Abstract
The hemimetabolous insect, Gryllus bimaculatus, has two compound eyes that begin to form in the embryo and increase in size five- to sixfolds during nymphal development. Retinal stemlike cells reside in the anteroventral proliferation zone (AVPZ) of the nymphal compound eye and proliferate to increase retinal progenitors, which then differentiate to form new ommatidia in the anterior region of the eye. Here, we introduce the morphology and development of the cricket eye first, and then we focus on the roles of retinal determination genes (RDGs) such as eyes absent (eya) and sine oculis (so) in Gryllus eye formation and growth. Since the principal function of the eye is photoreception, we finally summarize opsin photopigments in this species, broadening the roles of photoreception.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Compound eye
- Eyes absent
- Eye development
- Gryllus bimaculatus
- Hemimetabolous insect
- Ocellus
- Opsin
- RNA interference
- Sine oculis
1 Introduction: Morphology and Structure of the Cricket Eye
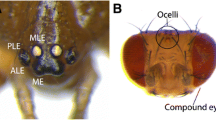
Crickets possess two kinds of visual organs: three dorsal ocelli innervated by the posterior part of the central brain and a pair of lateral compound eyes innervated by the optic lobes (Homberg 2004; Henze et al. 2012). Among the three dorsal ocelli, two lateral ones are positioned just dorsal to the antennal bases, whereas the median ocellus is located on the forehead (Fig. 4.1a). At present, the functions of these ocelli are not well studied in most insect species. However, in the two-spotted cricket, it is the lateral compound eyes that are the main visual organs and on which we mainly focus here.
Morphology of the cricket eye (Figure adapted from Takagi et al. 2012, except a). (a) Frontal view of a white-eye mutant cricket. A antenna (trimmed for better visualization), C compound eyes, L lateral ocelli, M median ocellus. Scale bar: 2 mm. (b) A longitudinal section of a cricket adult eye. (c) A diagrammatic illustration of an ommatidium of a cricket eye in a longitudinal section. (d) A diagrammatic illustration of an ommatidium of a cricket eye in a transverse section at the middle of the ommatidium. Retinular cells are numbered 1–8. Rhabdoms are indicated by striped patterns. Basement membrane (BM), axon (X), and optic lobe (OL) are evident in the eye. (c) and (d) are modified illustrations of Fig. 1A and D in Sakura et al. (2003) with permission. The scale of each image is arbitrary. Abbreviations: CC crystalline cones, CCT crystalline cone tracts, CL corneal lens, PC pigment cell, RC retinular cell, RD rhabdom, RE retina, BM basement membrane, X axon
Each compound eye is made up of approximately 7000 repeated crystalline units called ommatidia (Burghause 1979), which is substantially larger than the 750 ommatidia present in Drosophila (Wolff and Ready 1993). Each Gryllus ommatidium is composed of precisely arranged photoreceptors and nonneural support cells (Fig. 4.1b, c; Takagi et al. 2012; Sakura et al. 2003). Inside the ommatidium in the midline region, there are rhabdoms composed of tightly packed tubular membranes (microvilli) that contain the phototransduction proteins (Sakura et al. 2003; Fig. 4.1c), and these are surrounded by eight retinular cells (Takagi et al. 2012; Sakura et al. 2003; Fig. 4.1d). Overall, the general aspects of the cricket ommatidium appear to be structurally similar to those observed in the Drosophila compound eye.
2 Developmental Processes of the Cricket Eye
Holometabolous insects like Drosophila proceed through two phases of visual system development (Friedrich 2006; Kumar 2011): the embryonic phase, which generates the simple eyes of the larva, and the postembryonic phase that produces the adult specific compound eyes during late larval development and pupation. In contrast, in the basal hemimetabolous insects such as crickets and grasshoppers, the development of the compound eyes is continuous throughout embryogenesis and postembryogenesis (Friedrich 2006).
