Abstract
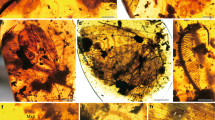
Gracillariidae, the family to which Epicephala belongs, is a large group of miniature moths with roughly 100 recognized genera and 2000 recognized species (De Prins and De Prins 2016). They have a global distribution and are found almost everywhere there are plants, except for extremely harsh environments (e.g., the arctic). Gracillariidae is one of several lepidopteran families that consist almost entirely of leaf-mining species, although the leaf-mining habit itself is known to occur in about 30 moth families (Powell et al. 1999). In most gracillariid species, early-instar larvae have remarkably flat head capsules without chewing mandibles, and feed exclusively on cell sap within the nongreen, epidermal layer of the leaf (sap feeder; Fig. 5.1). Later-instar larvae then feed on the palisade layer and finally the spongy layer of the leaf with functional mandibles, and excrete granular frass (tissue feeder; Fig. 5.1). The larvae of the genus Phyllocnistis are exceptional in that they spend all their instars as sap feeders in the leaf epidermal layer. Gracillariid moths are thus unique among insects in that they undergo hypermetamorphosis, a process by which some larval instars become functionally and morphologically distinct from other instars.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Active Pollination
- Epicephala
- Gracillariidae
- Flueggea
- Glochidion
- Ornixolinae
- Phyllanthus
- Phylogeny
- Proboscis
- Seed parasite
1 Diversity and Classification of Gracillariidae
Gracillariidae , the family to which Epicephala belongs, is a large group of miniature moths with roughly 100 recognized genera and 2000 recognized species (De Prins and De Prins 2016). They have a global distribution and are found almost everywhere there are plants, except for extremely harsh environments (e.g., the arctic). Gracillariidae is one of several lepidopteran families that consist almost entirely of leaf-mining species, although the leaf-mining habit itself is known to occur in about 30 moth families (Powell et al. 1999). In most gracillariid species, early-instar larvae have remarkably flat head capsules without chewing mandibles, and feed exclusively on cell sap within the nongreen, epidermal layer of the leaf (sap feeder ; Fig. 5.1). Later-instar larvae then feed on the palisade layer and finally the spongy layer of the leaf with functional mandibles, and excrete granular frass (tissue feeder ; Fig. 5.1). The larvae of the genus Phyllocnistis are exceptional in that they spend all their instars as sap feeders in the leaf epidermal layer. Gracillariid moths are thus unique among insects in that they undergo hypermetamorphosis , a process by which some larval instars become functionally and morphologically distinct from other instars.
Various feeding habits of gracillariid moths. (a) Early-instar Gibbovalva quadrifasciata (Acrocercopinae) larvae mining Cinnamomum doederleinii. Sap-feeding larvae mine the epidermal layer of the leaf and produce linear mines, and later tissue-feeding larvae consume the leaf tissue within the expanded blotch mine. (b) Late-instar Psydrocercops wisteriae (Acrocercopinae) larva-mining a leaflet of Wisteria floribunda. (c) Mine of Phyllocnistis sp. (Phyllocnistinae) on Ilex pedunculosa. Pyllocnistinae larvae are sap-feeders throughout the larval stage and only use the epidermal layer of the host leaf. (d, e) Tentiform mine of Phyllonorycter lyoniae (Lithocolletinae) viewed from adaxial (d) and abaxial (e) sides of the leaf. Phyllonorycter larvae line the inner walls of mines with silk on the abaxial side, causing the mine eventually to become tentiform as the silk dries and wrinkles. Note that the initial instars are sap-feeding (arrow). (f) Diphtheroptila scriptulata (Ornixolinae) larva mining Glochidion acuminatum. Many species of Ornixolinae expand the mine as they feed on the leaf tissue. (g, h) Caloptilia ryukyuensis (Gracillariinae) on Glochidion zeylanicum. Most Caloptilia species are sap-feeders as early instars (g), whereas late-instar larvae roll the leaf apex and feed on the leaf externally within the rolled leaf (h). (i) Galls of Caloptilia cecidophora (Gracillariinae) on Glochidion obovatum. This species is exceptional among Gracillariidae for its gall-inducing habit
Sap feeding is an apomorphy among Gracillariidae that clearly distinguishes them behaviorally from other leaf-mining Lepidoptera . The extent to which their evolutionary success has been facilitated by sap feeding is a fascinating question that merits further study. Sap-feeding instars are secondarily lost in Spinivalva (leaf miner of Passiflora ; Brito et al. 2013) and in several non-leaf-mining genera, including Epicephala .
