Abstract
The human endothelium forms a permeable barrier between the blood stream and surrounding tissues, strictly governing the passage of immune cells, fluids and metabolites. The regulation of cell–cell contact dynamics between endothelial cells is essential for this function and thus for the maintenance of vascular integrity. Intercellular adhesion within the endothelium is mainly dependent on adherens junctions, composed of cell–cell adhesion proteins such as VE-cadherin and nectin, and their associated proteins. Recent research points to a critical role of the actin cytoskeleton in endothelial integrity, by providing anchorage of adhesion complexes to the cell cortex. We could show that the F-actin-binding protein drebrin is a critical regulator of endothelial integrity, by linking nectin to the cortical actin cytoskeleton. In particular, the knockdown of drebrin leads to functional impairment of endothelial cells, characterized by rupturing of endothelial monolayers cultured under conditions mimicking vascular flow. This weakening of cell–cell contacts upon drebrin depletion is based on the destabilization of nectin at adherens junctions, followed by internalization and degradation in lysosomes. Conducting interaction studies, we showed that drebrin binds to nectin’s interaction partner afadin, thus linking the nectin/afadin system to the cortical F-actin network. Drebrin, containing binding sites for both afadin and F-actin, is thus uniquely equipped to stabilize nectin at adherens junctions, thereby preserving endothelial integrity. Collectively, these results contribute to the current understanding of cell–cell junction regulation, introducing a new function of drebrin as a stabilizer of endothelial integrity.
Parts of this book chapter have been published, in modified form, in the doctoral thesis of Kerstin Rehm “Drebrin preserves endothelial integrity by stabilizing nectin at adherens junctions,” 2013.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Drebrin in Monolayer-Forming Cells
Cell monolayers fulfill critical roles in multicellular organisms (Harris et al. 2012). Monolayers act by separating different tissues or compartments from each other, forming semipermeable barriers that can withstand mechanical forces. This is especially important in the case of epithelial and endothelial cells. The endothelium , in particular, serves as a semi-selective barrier between the bloodstream and the surrounding tissue, controlling the passage of leukocytes and solutes. Depending on the location of the endothelium in the vascular tree, the respective degree of permeability is quite variable. In this respect, specific and regulated anchorage between individual cells within monolayers is of major importance. Accordingly, dysregulation of cell–cell junctions compromises endothelial integrity and can thus lead to pathological scenarios such as thrombogenesis (Vestweber 2007; Muller 2003). In recent years, it has become increasingly clear that the various systems of intercellular cell–cell junctions (see Sect. 21.1.1) depend on the filamentous (F)-actin cytoskeleton for their stabilization. The regulation of F-actin dynamics is thus of major importance for the coherence and adaptability of the endothelium (Lampugnani 2010). This implies critical roles for F-actin-associated proteins in the maintenance of vascular integrity.
In this context, Peitsch et al. described drebrin E in a variety of cell types and tissues, also including epithelial and endothelial cells (Peitsch et al. 1999). Interestingly, drebrin levels are strongly increased in epidermal skin tumors, where it localizes to junctional areas. Of note, even at moderate levels of expression, such as in cultured keratinocytes, drebrin is enriched at adherens junctions (Peitsch et al. 2005). In line with these findings, confluent endothelial monolayers show a pronounced expression of drebrin E, with the majority being enriched at the cell cortex, where it associates with F-actin filaments, and preferentially near adherens junctions (Peitsch et al. 1999).
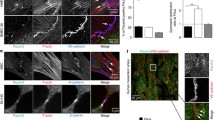
In our study, we were able to confirm the localization of drebrin to endothelial cell–cell junctions in human umbilical vein endothelial cells (HUVEC ) and focused in the following on the as yet unclear function of drebrin in endothelial junction regulation (Rehm 2013). In particular, we studied the dynamic behavior of endothelial monolayers that were cultivated under constant flow conditions of 15 dyne/cm2, thus mimicking flow conditions in medium-sized vessels (Paz et al. 2012). As expected, both control and drebrin-depleted cells aligned themselves along the direction of flow. However, in contrast to control monolayers that retained their integrity, monolayers of drebrin-depleted cells showed ruptures after 3 days, pointing to a role of drebrin in the maintenance of endothelial integrity especially under shear stress, probably through regulation of cell–cell junctions (Rehm et al. 2013) (Fig. 21.1).
