transporters to achieve the removal of metal ions from the cell hold great potential (Tong et al. 2004). The natural resistance-associated macrophage proteins (Nramp) family of transporters has been recently characterized from rice and Arabidopsis.
13.2.2 Phytochelatins for Metal Sequestering
The principal classes of metal chelators include phytochelatins, metallothioneins, organic acids, and amino acids. Phytochelatins (PCs) are small metal-binding peptides found in plants. Iso-PCs, a series of PC-like homologous chelating peptides, are reported with varying terminal amino acids and have a C-terminal modified residue other than glycine. The PC and iso-PC molecules form complexes with heavy metals like Cd. In addition to PC-Cd complex other PC-metal complexes include Ag, Cu, and As (Shah and Nongkynrih 2007).
In vitro experiments have shown that a series of metal-sensitive plant enzymes can tolerate a 10- to 1,000-fold concentration of Cd in the form of a PC complex than as free radical ion. PC reactivate metal poisoned plant enzymes such as nitrate reductase up to 1,000-fold better than chelators such as glutathione (GSH) or citrate, signifying the extraordinary sequestering potential of these peptides.
13.2.3 Metallothioneins
Metallothioneins (MTs) are metal-binding proteins that confer heavy metal tolerance and accumulation (Hamer 1986; Malin and Bülow 2001). These are low molecular mass cysteine rich proteins that were originally isolated as Cu, Cd, and Zn binding proteins in mammals. MTs are able to bind a variety of metals by the formation of mercaptide bonds between the numerous cysteine (Cys) residues present in the proteins and the metal, and it is the arrangement of these residues that in part determines the metal-binding properties of the MT proteins. Researchers successfully reported more than 50 MTs in different plants categorized into four types, types 1–4. These are based on the Cys arrangement. Although MTs are expressed throughout the plant, some have been found to be expressed in a tissue-specific manner. Transgenic plants expressing MTs have been created, and although these plants exhibited enhanced tolerance to high metal concentrations, the uptake of metals was not enhanced. To enhance higher plant metal sequestration, the yeast MT CUP1 was introduced into tobacco plants, and the cup 1 gene expression and Cu and Cd phytoextraction were determined. Overexpression of Cu inducible MT cup 1 also enhanced Cu tolerance in plants. In plants, a wide range of MT genes from various sources have been overexpressed including those from human, mouse, Chinese hamster, and yeast.
The vacuole is generally considered to be the main storage site for metals in yeast and plant cells and there is evidence that phytochelatin-metal complexes are pumped into the vacuole. The best characterized of the known vacuolar transporters and channels involved in metal tolerance are YCF1 from yeast, Saccharomyces cerevisiae. YCF1 is an MgATP-energized glutathione S-conjugate transporter responsible for vacuolar sequestration of compounds after their S-conjugation with glutathione. Overexpression of the YCF1 gene in Arbidobsis thaliana and the YCF1 proteins were found to be associated with the tonoplast and the plasma membrane. The vacuoles of the YCF1-transgenic plants exhibited a 4-fold higher rate of glutathione-Cd uptake than those of wild-type plants, indicating that expression of YCF1 strongly increases Cd transport activity. The transgenic plants showed improved resistance to both Cd and Pb and elevated metal content, characteristics desirable for phytoremediation.
13.2.4 Genes to Change the Oxidation State of Heavy Metals
Genes may be introduced that code for enzymes to change the oxidation state of heavy metals like Hg and selenium (Se). For instance, introduction of the bacterial merA gene encoding mercuric oxide reductase (Rugh et al. 1996), or that which converts metals into less toxic forms, such as enzymes that can methylate Se into dimethylselenate (Hansen et al. 1998). In both these cases, the resulting form of the metal is volatile, so that one can create a plant capable of metal remediation by phytovolatilization, another type of phytoremediation.
13.3 Genetically Modified Plants for Metal Uptake, Tolerance, and Detoxification
Several researchers have already reported encouraging results using plants bioengineered with increased heavy metal tolerance and uptake of heavy metals for the purpose of phytoremediation. The majority of these novel plants have only been tested under limited laboratory conditions and very few have been grown in the field (Table 13.1).