The seed-feeding habit of Epicephala is unique within Gracillariidae. Nevertheless, deviations from the typical leaf-mining habit occur in a number of groups. Caloptilia species, which are common herbivores of Glochidion plants (Chap. 7), are sap-feeders as early instars, but later-instar larvae construct leaf roll s in which they live for the rest of the larval period. Larvae inside leaf rolls feed externally on the inner portions of folded leaves (Fig. 5.1). Galling has evolved in a number of gracillariid genera independently, for example, in Caloptilia (Kumata 1966; Fig. 5.1), Borboryctis (Kumata et al. 1988), and Parectopa (Wise 1962). Some species mine plant parts other than the leaf, such as stems or branches ( Marmara , Dendrorycter , Spulerina ) or fruit peel ( Marmara , Spulerina ). Others are plant-borers that use the seed ( Epicephala , Conopomorpha ), bud ( Stomphastis , Conopobathra , Chileoptilia ; Vargas and Landry 2005), or stem gall induced by rust fungi ( Polysoma ; Bashford 2002). Presumably, many more feeding types await discovery. Although the ultimate factor facilitating transitions from leaf mining to alternative feeding habits remains unknown, escape from parasitoid attack is probably a major reason for its evolution, as the structures they construct (leaf roll), induce (gall), or utilize (seed, bud, or fungal gall) are often protective against oviposition by parasitoids.
Gracillariidae species generally have narrow diet s, and each specializes upon one or a few species in a single plant genus or, less commonly, in multiple related genera. Exceptions include Acrocercops transecta , which feeds on Juglandaceae and Ericaceae (Ohshima 2008); Calybites phasianipennella , which uses Polygonaceae and Myrsinaceae (Kumata 1982); and several stem- or fruit-feeding species with exceptionally wide host ranges spanning multiple families ( Marmara , Conopomorpha ). Hosts of Gracillariidae are found in 93 diverse angiosperm families, although only three gracillariid species are known to feed on monocots: Acrocercops maranthaceae (Maranthaceae ; Busck 1934), Marmara smilacisella (Smilacaceae ; Braun 1909), and Gibbovalva tricuneatella (Typhaceae ; Sugisima et al. 2005). Parectopa leucocyma , the only known gymnosperm leaf-miner, feeds on Agathis australis in New Zealand (Wise 1962), and several undescribed gracillariid species are found on Podocarpus and Gnetum in Japan and Southeast Asia, respectively. No gracillariids are known to attack fern s or bryophyte s. For reasons presently unknown, Gracillariidae predominantly use woody hosts, a pattern also observed in certain other groups of leaf-miners (e.g., Nepticulidae , Heliozelidae ). The scarcity of monocot hosts may thus reflect the rarity of woody monocots rather than a true lack of preference.
Gracillariidae species have traditionally been classified into four subfamilies: Gracillariinae , Lithocolletinae , Phyllocnistinae , and Oecophyllembiinae (Kumata et al. 1988a,b; Kumata 1998; note that the circumscription of subfamilies varies among authors). Among these families, Gracillariinae contains by far the largest number of species (>1380), and it has been further divided by Kumata et al. (1988a,b) into four genus groups: Parornix-, Acrocercops-, Gracillaria-, and Parectopa-groups. Kawahara et al. (2017) recently provided a robust phylogenetic framework for the family based on the sequences of 22 genes, and proposed a new classification consisting of eight subfamilies: Acrocercopinae , Gracillariinae , Lithocolletinae , Marmarinae , Oecophyllembiinae , Ornixolinae , Parornichinae , and Phyllocnistinae (Table 5.1).
Epicephala belongs to the Ornixolinae subfamily (corresponding to the Parectopa group of Kumata et al. 1988a,b), which, unlike other subfamilies that have high species diversity in temperate zones, is concentrated in the tropics. Therefore, the numbers of genera and species are likely to increase dramatically with further taxonomic studies. Ornixolinae is also unique among Gracillariidae in that it contains a disproportionately high number of non-leaf-mining species. In fact, the plant borers listed above ( Epicephala , Conopomorpha , Stomphastis , Conopobathra , Chileoptilia , and Polysoma ) all belong to Ornixolinae. Thus, there may have been an evolutionary precursor within the Ornixolinae lineage that enabled repeated transitions from leaf-mining to boring feeding habits, giving rise to Epicephala.
2 Phylogeny of Epicephala
The genus Epicephala was described by Meyrick (1881) based on specimens collected in Sydney, Australia. Meyrick, the founder of modern Microlepidoptera systematics, made the greatest contribution to Epicephala taxonomy, eventually describing more than 20 Epicephala species from various parts of the Old World . However, several of the species described by Meyrick (e.g., Stomphastis chalybacma ) were transferred to other genera after detailed examination of genital morphology by later authors. A few more species currently placed in Epicephala will likely be subject to such taxonomic rearrangements. Concurrently with Meyrick, Turner greatly advanced the knowledge of Epicephala fauna in Australia , naming 11 currently accepted species. He was the first to note that Epicephala are seed-feeders; he observed Epicephala adults emerging from the capsules of Glochidion ferdinandii in Brisbane, Australia (Turner 1913). He named the moth E. frugicola , but later synonymized the species with E. colymbetella , the type species of the genus. Later, Vári (1961) described six Epicephala species from South Africa and provided the first detailed illustrations of the genitalia for Epicephala (as well as for many other genera of Gracillariidae). The study of Epicephala slowed down during the late twentieth century, during which time only three species were described (from Russia , Nigeria , and the Marquesas Islands ).
