Abstract
Drebrin was first discovered by our group as “developmentally regulated brain protein” from the chicken optic tectum. Drebrin is an actin-binding protein, which is classified into two major isoforms produced by alternative splicing from a single DBN1 gene. The isoform predominantly expressed in the adult brain (drebrin A) is neuron specific, containing a neuron-specific sequence (Ins2) in the middle of the molecule. Drebrin A is highly concentrated in dendritic spines, and its accumulation level is regulated by synaptic activity. In contrast, drebrin E, which lacks Ins2, is found in widespread but not ubiquitous cell types in various tissues. The isoform conversion from drebrin E to drebrin A occurs in parallel with synaptogenesis. Drebrin decorating F-actin is found at the recipient side of cell-cell communication systems, such as gap junctions, adherens junctions, immunological synapses, and neuronal synapses. In addition, it is involved in the cellular mechanisms of cell migration, cell process formation, cancer metastasis, and spermatogenesis. Lack of drebrin leads to the dysfunction of cell-cell communication, resulting in aberrant migration of metastatic cancer cells, aberrant synaptic function in dementia, and rupture of endothelial integrity. Because drebrin forms a unique F-actin with a longer helical crossover, drebrin may create an F-actin platform for molecular assembly and play a pivotal role in intercellular communication.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Alternative splicing
- Cancer
- Cell migration
- Intercellular communication
- Physical property of actin filament
- Synaptogenesis
- Synaptic plasticity
1 Introduction
Drebrin was first discovered by our group as “developmentally regulated brain protein” from the chicken optic tectum in 1985 (Shirao and Obata 1985). In the first 15 years after the discovery, no other groups except us were interested in drebrin, which is expressed in the nervous tissue. During these years, we purified chicken and rat drebrins, raised polyclonal and monoclonal antibodies, and cloned DBN1 cDNAs. Consequently, we have identified major isoforms of drebrin in chicken, rodent, feline, and human expressed in the nervous tissue. We further clarified the genetic and biochemical properties of drebrin, such as actin-binding activity and phosphorylation. The expression of each isoform depends on the developmental stage. Because the isoform predominantly expressed in adult brain (drebrin A) is neuron specific, our later studies were mainly focused on drebrin A (Shirao et al. 2017).
In 1996, we found that drebrin A is highly concentrated in dendritic spines in adult rat brain, forms a complex with actin and myosin, and inhibits the actin-activated ATPase activity of myosin II (Hayashi et al. 1996). Thus, we proposed that drebrin may play a role in the structure-based plasticity of synapses through the actin-linked control of the actomyosin interaction in dendritic spines. In 1999, we successfully showed that exogenously expressed drebrin A specifically elongates dendritic spines of primary cultured neurons (Hayashi and Shirao 1999). This was the first report demonstrating that the manipulation of a single actin-binding protein in a neuron alters spine morphology. After these epoch-making findings, drebrin and the actin cytoskeleton in dendritic spines were thrown into the limelight. Since then we have shown the pivotal roles of drebrin in spine formation (Takahashi et al. 2003; Aoki et al. 2005) and synaptic plasticity (Takahashi et al. 2006; Mizui et al. 2014; Sekino et al. 2006). Nowadays, hundreds of spine-resident proteins have been found, but drebrin is still a key protein in modulating the actin cytoskeleton in dendritic spines (Sekino et al. 2007; Koganezawa et al. 2017).
Actin-binding proteins modulate the characteristics of the actin cytoskeleton and consequently regulate cell structures or produce the motile force of cells. Drebrin isoforms other than drebrin A are widely distributed in nonnervous tissues as well as the nervous tissue, not only in avian (Shirao and Obata 1986) and mammals (Shirao et al. 1994; Peitsch et al. 1999) but also in the soil amoebae (Luna et al. 1997). Furthermore, drebrin has been found at the recipient side of various intercellular communication systems, such as gap junctions, adherens junctions, immunological synapses, and neuronal synapses. This suggests the universal role of drebrin as an actin modulator.
How does drebrin change F-actin structures? Why does drebrin appear at the cell-cell communication sites? More generally, what is the physiological function of drebrin? This chapter will briefly introduce the key discoveries and proposals contributing to elucidating the above questions.
2 Historical Orientation
2.1 Background of Drebrin Study
The development of the brain is achieved by a combination of several fundamental processes, such as the proliferation and migration of neurons, the directed extension of nerve fibers, and synapse formation. Before 1960 classic morphological techniques were used for the study of brain development, because morphological structures of the brain dramatically change when each process occurs. In the 1960s and 1970s, developmental studies were accelerated by the progression of new technologies such as the autoradiography using tritiated thymidine. These new methods disclosed in detail the birth date of each neuron, the layer formation, and subsequent maturation in mammalian cerebral and cerebellar cortices and in the chicken optic tectum. However, the molecular mechanism of each process was not yet clarified.
To disclose the molecular mechanism of the brain development, the identification of the master proteins that govern each fundamental process was eagerly pursued. One approach was to select a key function in each developmental process and to look for the protein(s) that mediates that function. Adopting this approach, Edelman and his collaborators developed a specific immunological assay for molecules involved in cell adhesion (Brackenbury et al. 1977) and discovered cell adhesion molecules (CAMs) as key molecules in brain development (Hoffman et al. 1982).
Another approach was based on the conjecture that the master proteins are expressed at limited developmental stages in a restricted region of the brain. Sperry hypothesized the presence of two orthogonal gradients of molecules on retinal ganglion neurons that determine specific connections between retinal and tectal neurons (Sperry 1963), and Nirenberg’s group identified an antigen that is distributed in a dorsal-ventral topographic gradient in chick embryo retina by screening a library of monoclonal antibodies in 1981 (Trisler et al. 1981).
2.2 Discovery of Drebrin by Proteomics
In January 1982, we started seeking for yet-to-be-discovered master proteins in the developing brain. We surveyed the changes in the proteome of the developing brain using O’Farrell’s two-dimensional gel electrophoresis (2DGE) (O'Farrell 1975). The chicken optic tectum was chosen as the target region, because it is a uniform and regularly layered structure that develops correctly on a timetable, as revealed by Cowan and colleagues (LaVail and Cowan 1971a, b). After the electrophoresed gel was stained with Coomassie brilliant blue, 54 proteins were counted (Fig. 1.1). Most of them were found at the beginning (4-day embryo) and remained unchanged until adulthood. There were eight proteins that remarkably changed their staining intensities during embryonic development (Shirao and Obata 1985). These eight proteins were further classified into three groups. The first group was monotonically increasing proteins, including neurofilament proteins and drebrin A (adult-type isoform). The second group was monotonically decreasing proteins. The third group was intensely stained only at embryonic stages and was later named chicken drebrin E1 and E2 isoforms. Note that in mammals there is only one embryonic isoform named drebrin E, while chickens have two embryonic isoforms. The developmental changes in the amount of drebrins in the optic tectum are shown in Fig. 1.2. Drebrin isoforms were found with similar developmental changes in other brain regions. However, the time course of their changes varied from region to region. Even within the optic tectum, developmental changes in drebrin occur earlier in the rostral portion than in the caudal portion, which corresponds to the rostro-caudal gradient of histological development (LaVail and Cowan 1971a). Together, these results suggest that the changes in drebrin expression are paralleled with brain development, which are explained in detail in Part III of this book.