Monolayers of HUVEC depleted for drebrin show ruptures when cultured under constant flow. Images of HUVEC monolayers, treated with drebrin-specific (a, c) or control siRNA (b, d) and seeded in Microslides. Cells were submitted to constant fluid shear stress for 1–3 days. The enlarged images (ai–di) show boxed regions of (a–d). Arrows indicate the direction of flow. Note the rupture of drebrin knockdown monolayers at day 3. Bars, 10 μm in (ai–di), 100 μm in (a–d) (Rehm et al. 2013)
1.1 Different Sets of Cell–Cell Junctions Are Involved in Maintaining Monolayer Integrity
Within monolayers, cells are connected by a number of different junctional systems, which regulate endothelial barrier function, tissue integrity, as well as cell–cell communication (Wallez and Huber 2008; Lampugnani and Dejana 1997). These junctional systems, including tight junctions, adherens junctions, gap junctions, and desmosomes, have been studied especially in epithelial cells. In these cells, the different junctions follow a well-defined distribution along the intercellular cleft, with tight junctions being the most apical component, followed by adherens junctions and desmosomes. However, in endothelial cells, the junction types are less well organized and do not exhibit such a clear progression along the contact zone (Engelhardt and Wolburg 2004). Moreover, desmosomes are missing in endothelial cells, and instead, typical endothelial proteins such as platelet endothelial cell adhesion molecule-1 (PECAM-1) and intercellular adhesion molecule-2 (ICAM-2 ) are found, which contribute to cell–cell adhesion and also to angiogenesis or leukocyte extravasation (Muller et al. 1993; DeLisser et al. 1994; Lyck et al. 2003). Still, junctions of the epithelium and endothelium are highly homologous, which often allows a careful transfer of observations made in epithelial cell models also to endothelial cells.
1.1.1 Tight Junctions
In epithelial cells, tight junctions are found at the most apical position (Dejana et al. 2009). Their main function is the formation of an impermeable barrier for soluble molecules. In endothelial cells, the necessity for permeability control is not as absolute and can vary, depending on the location within the vascular tree (Bazzoni and Dejana 2004). The most prominent components of tight junctions are occludins, claudins, and junctional adhesion molecules (JAMs), with the latter also occurring in cells that do not form junctions (Bazzoni 2003). Occludins have four membrane-spanning regions, with their N- and C-termini both being intracellular. The 15 claudins identified so far show a similar architecture, with claudin-5 being the endothelial-specific member (Morita et al. 1999). Occludin and claudin can bind to different zonula occludentes (ZO) proteins (Tsukita et al. 1999; Tsukita and Furuse 1999), which primarily serve as linkers to the F-actin cytoskeleton and to other proteins (Stevenson et al. 1986).
1.1.2 Gap Junctions
In addition to the junctional complexes that serve as anchoring structures, gap junctions mediate communication between neighboring cells (Simon and Goodenough 1998). They constitute clusters of few to hundreds of intercellular channels, which are permeable for ions and metabolites, excluding molecules that exceed 1 kDa in size (Alexander and Goldberg 2003). Each channel is formed by transmembrane proteins that belong to the connexin (Cx) family, which consists of 20 members in humans, with Cx43, Cx40, and Cx37 being expressed in the endothelium. Connexins assemble into hexameric clusters, forming a hemichannel (connexon) in the plasma membrane, which aligns with another connexon of an adjacent cell, thus forming a pore that connects their cytoplasms (Bazzoni and Dejana 2004). This way, adjoined cells can share second messengers or metabolites and can give coordinated responses to extracellular stimuli. Of note, Butkevich et al. found drebrin at gap junctions of green monkey kidney epithelial cells. Moreover, siRNA-mediated depletion of drebrin led to impaired cell–cell coupling and internalization of connexin-43, thus destabilizing gap junctions (Butkevich et al. 2004).