13.3.1 Arabidopsis
Arsenic is an extremely toxic metalloid pollutant and the decontamination of polluted sites can be environmentally destructive. It is a lethal poison that is released into the environment from natural processes and via arsenic-based chemicals. Genetically engineered Arabidopsis plants can sequester As from the soil. These plants can transport As aboveground, reduce it to arsenite, and sequester it in thiol-peptide complexes (Dhankher et al. 2002). By coexpressing two bacterial genes, arsenate reductase (ArsC) and γ-glutamylcysteine synthetase (γ-ECS) in Arabidopsis plants, it was observed that plants expressing SRS1p/ArsC and ACT2p/γ-ECS together showed substantially greater As tolerance than wild-type plants or plants expressing γ-ECS alone. In addition, when grown on As, these plants accumulated 4- to 17-fold greater fresh shoot weight and accumulated 2- to 3-fold more As per gram of tissue than wild-type plants or plants expressing γ-ECS or ArsC alone.
Extensive progress has also been achieved in identifying genes and proteins involved in uptake of iron (Fe) by yeast and plants. As described earlier, the utility of the yeast protein YCF1, a protein which detoxifies Cd by transporting it into vacuoles, has been implemented for the remediation of Cd and Pb. Transgenic A. thaliana plants overexpressing YCF1 showed an enhanced tolerance and accumulated greater amounts of Cd and Pb.
The close relationship between Arabidopsis halleri, a metal tolerant and hyperaccumulating relative of the biological model species A. thaliana, has recently allowed the use of A. thaliana GeneChips to compare gene expression levels between A. halleri and the nontolerant A. thaliana and, consequently, permitted the identification of genes potentially involved in metal tolerance and/or hyperaccumulation. The complete annotation of the A. thaliana genome sequence provides a solid foundation for comparative mapping studies within the Brassicaceae family. The genome organization of A. thaliana has already been compared with those of several species like Arabidopsis lyrata, Arabidopsis petraea and Capsella rubella. Moreover, A. halleri is a species that has undergone natural selection for Zn tolerance. Isolation of the quantitative trait loci (QTL) associated with this trait holds great promise for the identification of the main genes responsible for this adaptation.
13.3.2 Brassica
Brassica juncea was genetically engineered to investigate rate-limiting factors for glutathione and phytochelatin production. To achieve this Escherichia coli gshII gene was introduced. Cd-treated GS plants had higher concentrations of glutathione, phytochelatin, thiol, sulphur, and calcium than wild-type plants (Zhu et al. 1999). A study showed that γ-glutamylcysteine synthetase inhibitor, L-buthionine-[S,R]-sulphoximine (BSO), dramatically increased As sensitivity both in nonadapted and As-hypertolerant plants, showing that phytochelatin-based sequestration is essential for both normal constitutive tolerance and adaptative hypertolerance to this metalloid (Schat et al. 2002).
Astragalus bisulcatus is a native plant that has the capacity to grow on Se containing soils and accumulate Se to high concentrations but it has a slow growth rate. It has been proposed that in A. bisulcatus selenocysteine methyltransferase (SMT) converts the amino acid SeCys into the nonprotein amino acid (MetSeCys). By incorporating gene for SMT from the Se hyperaccumulator A. bisulcatus, it diverted the flow of Se from the Se amino acids that may otherwise be incorporated into protein, leading to alterations in enzyme structure, function, and toxicity. Transgenic plants overexpressing SMT show enhanced tolerance to Se, particularly selenite, and produced 3- to 7-fold more biomass than wild type and 3-fold longer root lengths (LeDuc et al. 2004). Indian mustard plants overexpressing the A. bisulcatus smt gene, exhibited a greatly increased accumulation of MetSeCys and tolerance to Se compounds, in particular selenite.
13.3.3 Tobacco
Scientists at the University of Cambridge expressed the bacterial gene encoding pentaerythritol tetranitrate reductase in transgenic tobacco, conferring the ability to survive on growth media containing otherwise toxic levels of the nitrate ester class of explosives. Further analysis also demonstrated an enhanced degradation of these compounds by transgenic tobacco plants relative to untransformed seedlings.