2.3 Purification of Drebrin
In 1985, we succeeded to purify drebrin E1 and E2 from embryonic day (ED) 11 chicken brains (Shirao and Obata 1985). We used the 2DGE assay and found that all drebrins were recovered in the same fractions by various purification methods such as isoelectric precipitation, ammonium sulfate precipitation, and ion-exchange chromatography. Gel electrophoresis peptide mapping using the Staphylococcus aureus V8 protease demonstrated structural homology of drebrins. Using radiolabeled methionine, we confirmed that changes in the amount of drebrin in 2DGE are due to changes in drebrin synthesis, but not in drebrin degradation.
During these purification steps, contamination of actin in the drebrin fraction seemed constant at each step. In addition, all drebrin isoforms are acidic proteins (isoelectric point around 4.2), while one of the common features of actin cytoskeleton-related proteins such as calmodulin and troponin is a low isoelectric point. Furthermore, drebrins are heavily phosphorylated proteins on ED5 and ED11 in the optic tectum. Collectively, the results suggested that drebrin isoforms are closely related phosphoproteins associated with the actin cytoskeleton. It is known that the actin-binding activity is the most important function of drebrin, which is discussed below. The actin-binding activity has recently been suggested to be modified by phosphorylation (Worth et al. 2013), which plays a role in cell migration (Tanabe et al. 2014) and neuritogenesis (Geraldo et al. 2008).
2.4 Antibodies Against Drebrin
In 1986, we succeeded to produce polyclonal and monoclonal antibodies against electrophoretically purified drebrin E1 and E2. The resulting antibodies included monoclonal antibody (mAb) M2F6, which specifically recognizes all drebrins (E1, E2, and A) (Shirao and Obata 1986). Ever since, mAb M2F6 has been used for drebrin studies as the standard antibody, because its epitope is located in the common sequence of all drebrin isoforms and is conserved from avian to mammals.
We immunohistochemically stained frozen sections of chick embryo at various developmental stages using mAb M2F6 (Shirao and Obata 1986). Drebrin first appeared on ED2 in the myotome. In the early developmental stages of the optic tectum, drebrin is widely distributed in the neuronal somata and processes. As developmental stages proceed, drebrin highly accumulates in the tip of the growing cell processes, namely, the axonal and dendritic growth cones. On the other hand, in the adult, the drebrin immunostaining pattern is dot-like in the neuropil region, suggesting that drebrin is localized at the synapses. Then, we further analyzed the subcellular localization of drebrin using immunoelectron microscopy and found that drebrin is localized in the dendritic spine in the adult brain (Shirao et al. 1987). However, at this point, it was not yet clarified whether the developmental changes in drebrin subcellular distribution depend on each isoform or not.
2.5 Cloning of DBN1 cDNAs
Using the antibodies, we then screened a λgt11 cDNA library from a 10-day-old chicken embryo and isolated a cDNA clone of DBN1 (gDcw1) (Shirao et al. 1988). Then, using gDcw1 we isolated the full-length cDNAs of drebrin E1, E2, and A (Kojima et al. 1988; Kojima et al. 1993). In 1993, we further elucidated that chicken drebrin E1, E2, and A are translated from three mRNAs that are produced from a single DBN1 gene by alternative splicing (Kojima et al. 1993). Among the drebrin isoforms, drebrin E1 (564 amino acids) is expressed in the earliest developmental stages. Drebrin E2 (607 amino acids) has an additional 43-amino-acid insertion sequence (Ins1) in the middle of drebrin E1. Drebrin A (653 amino acids) has another 46-amino-acid sequence (Ins2) inserted right in front of Ins1.
In parallel with the above study, we cloned the cDNAs of rat drebrin A (707 amino acids) (Shirao et al. 1992) and human drebrin E (649 amino acids) (Toda et al. 1993). The overall amino acid identity between chicken and rat drebrin A is 60%. In particular, the homology of the N-terminal half including the Ins2 sequence (1–364 amino acid residues in rat) and two short regions in the C-terminus (581–599 and 650–707 residues) is greater than 80% (Kojima et al. 1993). The regions are also well conserved in human. It has been shown that the rat, mouse, and human Ins2 sequence is almost identical. So far, the drebrin A isoform, which contains the Ins2 sequence, including s-drebrin A (Jin et al. 2002), has only been detected in neurons from chicken to human, and its expression depends on brain maturation. The schematic structure of drebrin E and A is shown in Fig. 1.3.
Schematic representation of domain structures of rat drebrin. Drebrin A has “Ins2” insertion that is not present in drebrin E. Rat drebrin E corresponds to chick drebrin E2. ADF-H is described as drADF-hd in Chap. 5. Actin-binding region 1 (AB1) is comparable to coiled-coil domain (CC) and helical-charged motif (HCM) in Chaps. 4 and 5, respectively. Actin-binding region 2 is comparable to helical domain (Hel), drebrin actin-binding domain (drABD) and minimal actin-remodeling region in Chaps. 3, 4 and 21, respectively. C1, C2, and C3 are conserved regions between chicken and rodent, and V1 and V2 are variable regions. C1 is further subdivided into C1a, C1b, and C1c (see Chap. 2). Spikar binds to ADF-H. 3,5-Bistrifluoromethyl pyrazole (BTP) binds to AB2 region. Cyclin-dependent kinase 5 (CDK5) phosphorylates S142 and S342. Phosphatase and tensin homologue (PTEN) dephosphorylates S647
It is still unclear why rodents have one embryonic isoform, while chickens have two. The chicken Dbn1 gene is over 15.4 kb, and the exons encoding the Ins1 and Ins2 sequences are separate (Kojima et al. 1993). In contrast, the mouse Dbn1 gene contains exon 12a encoding the Ins2 sequence, which directly connects to exons12b and 12c (see details in Chap. 2). Either amino acid sequence of the chicken E2 or mouse E different from comparable drebrin A is only Ins2 (Jin et al. 2002). Therefore, mammalian drebrin E is thought to be homologous to chicken drebrin E2, but not drebrin E1.