The taxonomy of Epicephala has attracted renewed interest since the discovery of obligate pollination mutualism (Kato et al. 2003). Li and colleagues, working on Epicephala found in China , described 20 species associated with Glochidion, Breynia, and Phyllanthus, and provided detailed descriptions of adult behavior and morphology for some of them. Kawakita and Kato (2016) reviewed the Japanese fauna of Epicephala and described seven species. As of April 2016, the genus contains 64 species (Table 5.2; Fig. 5.2). However, ecological, molecular, and biogeographical data suggest that the genus contains several hundred species (Kawakita 2010). For example, some clades of Epicephala are confined to Madagascar or New Caledonia , where none of the described Epicephala species occurs (Kawakita and Kato 2009). These regions are known for hotspots of Phyllanthus diversity, and thus potentially have large numbers of undescribed Epicephala species. There is also a high level of Phyllanthus diversity in the New World , where Epicephala has not been previously recorded; furthermore, as detailed in the following section, Epicephala is also prevalent in the Neotropics . Accelerating the taxonomy of Epicephala at a global scale is therefore critical for facilitating the ecological and evolutionary study of this model group.
Representative specimens of the nine Epicephala species in Japan. Variation in wing pattern among species is low, and is of minor importance in morphological identification. Variation in size largely reflects host seed size. The wing pattern of E. parasitica is sexually dimorphic, so specimens of both sexes are shown for this species. (a) E. anthophilia (Amami Island, Kagoshima, ♀, host: Glochidion acuminatum). (b) E. bipollenella (Henoko, Okinawa, ♀, host: G. zeylanicum). (c) E. lanceolatella (Cape Hedo, Okinawa, ♀, host: G. lanceolatum). (d) E. perplexa (Cape Hedo, Okinawa, ♀, host: G. lanceolatum). (e) E. obovatella (Tomogashima, Wakayama, ♂, host: G. obovatum and G. rubrum). (f) E. corruptrix (Takae, Okinawa, ♀, host: G. obovatum and G. rubrum). (g) E. vitisidaea (Yona, Okinawa, ♀, host: Breynia vitis-idaea). (h) E. parasitica (Yonaguni Island, Okinawa, ♀, host: Phyllanthus lepidocarpus). (i) E. parasitica (Hateruma Island, Okinawa, ♂, host: P. lepidocarpus). (j) E. nudilingua (Watarase-yusuichi, Tochigi, ♀, host: P. ussuriensis). Scale bar: 5 mm
Figure 5.3 shows the most recent analysis of the phylogenetic relationships among Epicephala (Kawakita and Kato 2016). Seven clades can be recognized, each of which is generally associated with a particular clade of Phyllantheae. An exception is Clade 2, which consists of species associated with herbaceous Phyllanthus belonging to various Phyllanthus subgenera. Conopomorpha flueggella , which is a nonpollinating seed-feeder of Flueggea suffruticosa , is depicted as a sister to Epicephala in Fig. 5.3. However, the phylogenetic position of C. flueggella is contentious because the species is sometimes placed as a sister to Clade 7 (Fig. 5.3). Conopomorpha flueggella clearly lacks pollination behavior, and lays eggs in buds or young fruits as well as flowers (Fig. 5.4). It also lacks an ovipositor , placing its eggs on the surface of the ovule (Fig. 5.4). Interestingly, ovipositing females sometimes take nectar with their proboscises prior to oviposition (Fig. 5.4) in a manner similar to Epicephala’s use of its proboscis to pollinate. The nectaring behavior of C. flueggella may have been the evolutionary precursor for active pollination; however, this question requires a robust understanding of the phylogenetic placement of C. flueggella with respect to Epicephala. At present, it is equally probable that the behavior of C. flueggella represents a secondary loss of pollination behavior. A hatched C. flueggella larva usually consumes all six seeds contained in each fruit of Flueggea suffruticosa (Fig. 5.4).
Phylogeny of Epicephala. The tree is based on a molecular phylogenetic analysis by Kawakita and Kato (2009). Host plant associations are provided below clade numbers. The phylogenetic position of Conopomorpha flueggella is inconsistent among analyses; an alternative placement as a sister to Clade 7 is also indicated. Clade triangle size is proportional to the estimated number of species in each clade. Major evolutionary events are listed on branches. Lineages indicated in grey do not possess the pollination behavior
A nonpollinating, seed-parasitic moth, Conopomorpha flueggella. (a) Female moth depositing an egg underneath the horizontally spread styles. (b) Female moth taking nectar from nectary at the base of the ovary. Note that the moth proboscis lacks sensilla and does not bear pollen (arrow). A droplet of nectar collected at the base of the ovary and protruded through the tepals can be seen (arrow). (c) Moth egg laid externally on the surface of the ovary. (d) Mature fruit with exit hole excavated by moth larva. Seeds are usually entirely destroyed in such fruits
Conopomorpha flueggella was described by Hu et al. (2011), who placed it in the genus Conopomorpha based mainly on wing morphology. However, the true Conopomorpha , which include species that attack seeds of tropical fruit trees such as lychee and longan (Sapindaceae ) and cacao (Malvaceae ), are distantly related to Conopomorpha flueggella or Epicephala within Ornixolinae. Therefore, C. flueggella can likely be more accurately placed in a separate genus.