1.1.3 Adherens Junctions
Adherens junctions (AJ) are molecular assemblies of proteins, which provide intercellular adhesion. Among the most prominent components are cadherins , with VE (vascular endothelial)-cadherin being the only one of over 350 cadherins that is expressed exclusively in endothelial cells (Hulpiau and van Roy 2011). VE-cadherin is part of the subfamily of classical cadherins, which all share six conserved extracellular cadherin domains responsible for calcium-dependent dimerization (Boggon et al. 2002). Besides their main function of mediating adhesion, cadherins play a role in intracellular signaling, as their cytoplasmic tail region interacts with a variety of proteins. Of major importance is p120-catenin, which binds to the juxtamembrane region of cadherins, preventing their clathrin-related endocytosis through stabilization at the membrane (Davis et al. 2003; Chiasson et al. 2009). Upon the release of p120-catenin, it is able to translocate into the nucleus to regulate transcription, displaying a dual role in the cell that is common for many junction-associated proteins (Cavallaro and Dejana 2011; Yap et al. 1997). Another prominent binding partner of cadherins is β-catenin , which was thought to link cadherins to F-actin through α-catenin (Gates and Peifer 2005). However, this model has been challenged, for example, through FRAP experiments showing that F-actin has a more dynamic behavior than α-catenin, which excludes the possibility of a stable complex (Yamada et al. 2005; Drees et al. 2005). Accordingly, Drees et al. showed that α-catenin either binds to β-catenin/cadherin or to F-actin but not to both at the same time (Drees et al. 2005; Weis and Nelson 2006).
Nectins constitute a further group of AJ-localized intercellular adhesion molecules (Lopez et al. 1995). They work independently of Ca2+ and consist of a cytoplasmic tail region, a single transmembrane region and three extracellular immunoglobulin-like loops involved in cis- and trans-oligomerization. Comparable to cadherins, nectins initially form lateral homo (or hetero-)-cis-dimers, followed by trans-interactions with a dimer of the adjacent cell (Satoh-Horikawa et al. 2000; Reymond et al. 2001). Currently, four members of the nectin family have been identified (nectin-1, 2, 3, and 4), with several respective splice variants (Satoh-Horikawa et al. 2000; Reymond et al. 2001). The major nectin isoforms in endothelial cells, including HUVEC, are nectin-2 and nectin-3 (Lopez et al. 1998). The cytoplasmic tail of nectins contains the motif E/A-X-Y-V, which binds the Postsynaptic density protein-95/Drosophila disc large tumor suppressor/Zonula occludens-1 protein (PDZ) domain of their typical binding partner afadin (Mandai et al. 1997).
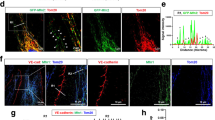
Afadin (or l-Afadin) is a multivalent adaptor protein featuring a variety of functional domains, two Ras-associated domains (RA), one dilute (DIL) domain, a fork head-associated (FHA) domain, a PDZ domain, three proline-rich regions (PR), and an F-actin-binding C-terminus, allowing interactions with a variety of other proteins (Takahashi et al. 1999; Takai et al. 2008) (Fig. 21.2). Afadin is thought to serve as a connector between the two main adhesion systems, nectins, and cadherins, by binding nectin and several of the cadherin-associated proteins. Among others, it can bind to α-catenin , which is mainly localized at cadherin-based junctions and to ponsin, which then binds vinculin (Pokutta et al. 2002; Mandai et al. 1999).