The morphological changes of the developing retina in Gryllus bimaculatus are shown in Fig. 4.2. All eye components originate from the eye lobes, a pair of distinct embryonic anlagen derived from the lateral neuroectoderm of the embryonic procephalon. The main gene expressed in this region is dachshund (Gb′ dac ) , a retinal determination transcription factor that will be discussed later (Inoue et al. 2004). During embryonic development, the eye primordia can be observed in the head at stage 11 (Mito and Noji 2008; Takagi et al. 2012; Fig. 4.2a, b). Note that distinct retinal structures are not yet visible at this stage (Fig. 4.2d). Later on, between stages 11 and 14, the morphogenetic furrow is formed (Takagi et al. 2012; Fig. 4.2c), similar to the previous reports in the grasshopper (Friedrich and Benzer 2000). Finally, at stage 14, corneal lenses, crystalline cones, and the retina consisting of pigment cells, retinular cells, and rhabdoms (Fig. 4.1b) can all be visualized in the differentiating primordium (Fig. 4.2e).
Morphological changes during development of the cricket eye (Figure adapted from Takagi et al. 2012). (a) A scanning electron micrograph of a cricket embryo at stage 11. The arrow indicates a developing eye. Anterior is upward. (b) A high-magnification image of an embryonic head. The arrow indicates the morphogenetic furrow formed between A and P. A anterior, P posterior. (c) A developing eye stained with phalloidin to visualize F-actin in morphogenetically active cells. The arrow indicates the morphogenetic furrow. (d) A sagittal section of an embryonic eye at stage 11 (before formation of the morphogenetic furrow). ECD ectoderm, MSD mesoderm. (e) A section of an embryonic eye at stage 14, where ommatidia are formed. CC crystalline cones, RE retina. (f) A section of a nymph at the second instar. Scale bars: 1mm in (a), 250 μm in (b)
During postembryonic development, the Gryllus first instar nymphs hatch out with small compound eyes, which will continue to grow throughout the subsequent nymphal stages. Additional rows of ommatidia are formed during each molt. By the second nymphal stage, the main eye structures have become fairly well established (Fig. 4.2f). The compound eyes continue to develop at the anterior margin during the third, fourth, and fifth instar nymphal stages (Takagi et al. 2012; Fig. 4.3a–c), similar to previous findings in other hemimetabolous insects (Anderson 1978). The anterior-most margin of nymphal eyes becomes filled with highly compacted cells, some of which may be retinal stem cells (Anderson 1978; Dong and Friedrich 2010). The thymidine analogue incorporation assays performed during third nymphal stage show that these cells are actually proliferating (Takagi et al. 2012). At this point, the anterior ventral proliferation zone (AVPZ), which is responsible for continuous growth of the compound eye, is formed at the anterior ventral margin of the nymphal eye primordium (Fig. 4.3d) .
Morphological changes of cricket nymphal eyes from the third to fifth instar (Figure adapted from Takagi et al. 2012). (a) A sagittal section of a nymphal eye at the third instar. (b) A sagittal section of a nymphal eye at the fourth instar. (c) A sagittal section of a nymphal eye at the fifth instar. (d) Illustration of the anteroventral proliferation zone (AVPZ, green) in the nymphal eye (blue) of a white-eyed cricket. D dorsal, V ventral, A anterior, P posterior. Scale bar: 100 μm in (a-c)
3 Retinal Determination Genes and Their Roles in Cricket Eye Formation
The extensive studies of Drosophila eye development have established the framework of the retinal determination gene (RDG) network (Fig. 4.4; Kumar 2011; Jemc and Rebay 2007). A crucial transcription factor for eye formation is Pax6, a member of the paired-box homeodomain proteins in vertebrates as well as in insects (Wawersik and Maas 2000). The Drosophila Pax6 homologs are eyeless (ey ) and twin of eyeless ( toy ) that synergistically pattern the developing fly eye. Their targets include sine oculis (so), eyes absent (eya), and dachshund (dac, Fig. 4.4). Loss-of-function mutations of ey, toy, so, eya, and dac can result in a small eye or the lack of the eye in the adult, while ectopic expression of these genes, either alone or in combination, can induce ectopic eye formation, suggesting that these eye-forming genes act at the very early stages of eye development (Halder et al. 1995; Bonini et al. 1997; Chen et al. 1997; Pignoni and Zipursky 1997; Shen and Mardon 1997; Czerny et al. 1999; Kronhamn et al. 2002; Jang et al. 2003). However, very little is known about the RDG networks in basal insects. In order to understand the mechanisms underlying the continuous eye development in the hemimetabolous mode of development, it is critical to characterize the functions of the RDGs more precisely in these species.