The basal-most Epicephala are the clade of moths associated with the Phyllanthus section Gomphidium in New Caledonia (Fig. 5.3). They clearly exhibit active pollination behavior (Chap. 3), but lack the sensilla on the proboscis and ovipositor that characterize the derived members of Epicephala. As such, they retain the morphology of the earliest Epicephala to acquire active pollination behavior. They lay eggs on the surface of the pistils (Fig. 5.5), and a single hatched larva consumes all the seeds contained in each fruit. However, moth mortality is very high, probably owing to desiccation during the egg stage. This high mortality is necessary for a fraction of the seeds to remain intact despite destructive seed-feeding by the larvae (Chap. 3). Because there is a large morphological disparity between moths of this clade and those of the derived Epicephala, especially with respect to the female genitalia, the former should probably be placed in a separate genus. However, determining whether it is congeneric with C. flueggella requires a closer examination of morphology and a better resolution of the basal phylogenetic relationships.
Difference in the placement of eggs by Epicephala moths with and without ovipositors. Roughly half of the species associated with New Caledonian Phyllanthus (Clade 7 in Fig. 5.3) do not possess ovipositors and oviposit externally (a, b) whereas species of derived clades have well-developed ovipositors and lay eggs internally in floral tissue (c, d). (a) Female flower of Phyllanthus kouaouaensis with an Epicephala moth egg laid externally on style surface (arrow). (b) Female flowers of P. cf. koniamboensis with Epicephala eggs. (c) Female E. eriocarpa with extended ovipositor (arrow). (d) Vertical section of Glochidion zeylanicum female flower containing an Epicephala moth egg (arrow). Ova, ovary; ovu, ovule; st, style; te, tepal
The remaining moths can safely be placed in Epicephala, and share the female ovipositor as a morphological synapomorphy (Clades 1–6; Figs. 5.3, 5.5). Ovipositors are not known in any other Gracillariidae genus; thus, aside from pollination behavior, they represent the trait that best characterizes Epicephala. The development of the ovipositor is most likely an adaptation for laying eggs internally in floral tissue, thereby avoiding egg desiccation (Fig. 5.5).
Interestingly, there is considerable variation in egg placement behavior among Epicephala species. Most Epicephala species associated with Glochidion lay eggs either through the apical pit of the stylar column into the stylar tissue or laterally through the ovary wall on the surface of the ovule (Fig. 5.6). This difference in oviposition mode is reflected in morphology; the ovipositor s of laterally ovipositing species are distinctly more angular than those of apically ovipositing species (Kawakita and Kato 2016; Fig. 5.6).
Oviposition behavior (a–f) and ovipositor morphology (g–l) of six Epicephala species associated with Glochidion in Japan. (a) E. anthophilia ovipositing through stylar pit of G. acuminatum flower. (b) E. bipollenella ovipositing through stylar pit of G. zeylanicum flower. (c) E. lanceolatella ovipositing through stylar pit of G. lanceolatum flower. (d) E. perplexa ovipositing through lateral ovary wall of G. lanceolatum flower. The ovipositor of this species penetrates both the tepal and the ovary wall. (e) E. obovatella ovipositing through lateral ovary wall of G. obovatum flower. The ovipositor of this species is inserted directly into the ovary and does not penetrate the tepal. (f) E. corruptrix ovipositing through ovary wall of G. rubrum flower. Similar to that of E. obovatella, the ovipositor of this species also does not penetrate the tepal. (g) E. anthophilia. (h) E. bipollenella. (i) E. lanceolatella. (j) E. perplexa. (k) E. obovatella. (l) E. corruptrix. Note that the ovipositors of species that oviposit through lateral ovary walls are distinctly angular
Certain species associated with Phyllanthus and Glochidion lay eggs in the pedicels of female flowers (Chap. 10). In such species, the hatched larvae initially bore through the pedicel to enter the ovary. Epicephala vitisidaea and E. mirivalvata lay eggs in the narrow space between the sepals and ovary of their host Breynia plants, having thus reverted to external oviposition (Kawakita and Kato 2004; Zhang et al. 2012a), although both species have retained functional ovipositors. Because Phyllantheae plants are known to abort selectively those flowers with heavy egg loads and abortion is likely based on the extent of mechanical damage to flowers (Chap. 9), external oviposition in these species may have evolved to circumvent the abortion response in their host plants.