Drebrin and afadin interact through their polyproline and PR1-2 regions. Upper scheme: Drebrin domain structure, including ADF-homology region (aa 8–134), coiled coil region involved in homodimerization and F-actin binding (Peitsch et al. 2001) (CC, aa 176–256), minimal actin-remodeling region (Hayashi et al. 1999) (MAR, aa 233–317), and polyproline region (PP, aa 364–417). Lower scheme: Afadin domain structure, including RA regions involved in Rap1 binding (aa 30–347), FHA region (aa 371–487), DIL region (aa 647–892), PDZ region involved in nectin binding (aa 1016–1100), PR1-2 region containing two polyproline stretches (aa 1219–1399), and FAB region involved in F-actin binding (aa 1691–1829), containing a third proline-rich stretch (PR3). Middle box: Illustration of direct interaction of drebrin’s PP and afadin’s PR1–2 regions. Western blots of pulldown assay using drebrin–PP fused to MBP (MBP-drebrin-PP) or MBP as control immobilized on amylose resin beads, incubated with afadin-PR1-2 fused to GST (GST-afadin-PR1-2). ± indicates presence of respective components in experiments. Western blots developed with indicated antibodies (Rehm 2013; Rehm et al. 2013)
2 Junctional Integrity Depends on the F-actin Cytoskeleton
The cytoskeleton of cells is essential for their morphology, migration, and cytokinesis and also for intracellular transport, endo- and exocytosis, as well as a large variety of other processes (Pollard and Borisy 2003). It is comprised of three systems, microtubules, intermediate filaments, and F-actin, each associated with numerous specific accessory and regulatory proteins (Dudek and Garcia 2001). Actin is the central cytoskeletal element of endothelial cells, which comprises up to 15% of total protein content. In confluent endothelial cells, F-actin is found primarily beneath the plasma membrane, forming the pool of “cortical F-actin ” and interacting with cell–cell adhesion complexes through respective adaptor proteins. The presence of this circumferential cortical F-actin ring has been shown to be essential for adhesion in general; if disrupted by Latrunculin A or cytochalasin D, junction integrity is lost (Shen and Turner 2005; Ivanov et al. 2005; Yamazaki et al. 2007; Quinlan and Hyatt 1999).
The formation of cell–cell junctions is usually initiated between migrating cells, which form characteristic lamellipodia with unevenly distributed adhesion molecules at their leading edges (Yap et al. 1997; Hoelzle and Svitkina 2012). This initial assembly of nascent cell–cell contacts is independent of F-actin. However, once this contact is established, the further maturation of junctions relies on an intact cortical actin cytoskeleton (Chu et al. 2006; Chu et al. 2004). Furthermore, linkage to F-actin also influences the strength of the respective intercellular adhesion (Mege et al. 2006). Quickly after initial adhesion site formation at the lamellipodia, the actin-related protein (Arp) 2/3 complex is deactivated, or potentially repressed by an enrichment of α-catenin (Drees et al. 2005; Pokutta and Weis 2007). Consequently, other actin-binding proteins, among them vasodilator-stimulated phosphoprotein) and Mena, are subsequently recruited through α-catenin, leading to the formation of thicker F-actin cables needed to stabilize the developing junctions (Drees et al. 2005; Krause et al. 2003; Scott et al. 2006).
2.1 Adaptor Proteins Link Cortical F-actin to Junctional Proteins
The cortical actin cytoskeleton is crucial for the maintenance of cell–cell junctions. However, many proteins that form intercellular oligomers do not directly interact with F-actin. Therefore, the presence of linker proteins connecting intercellular adhesion proteins to F-actin is especially important.
Prominent examples of such adaptor proteins are α-catenin, VASP, Epithelial protein lost in neoplasm (EPLIN), or afadin, all of which are involved in anchoring adhesion systems to F-actin. Their importance is underlined by different studies, which show how their loss leads to severe defects in junction formation and maintenance (Vasioukhin et al. 2000; Kwiatkowski et al. 2010; Pappas and Rimm 2006; Abe and Takeichi 2008). The underlying reasons for these defects in proper junction formation are varied, depending on the function of the respective protein. Colon carcinoma cell lines deficient in α-catenin were not able to maintain their adhesive properties, due to an inability of forming cadherin-mediated contacts (Pappas and Rimm 2006). VASP activity was shown to be necessary both for F-actin accumulation and its assembly at cell–cell contacts, and sequestering VASP through relocating it to mitochondria led to defects in F-actin organization at contact sites (Scott et al. 2006), while VASP knockdown led to enhanced endothelial permeability (Schlegel et al. 2008; Reinhard et al. 2001). In afadin-depleted epithelial cells, adherens junctions do not form due to defective recruitment of cadherins to initial adherens junction sites, underlining the importance of afadin during initial junction formation (Tachibana et al. 2000). EPLIN is another linker of the cadherin–catenin complex to F-actin and is also needed for the stabilization of cortical actin fibers. Accordingly, siRNA-mediated knockdown of EPLIN in colon adenocarcinoma cell lines led to misorganization of the cortical F-actin, with E-cadherin showing only a punctate accumulation at cell–cell contact zones (Abe and Takeichi 2008; Chervin-Petinot et al. 2012).