2.6 Actin-Binding Activity of Drebrin
In 1994, the first solid proof for the actin-binding activity of drebrin E was reported (Ishikawa et al. 1994). We improved the purification method of drebrin E from rat brain to get enough protein for biochemical characterization. We have shown that drebrin E does not bind to actin monomers (G-actin) but binds to F-actin with a stoichiometry of 1:5 (drebrin E: actin) and an apparent dissociation constant (K d) of 1.2 × 10−7 M.Footnote 1 Drebrin E competitively binds to F-actin with tropomyosin, fascin, and α-actinin.
On the other hand, drebrin A was not successfully purified from brain tissue for biochemical analyses, although we knew that drebrin A precipitated with F-actin. To examine whether drebrin A binds to F-actin similarly to drebrin E, we transfected various cell lines with GFP-tagged drebrin A cDNA and found that drebrin A and F-actin colocalize within a transfected cell and that tropomyosin disappears from drebrin A-bound F-actin (Shirao et al. 1994). This suggested that drebrin A also competitively binds to F-actin with tropomyosin. However, we had to wait until Ishikawa’s group succeeded in purifying bacterially expressed drebrin A (Ishikawa et al. 2007) (more than 10 years) before we could elucidate the exact actin-binding property of drebrin A.
2.7 Drebrin Modifies the Actin Cytoskeleton in Dendritic Spines
As mentioned previously in the chicken optic tectum and cerebellum, drebrin A is mainly localized in dendritic spines (Shirao et al. 1987; Shirao and Obata 1986). In 1996, we studied the detailed localization of drebrin in the rat brain (Hayashi et al. 1996). Drebrin immunostaining is distributed in a dot-like pattern in the rat brain similarly to the chicken brain, suggesting a dendritic spine localization of drebrin. However, the drebrin immunostaining intensity did not exactly correlate with the intensity of synaptophysin, a synaptic marker protein. Interestingly, immunoblot analysis showed that drebrin E was uniformly distributed at a low level throughout the brain, but drebrin A was expressed differentially, as it is abundant in the forebrain but present only at low levels in the cerebellar cortex. The high concentration of drebrin A in the forebrain suggests that the role of drebrin in the adult brain is related to learning and memory, which is characteristic of the forebrain. Electron microscopy showed that drebrin is localized at postsynaptic sites. Confocal microscopy of double labeling of drebrin and synaptophysin at the cerebral cortex showed a clear separation between synaptophysin-labeled presynaptic terminals and drebrin-labeled postsynaptic spines (Fig. 1.4a) (Hayashi et al. 1996).
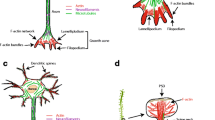
Cellular functions of drebrin at a glance. (a) Drebrin forms stable F-actin pool at postsynaptic sites of excitatory synapses (Aoki et al. 2005). (b) Drebrin stabilizes nectin via afadin at adherens junctions (Rehm et al. 2013) and regulates gap junction via connexin (Butkevich et al. 2004). (c) Drebrin is not localized at vinculin-positive focal adhesions (Peitsch et al. 1999), but some focal adhesions are stabilized by drebrin (Ikeda et al. 1996). (d) Association of drebrin and chemokine receptor CXCR4 is enhanced by antigenic stimulation at immunological synapses (Perez-Martinez et al. 2010) and inhibits the entry of HIV-1 at virological synapses (Gordon-Alonso et al. 2013) and endocytosis (Li et al. 2017). Drebrin is also necessary for the store-operated Ca2+ channel function (Mercer et al. 2010) but inhibits TRP channel activity (Stiber et al. 2016). (e) Spikar, a transcription coactivator, enters into nucleus when it dissociates from drebrin (Yamazaki et al. 2014). (f) Drebrin is a specific component of small GTP-binding protein ARF-dependent actin pool on the Golgi complex (Fucini et al. 2000). (g) Drebrin is localized at the juxtanuclear drebrin-enriched zone, which is purportedly concerned with cell migration (Peitsch et al. 2006). (h) Drebrin mediates ectosome release from the tip of cilia (Nager et al. 2017). (i) Drebrin is localized at the transitional zone between lamellipodia and microtubules at the tip of cell processes (Geraldo et al. 2008; Mizui et al. 2009). Abbreviations: ARF ADP-ribosylation factor, BTC basal transcription complex, cSMAC central supramolecular activation cluster, ECM extracellular matrix, HIV human immunodeficiency virus, NT neurotransmitter, TRP transient receptor potential. Long and normal helical pitches of F-actin are 40 nm and 36 nm, respectively
Next, we examined the association of drebrin with the spine cytoskeleton. Actin, gelsolin, and myosin I, II, and V were immunoprecipitated as drebrin-containing cytoskeleton. On the other hand, microtubule-associated protein 2 (MAP2), fodrin, caldesmon, α-actinin, fascin, and tropomyosin, which are all detectable in the brain, were not detected within the immunoprecipitates. Thus, drebrin forms a distinct cytoskeletal complex with actin, gelsolin, and myosins in dendritic spines. Furthermore, we found that drebrin inhibits the actin-activated ATPase activity of myosin II (Hayashi et al. 1996). This suggests that drebrin regulates the actin-myosin interaction by the actin-linked control. It is known that caldesmon and tropomyosin, which have inhibitory effects on the actomyosin interaction similarly to drebrin, carry out the actin-linked control in smooth muscles (Ngai and Walsh 1984; Yamaguchi et al. 1984; Bugyi et al. 2010). Drebrin may take the place of caldesmon or tropomyosin in the actin-linked control of the actomyosin interaction in dendritic spines and may play a role in the structure-based plasticity of synapses.
To examine this hypothesis, we introduced excessive drebrin A into primary cultures of cortical neurons and analyzed its effect on the spine shape. In 1999, we reported that exogenous GFP-tagged drebrin A localized at the spines and induced spines to elongate (Hayashi and Shirao 1999). This was the first direct evidence that manipulation of a single actin-binding protein in a neuron specifically alters dendritic spine morphology. Although the underlining molecular mechanism was not clarified, we proposed the hypothesis that drebrin gives a unique character to the actin cytoskeleton bound to it (Shirao and Sekino 2001).