13.3.4 Tomato
When bacterial gene 1-aminocyclopropane-1- carboxylic acid (ACC) deaminase was expressed in tomato plants, it showed enhanced metal accumulation and tolerance levels for a range of heavy metals (Cd, Cu, Ni, Mg, Pb, and Zn) than untransformed plants (Grichko et al. 2000).
13.3.5 Pteris vittata
Pteris vittata can effectively remove As metalloid from soil. For example, in soil contaminated with As at a concentration of 97 ppm, the older fronds of the fern had As concentrations of up to 3894 μg per gram of tissue. Less than 168 μg As per gram was found in the root tissue. More than 95 % of the As removed from the soil by the fern was translocated to the aboveground biomass (Doty 2008). Unfortunately, this plant species grows well only in warm, humid environments.
13.3.6 Poplar and Willow
Although the research on several hyperaccumulating species is promising, the species themselves are too small and slow-growing for some phytoremediation applications. For this reason, high-biomass crops such as poplar and willow are being studied for phytoremediation of metals. Poplar and willow are not hyperaccumulators as they do not concentrate metals to high concentrations, but because of their greater biomass and deep root systems, they are also effective remediators of metal contamination. Poplar plant transformation was conducted using Agrobacterium-mediated transfer of genes. Transgenic poplar plantlets which express genes of interest were then multiplied by cuttings. After multiple-year field trials, it can be confirmed whether transgenic poplar plants are indeed improved in the capacity to remediate the polluted environment, thus suitable for actual phytoremediation. There is a possibility that plants with single gene insertion may not be sufficiently improved in the capacity to remediate the actual field. To make the process more effective, multiple genes with different and synergistic mechanisms of phytoremediation are expressed at the same time. To develop more and better plants for phytoremediation, the search for new genes with different functions and stacking them (introducing many genes into the same line of plants) will hopefully further improve the plants’ capacity to clean up the environment.
Willow was specifically suggested for phytoremediation of heavy-metal contaminated lands because of its unique ability to re-grow readily after its shoots have been harvested, a distinctive trait of willow. Further there is substantial genetic diversity of willow, with over 450 Salix species. Willow plants (Salix matsudana × Salix alba NZ1040) grown in soil contaminated with Cd at concentrations commonly found in agricultural sites fertilized with Cd-containing fertilizers accumulated Cd in the aboveground tissues with BCFs of about 10 (Doty 2008).
13.4 Challenges
To improve the process of phytoremediation using genetic modifications success has been achieved, and these processes have great potential for field applications, the only constraint being public acceptance of genetically modified organisms.
The key concerns in remediating contaminated sites by transgenic plants include:
-
1.
Characterization of vector system, its stability, and expression in the plant cell.
-
2.
Avoidance of unwanted properties encoded by introduced DNA like toxic, pathogenic, or deleterious functions.
-
3.
Potential risks to birds and insects that might feed on these plants.
-
4.
Proper disposal of plant biomass containing high concentrations of hazardous substances, particularly metals.
-
5.
Competitiveness of transgenic to wild type.
-
6.
The ability to outcross with related species (particularly wild relatives).
13.4.1 Complexity of Phytoremediation in the Field
Uneven distribution of contaminants is the frequently encountered challenge in field studies, including ‘‘hot spots’’ across a site. In laboratory and greenhouse experiments, soils generally have uniform matrix. This may not be possible in the field even if the site is extensively tilled prior to planting; this spatial heterogeneity of initial contaminant levels results in data scatter, which can make it difficult to statistically show significant treatment effects for field trials. This is also problematic in terms of regulatory standards because remediation success is often judged on a point-by-point basis rather than an average of data points from across the site. Each and every point should meet the regulatory targets; otherwise, the whole site fails.
Other factors such as soil pH, moisture content, root structure, soil structure, organic composition of the soil, and microbial activity often exhibit spatial variability at a given site and can change over time. One way to mitigate the problem of spatial variability is to increase the number of samples analyzed per treatment plot. However, extensive sampling can lead to highly variable data that is difficult to interpret.