Retinal determination gene (RDG) network as revealed by Drosophila studies (Figure modified from Kumar 2011 and Jemc and Rebay 2007). Black arrows represent direct transcriptional regulation or undetermined genetic regulatory pathways. Blue arrows depict protein-protein interactions. Mammalian homologs are shown on the right
3.1 Toy, Ey, and Dac
The Gryllus homologs of toy and ey have been cloned, and their expression patterns were localized in the eye primordium (S. N., H. O., unpublished data). However, neither targeting the individual genes with RNAi nor dual RNAi against both genes has any effects on eye formation and generates only wild-type morphology.
During embryonic eye development, Gb′dac expression is first observed in the lateral head region that corresponds to the eye primordium and a part of the deutocerebrum, the second part of the three-partite arthropod brain. Before the formation of compound eyes, the expression becomes restricted to the posterior region of the eye anlage. The expression then moves anteriorly as the morphogenetic furrow progresses (Inoue et al. 2004). This Gryllus expression pattern of dac corresponds very well to that observed in Drosophila eye imaginal disc, although these two insect species are markedly different in phylogeny (Takagi et al. 2012). Similar to the situation with toy and ey, no noticeable RNAi phenotype against Gb′dac has been observed (S. N., H. O., unpublished data). Recent work in Tribolium showed that only triple knockdowns (including toy, ey, and dac) result in the loss of compound eyes (Yang et al. 2009). Taken together, relationship among Toy, Ey, and Dac in embryonic eye development awaits further elucidation, but is likely modified in crickets as compared to that in Drosophila (Fig. 4.4).
3.2 Eya and So
Gryllus homologs of sine oculis (Gb′so) and eyes absent (Gb′eya) are expressed during embryonic eye development (Takagi et al. 2012). Their expression domains appear to overlap with the procephalic expression regions of the Gryllus homologs of hedgehog (Gb′hh) and wingless (Gb′wg) at stage 4 (Miyawaki et al. 2004). Parental RNAi (paRNAi) against Gb′eya results in smaller eye primordia or no embryonic eyes (see Fig. 4.5 from Takagi et al. 2012). Almost half of Gb′eya RNAi embryos have neither compound eyes nor ocelli. In contrast, the embryonic phenotypes of Gb′so RNAi are very subtle, possibly due to the insufficient depletion of its transcripts (Takagi et al. 2012).
Gb′eya is required for formation of embryonic eyes and ocelli of the hemimetabolous cricket as revealed by parental RNAi (Figure adapted from Takagi et al. 2012). (a, a′ and a″) Control embryos. (a) Lateral view; (a′) High magnification of the head region of (a); (a″) Dorsal view. Arrowheads indicate three ocelli. (b, b′ and b″) A Gb′eya RNAi embryo. Neither eye nor ocellus was observed. Scale bars: 300 μm
During later embryogenesis, around stage 14, Gb′eya and Gb′so are expressed in the posterior region of the morphogenetic furrow (MF). These expression patterns are very similar to those observed in the Drosophila eye imaginal disc (Callaerts et al. 2006). Since it has been reported that Ey- and Toy-dependent activation of eya, so, and dac locks the cells in a retina-committed state (Chen et al. 1999; Curtiss and Mlodzik 2000; Bessa et al. 2002), it is tempting to postulate that Gb′eya and Gb′so in the posterior region of the cricket embryonic eye may be involved in keeping cells in a similar retinal-committed state.