After these epoch-making findings, drebrin and the actin cytoskeleton in dendritic spines were thrown into the limelight, and in 2000 several papers focusing on drebrin localization were published by other groups (Allison et al. 2000; Conroy et al. 2000; Shoop et al. 2000) using the anti-drebrin monoclonal antibody, M2F6, developed in our laboratory. Since then, mAb M2F6 has been used in drebrin studies as the standard antibody worldwide.
2.8 Advances in Drebrin Studies in the Twenty-First Century
In the last 18 years, numerous drebrin functions related to various physiological phenomena, such as cell migration, synaptogenesis, and synaptic plasticity, have been elucidated extensively.
In 2006, we found the N-methyl-d-aspartate (NMDA) receptor activity-dependent change in drebrin localization (Sekino et al. 2006) and proposed the hypothesis that the drebrin localization change modifies the association of F-actin with other spine-resident actin-binding proteins, consequently leading to changes in spine morphology during synaptic plasticity (Sekino et al. 2006). However, it was difficult to discriminate neuronal F-actin from the glial one in a cortical mixed culture. To achieve precise studies about drebrin A and F-actin distribution in neurons, we innovated Banker’s method (Kaech and Banker 2006) for our neuronal culture technique. In this method embryonic hippocampal neurons plated on a coverslip were cultured over a glial sheet in a dish. By immunocytochemically analyzing the pure neuronal culture on the coverslip, we could analyze the distribution of drebrin and F-actin without interference of the glial F-actin.
In two days in vitro (DIV) neurons, drebrin E but not A was concentrated in the growth cone, where F-actin predominantly localized in the peripheral half and microtubules localized in the central half. Drebrin E is localized only in the proximal area of the F-actin-rich region, which is the transitional zone to the central microtubule-rich region (Geraldo et al. 2008). Interestingly, the retrograde flow of drebrin-decorated F-actin was slower than that of other F-actins (Mizui et al. 2009).
In seven DIV neurons, drebrin immunostaining and F-actin were observed discontinuously at the submembranous region of the dendritic shaft and as a fibrous pattern in filopodia, except for the tip where only F-actin was observed. In 21 DIV neurons, strong drebrin immunostaining was colocalized with F-actin at the dendritic spines, but the colocalization at the dendritic shafts was decreased (Takahashi et al. 2003).
In 2008, we found that migrating neurons contain drebrin in their cell body even in the adult, which is different from the typical synaptic localization of drebrin A. Therefore, we hypothesized that migrating neurons contain drebrin E, but not drebrin A. Unfortunately, a drebrin E-specific antibody has not been successfully produced, yet, although a drebrin A-specific antibody (DAS2) was available. Therefore, it was difficult to determine whether the drebrin isoform distributed in migrating cell somata is drebrin E. Thus, we developed an image subtraction method using mAb M2F6 and DAS2 to identify the subcellular distribution of drebrin E (Song et al. 2008). Using this method, we found that drebrin E was widely distributed within the migrating neuron until drebrin A appeared. Once drebrin A is expressed in a developing neuron, drebrin E is no longer observed in the cell soma but is distributed in cell processes (Song et al. 2008).
As we mentioned previously, drebrin is also expressed in nonnervous tissues (Shirao and Obata 1986). The drebrin isoforms expressed in non-neuronal cells are only the embryonic types of drebrin, which do not have the Ins2 sequence in the molecule; hence, drebrin A, which has the Ins2 sequence, is not expressed in these cells. Many studies have been recently reported in relation to drebrin’s role in various cellular functions, such as cell migration, cell process formation, intercellular communication, cancer metastasis, and spermatogenesis. These studies are discussed in more detail in specific chapters of this book, although some of them are also highlighted in the following section of this chapter (Fig. 1.4).
3 Physiological Roles of Drebrin
3.1 Morphogenetic Activity of Drebrin
One of the most striking features of drebrin function is the cell morphogenetic activity. In 1992, we demonstrated that overexpression of exogenous drebrin A in cultured fibroblasts induced many filopodia-like processes around the cell body, and some of them became highly branched long processes similar to neuronal dendrites (Fig. 1.5a) (Shirao et al. 1992). Time-lapse recording analysis of the transfected cells indicated that microvilli protruded from the cell bodies seldom retracted, but elongated and fused side-by-side, consequently forming neurite-like cell processes.
Immunocytochemistry and immunoelectron microscopy of drebrin A transfected fibroblasts. (a) and (b) Transfected cells were subjected to immunostaining with anti-drebrin antibody (Mab M2F6). Intensely stained cell was neuron-like with round thick cell bodies and highly branched processes extending from the cell perimeter (a). Within a transfected cell, immunofluorescence of drebrin staining colocalized with F-actin forms thick curving bundles (b). C–G, Fine structures of drebrin A transfected fibroblasts. A neurite-like process of the transfectant showing microspikes (c). The round cell body shows occasional microvilli on its surface (d). Cell process containing microtubules and intermediate filaments in the central region (e). A microspike containing microfilaments connected to those in the cell process (f). Bundles of microfilaments were shown in the submembranous region of the cell process (g). Scale bars: (a), 40 μm; (c) and (d), 5 μm; (e), (f), and (g), 0.1 μm. (c) to (g) were reprinted from Inoue and Shirao (1997)
We examined the ultrastructural changes associated with process formation in drebrin A cDNA-transfected L cells by electron microscopy. Non-transfected L cells were large and epitheloid or spindle shaped with lamellipodia on the cell borders. Many short microvilli were present diffusely on cell surfaces, but not on lamellipodia. Surface and intracellular structures showed a diffused arrangement and had no special organization, except for stress fibers. On the other hand, transfected cells had round or polygonal cell bodies and neurite-like cell processes. The surfaces of the cell bodies were smooth overall, although some microvilli were observed. The neurite-like cell processes had numerous microspikes on them. In the cell processes, mitochondria, microtubules, and intermediate filaments, as well as the submembranous F-actin bundles, were observed. In contrast, F-actins were only predominant structures in the microspikes (Fig. 1.5c–f) (Inoue and Shirao 1997).
We then immunocytochemically examined the distribution of drebrin A and F-actin within the cell. In the transfected cells, tropomyosin disappeared from F-actin, and drebrin A-decorated F-actin formed thick curving bundles that were different from the straight tropomyosin-binding stress fibers (Fig. 1.5b) (Shirao et al. 1994). How are thick curving F-actin bundle formed? Does drebrin A have F-actin-bundling activity?
Although drebrin E purified from brain tissue did not exhibit any actin-bundling activity (Ishikawa et al. 1994), bacterially expressed drebrin E and A did exhibit actin-bundling activity. In addition, molecular dissection of drebrin revealed two actin-binding regions (AB1 and AB2 in Fig. 1.3), and the fragment containing both regions had actin-bundling activity (Worth et al. 2013). These results suggest that drebrin has actin-bundling activity, and the bundled drebrin-decorated F-actin forms thick curving structures.