However, the adaptive significance of other oviposition modes is less clear. In cases where two or more Epicephala species co-occur on the same Phyllantheae host, the different species exhibit different oviposition behaviors (Kawakita et al. 2015; Kawakita and Kato 2016). This may indicate that different oviposition strategies are necessary for stable coexistence on shared hosts.
Another distinguishing characteristic of the core Epicephala clade is the numerous sensilla on the proboscis es of females (Fig. 5.7). Sensilla are absent in males, and a sensilla-bearing proboscis is not known in any other genera of Gracillariidae. Thus, we can infer that the function of the sensilla is linked to active pollination. Because most pollen is held on the basal area of the proboscis of pollen-carrying females where the density of sensilla is highest, females are able to carry more pollen than would be possible in the absence of such a structure (Fig. 5.7). Support for the interpretation that the sensilla assist in pollination comes from the absence of sensilla in species that have secondarily lost the pollination behavior. For example, of the six major lineages of the core Epicephala clade, the herbaceous Phyllanthus clade consists of species that lay eggs in young fruits of herbaceous Phyllanthus and thus do not pollinate flowers. Accordingly, the sensilla on their proboscises are lost entirely or are rudimentary (Fig. 5.7). The Phyllanthus reticulatus clade represents another Epicephala lineage that lost the sensilla. Of the six species associated with plants of the Phyllanthus reticulatus species complex in Taiwan, three induce gall formation on female flowers, although two of them retain pollination behavior. Sensilla are completely lost in the three gall-inducing species, probably because selection to transfer pollen is relaxed or absent owing to their ability to induce galls (Chap. 11).
Proboscis sensilla. (a, b) Scanning electron micrographs of female (a) and male (b) proboscises of actively pollinating Epicephala species (Epicephala sp. associated with Phyllanthus reticulatus). Note that numerous sensilla are present on the female proboscis, whereas they are absent on the male proboscis. (c) E. anthophilia female actively depositing pollen on G. acuminatum stigma in Amami Island, Japan. Sensilla on moth proboscises allow more pollen to be held on the proboscis. (d) Proboscis of female E. bipollenella bearing sensilla. (e) Proboscis of female E. nudilingua, a nonpollinating species, lacking sensilla. (f) E. parasitica, a nonpollinating species, ovipositing in a young fruit of P. lepidocarpus in Ishigaki Island, Japan. (g) Another nonpollinating species (E. sp.) ovipositing in a very young fruit of P. amarus through leaves folded at night in Thakhek, Laos. Scale bar: 0.1 mm
As mentioned in the previous section, gall-inducers have arisen repeatedly in the course of gracillariid evolution, but the evolution of galling may be particularly common in Epicephala. In the Ryukyu Archipelago of southern Japan, E. corruptrix is associated with two Glochidion species ( G. obovatum and G. rubrum ). On both hosts, E. corruptrix exhibits pollination behavior, but the resulting fruits do not develop normally. Instead, the locule in which the larva develops grows irregularly and eventually becomes a gall (Fig. 5.8). Populations of G. obovatum and G. rubrum associated with E. corruptrix suffer very low seed production. This contrasts sharply with populations elsewhere in their ranges, where the plants are pollinated by non-gall-inducing E. obovatella , and produce large numbers of normal fruits and seeds. Epicephala corruptrix is distantly related to the gall-inducers of the Phyllanthus reticulatus species complex, so the galling habit has independently evolved at least twice in Epicephala. Similar gall-like development is found in P. humbertii in Madagascar and P. cuscutiflorus in Australia. It is therefore interesting to consider how many other lineages of Epicephala evolved the gall-inducing ability. The adaptive significance of galling is still unclear, but is probably linked to escape from parasitoid attack (Chap. 11).
Fruits and galls produced by Epicephala species on Glochidion obovatum. (a) Fruit produced after pollination by E. obovatella. (b) Gall induced on female flower by E. corruptrix. (c) Cross-section of the gall induced by E. corruptrix. Arrow indicates the galled locule with feeding traces of Epicephala larva. Note that the irregularly developed ovules of the galled locule have merged and become indistinguishable from septa. Scale bar: 2 mm
3 Global Diversity of Epicephala
The genus Epicephala has thus far been described only in the Old World tropics , but they are also prevalent in the Neotropics . Observation of Phyllanthus in Cuba and Jamaica indicate that several species in the subgenus Xylophylla are associated with seed-feeding Epicephala. Some of the these Phyllanthus species (e.g., P. chamaecrystoides , P. myrtilloides ) bear female flowers with fused styles characteristic of Epicephala-pollinated plants in the Old World, whereas others possess bifid and spread styles indicative of pollination by nonspecialized insects (e.g., P. angustifolius , P. nutans ). Whether obligate pollination mutualism occurs in Xylophylla is still unclear, but the outcome of association between Xylophylla and Epicephala appears to be highly variable among species. There are roughly 60 species of Xylophylla, with the highest concentration of species being in the Caribbean islands, although the subgenus occurs as far south as Andean Peru.