The expression patterns of Gb′eya and Gb′so were also examined in Gryllus nymphs. In situ hybridization of the fifth instar eye shows that Gb′eya is expressed intensely in the AVPZ of the nymphal eye primordium (Figs. 4.6a; compare with Fig. 4.6b) as well as in the pigment cells. In contrast, the Gb′so could not be detected (Takagi et al. 2012). These expression studies were followed up with the nymphal RNAi (nyRNAi) at the third or fourth instar stage using a white-eye mutant line (Fig. 4.6c). This line is highly inbred to reduce genetic background differences and to observe color changes in the eyes when making transgenic lines (Nakamura et al. 2010) or RNAi experiments (Takagi et al. 2012). In Gb′eya nyRNAi experiments with low amounts of dsRNA administered (2 μM conc.), the white eyes turn black in the immediate nymphal stage. The eyes of later nymphs and adults, though, revert back to white. When the higher dosage of Gb′eya dsRNA (20 μM) is injected into third instar nymphs, not only do their eyes turn black, but a small-eye phenotype can also be observed (Fig. 4.6d). Consistent with these results, when a 100-fold lower concentration of dsRNA is used (0.2 μM), the frequency of such severe phenotypes is greatly reduced. In Gb′so nyRNAi injections with the highest concentration of dsRNA (20 μM), there is a significant increase of black pigmentation in the eyes (Fig. 4.6e), resulting in dull-white eyes. However, the small-eye phenotype is never observed. Histological analysis shows that the darkening of the eyes is likely due to the formation of a brown cuticular layer at the surface of the eye, which is usually produced by the epidermal cells, instead of the cornea (Takagi et al. 2012). Since eya is normally expressed in the pigment cells abutting the corneal lens (CL; Fig. 4.1c), it is likely that reduction of eya expression in CL leads to transformation of the cornea to epidermal cells, which produce cuticle .
Effects of Gb′eya or Gb′so RNAi on development of the nymphal eye (Figure adapted from Takagi et al. 2012). (a) Expression of Gb′eya in the AVPZ (brown, arrow) of the nymphal eye at the fifth instar as revealed by in situ hybridization. (b) A sense probe of Gb′eya does not give rise to brown staining in the AVPZ. (c) A control white adult eye. (c′) A sagittal section of a control nymphal eye at the fifth instar stained with hematoxylin and eosin (HE). AVPZ is shown by bracket. (d) A severe phenotype of Gb′eya nyRNAi nymph injected with dsRNA at the seventh instar. (d′) A sagittal section of the Gb′eya nyRNAi eye with a severe phenotype, stained with HE. A typical AVPZ is not observed and retinal structure is disrupted. (e) A severe phenotype of Gb′so nyRNAi nymph at the seventh instar. The same defects are observed in the mild Gb′eya nyRNAi eye. Scale bars: 100 μm in (a, b), 2 mm in (c-e)
Severe cases of Gb′eya RNAi exhibit significant reduction in the size of compound eyes in addition (Fig. 4.6d; compare with Fig. 4.6c). Cell proliferation assays show that the cells in S phase are either completely absent in Gb′eya or have greatly reduced numbers in Gb′so, resulting in a dramatically smaller AVPZ in the fifth instar stage (Fig. 4.6d′; compare with Fig. 4.6c′) (Takagi et al. 2012). Combined, these results indicate that Gb′eya and Gb′so are essential for cell proliferation and AVPZ growth during postembryonic eye development. During eye development, eya and so are known to be components of a group of transcription factors that play an important role in the specification of the eye field not only in Drosophila but also in mammals as well (Oliver et al. 1995, 1996; Xu et al. 1999). Moreover, they function as a complex in both eye specification and differentiation (Pignoni et al. 1997; Kumar 2011; Fig. 4.4). Therefore, it is reasonable to assume that they might also act as a complex during eye development in Gryllus bimaculatus. In light of the RNAi experiments in Gryllus and Tribolium (Yang et al. 2009), the Eya/So complex is evolutionarily conserved and required for retinal differentiation, maintenance of the retinal region, and cornea formation during eye development in insects in general.