However, many cell lines express drebrin E without forming the thick curving F-actin bundles and keep straight tropomyosin-binding stress fibers. In addition, overexpression of AB2 region is enough to induce the thick actin bundles in Chinese hamster ovary (CHO) cells (Hayashi et al. 1999). Therefore, we propose that drebrin has actin-bundling activity under specific conditions. In the transfected CHO cells, overexpressed drebrin AB2 region may induce the specific conditions and allow endogenous drebrin E to bundle drebrin-decorated F-actins.
In 2009, we reported that drebrin-decorated F-actin shows slow treadmilling (Mizui et al. 2009). In 2011, Sharma et al. reported that drebrin induces remodeling of F-actin (Sharma et al. 2011). Drebrin-decorated F-actin shows unexpected unique physical and chemical characteristics, such as a longer helical crossover (40 nm), higher heat stability, and slower depolymerizing speed (Sharma et al. 2011; Mikati et al. 2013). Furthermore, the cooperative binding activity of drebrin to F-actin has been elucidated (Sharma et al. 2012). This suggests that the binding of drebrin to F-actin leads to the exclusion of other existing actin-binding proteins from F-actin and consequently the formation of F-actin fully covered by drebrin.
Thus, one possible underlining mechanism of the drebrin morphogenetic activity is that drebrin-decorated F-actin responds differently to various actin-regulating signals, resulting in the morphological change of cells. On the other hand, it is known that drebrin-decorated F-actin can link to microtubules (see below). Therefore, another possible mechanism is that submembranous drebrin-decorated F-actin bundles in the drebrin A cDNA-transfected cells enable microtubules to intrude into microvilli and consequently form cell processes.
3.2 Drebrin’s Function in Synaptogenesis
During development, the expression of drebrin A in the brain is paralleled with synapse formation. Electron microscopy analysis of the developing rat brain has demonstrated that drebrin A locates at dendritic membranes at the initial contact sites between axons and dendrites (Aoki et al. 2005). In addition, we have shown using primary hippocampal cultured neurons that inhibition of drebrin A expression resulted in the delay of synapse formation as well as the inhibition of postsynaptic protein accumulation, such as postsynaptic density protein 95 (PSD-95), Ca2+/calmodulin-dependent protein kinase II (CaMKII), spikar, and glutamate receptors (Takahashi et al. 2003; Yamazaki et al. 2014). It has been indicated that spikar (Yamazaki et al. 2014) and CaMKII (Yamazaki et al. submitted) accumulate by their direct binding activity to drebrin, while PSD-95 is likely to accumulate through the drebrin-Homer-Shank complex (Shiraishi-Yamaguchi et al. 2009; Tu et al. 1999).
How does drebrin A accumulate at postsynaptic sites? We have shown that synaptic activity governs drebrin A accumulation at postsynaptic sites (Takahashi et al. 2009). We analyzed drebrin dynamics within individual spines using the fluorescence recovery after photobleaching (FRAP) technique and found that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activity promotes drebrin A stabilization. This suggests the AMPA receptor-dependent appearance of the drebrin A-decorated F-actin complex at postsynaptic sites, which may function as a platform for molecular assembly of other postsynaptic proteins such as PSD-95, CaMKII, spikar, and glutamate receptors (Fig. 1.6).
Diagram of excitatory postsynaptic maturation. (a) Dynamic F-actin (normal helical pitch F-actin) is transported at cortical cytoplasm of dendritic shaft and dendritic filopodia. (b) Drebrin A accumulates at presumptive postsynaptic sites through AMPA receptor activity and forms drebrin A-decorated F-actin (long helical pitch F-actin). Note that drebrin E frequently binds to the long helix F-actin, but rarely to normal F-actin in vivo. (c) Long helical pitch F-actin builds up a platform for assembly of glutamate receptors, CaMKII, spikar, and scaffold proteins such as PSD-95. (d) Postsynaptic density structure is constructed in a mature spine, and drebrin A-decorated F-actin is located relatively far from the postsynaptic membrane in a fully mature spine
3.3 Drebrin’s Function in Synaptic Plasticity
It has been shown that stable and dynamic F-actins localize at the core and periphery of dendritic spines, respectively (Honkura et al. 2008). As we mentioned, drebrin-decorated F-actin is stable with slow treadmilling and a longer helical crossover. Super resolution microscopy and electron microscopy (see details in Chap. 8) studies have shown that drebrin is located at the center of dendritic spines, indicating that stable F-actin at the core of dendritic spines consists of drebrin-decorated F-actin.
We have recently demonstrated that NMDA receptor-dependent Ca2+ influx activates myosin II ATPase and consequently induces the exodus of drebrin A-decorated F-actin from dendritic spines, resulting in the initiation of morphological synaptic plasticity (Mizui et al. 2014).
Taken together, we propose that synapses show morphological stability and reactivity when drebrin A-decorated F-actin is present and absent, respectively. When NMDA receptor is activated by a strong input that will induce synaptic plasticity (Hebbian plasticity), the spine gets the reactivity, because of the drebrin exodus from dendritic spines. After the spines have changed their morphology, drebrin reforms drebrin A-decorated F-actin at the core region and gives the morphological stability to the spines. This idea is consistent with our recent finding that synaptic plasticity is impaired in drebrin A-specific knockout (DAKO) mice (Kojima et al. 2016).
In addition to the aforementioned Hebbian synaptic plasticity, the brain has a homeostatic synaptic plasticity, which is a compensatory adjustment of synaptic excitability relative to the network activity. In 2001, we proposed that NMDA receptor accumulates through a specific trafficking system associated with drebrin and is anchored to F-actin via α-actinin at the postsynaptic membrane (Shirao and Sekino 2001). Moreover, 5 years later we showed that drebrin is involved in the mechanism of activity-dependent synaptic targeting of NMDA receptors using cultured neurons (Takahashi et al. 2006). Furthermore, Aoki et al. showed using DAKO mice that the lack of drebrin A results in the loss of homeostatic plasticity of NMDA receptor in vivo (Aoki et al. 2009).
In the brain of Alzheimer’s disease patients, drebrin disappears from dendritic spines even though the synapses are still there (Harigaya et al. 1996). Moreover, the decrease in drebrin is observed in relation to the cognitive impairment associated with normal aging (Hatanpaa et al. 1999). The loss of drebrin A means loss of stable drebrin A-decorated F-actin, suggesting that the compatibility between the stability and the reactivity of synapses dissociates in Alzheimer’s disease.