Agronomic techniques are often employed prior to planting. Tilling and addition of nutrients and organic matter are generally designed to improve soil texture and composition to facilitate plant and/or microbial growth. They also cause changes in soil pH and oxygen content, which can affect the bioavailability, hence degradation of contaminants even in bulk (nonrhizosphere) soil. Nutrients in the bulk soil, such as nitrogen and phosphorus, will positively affect the indigenous microbial populations, including contaminant degraders. Aeration of field soil increases its oxygen concentration, which promotes oxidative degradation of numerous organic contaminants. This may also lead to photo-oxidation of previously buried organics. Hence, there is often a decrease in contaminant concentrations in bulk soil (generally used as the negative control) as well as in treatment plots. As a result it is difficult to statistically show that phytoremediation is superior to natural attenuation, particularly in the initial stages of a field trial. Less remediation is thought to occur in the rhizosphere (2 mm zone extending from the roots), and much less in bulk soil. Determining the boundary between rhizosphere and bulk soil is almost impossible. Furthermore, it is impractical to definitively separate fine roots, soil directly in contact with the roots, and rhizosphere soil into different samples for analyses. This can present an analytical challenge for researchers endeavoring to show PHC and PCB remediation where rhizoremediation is thought to occur at the root–soil boundary (rhizoplane). There is evidence that some plants can provide the impetus for movement of hydrophobic organics such as PAHs from bulk soil into the rhizosphere (Liste and Alexander 2000). Mobilization of PAHs may be accelerated by the release of organic acids from roots, which putatively increases PAH solubility and bioavailability.
13.4.2 Physical Restrictions and Stress Factors
Despite increasing interest in situ remediation of contaminants, the number of reports pertaining to phytoremediation appears to be declining, suggesting a decrease in the activity for this area of research. Numerous unsuccessful and inconclusive field trial reports provide some insight into the challenges that researchers have encountered in this field (Gerhardt et al. 2009). The slower rate of remediation compared to other strategies, such as excavation and ex situ treatment, is a frequently cited disadvantage of phytoremediation. This may be probably because plant growth is hard to achieve in heavily impacted soils, thereby limiting catabolism of contaminants. Factors that affect phytoremediation in the field are not encountered in the laboratory or greenhouse. These include stressors like plant pathogens; herbivore (insects and/or animals); variations in temperature, nutrients and precipitation; and competition from weed species that are better adapted to the site. Any of these abiotic or biotic stressors can slow down or prevent plant growth in the field. For example, attempted phytoremediation of PHC and other organics at a hydrocarbon burn facility at NASA Kennedy space center failed due to drought and competition from weeds (http://www.cluin.org).
There are other physical challenges that limit the use of phytoremediation. For instance, increasing the moisture content of hydrophobic PHC-contaminated soils can be problematic once they have become dry, which can prevent germination and seedling growth. Another problem is faced when the contaminated soil is deeper than the rooting zone, e.g., average root depth of herbaceous plants is 50 cm. In that case, there is a requirement of excavation prior to phytoremediation. Trees have deeper roots (average 3 m, with much longer roots in some species) which can facilitate remediation at greater depths without excavation. Dendroremediation (phytoremediation using trees) of explosives, e.g., TNT and TCE from soil and groundwater has shown great promise (Van Aken et al. 2004). However, here the challenge is that it can be difficult to grow and establish trees in contaminated soils. The process is quite long and they take several years to attain sufficient biomass for efficient rates of phytoremediation. Further, in case of trees there may be a disposal problem if the roots and wood are deemed to be contaminated after completion of remediation.
13.4.3 In-field Performance of Genetically Modified Organisms
Genetically engineered microbial strains often fail to compete with native microbes in the rhizosphere, and their numbers diminish to levels that merely support remediation.