The widespread occurrence of Epicephala in the New World is further demonstrated by the presence of Epicephala larvae and pupae on herbarium specimens (Fig. 5.9). Because plant specimens bearing fruits may sometimes contain Epicephala larvae at the time of collection, the larvae are occasionally found on Phyllanthus specimens, especially those in which the capsules dehisced while being dried. The larvae of most Epicephala species have a characteristic red color with narrow white bands, and cannot be mistaken for those of other Lepidoptera. Mature larvae inside fruits may also spin cocoon s on the edges of leaves before they are completely dried. While spinning cocoons, the larvae of Epicephala excrete bubblelike balls from the anus, grab them with the mandibles, and attach them to the surface of the cocoon through a hole punched from inside the cocoon (Fig. 5.9). Although the adaptive role of ball production is unknown, such a habit is known only in Epicephala and several related genera of the Ornixolinae . Therefore, the presence of such cocoons on herbarium specimens provides reliable evidence of an association with Epicephala.
Larva and cocoon of Epicephala moth. (a) Larva of Epicephala sp. associated with P. reticulatus in Taiwan. The number and position of white bands varies among species. (b) Cocoon of E. bipollenella whose surface is decorated with bubbles. The moth has emerged from the cocoon, and the exuvia can be seen. (c) Herbarium specimen of Phyllanthus mocinianus at the herbarium of the University of California, Davis. The herbarium sheet is attached with an envelope (arrow) containing detached plant parts (mostly dehisced capsules and seeds), among which Epicephala larvae are sometimes found. (d) Dried Epicephala larva found inside the envelope. (e) An Epicephala cocoon found on a detached leaf in the envelope
Epicephala larvae and cocoons are often found on specimens of Phyllanthus section Nothoclema (subgenus Conami; Fig. 5.9; Table 5.3). The group contains 10 species distributed from Mexico to Argentina, and is often a prominent component of the local flora. The female flowers of the Nothoclema species have spread styles and do not appear to be specialized to Epicephala; thus, it would be interesting to clarify the pollination system of Nothoclema plants to understand whether obligate pollination mutualism occurs in the New World. One surprising finding was an association between Epicephala and Flueggea elliptica (Table 5.3), a plant that is only known from a small coastal area in southernmost Ecuador . The Old World Flueggea suffruticosa is host to Conopomorpha flueggella and Epicephala relictella , but other Flueggea species, such as the widespread and abundant F. virosa , have never been found hosting Epicephala. The Flueggea–Epicephala association in the New World is probably phylogenetically independent from that found in Asia, but determining where the Ecuadorian species belong within the Epicephala phylogeny is critical for the global understanding of Epicephala diversity and evolution.
Finally, one exciting possibility is the occurrence of obligate pollination mutualism on the tepuis of the Guiana Highlands . The tepui s are table-top mountains of granite arenite sandstone rising abruptly from the Amazonian rainforest that are host to a spectacular array of endemic plants and animals. The majority of the tepuis occur in Venezuela and Western Guyana , but some are also found in Colombia , Suriname , French Guiana , and in northernmost Brazil .
The Phyllanthus species found in the Guiana Highlands are grouped into a well-defined section, Microglochidion (Webster and Carpenter 2002, 2008). Many species possess elongated, nonbifid, and fused styles, which strikingly resemble those of Glochidion or New Caledonian Phyllanthus. Although neither larvae nor cocoons of Epicephala were found on herbarium specimens, the seeds of several Microglochidion species have holes that are typical of those made by Epicephala larvae, suggesting that the association with Epicephala is widespread among the ~10 species of Guiana Highland Phyllanthus (Table 5.3). It is exciting to think of the possibility that obligate pollination mutualism, which probably originated in the Old World, has reached some of the most exotic biota on earth and produced an impressive number of endemic species.