4 Photoreception and Expression of Opsins in the Cricket Eye
All eye types, irrespective of their level of morphological complexity or modification in regard to individual lifestyle and environmental conditions, have a primary function in animal vision or photoreception. Previous studies in Gryllus demonstrated the existence of three spectral classes of photoreceptors in its compound eyes with maximal absorption levels observed at 332 nm (ultraviolet; uv), 445 nm (blue), and 515 nm (green; Zufall et al. 1989). A different situation was observed in ocelli, with only two classes of photoreceptors being reported: green- and uv-sensitive at the peak absorbance of ~511 nm and ~351 nm, respectively (Henze et al. 2012). There are four opsin genes that are responsible for photoreception in crickets: one in the short-wavelength (SW) , one in the middle-wavelength (MW) , and two in the long-wavelength (LW) clade of the phylogenetic tree (Henze et al. 2012). The Gb′SW, MW , and two LW opsin genes are termed as UV , blue , green A , and green B , respectively. Of the four genes, only the green B is expressed in all eye regions except for the dorsal rim area (DRA) (Henze et al. 2012; Tamaki et al. 2013), while the others exhibit differential expressions. The DRA is a specialized region at the dorsal-frontal margin of the compound eye and is a non-imaging eye region (Labhart and Meyer 1999). The UV opsin is expressed in the DRA and in the remainder of the eye excluding a portion in the ventral retina. The blue opsin is expressed in the DRA and the ventral portion of the retina. Thus, UV, blue, and green B opsins are likely responsible for the three wavelengths that are absorbed by the cricket ommatidia. With the regard to ocelli, only green A is expressed in all three ocelli types, while UV opsin is not. Therefore, it is presumed that an additional ocellus-specific opsin should be present in crickets. The preliminary analysis of the Gryllus genome has identified two additional opsin family members (TM and SN, unpublished data): an Rh7 -like gene and pteropsin (Fig. 4.7), which is an invertebrate opsin 3 (Opn3) homolog. The photosensitivity of Rh7 has not been characterized in any invertebrate at present, whereas a single study of mosquito Opn3 homolog revealed it to be a green-sensitive photopigment (Koyanagi et al. 2013).
Structure of Gb′opsin genes deduced from the cricket genome database. There are at least six opsin genes in the genome of Gryllus bimaculatus. OpsinLWa (green A) and OpsinLWb (green B) genes are located tail to tail in the reverse orientation, suggesting that they were produced by a recent gene duplication. The cricket Rh7-like gene has three exons, which is conserved among Rh7-like genes of other species. The genomic structure for a cricket pteropsin is preliminary, because the putative exon 1 and exon 2 are in different scaffolds and exon 5 does not contain a termination codon at present (2013.12 version)
While the data is lacking in Gryllus, the functions of opsin genes have been examined via RNAi in another cricket, Modicogryllus siamensis (Tamaki et al. 2013). Interestingly, the loss of function of the UV opsin causes photoperiod -dependent changes in the durations of nymphal development in this species. Hence, some of the opsin genes may also play a role in photoperiodic responses in insects, although such photoperiodicity is not observed in Gryllus bimaculatus. It is also tempting to examine the role of opsins expressed in the ocelli by RNAi or genome-editing techniques developed recently (Chap. 21).