Degradation of organic contaminants generally requires the concerted action of numerous enzymes and it is generally impractical to introduce all the genes required for degradation of an organic contaminant into a single plant or microbial genome. It can be difficult to stably maintain even a single gene in transformed or recombinant organisms and the desired trait, such as enhanced degradative capacity, is often lost. For example in plants, silencing of transgenes, via mechanisms such as cytosine methylation, makes the use of this technology inherently unpredictable.
Though genetically modified organisms in the laboratory and greenhouse for phytoremediation has made considerable progress, regulatory restrictions for in situ applications have prevented any substantial accumulation of field data. There is the potential for inserted genetic material to be transferred to indigenous populations if genetically modified organisms are used without adequate containment systems. Further, recombinant strains that contain antibiotic resistance genes from the cloning procedures cannot be released in the environment. Besides, there has also been low public acceptance of genetic engineering.
13.4.4 Regulatory Acceptability
The issue of regulatory acceptability for phytoremediation of hazardous waste is to ensure public safety and to protect the environment. The Government regulators must be convinced that a given remedial strategy will diminish contaminant toxicity, mobility, and/or concentration before it is approved for use in the field. Bioaccumulation of hazardous compounds in plants is one consideration. In situations where plants do not catabolize the contaminants, provisions may be required for removal of contaminated plant materials as part of a remedial plan. Another consideration is the potential ecological risks of introducing non-native plant and microbial species into field sites. These species can move from the contaminated site by displacing, or hybridizing with native species.
Phytoremediation of organic compounds often takes place in the rhizosphere of fine roots that have a high turnover rate. These roots have a very short life and the decaying root tissue is converted into bulk soil during humification. Contributions of organic matter from the degraded root tissue can complicate sample analyses due to the structural similarities to compounds being remediated. Most of the regulatory agencies for example, United States Environmental Protection Agency (EPA) and Canadian Council of Ministers of the Environment (CCME), do not distinguish between petrogenic and phytogenic carbon compounds. This means that in the case of PHC remediation naturally occurring organic matter that is co-extracted with organic contaminants can easily lead to exaggerated PHC levels in soil samples.
13.5 Overcoming the Challenges
13.5.1 Chloroplast Engineering
Transformation of chloroplast prevents the escape of transgenes via pollen to related weeds and crops. This method was used to stably integrate the bacterial merAB operon into the chloroplast genome of tobacco. The resulting plants were substantially more resistant to highly toxic organic mercury (Bizily et al. 2000), in the form of phenyl mercuric acetate, than wild type. Other important advantages of chloroplast transformation include the fact that codon optimization is not required to improve expression of bacterial transgenes.
13.5.2 Minimizing Ecological Risk
To minimize the risks of introducing non-native biota (including genetically modified species) into an ecosystem, the best strategy is to use native species for phytoremediation. It can be applied to microbial species that are used to facilitate plant growth and/or degrade contaminants (Davison 2005). Using native plant species can serve the dual purpose of remediation as well as native habitat reconstruction/reclamation (for microbe-assisted phytoremediation), which may be required after successful remediation.
To minimize ecological risks from non-native (transgenic and nontransgenic) phytoremediation species, a biological containment system is often employed. For example, genes can be introduced to prevent propagation, or to render a species overly sensitive to abiotic stressors such as temperature changes or chemicals. Multiple transgenes are employed to prevent gene flow between the introduced species and other species in the environment. This containment system can be reinforced by adding mitigator genes linked to the primary transgene. Mitigators genes confer non-deleterious traits to the phytoremediation species, but are harmful to relate species in case of gene transfer. Alternatively, they can prevent the phytoremediation species from successfully competing outside the contaminated site.
13.5.3 Overcoming the Challenge of Plant Stress
Naturally occurring endophytic bacteria have been found to degrade organic contaminants such as nitro-substituted explosives. In contrast to root-colonizing microbes, endophytes colonize internal plant tissues, including root, leaf, and vascular tissues. Inoculating plants with endophytes can thwart competitive pressures in the rhizosphere as well as some of the challenges that limit effectiveness of root colonizers, including dependence on specific soil pH, temperature, and soil moisture content for optimum growth. Field trials will be required to assess the effectiveness of the use of endophytes.