2.2 Drebrin Acts as a Stabilizer for Nectins, by Linking Afadin to Cortical F-actin
It is well described that cadherins are linked to the F-actin cytoskeleton. Also, the roles of the different adaptor proteins through which this linkage is established have been explored in depth (Pappas and Rimm 2006; Abe and Takeichi 2008; Tachibana et al. 2000). In contrast, knowledge on how other adherens junction components, in particular nectins , are stabilized in the junctional area is scarce. This is in part based on the fact that nectins are not well characterized in the endothelium in general and are usually only mentioned in the context of initial junction formation (Takahashi et al. 1999; Mizoguchi et al. 2002; Rikitake et al. 2012; Reymond et al. 2000; Ikeda et al. 1999; Mueller et al. 2003). In our work, we could show that drebrin acts by stabilizing the nectin/afadin system through linking it to the cortical F-actin network. These data revealed a novel function of drebrin in the endothelium, as an adaptor protein for the nectin/afadin system (Rehm et al. 2013). In addition, our work showed that nectins are not only necessary for the formation of nascent cell–cell contacts but are also of major importance for the maintenance of established adherens junctions, especially under conditions of enhanced shear stress such as vascular flow (Fig. 21.1).
In our study, we could show that siRNA-mediated depletion of drebrin leads to functional impairments of endothelial monolayers, as demonstrated by a decrease of transendothelial electrical resistance (TER) and also by rupturing of HUVEC monolayers cultured under constant unidirectional flow conditions (Fig. 21.1). The observed weakening of cell–cell contacts upon drebrin depletion is characterized by a specific loss of nectin-2 from adherens junctions, whereas other junctional proteins such as connexin-43, VE-cadherin, occludin, or PECAM-1 remain apparently unaffected (Rehm et al. 2013). Further experiments showed that, in the absence of drebrin, nectin is endocytosed and subsequently degraded in lysosomes, pointing to an important role of drebrin in the stabilization of nectin at the junctional area. The importance of drebrin for nectin’s presence at junctions was further underlined by rescue experiments, where reexpression of siRNA-insensitive drebrin led to recovery of nectin at junctions.
In co-immunoprecipitation experiments, we could show that drebrin does not interact with nectin directly but with its most prominent intracellular binding partner, afadin. Direct binding of drebrin and afadin is mediated through their polyproline and PR1–2 regions, as shown by glutathione S-transferase (GST)-pulldown experiments using bacterially expressed domain constructs of both proteins fused to GST or maltose-binding protein (MBP) (Fig. 21.2). Moreover, confocal microscopy studies revealed strong binding between drebrin and afadin also in a cellular context, as drebrin’s polyproline region fused to a mitochondrial targeting signal is sufficient to relocalize endogenous afadin to the outer membrane of mitochondria. The close association of afadin and drebrin was further demonstrated by FRAP experiments, which showed that afadin’s mobility at the junctional area is enhanced under drebrin depletion. The fact that afadin remains at junctions, in contrast to nectin, is most probably due to accessory binding by other proteins, such as ZO-1 or α-catenin (Tachibana et al. 2000; Takai and Nakanishi 2003).
Furthermore, we could demonstrate that drebrin maintains junctional integrity through its ability to link the nectin/afadin system to the cortical F-actin network. Being equipped with an F-actin-binding module (CC-region) and the afadin-binding polyproline region, drebrin is able to anchor afadin to the cortical actin cytoskeleton. As afadin can bind to nectin through its PDZ region, this results in a chain of protein interactions, with the sequence F-actin-drebrin-afadin-nectin. Nectin is thus indirectly stabilized by drebrin at junctions through drebrin’s dual ability to bind afadin and F-actin (Fig. 21.3).