Literature Cited
Bashford R (2002) The insect fauna inhabiting Uromycladium (Uredinales) rust galls on silver wattle (Acacia dealbata) in Tasmania. Aust Entomol 29:81–95
Bland KP (1980) Nigerian Gracillariidae. J Lepidop Soc 34:25–35
Braun AF (1909) Notes on Chamber’s species of Tineina. Entomol News 20:428–434
Brito R, Gonçalves GL, Vargas HA, Moreira GR (2013) A new Brazilian Passiflora leafminer: Spinivalva gaucha, gen. n., sp. n. (Lepidoptera, Gracillariidae, Gracillariinae), the first gracillariid without a sap-feeding instar. Zookeys 17:1–26
Busck A (1934) Microlepidoptera of Cuba. Entomol Am 13:151–217
Clarke JFG (1986) Pyralidae and microlepidoptera of the Marquesas Archipelago. Smithson Contr Zool 416:1–485
De Prins J, De Prins W (2005) World catalogue of insects, Volume 6: Gracillariidae (Lepidoptera). Apollo Books, Svendborg
De Prins J, De Prins W (2016) Global taxonomic database of Gracillariidae (Lepidoptera). Available at: http://www.gracillariidae.net
Diakonoff A (1955) Microlepidoptera of New Guinea. Results of the third Archbold Expedition (American-Netherlands Indian Expedition 1938–1939). Part V. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, Afdeling Natuurkunde 50:1–210
Fletcher TB (1921) Life-histories of Indian Insects. Microlepidoptera. VI. Gracillariadae [sic]. Mem Dept Agr ic India Entomol Ser 6:1–217
Fletcher TB (1933) Life histories of Indian Microlepidoptera (Second Series). Cosmopterygidae to Neopseustidae. Scientific monograph, vol 4. Imperial Council of Agricultural Research, New Delhi, pp 1–85
Hu BB, Wang SX, Zhang J, Li HH (2011) Taxonomy and biology of two seed-parasitic gracillariid moths (Lepidoptera, Gracillariidae), with description of one new species. ZooKeys 83:43–56
Kato M, Takimura A, Kawakita K (2003) An obligate pollination mutualism and reciprocal diversification in the tree genus Glochidion (Euphorbiaceae). Proc Natl Acad Sci U S A 100:5264–5267
Kawahara AY, Sohn J-C, De Prins J, Cho S (2010) Five species of Gracillariidae (Lepidoptera) new to Korea. Entomol Res 40:131–135
Kawahara AY, Plotkin D, Ohshima I, Lopez-Vaamonde C, Houlihan P, Breinholt JW, Kawakita A, Xiao L, Regier JC, Davis DR, Kumata T, Sohn J-C, De Prins J, Mitter C (2017) A molecular phylogeny and revised higher-level classification for the leaf-mining moth family Gracillariidae and its implications for larval host use evolution. Syst Entomol 42:60–81
Kawakita A, Kato M (2009) Repeated independent evolution of obligate pollination mutualism in the Phyllantheae–Epicephala association. Proc R Soc B 276:417–426
Kawakita A, Kato M (2016) Revision of the Japanese species of Epicephala Meyrick with descriptions of seven new species (Lepidoptera, Gracillariidae). ZooKeys 568:87–118
Kuznetzov VI, Baryshnikova SV (2001) New and little-known Asian species of the leaf miners (Lepidoptera, Gracillariidae). Trudy Zoologicheskogo Instituta, Rossijskaya Akademija Nauk 291:31–46
Kawakita A, Mochizuki K, Kato M (2015) Reversal of mutualism in a leafflower–leafflower moth association: the possible driving role of a third-party partner. Biol J Linn Soc 116:507–518
Kumata T (1966) Descriptions of twenty new species of the genus Caloptilia Hübner from Japan including the Ryukyu Islands (Lepidoptera: Gracillariidae). Insecta Matsumurana 29:1–21
Kumata T (1982) A taxonomic revision of the Gracillaria group occurring in Japan (Lepidoptera: Gracillariidae). Insecta Matsumurana 26:1–186
Kumata T (1998) Japanese species of the subfamily Oecophyllembiinae Real et Balachowsky. (Lepidoptera: Gracillariidae), with descriptions of a new genus and eight new species. Insecta Matsumurana 54:77–131
Kumata T, Kuroko H, Ermolaev VP (1988a) Japanese species of the Acrocercops-group (Lepidoptera: Gracillariidae). Part II. Insecta Matsumurana 40:1–133
Kumata T, Kuroko H, Ermolaev VP (1988b) Japanese species of the Acrocercops-group (Lepidoptera: Gracillariidae). Part I. Insecta Matsumurana 38:1–111
Kuznetzov VI (1979) A review of the genera of Gracillariidae (Lepidoptera) of the Palaearctic fauna. Entomol Obozr 58:835–856
Li H, Yang X (2015) Three new species of Epicephala Meyrick (Lepidoptera, Gracillariidae) associated with Phyllanthus microcarpus (Benth.) (Phyllanthaceae). ZooKeys 484:71–81
Li H, Zhang Z (2016) Five species of the genus Epicephala Meyrick, 1880 (Lepidoptera: Gracillariidae) from China. Zootaxa 4084:391–405
Li H, Wang Z, Hu B (2015) Four new species of Epicehala Meyrick, 1880 (Lepidoptera, Gracillariidae) associated with two species of Glochidion (Phyllanthaceae) from Hainan Island in China. ZooKeys 508:53–67