13.5.4 Monitoring, Sampling, and Analyzing Experimental Data from the Field
To demonstrate adequate performance of remediation systems in the field, government agencies are focusing on issues that address the inconsistencies in experimental protocols, particularly regarding which analytical parameters need to be measured. Remediation Technologies Development Forum, a group with government, industry, and academic partners has developed the protocol of phytoremediation of PHCs in soil in the 1990s. This forum intends to develop protocols that would allow comparative tests of remedial strategies at numerous and varied geographical locations. These protocols recommend standards for plot size; number of replications; choice of plant species; plant and soil sampling procedures; microbial and hydrocarbon analyses; statistical treatment of data; time-points and end-point (3 years). A three-year end-point is considered more realistic than remediation in a single growing season. Establishing a longer time frame for field trials is always advantageous for researchers because ambiguous results are often observed in short-term field studies as a result of tilling and amending the soil at the onset of a field trial. The three-year frame duration also allows for assessment of the phytoremediation system and improvement of methods between field seasons. If any problems in the field are encountered, laboratory and greenhouse experiments can be conducted to resolve the issues prior to the onset of the next field season.
One way to facilitate statistical analysis of field data is to use particularly recalcitrant compounds in the soil samples to normalize the data. These are called internal markers (also called “conservative biomarkers”). They are not generally degraded to any appreciable degree during phytoremediation. Concentrations of individual contaminants can be directly compared (normalized) to the internal marker. The relative ratio of a specific compound to the internal marker decreases as remediation occurs. For example, Hopanes, compounds found in crude oil, can be used as biomarkers for PHC remediation, including PAHs.
The methods developed to distinguish biologically derived organic compounds from organic contaminants can be used for chemical fingerprinting to assess liability after accidental release of chemicals into the environment. These methods can also be used to distinguish plant-derived hydrocarbons from petrogenic hydrocarbons for assessing phytoremediation. Although these methods are not widely employed in the phytoremediation field, in case of PHC phytoremediation it becomes necessary to distinguish contamination from naturally occurring and anthropogenic hydrocarbons (i.e., background hydrocarbons). A number of hydrocarbon indices have been derived to assess the source of environmental hydrocarbons, including the carbon preference index, average chain length, and various n-alkane/acyclic isoprenoid ratios.
13.6 Future Outlook
The phytoremediation technology is still in its early development stages and full scale applications are still limited. The study and use of genetic modifications must be performed to determine the true costs and benefits of this technology to the ecosystem as a whole. Progress in the field of molecular genetics will allow the analysis of metal hyperaccumulator plants and should provide new insights into metabolic detoxification processes and identify tolerant genes, thus providing more information about the genomes of these model organisms used for this technology.
The genomics can accelerate the discovery of genes that confer key traits, allowing their modification. In addition, metabolomics can provide biochemical and physiological knowledge about network organization in plants subject to toxic metal stress, providing a much more detailed understanding of the molecular basis of hyperaccumulation.
Identification of many signaling pathways and proteins within the stress response network can contribute to the cellular stress response. The development of DNA and RNA microarray chip technologies in systematic genome mapping, sequencing, functioning, and experimentation may allow the identification and genotyping of mutations and polymorphisms. This will provide a better insight into structure–function interaction of genome complexity under toxic metal stress. For example, Mitogen-Activated Protein Kinase (MAPK) pathways are activated in response to metal stress, which encourages new strategies for improving plant tolerance to heavy metals and phytoremediation.
Molecular genetics approaches such as insertion mutagenesis can be used to identify genes involved in hyperaccumulation. Similarly, transposon tagged plants can be screened to identify mutants impaired in the ability to accumulate metals. Recently, considerable progress has been made in identifying plant genes encoding metal ion transporters with important functions in cation transport and homeostasis.
Modern molecular techniques, bioinformatics, and computational techniques are effective tools for detailed structure–function genome analysis. Approaches allowing recombination hotspots to be highlighted will further aid plant breeding efforts. It is clear that both fundamental and applied research must be carried out in association, since the lack of the basic understanding will make it difficult to exploit many of the recent advances in plant molecular biology.