Drebrin binds afadin and anchors nectin to the F-actin cytoskeleton in endothelial cells. (a) Drebrin binds to F-actin with high affinity through its coiled coil (CC) region and binds via its polyproline (PP) region to afadin’s PR1–2 regions, while afadin’s PDZ domain binds nectin. Nectin is thus stabilized at the junctional region, preserving endothelial integrity . (b) The absence of drebrin and/or afadin leads to the loss of nectin’s indirect anchorage to the actin cytoskeleton . Nectins are subsequently internalized and degraded in lysosomes, resulting in impaired endothelial integrity. (c) Nectin can be artificially stabilized at junctions even in the absence of both afadin and drebrin upon the overexpression of constructs containing the afadin PDZ region and drebrin’s CC region, reestablishing proper anchorage to the actin cytoskeleton
In order to verify that the linkage of nectin to F-actin is indeed essential for monolayer integrity , we generated a variety of minimal rescue constructs and expressed them in cells under knockdown of both drebrin and afadin. Remarkably, minimal constructs containing only afadin’s PDZ region coupled to drebrin´s F-actin-binding region, or to lifeact, were able to fully rescue nectin at adherens junctions. These results showed that drebrin functions indeed by providing indirect linkage for nectin to F-actin. At the same time, the construct containing lifeact instead of drebrin’s F-actin-binding region demonstrated that only binding to F-actin per se is necessary and that the drebrin-provided anchorage is not based on a specific property of the drebrin–actin interface. Still, drebrin is uniquely equipped to stabilize nectin at endothelial junctions, as it contains binding sites for both afadin and F-actin. This enables drebrin to function as a linker for the nectin/afadin system to the cortical actin cytoskeleton and thus preserve endothelial integrity. Collectively, these results contribute to the current understanding of cell–cell junction regulation in the endothelium, and especially under vascular flow. In particular, the newly identified interaction between drebrin and afadin is shown to be crucial for junctional integrity, not only for junction formation but also during the maintenance of junctions under steady-state conditions (Rehm et al. 2013).
References
Abe K, Takeichi M (2008) EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A 105(1):13–19. doi:10.1073/pnas.0710504105
Alexander DB, Goldberg GS (2003) Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem 10(19):2045–2058
Bazzoni G (2003) The JAM family of junctional adhesion molecules. Curr Opin Cell Biol 15(5):525–530
Bazzoni G, Dejana E (2004) Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84(3):869–901. doi:10.1152/physrev.00035.2003
Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L (2002) C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296(5571):1308–1313. doi:10.1126/science.1071559
Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I (2004) Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr Biol 14(8):650–658. doi:10.1016/j.cub.2004.03.063
Cavallaro U, Dejana E (2011) Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol 12(3):189–197. doi:10.1038/nrm3068
Chervin-Petinot A, Courcon M, Almagro S, Nicolas A, Grichine A, Grunwald D, Prandini MH, Huber P, Gulino-Debrac D (2012) Epithelial protein lost in neoplasm (EPLIN) interacts with alpha-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem 287(10):7556–7572. doi:10.1074/jbc.M111.328682
Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP (2009) p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell 20(7):1970–1980. doi:10.1091/mbc.E08-07-0735
Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S (2004) Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol 167(6):1183–1194. doi:10.1083/jcb.200403043
Chu YS, Eder O, Thomas WA, Simcha I, Pincet F, Ben-Zéev A, Perez E, Thiery JP, Dufour S (2006) Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J Biol Chem 281(5):2901–2910. doi:10.1074/jbc.M506185200
Davis MA, Ireton RC, Reynolds AB (2003) A core function for p120-catenin in cadherin turnover. J Cell Biol 163(3):525–534. doi:10.1083/jcb.200307111
Dejana E, Tournier-Lasserve E, Weinstein BM (2009) The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16(2):209–221. doi:10.1016/j.devcel.2009.01.004
DeLisser HM, Newman PJ, Albelda SM (1994) Molecular and functional aspects of PECAM-1/CD31. Immunol Today 15(10):490–495. doi:10.1016/0167-5699(94)90195-3
Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI (2005) Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123(5):903–915. doi:10.1016/j.cell.2005.09.021
Dudek SM, Garcia JG (2001) Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91(4):1487–1500