3.4 Drebrin’s Function in Intercellular Communication
Various drebrin-associated proteins have been found thick and fast. Interestingly, drebrin and its partner proteins are often found in the structure for cell-cell communication in recipient cells as well as the postsynaptic structure of neurons. Connexin 43 binds to drebrin E and forms a supramolecular complex of drebrin-decorated F-actin at the gap junction in astrocytes (Butkevich et al. 2004) and in migrating neurons (Ambrosi et al. 2016). At adherens junctions, drebrin E binds to afadin and acts as a stabilizer of nectins by linking the nectin-afadin complex to cortical F-actin and thus preserves endothelial integrity under shear stress (Fig. 1.4b) (Rehm et al. 2013).
In CD4+ T cells, the chemokine receptor, CXCR4, is associated with drebrin E (Fig. 1.4C) (Perez-Martinez et al. 2010). This association is enhanced by antigenic stimulation and is necessary for complete activation of CD4+ T cells. Furthermore, drebrin negatively regulates human immunodeficiency virus (HIV)-1 infection of CD4+ T lymphocytes, where CXCR4 is known as an HIV-1 co-receptor (Gordon-Alonso et al. 2013). Interestingly, drebrin E is a target of the immunosuppressant 3,5-bistrifluoromethyl pyrazole (BTP), which inhibits calcium influx into cells. Drebrin is necessary for the store-operated Ca2+ entry (Fig. 1.4c) (Mercer et al. 2010). Particularly, in mast cells, drebrin E regulates allergic responses, namely, the FcεRI-mediated increase in intracellular Ca2+ and the IgE-mediated histamine release (Law et al. 2015).
In the testis, drebrin E plays a role through association with the Arp2/3 complex at the ectoplasmic specialization, conferring plasma membrane plasticity, accommodating changes in spermatid shape, promoting germ cell transport, and inducing junction restructuring (Li et al. 2011).
Taken together, drebrin E functions as a dynamic linker between membrane proteins and submembranous F-actin structures, to regulate the intercellular communication.
3.5 Drebrin-Decorated F-Actin Can Link to Microtubules
Dynamic interactions between F-actin and microtubules underlie various cellular processes; however, the molecular mechanism involved was unknown. Drebrin-decorated F-actins in neurons are localized in adjoining zones to the microtubule-rich region; e.g., the dendritic spines (Fig. 1.4a) are in contact with the microtubule-rich dendritic shaft, and the transitional zone of the axonal growth cone (Fig. 1.4i) is between F-actins and microtubules. This characteristic localization suggests that drebrin may link microtubules to F-actin. In 2008, it was reported that drebrin binds to the microtubule plus-end binding protein, EB3, indicating that drebrin-decorated F-actin can link to microtubules via EB3 (Geraldo et al. 2008).
3.6 Drebrin and Cancer
Cancer cells break the intercellular connection found in normal cells and show motility ability as well as a dramatic change in cellular morphology. Because drebrin activity is involved in these cellular processes, the role of drebrin in carcinogenesis has been actively studied. In 2005, the change in drebrin E expression in skin cancers and their precursors was reported (Peitsch et al. 2005). Of particular note is the fact that drebrin E is markedly overexpressed in metastatic cancer cells (Lin et al. 2014). Recently, it has been reported that drebrin is critical for progranulin-dependent activation of the Akt and MAPK pathways and modulates motility, invasion, and anchorage-independent growth in bladder carcinomas (Xu et al. 2015).
4 Perspective and Future Directions
The compatibility between the stability and the reactivity of synapses plays a pivotal role in synaptic plasticity. Dendritic filopodia seem too motile to achieve this compatibility. On the other hand, dendritic spines are suitable for keeping this compatibility. Drebrin is present both in filopodia and spines, and the drebrin isoform change from E to A occurs in parallel with the change of filopodia into spines. Furthermore, inhibition of drebrin isoform conversion forms aberrant synapses, which impairs long-term potentiation (LTP). The difference between the two isoforms is the 46-amino-acid sequence, Ins2, which is inserted in the middle of the drebrin molecule by alternative splicing. It is of great interest to elucidate the role of this sequence in drebrin function. In addition, elucidation of the alternative splicing mechanism of drebrin may make an even greater impact on developmental neurobiology, because many brain proteins change their isoform by alternative splicing simultaneously with drebrin around the synaptogenesis period.
Drebrin forms slow treadmilling F-actin with a longer helical crossover. It is now believed that the structural heterogeneity of F-actin has an important physiological role by affecting the recruitment and occupancy of actin regulators. Drebrin-decorated F-actin is often found at the recipient side of various cell-cell communication systems, and the lack of drebrin leads to the dysfunction of cell-cell communication. In the brain, drebrin is postsynaptic, and its deficiency leads to dementia. In the immune system, drebrin is in the immunological synapses, regulating viral infection and modifying allergic responses. In carcinogenesis, drebrin is at intercellular adherens junctions, and its expression level is associated with cancer metastasis. Therefore, we propose that drebrin-decorated F-actin creates a unique platform for molecular assembly necessary for intercellular communication.
Notes
- 1.
This K d was quantified by densitometry of a Coomassie blue-stained SDS-PAGE gel for F-actin co-sedimentation assay. Therefore, we cannot exclude the possibility that the K d of drebrin and F-actin is less than 10 nM (see details in Chap. 3).