13.7 Conclusion
It is evident that phytoremediation has benefits to restore balance to a stressed environment, but it is important to proceed with caution before it is applied on a larger scale. The results already obtained have indicated that the plants are effective and could be used in toxic metal/organic contaminants remediation. Although it appears to be a widely accepted technology amongst scientists, engineers, and regulators, it is important that public awareness about it is considered. Clear and precise information should be made available to the general public to enhance its acceptability as a global sustainable technology.
References
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton, pp 85–107
Bizily SP, Ruch CL, Meagher RB (2000) Phytodetoxification of hazardous organo-mercurials by genetically engineered plants. Nat Biotechnol 18:213–217
Chaney RL, Malik M, Li YM, Brown S, Brewer EP, Angel JS, Baker AJ (1997) Phytoremediation of soil material. Curr Opin Biotechnol 8:279–284
Davison J (2005) Risk mitigation of genetically modified bacteria and plants designed for bioremediation. J Ind Microbiol Biotechnol 32:639–650
Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γglutamylcysteine synthetase expression. J Nat Biotechnol 20:1140–1145
Doty SL, Shang QT, Wilson AM, Moore AL, Newman LA, Strand SE, Gordon MP (2000) Enhanced metabolism of halogenated hydrocarbons in transgenic plants contain mammalian P450 2E1. Proc Natal Acad Sci USA 97:6287–6291
Doty SL, James CA, Moore AL, Vajzovic A, Singleton GL, Ma C, Khan Z, Xin G, Kang JW, Park JY, Meilan R, Strauss SH, Wilkerson J, Farin F, Strand SE (2007) Enhanced phytoremediation of volatile environmental pollutants with transgenic trees. Proc Natl Acad Sci USA 104(43):16816–16821
Doty SL (2008) Tansley review: enhancing phytoremediation through the use of transgenics and endophytes. New Phytol 179:318–333
Dowling DN, Doty SL (2009) Improving phytoremediation through biotechnology. Curr Opin Biotechnol 20:1–3
Eide D, Broderius M, Fett JM, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci 93(11):5624–5628
Flocco CG, Lindblom SD, Smits EAHP (2004) Overexpression of enzymes involved in glutathione synthesis enhances tolerance to organic pollutants in Brassica juncea. Int J Phytoremediat 6:289–304
French CJ, Rosser SJ, Davies GJ, Nicklin S, Bruce NC (1999) Biodegradation of explosives by transgenic plants expressing pentaerythritol tetranitrate reductase. Nat Biotechnol 17:491–494
Fulekar MH, Singh A, Bhaduri AM (2009) Genetic engineering strategies for enhancing phytoremediation of heavy metals. Afr J Biotechnol 8(4):529–535
Gandia-Herrero F, Lorenz A, Larson T, Graham IA, Bowles J, Rylott EL et al (2008) Detoxification of the explosive 2,4,6- trinitrotoluene in Arabidopsis: discovery of bifunctional O and C-glucosyltransferases. Plant J 56:963–974. doi:10.1111/j.1365-313X.2008.03653.x
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176:20–30
Grichko VP, Filby B, Glick BR (2000) Increased ability of transgenic plants expressing the enzyme ACC deaminase to accumulate Cd, Co., Cu, Ni, Pb and Zn. J Biotechnol 81:45–53
Hamer DH (1986) Metallothioneins. Ann Rev Biochem 55:913–951
Hansen D, Duda P, Zayed AM, Terry N (1998) Selenium removal by constructed wetlands: role of biological volatilization. Environ Sci Technol 32:591–597
Hirose S, Kawahigashi H, Ozawa K, Shiota N, Inui H, Ohkawa H et al (2005) Transgenic rice containing human CYP2B6 detoxifies various classes of herbicides. J Agric Food Chem 53:3461–3467
Kawahigashi H, Hirose S, Inui H, Ohkawa H, Ohkawa Y (2003) Transgenic rice plants expressing human CYP1A1 exudes herbicide metabolites from their roots. Plant Sci 165:373–381