Engelhardt B, Wolburg H (2004) Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol 34(11):2955–2963. doi:10.1002/eji.200425327
Gates J, Peifer M (2005) Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell 123(5):769–772. doi:10.1016/j.cell.2005.11.009
Harris AR, Peter L, Bellis J, Baum B, Kabla AJ, Charras GT (2012) Characterizing the mechanics of cultured cell monolayers. Proc Natl Acad Sci U S A 109(41):16449–16454. doi:10.1073/pnas.1213301109
Hayashi K, Ishikawa R, Kawai-Hirai R, Takagi T, Taketomi A, Shirao T (1999) Domain analysis of the actin-binding and actin-remodeling activities of drebrin. Exp Cell Res 253(2):673–680. doi:10.1006/excr.1999.4663
Hoelzle MK, Svitkina T (2012) The cytoskeletal mechanisms of cell–cell junction formation in endothelial cells. Mol Biol Cell 23(2):310–323. doi:10.1091/mbc.E11-08-0719
Hulpiau P, van Roy F (2011) New insights into the evolution of metazoan cadherins. Mol Biol Evol 28(1):647–657. doi:10.1093/molbev/msq233
Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y (1999) Afadin: a key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J Cell Biol 146(5):1117–1132
Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA (2005) Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 16(6):2636–2650. doi:10.1091/mbc.E05-01-0043
Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB (2003) Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol 19:541–564. doi:10.1146/annurev.cellbio.19.050103.103356
Kwiatkowski AV, Maiden SL, Pokutta S, Choi HJ, Benjamin JM, Lynch AM, Nelson WJ, Weis WI, Hardin J (2010) In vitro and in vivo reconstitution of the cadherin-catenin-actin complex from Caenorhabditis elegans. Proc Natl Acad Sci U S A 107(33):14591–14596. doi:10.1073/pnas.1007349107
Lampugnani MG (2010) Endothelial adherens junctions and the actin cytoskeleton: an ‘infinity net’? J Biol 9(3):16. doi:10.1186/jbiol232
Lampugnani MG, Dejana E (1997) Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol 9(5):674–682
Lopez M, Eberle F, Mattei MG, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P (1995) Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene 155(2):261–265
Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P (1998) The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood 92(12):4602–4611
Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B (2003) T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood 102(10):3675–3683. doi:10.1182/blood-2003-02-0358
Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y (1997) Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol 139(2):517–528
Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, Takai Y (1999) Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell–cell and cell-matrix adherens junctions. J Cell Biol 144(5):1001–1017
Mege RM, Gavard J, Lambert M (2006) Regulation of cell–cell junctions by the cytoskeleton. Curr Opin Cell Biol 18(5):541–548. doi:10.1016/j.ceb.2006.08.004
Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, Tsutsumi T, Sonoda S, Ide C, Takai Y (2002) Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol 156(3):555–565. doi:10.1083/jcb.200103113
Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147(1):185–194
Mueller S, Rosenquist TA, Takai Y, Bronson RA, Wimmer E (2003) Loss of nectin-2 at Sertoli-spermatid junctions leads to male infertility and correlates with severe spermatozoan head and midpiece malformation, impaired binding to the zona pellucida, and oocyte penetration. Biol Reprod 69(4):1330–1340. doi:10.1095/biolreprod.102.014670
Muller WA (2003) Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 24(6):327–334
Muller WA, Weigl SA, Deng X, Phillips DM (1993) PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 178(2):449–460
Pappas DJ, Rimm DL (2006) Direct interaction of the C-terminal domain of alpha-catenin and F-actin is necessary for stabilized cell–cell adhesion. Cell Commun Adhes 13(3):151–170. doi:10.1080/15419060600726142
Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D’Amore PA (2012) Role of shear-stress-induced VEGF expression in endothelial cell survival. J Cell Sci 125:831–843. doi:10.1242/jcs.084301
Peitsch WK, Grund C, Kuhn C, Schnolzer M, Spring H, Schmelz M, Franke WW (1999) Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol 78(11):767–778
Peitsch WK, Hofmann I, Pratzel S, Grund C, Kuhn C, Moll I, Langbein L, Franke WW (2001) Drebrin particles: components in the ensemble of proteins regulating actin dynamics of lamellipodia and filopodia. Eur J Cell Biol 80(9):567–579
Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW (2005) Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes. J Invest Dermatol 125(4):761–774. doi:10.1111/j.0022-202X.2005.23793.x