References
Allison DW, Chervin AS, Gelfand VI, Craig AM (2000) Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J Neurosci 20:4545–4554
Ambrosi C, Ren C, Spagnol G, Cavin G, Cone A, Grintsevich EE, Sosinsky GE, Sorgen PL (2016) Connexin43 forms Supramolecular complexes through non-overlapping binding sites for drebrin, tubulin, and ZO-1. PLoS One 11:e0157073
Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T (2005) Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol 483:383–402
Aoki C, Kojima N, Sabaliauskas N, Shah L, Ahmed TH, Oakford J, Ahmed T, Yamazaki H, Hanamura K, Shirao T (2009) Drebrin a knockout eliminates the rapid form of homeostatic synaptic plasticity at excitatory synapses of intact adult cerebral cortex. J Comp Neurol 517:105–121
Brackenbury R, Thiery JP, Rutishauser U, Edelman GM (1977) Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem 252:6835–6840
Bugyi B, Didry D, Carlier MF (2010) How tropomyosin regulates lamellipodial actin-based motility: a combined biochemical and reconstituted motility approach. EMBO J 29:14–26
Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I (2004) Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr Biol 14:650–658
Conroy WG, Ogden LF, Berg DK (2000) Cluster formation of alpha7-containing nicotinic receptors at interneuronal interfaces in cell culture. Neuropharmacology 39:2699–2705
Fucini RV, Navarrete A, Vadakkan C, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M (2000) Activated ADP-ribosylation factor assembles distinct pools of actin on golgi membranes. J Biol Chem 275:18824–18829
Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR (2008) Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol 10:1181–1189
Gordon-Alonso M, Rocha-Perugini V, Alvarez S, Ursa A, Izquierdo-Useros N, Martinez-Picado J, Munoz-Fernandez MA, Sanchez-Madrid F (2013) Actin-binding protein drebrin regulates HIV-1-triggered actin polymerization and viral infection. J Biol Chem 288:28382–28397
Harigaya Y, Shoji M, Shirao T, Hirai S (1996) Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's disease. J Neurosci Res 43:87–92
Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI (1999) Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol 58:637–643
Hayashi K, Shirao T (1999) Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci 19:3918–3925
Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T (1996) Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci 16:7161–7170
Hayashi K, Ishikawa R, Kawai-Hirai R, Takagi T, Taketomi A, Shirao T (1999) Domain analysis of the actin-binding and actin-remodeling activities of drebrin. Exp Cell Res 253:673–680
Hoffman S, Sorkin BC, White PC, Brackenbury R, Mailhammer R, Rutishauser U, Cunningham BA, Edelman GM (1982) Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem 257:7720–7729
Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H (2008) The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 57:719–729
Ikeda K, Kaub PA, Asada H, Uyemura K, Toya S, Shirao T (1996) Stabilization of adhesion plaques by the expression of drebrin A in fibroblasts. Brain Res Dev Brain Res 91:227–236
Inoue HK, Shirao T (1997) Neurite formation induced in neuroblastoma cells and genetically altered non-neuronal cells. J Electron Microsc 46:497–502
Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, Kohama K (1994) Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem 269:29928–29933
Ishikawa R, Katoh K, Takahashi A, Xie C, Oseki K, Watanabe M, Igarashi M, Nakamura A, Kohama K (2007) Drebrin attenuates the interaction between actin and myosin-V. Biochem Biophys Res Commun 359:398–401
Jin M, Tanaka S, Sekino Y, Ren Y, Yamazaki H, Kawai-Hirai R, Kojima N, Shirao T (2002) A novel, brain-specific mouse drebrin: cDNA cloning, chromosomal mapping, genomic structure, expression, and functional characterization. Genomics 79:686–692
Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415
Koganezawa N, Hanamura K, Sekino Y, Shirao T (2017) The role of drebrin in dendritic spines. Mol Cell Neurosci. doi:10.1016/j.mcn.2017.01.004
Kojima N, Kato Y, Shirao T, Obata K (1988) Nucleotide sequences of two embryonic drebrins, developmentally regulated brain proteins, and developmental change in their mRNAs. Brain Res 464:207–215
Kojima N, Shirao T, Obata K (1993) Molecular cloning of a developmentally regulated brain protein, chicken drebrin A and its expression by alternative splicing of the drebrin gene. Brain Res Mol Brain Res 19:101–114
Kojima N, Yasuda H, Hanamura K, Ishizuka Y, Sekino Y, Shirao T (2016) Drebrin A regulates hippocampal LTP and hippocampus-dependent fear learning in adult mice. Neuroscience 324:218–226
LaVail JH, Cowan WM (1971a) The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res 28:391–419
LaVail JH, Cowan WM (1971b) The development of the chick optic tectum. II. Autoradiographic studies. Brain Res 28:421–441
Law M, Lee Y, Morales JL, Ning G, Huang W, Pabon J, Kannan AK, Jeong AR, Wood A, Carter C et al (2015) Cutting edge: drebrin-regulated actin dynamics regulate IgE-dependent mast cell activation and allergic responses. J Immunol 195:426–430
Li B, Ding S, Feng N et al (2017a) Drebrin restricts rotavirus entry by inhibiting dynamin-mediated endocytosis. Proc Natl Acad Sci U S A 114:E3642–E3651
Li MW, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, Bonanomi M, Silvestrini B, Cheng CY (2011) Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis 1:123–136
Lin Q, Tan HT, Lim TK, Khoo A, Lim KH, Chung MC (2014) iTRAQ analysis of colorectal cancer cell lines suggests Drebrin (DBN1) is overexpressed during liver metastasis. Proteomics 14:1434–1443
Luna EJ, Pestonjamasp KN, Cheney RE, Strassel CP, Lu TH, Chia CP, Hitt AL, Fechheimer M, Furthmayr H, Mooseker MS (1997) Actin-binding membrane proteins identified by F-actin blot overlays. Soc Gen Physiol Ser 52:3–18
Mercer JC, Qi Q, Mottram LF, Law M, Bruce D, Iyer A, Morales JL, Yamazaki H, Shirao T, Peterson BR et al (2010) Chemico-genetic identification of drebrin as a regulator of calcium responses. Int J Biochem Cell Biol 42:337–345
Mikati MA, Grintsevich EE, Reisler E (2013) Drebrin-induced stabilization of actin filaments. J Biol Chem 288:19926–19938
Mizui T, Kojima N, Yamazaki H, Katayama M, Hanamura K, Shirao T (2009) Drebrin E is involved in the regulation of axonal growth through actin-myosin interactions. J Neurochem 109:611–622
Mizui T, Sekino Y, Yamazaki H, Ishizuka Y, Takahashi H, Kojima N, Kojima M, Shirao T (2014) Myosin II ATPase activity mediates the long-term potentiation-induced exodus of stable F-actin bound by drebrin A from dendritic spines. PLoS One 9:e85367
Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, Nachury MV (2017) An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168(252–263):e214