5 Conclusion
In this review, we summarized the morphology, development, and opsin photopigments of the Gryllus eye. Crickets have two types of eyes, ocelli and compound eyes, the latter of which are the main visual organs in this species. In the basal hemimetabolous insect, the development of the compound eyes continues from embryogenesis (patterning) to postembryogenesis (differentiation and growth). The anterior ventral proliferation zone (AVPZ), in which retinal stemlike cells reside, is responsible for continuous growth of the compound eye. The AVPZ in the cricket nymphal eye is reminiscent of the stem cell regions of some vertebrate retinas, the ciliary marginal zone (CMZ; for a review, see Kubo and Nakagawa 2008). Although retinal determination genes (RDGs) such as ey, toy, so, eya, and dac are expressed during cricket eye development, there seems to be divergence in their roles between crickets and holometabolous insects. eya and so are likely fundamental to cricket eye development rather than Pax6 homologs, which is different from that in Drosophila, where ey, toy, and dac functions at the top of the early eye patterning. In spite of its diversity in morphological complexity, the eye has a primary function in photoreception. Genomic analysis of Gryllus bimaculatus has revealed all the members of opsin photopigments. Their unidentified roles can now be elucidated by RNAi, transgenesis, or RNA-guided genome-editing systems in this species and will expand our understanding of vision in the hemimetabolous insects .
References
Anderson H (1978) Postembryonic development of the visual system of the locust. Schistocerca gregaria. II. An experimental investigation of the formation of the retina-lamina projection. J Embryol Exp Morphol 46:47–170
Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS (2002) Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev 16:2415–2427
Bonini NM, Bui QT, Gray-Board GL, Warrick JM (1997) The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development 124:4819–4826
Burghause F (1979) Die structurelle spezialisierung des dosalen augenteils der Grillen (Orthoptera, Gryllidae). Zool Jb Physioll Bd 83:S502–S525
Callaerts P, Clements J, Francis C, Hens K (2006) Pax6 and eye development in Arthropoda. Arthropodan Struct Dev 35:379–391
Chen R, Amoui M, Zhang Z, Mardon G (1997) Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell 91:893–903
Chen R, Halder G, Zhang Z, Mardon G (1999) Signaling by the TGF-beta homolog decapentaplegic functions reiteratively within the network of genes controlling retinal cell fate determination in Drosophila. Development 126:935–943
Curtiss J, Mlodzik M (2000) Morphogenetic furrow initiation and progression during eye development in Drosophila: the roles of decapentaplegic, hedgehog and eyes absent. Development 127:1325–1336
Czerny T, Halder G, Kloter U, Souabni A, Gehring WJ, Busslinger M (1999) Twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol Cell 3:297–307
Dong Y, Friedrich M (2010) Enforcing biphasic eye development in a directly developing insect by transient knockdown of single eye selector genes. J Exp Zool B Mol Dev Evol 314:104–114
Friedrich M (2006) Continuity versus split and reconstitution: exploring the molecular developmental corollaries of insect eye primordium evolution. Dev Biol 299:310–329
Friedrich M, Benzer S (2000) Divergent decapentaplegic expression patterns in compound eye development and the evolution of insect metamorphosis. J Exp Zool 288:39–55
Halder G, Callaerts P, Gehring WJ (1995) New perspectives on eye evolution. Curr Opin Genet Dev 5:602–609
Henze MJ, Dannenhauer K, Kohler M, Labhart T, Gesemann M (2012) Opsin evolution and expression in arthropod compound eyes and ocelli: insights from the cricket Gryllus bimaculatus. BMC Evol Biol 12:163
Homberg U (2004) Multisensory processing in the insect brain. In: Christensen TA (ed) Methods in insect sensory neuroscience. CRC Press, Boca Raton, pp 3–25
Inoue Y, Miyawaki K, Terasawa T, Matsushima K, Shinmyo Y, Niwa N, Mito T, Ohuchi H, Noji S (2004) Expression patterns of dachshund during head development of Gryllus bimaculatus (cricket). Gene Expr Patterns 4:725–731