Pokutta S, Weis WI (2007) Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu Rev Cell Dev Biol 23:237–261. doi:10.1146/annurev.cellbio.22.010305.104241
Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI (2002) Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem 277(21):18868–18874. doi:10.1074/jbc.M201463200
Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112(4):453–465
Quinlan MP, Hyatt JL (1999) Establishment of the circumferential actin filament network is a prerequisite for localization of the cadherin-catenin complex in epithelial cells. Cell Growth Differ 10(12):839–854
Rehm K (2013) Drebrin preserves endothelial integrity by stabilizing nectin at adherens junctions. Dissertation/ Doctoral thesis at the Universität Hamburg, Institut für Medizinische Mikrobiologie, Virologie und Hygiene at the Universitätsklinikum Hamburg-Eppendorf
Rehm K, Panzer L, van Vliet V, Genot E, Linder S (2013) Drebrin preserves endothelial integrity by stabilizing nectin at adherens junctions. J Cell Sci 126(Pt 16):3756–3769. doi:10.1242/jcs.129437
Reinhard M, Jarchau T, Walter U (2001) Actin-based motility: stop and go with Ena/VASP proteins. Trends Biochem Sci 26(4):243–249
Reymond N, Borg JP, Lecocq E, Adelaide J, Campadelli-Fiume G, Dubreuil P, Lopez M (2000) Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene 255(2):347–355
Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M (2001) Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem 276(46):43205–43215. doi:10.1074/jbc.M103810200
Rikitake Y, Mandai K, Takai Y (2012) The role of nectins in different types of cell–cell adhesion. J Cell Sci 125(Pt 16):3713–3722. doi:10.1242/jcs.099572
Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y (2000) Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell–cell adhesion activities. J Biol Chem 275(14):10291–10299
Schlegel N, Burger S, Golenhofen N, Walter U, Drenckhahn D, Waschke J (2008) The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am J Physiol Cell Physiol 294(1):C178–C188. doi:10.1152/ajpcell.00273.2007
Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS (2006) Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell 17(3):1085–1095. doi:10.1091/mbc.E05-07-0644
Shen L, Turner JR (2005) Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16(9):3919–3936. doi:10.1091/mbc.E04-12-1089
Simon AM, Goodenough DA (1998) Diverse functions of vertebrate gap junctions. Trends Cell Biol 8(12):477–483
Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103(3):755–766
Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y (2000) Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 150(5):1161–1175. doi:10.1083/jcb.150.5.1161
Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y (1999) Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol 145(3):539–549
Takai Y, Nakanishi H (2003) Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 116(Pt 1):17–27
Takai Y, Miyoshi J, Ikeda W, Ogita H (2008) Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9(8):603–615. doi:10.1038/nrm2457
Tsukita S, Furuse M (1999) Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol 9(7):268–273
Tsukita S, Furuse M, Itoh M (1999) Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol 11(5):628–633
Vasioukhin V, Bauer C, Yin M, Fuchs E (2000) Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100(2):209–219
Vestweber D (2007) Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev 218:178–196. doi:10.1111/j.1600-065X.2007.00533.x
Wallez Y, Huber P (2008) Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778(3):794–809. doi:10.1016/j.bbamem.2007.09.003
Weis WI, Nelson WJ (2006) Re-solving the cadherin-catenin-actin conundrum. J Biol Chem 281(47):35593–35597. doi:10.1074/jbc.R600027200
Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ (2005) Deconstructing the cadherin-catenin-actin complex. Cell 123(5):889–901. doi:10.1016/j.cell.2005.09.020
Yamazaki D, Oikawa T, Takenawa T (2007) Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell–cell adhesion. J Cell Sci 120(Pt 1):86–100. doi:10.1242/jcs.03311
Yap AS, Brieher WM, Gumbiner BM (1997) Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 13:119–146. doi:10.1146/annurev.cellbio.13.1.119
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Rehm, K., Linder, S. (2017). Drebrin’s Role in the Maintenance of Endothelial Integrity. In: Shirao, T., Sekino, Y. (eds) Drebrin. Advances in Experimental Medicine and Biology, vol 1006. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56550-5_21
Download citation
DOI: https://doi.org/10.1007/978-4-431-56550-5_21
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56548-2
Online ISBN: 978-4-431-56550-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)