Ngai PK, Walsh MP (1984) Inhibition of smooth muscle actin-activated myosin Mg2+−ATPase activity by caldesmon. J Biol Chem 259:13656–13659
O’Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Peitsch WK, Grund C, Kuhn C, Schnolzer M, Spring H, Schmelz M, Franke WW (1999) Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol 78:767–778
Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW (2005) Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes. J Invest Dermatol 125:761–774
Peitsch WK, Bulkescher J, Spring H, Hofmann I, Goerdt S, Franke WW (2006) Dynamics of the actin-binding protein drebrin in motile cells and definition of a juxtanuclear drebrin-enriched zone. Exp Cell Res 312:2605–2618
Perez-Martinez M, Gordon-Alonso M, Cabrero JR, Barrero-Villar M, Rey M, Mittelbrunn M, Lamana A, Morlino G, Calabia C, Yamazaki H et al (2010) F-actin-binding protein drebrin regulates CXCR4 recruitment to the immune synapse. J Cell Sci 123:1160–1170
Rehm K, Panzer L, van Vliet V, Genot E, Linder S (2013) Drebrin preserves endothelial integrity by stabilizing nectin at adherens junctions. J Cell Sci 126:3756–3769
Sekino Y, Tanaka S, Hanamura K, Yamazaki H, Sasagawa Y, Xue Y, Hayashi K, Shirao T (2006) Activation of N-methyl-D-aspartate receptor induces a shift of drebrin distribution: disappearance from dendritic spines and appearance in dendritic shafts. Mol Cell Neurosci 31:493–504
Sekino Y, Kojima N, Shirao T (2007) Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int 51:92–104
Sharma S, Grintsevich EE, Phillips ML, Reisler E, Gimzewski JK (2011) Atomic force microscopy reveals drebrin induced remodeling of f-actin with subnanometer resolution. Nano Lett 11:825–827
Sharma S, Grintsevich EE, Hsueh C, Reisler E, Gimzewski JK (2012) Molecular cooperativity of drebrin1-300 binding and structural remodeling of F-actin. Biophys J 103:275–283
Shiraishi-Yamaguchi Y, Sato Y, Sakai R, Mizutani A, Knopfel T, Mori N, Mikoshiba K, Furuichi T (2009) Interaction of Cupidin/Homer2 with two actin cytoskeletal regulators, Cdc42 small GTPase and drebrin, in dendritic spines. BMC Neurosci 10:25
Shirao T, Obata K (1985) Two acidic proteins associated with brain development in chick embryo. J Neurochem 44:1210–1216
Shirao T, Obata K (1986) Immunochemical homology of 3 developmentally regulated brain proteins and their developmental change in neuronal distribution. Brain Res 394:233–244
Shirao T, Sekino Y (2001) Clustering and anchoring mechanisms of molecular constituents of postsynaptic scaffolds in dendritic spines. Neurosci Res 40:1–7
Shirao T, Inoue HK, Kano Y, Obata K (1987) Localization of a developmentally regulated neuron-specific protein S54 in dendrites as revealed by immunoelectron microscopy. Brain Res 413:374–378
Shirao T, Kojima N, Kato Y, Obata K (1988) Molecular cloning of a cDNA for the developmentally regulated brain protein, drebrin. Brain Res 464:71–74
Shirao T, Kojima N, Obata K (1992) Cloning of drebrin A and induction of neurite-like processes in drebrin-transfected cells. Neuroreport 3:109–112
Shirao T, Hayashi K, Ishikawa R, Isa K, Asada H, Ikeda K, Uyemura K (1994) Formation of thick, curving bundles of actin by drebrin A expressed in fibroblasts. Exp Cell Res 215:145–153
Shirao T, Hanamura K, Koganezawa N, Ishizuka Y, Yamazaki H, Sekino Y (2017) The role of drebrin in neurons. J Neurochem 141(6):819–834
Shoop RD, Yamada N, Berg DK (2000) Cytoskeletal links of neuronal acetylcholine receptors containing alpha 7 subunits. J Neurosci 20:4021–4029
Song M, Kojima N, Hanamura K, Sekino Y, Inoue HK, Mikuni M, Shirao T (2008) Expression of drebrin E in migrating neuroblasts in adult rat brain: coincidence between drebrin E disappearance from cell body and cessation of migration. Neuroscience 152:670–682
Sperry RW (1963) Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A 50:703–710
Stiber JA, Wu JH, Zhang L, Nepliouev I, Zhang ZS, Bryson VG, Brian L, Bentley RC, Gordon-Weeks PR, Rosenberg PB et al (2016) The actin-binding protein drebrin inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol 36:984–993
Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T (2003) Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci 23:6586–6595
Takahashi H, Mizui T, Shirao T (2006) Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J Neurochem 97(Suppl 1):110–115
Takahashi H, Yamazaki H, Hanamura K, Sekino Y, Shirao T (2009) Activity of the AMPA receptor regulates drebrin stabilization in dendritic spine morphogenesis. J Cell Sci 122:1211–1219
Tanabe K, Yamazaki H, Inaguma Y, Asada A, Kimura T, Takahashi J, Taoka M, Ohshima T, Furuichi T, Isobe T et al (2014) Phosphorylation of drebrin by cyclin-dependent kinase 5 and its role in neuronal migration. PLoS One 9:e92291
Toda M, Shirao T, Minoshima S, Shimizu N, Toya S, Uyemura K (1993) Molecular cloning of cDNA encoding human drebrin E and chromosomal mapping of its gene. Biochem Biophys Res Commun 196:468–472
Trisler GD, Schneider MD, Nirenberg M (1981) A topographic gradient of molecules in retina can be used to identify neuron position. Proc Natl Acad Sci U S A 78:2145–2149
Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M et al (1999) Coupling of mGluR/Homer and PSD-95 complexes by the shank family of postsynaptic density proteins. Neuron 23:583–592
Worth DC, Daly CN, Geraldo S, Oozeer F, Gordon-Weeks PR (2013) Drebrin contains a cryptic F-actin-bundling activity regulated by Cdk5 phosphorylation. J Cell Biol 202:793–806
Xu SQ, Buraschi S, Morcavallo A et al (2015) A novel role for drebrin in regulating progranulin bioactivity in bladder cancer. Oncotarget 6:10825–10839
Yamaguchi M, Ver A, Carlos A, Seidel JC (1984) Modulation of the actin-activated adenosinetriphosphatase activity of myosin by tropomyosin from vascular and gizzard smooth muscles. Biochemistry 23:774–779
Yamazaki H, Kojima N, Kato K, Hirose E, Iwasaki T, Mizui T, Takahashi H, Hanamura K, Roppongi RT, Koibuchi N et al (2014) Spikar, a novel drebrin-binding protein, regulates the formation and stabilization of dendritic spines. J Neurochem 128:507–522
Yamazaki H, Sasagawa Y, Yamamoto H, Shirao T. CaMKIIβ is localized in dendritic spines as both drebrin-dependent and drebrin-independent pools (submitted)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Shirao, T., Sekino, Y. (2017). General Introduction to Drebrin. In: Shirao, T., Sekino, Y. (eds) Drebrin. Advances in Experimental Medicine and Biology, vol 1006. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56550-5_1
Download citation
DOI: https://doi.org/10.1007/978-4-431-56550-5_1
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56548-2
Online ISBN: 978-4-431-56550-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)