Meyrick E (1881) Descriptions of Australian micro-Lepidoptera. III Tineina Proc Linnean Soc NSW 5(132–182):204–271
Meyrick E (1908a) Descriptions of Indian Micro-Lepidoptera. VIII. J Bombay Nat Hist Soc 18:806–832
Meyrick E (1908b) Descriptions of African Micro-Lepidoptera. Proc Zool Soc London 47:716–756
Meyrick E (1910) Notes and descriptions of Indian Micro-Lepidoptera. Rec Indian Mus 5:217–232
Meyrick E (1918) Exotic Microlepidoptera. Exotic Microlepidoptera (Marlborough) 2:161–192
Meyrick E (1922) Exotic Microlepidoptera. Exotic Microlepidoptera (Marlborough) 2:545–576
Meyrick E (1927) Micro-Lepidoptera. Insects of Samoa 3:65–116. British Museum of Natural History, London
Meyrick E (1930) Exotic microlepidoptera. Exotic Microlepidoptera (Marlborough) 3:577–608
Meyrick E (1931) Exotic microlepidoptera. Exotic Microlepidoptera (Marlborough) 4:161–192
Meyrick E (1935) Exotic microlepidoptera. Exotic Microlepidoptera (Marlborough) 4:577–608
Meyrick E (1936) Exotic microlepidoptera. Exotic Microlepidoptera (Marlborough) 5:1–64
Ohshima I (2008) Host race formation in the leaf-mining moth Acrocercops transecta (Lepidoptera: Gracillariidae). Biol J Linn Soc 93:135–145
Ralimanana H, Hoffmann P (2011) Taxonomic revision of Phyllanthus (Phyllanthaceae) in Madagascar and the Comoro Islands I: synopsis and subgenera Isocladus, Betsileani, Kirganelia and Tenellanthus. Kew Bull 66:331–335
Robinson GS, Tuck KR, Shaffer M (1994) A field guide to the smaller moths of South-East Asia. Malaysian Nature Society, Kuala Lumpur, pp 1–309
Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW, Hernández LM (2001) Hostplants of the moth and butterfly caterpillars of the Oriental Region. The Natural History Museum, London, pp 1–744
Stainton HT (1856) Descriptions of three species of Indian Micro-Lepidoptera. Trans Entomol Soc Lond NS 3:301–304
Stainton HT (1859) Descriptions of twenty-five species of Indian Micro-Lepidoptera. Trans Entomol Soc Lond NS 5:111–126
Sugisima K, Kumata T, Tominaga S (2005) Discovery of Acrocercops tricuneatella (Gracillariidae, Gracillariinae) from Ryukyu, southern Japan, and its appropriate generic placement. Trans Lepid Soc Jap 56:257–265
Turner AJ (1894) Descriptions of Micro-Lepidoptera from Moreton Bay. Trans Proc R Soc S Aust 18:120–138
Turner AJ (1896) Descriptions of Micro-Lepidoptera from Queensland. Trans Proc R Soc S Aust 20:1–34
Turner AJ (1900) New Micro-Lepidoptera, mostly from Queensland. Trans Proc R Soc S Aust 24:6–23
Turner AJ (1913) Studies in Australian Microlepidoptera. Proc Linnean Soc NSW 38:174–228
Turner AJ (1940) A revision of the Australian Gracilariidae [sic] (Lepidoptera). Trans Proc R Soc S Aust 64:50–69
Turner AJ (1947) Contributions to our knowledge of Australian Microlepidoptera. Proc R Soc Queensl 57:65–74
Vargas HA, Landry B (2005) A new genus and species of Gracillariidae (Lepidoptera) feeding on flowers of Acacia macracantha Willd. (Mimosaceae) in Chile. Acta Entomol Chil 29:47–57
Vári L (1961) South African Lepidoptera. volume 1: Lithocolletidae. Transvaal Museum, Pretoria
Webster GL, Carpenter KJ (2002) Pollen morphology and phylogenetic relationships in neotropical Phyllanthus (Euphorbiaceae). Bot J Linn Soc 138:325–338
Webster GL, Carpenter KJ (2008) Pollen morphology and systematics of palaeotropical Phyllanthus and related genera of Phyllanthinae (Euphorbiaceae). Bot J Linn Soc 157:591–608
Wise KAJ (1962) Parectopa leucocyma (Meyrick) (Lepidoptera: Gracillariidae) rediscovered as a leaf-miner of kauri (Agathis australis Salisb.) Trans R Soc NZ Zool 1:373–375
Zhang J, Wang SX, Li HH, Hu BB, Yang XF, Wang ZB (2012a) Diffuse coevolution between two Epicephala species (Gracillariidae) and two Breynia species (Phyllanthaceae). PLoS ONE 7:e41657
Zhang J, Hu B, Wang S, Li H (2012b) Six new species of Epicephala Meyrick, 1880 (Lepidoptera: Gracillariidae) associated with Phyllanthaceae plants. Zootaxa 3275:43–54
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Kawakita, A., Kato, M. (2017). Diversity of Pollinator Moths. In: Kato, M., Kawakita, A. (eds) Obligate Pollination Mutualism. Ecological Research Monographs. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56532-1_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-56532-1_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56530-7
Online ISBN: 978-4-431-56532-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)