Jang CC, Chao JL, Jones N, Yao LC, Bessarab DA, Kuo YM, Jun S, Desplan C, Beckendorf SK, Sun YH (2003) Two Pax genes, eye gone and eyeless, act cooperatively in promoting Drosophila eye development. Development 130:2939–2951
Jemc J, Rebay I (2007) The eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu Rev Biochem 76:513–538
Koyanagi M, Takada E, Nagata T, Tsukamoto H, Terakita A (2013) Homologs of vertebrate Opn3 potentially serve a light sensor in nonphotoreceptive tissue. Proc Natl Acad Sci U S A 110:4998–5003
Kronhamn J, Frei E, Daube M, Jiao R, Shi Y, Noll M, Rasmuson-Lestander A (2002) Headless flies produced by mutations in the paralogous Pax6 genes eyeless and twin of eyeless. Development 129:1015–1026
Kubo F, Nakagawa S (2008) Wnt signaling in retinal stem cells and regeneration. Dev Growth Differ 50:245–251
Kumar JP (2011) My what big eyes you have: how the Drosophila retina grows. Dev Neurobiol 71(12):1133–1152. doi:10.1002/dneu.20921
Labhart T, Meyer EP (1999) Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47:368–379
Mito T, Noji S (2008) The two-spotted cricket Gryllus bimaculatus: an emerging model for developmental and regeneration studies. CSH protoc. 2008, pdb emo110
Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, Noji S (2004) Involvement of wingless/armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev 121:119–130
Nakamura T, Yoshizaki M, Ogawa S, Okamoto H, Shinmyo Y, Bando T, Ohuchi H, Noji S, Mito T (2010) Imaging of transgenic cricket embryos reveals cell movements consistent with a syncytial patterning mechanism. Curr Biol 20:1641–1647
Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P (1995) Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121:4045–4055
Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P (1996) Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mech Dev 60:233–239
Pignoni F, Zipursky SL (1997) Induction of Drosophila eye development by decapentaplegic. Development 124:271–278
Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL (1997) The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91:881–891
Sakura M, Takasuga K, Watanabe M, Eguchi E (2003) Diurnal and circadian rhythm in compound eye of cricket (Gryllus bimaculatus): changes in structure and photon capture efficiency. Zool Sci 20:833–840
Shen W, Mardon G (1997) Ectopic eye development in Drosophila induced by directed dachshund expression. Development 124:45–52
Takagi A, Kurita K, Terasawa T, Nakamura T, Bando T, Moriyama Y, Mito T, Noji S, Ohuchi H (2012) Functional analysis of the role of eyes absent and sine oculis in the developing eye of the cricket Gryllus bimaculatus. Dev Growth Differ 54:227–240
Tamaki S, Takemoto S, Uryu O, Kamae Y, Tomioka K (2013) Opsins are involved in nymphal photoperiodic responses in the cricket Modicogryllus siamensis. Physiol Entomol 38:163–172
Wawersik S, Maas RL (2000) Vertebrate eye development as modeled in Drosophila. Hum Mol Genet 9:917–925
Wolff T, Ready DF (1993) Pattern formation in the Drosophila retina. In: Bate M, Martinez-Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 1277–1325
Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R (1999) Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23:113–117
Yang X, Zarinkamar N, Bao R, Friedrich M (2009) Probing the Drosophila retinal determination gene network in Tribolium (I): the early retinal genes dachshund, eyes absent and sine oculis. Dev Biol 333:202–214
Zufall F, Schmitt M, Menzel R (1989) Spectral and polarized-light sensitivity of photoreceptors in the compound eye of the cricket (Gryllus bimaculatus). J Comp Physiol A 164:597–608
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Ohuchi, H., Bando, T., Mito, T., Noji, S. (2017). Eye Development and Photoreception of a Hemimetabolous Insect, Gryllus bimaculatus . In: Horch, H., Mito, T., Popadić, A., Ohuchi, H., Noji, S. (eds) The Cricket as a Model Organism. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56478-2_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-56478-2_4
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56476-8
Online ISBN: 978-4-431-56478-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)