Kawahigashi H, Hirose S, Ohkawa H, Ohkawa Y (2006) Phytoremediation of herbicide atrazine and metolachlor by transgenic rice plants expressing human CYP1A1, CYP2B6 and CYP2C19. J Agric Food Chem 54:2985–2991
LeDuc DL, Tarun AS, Montes-Bayon M, Meija J et al (2004) Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol 135:377–383
Liste HH, Alexander M (2000) Accumulation of phenanthrene and pyrene in rhizosphere soil. Chemosphere 40:11–14
Lu YP, Li ZS, Rea PA (1997) AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc Natl Acad Sci USA 94:8243–8248
Malin M, Bülow L (2001) Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19:67–72
Monti MR, Smania AM, Fabro G, Alvarez ME, Argarana CE (2005) Engineering Pseudomonas fluorescens for biodegradation of 2,4-dinitrotoluene. Appl Environ Microbiol 71:8864–8872
Oller ALW, Agostini E, Talano MA, Capozucca C, Milrad SR, Tigier HA et al (2005) Overexpression of a basic peroxidase in transgenic tomato (Lycopersicon esculentum Mill. cv. Pera) hairy roots increases phytoremediation of phenol. Plant Sci 169:1102–1111
Phytoremediation at the hydrocarbon burn facility at NASA Kennedy Space Center in Florida http://www.cluin.org/products/phyto/search/phyto_details. cfm? ProjectID= 25 (July 22, 2008)
Rugh CL, Wilde D, Stack NM, Thompson DM, Summers AO, Meagher RB (1996) Mercuric ion reduction and resistancein transgenic Arabidopsis thaliana plants expressing a modified bacterial merA gene. Proc Natl Acad Sci USA 93:3182–3187
Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker PM (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot 53:2381–2392
Shah K, Nongkynrih J (2007) Metal hyperaccumulation and bioremediation. Biol Plant 51(4):618–634
Tong YP, Kneer R, Zhu YG (2004) Vacuolar compartmentalization: a second-generation approach to engineering plants for phytoremediation. Trends Plant Sci 9:7–9
Van Aken B, Yoon JM, Schnoor JL (2004) Biodegradation of nitro-substituted explosives 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, and octahydro-1,3,5,7-tetranitro-1,3,5-tetrazocine by a phytosymbiotic Methylobacterium sp. associated with poplar tissues (Populus deltoides × nigra DN34). Appl Environ Microbiol 70:508–517
Villacieros M, Whelan C, Mackova M, Molgaard J, Sánchez-Contreras M, Lloret J, Aguirre de Cárcer D, Oruezábal RI, Bolaños L, Macek T, Karlson U, Dowling DN, Martín M, Rivilla R (2005) Polychlorinated biphenyl rhizoremediation by Pseudomonas fluorescens F113 derivatives, using a Sinorhizobium meliloti nod system to drive bph gene expression. Appl Environ Microbiol 71:2687–2694
Wang GD, Li QJ, Luo B, Chen XY (2004) Ex planta phytoremediation of trichlorophenol andphenolic allelochemicals via engineered secretory laccase. Nat Biotechnol 22:893–897
Yamada T, Ishige T, Shiota N, Inui H, Ohkawa H, Ohkawa Y (2002) Enhancement of metabolizing herbicides in young tubers of transgenic potato plants with the rat CYP1A1 gene. Theor Appl Genet 105:515–520
Yee DC, Maynard JA, Wood TK (1998) Rhizoremediation of trichloroethylene by a recombinant, root-colonizing Pseudomonas fluorescens strain expressing toluene ortho-monooxygenase constitutively. Appl Environ Microbiol 64:112–118
Zhu YL, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing glutamylcysteine synthetase. Plant Physiol 121:1169–1177
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Baunthiyal, M. (2014). Engineering Plants for Phytoremediation. In: Ravi, I., Baunthiyal, M., Saxena, J. (eds) Advances in Biotechnology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1554-7_13
Download citation
DOI: https://doi.org/10.1007/978-81-322-1554-7_13
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1553-0
Online ISBN: 978-81-322-1554-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)