Abstract
Homochirality is essential for the development and maintenance of life. Until relatively recently, the homochirality of amino acids in living systems was believed to be maintained with the exception of the presence of d-amino acids in the cell wall of microorganisms. However, d-amino acids were recently found in various higher organisms in proteins and peptides and as free amino acids. In proteins, d-aspartate (Asp) residues have been detected in various tissues such as the eye lens, teeth, bone, aorta, ligament, brain, and skin of elderly individuals, and thus d-amino acids can no longer be considered as uncommon in living organisms. The presence of d-amino acids may change the higher-order structure of proteins, and this may be the cause of age-related diseases including cataract and Alzheimer’s disease. d-Asp in aged tissues of living organisms is thought to result from the spontaneous racemization of the Asp residues. The racemization of Asp residues in proteins does not occur uniformly but does so at specific residues on the basis of the sequence context or structural considerations. Therefore, it is necessary to determine the nature of Asp residues at specific sites within particular proteins. However, the detection of d-amino acids in proteins to date has been complex and difficult. This review deals with 1) the presence of d-aspartate (Asp) residues in protein of living tissues, 2) the mechanism of d-Asp formation in protein under physiological conditions, 3) the influence of d-Asp on protein structure and function, and 4) recent advances in d-amino acid analysis in protein.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Amino acids contain one (or more) asymmetric tetrahedral carbon atoms. Therefore, the molecules can exist in two nonsuperimposable mirror image forms, that is, they can be right-handed (d-enantiomer) and left-handed (l-enantiomer) structures. It is thought that equal amounts of d- and l-amino acids existed on primeval earth before the emergence of life. However, during the stage of chemical evolution, only l-amino acids were selected for polymerization and formation of peptides and proteins after which life emerged. Although the chemical and physical properties of l-amino acids and d-amino acids are very similar with the exception of their optical character, the reasons for the elimination of d-amino acids and why all living organisms are now composed predominantly of l-amino acids are not well understood. However, it is clear that only one of the enantiomers could be selected because proteins, which consist of many amino acid diastereoisomers, would not be able to fold into proper structures in a manner similar to current proteins. Therefore, homochirality is essential for the development and maintenance of life. Once the l-amino acid world was established, d-amino acids were excluded from living systems. Consequently, there has been little study of the presence and function of d-amino acids in living organisms.
d-Amino acids, however, were recently found in various living higher organisms in the form of free amino acids, peptides, and proteins. Free d-aspartate (Asp) and d-serine (Ser) are present and may have important physiological function in mammals. Free d-Asp may play a role as a novel messenger in the maturation and differentiation of tissues (Katane and Homma* 2011), while free d-Ser is found in the brain (Hashimoto et al. 1992, 1993) and functions as a co-agonist of N-methyl-d-aspartate (NMDA) receptors (Hashimoto et al. 1993). Small peptides containing one d-amino acid have been found in various vertebrates and invertebrates. Dermorphin is the first d-amino acid-containing peptide found which was isolated from the skin of a frog (Phyllomedusinae from South and Middle America) and is an opioid peptide with the sequence Y [d-Ala]-FGYP (Montecucchi et al. 1981). The activity which is about 1000 times greater than that of morphine is lost by substituting l-Ala for d-Ala (Broccardo et al. 1981). Many small peptides that contain d-amino acids are described in another excellent review (Jolles 1998).
In proteins, d-Asp residues have been widely detected in various tissues such as eye lens (Masters et al. 1977; Fujii et al. 1994a, b; Fujii et al. 2011), teeth (Helfman and Bada 1976; Masuda et al. 2002), bone (Ritz et al. 1996; Cloos and Fledelius 2000), aorta (Powell et al. 1992), ligament (Ritz-Timme et al. 2003), brain (Roher et al. 1993), and skin (Fujii et al. 2002; Ritz-Timme et al. 2003) of elderly individuals. The presence of d-Asp in aged tissues of living organisms is a result of spontaneous racemization of the Asp residues in these particular proteins. Most researchers held the view that l-amino acids in proteins could never change to d-isomers under the physical conditions of the living body because proteins were believed to be difficult to modify chemically, since selection during evolution before the emergence of life worked to ensure such molecules had very stable properties. This general idea had no real basis in scientific fact but became established because d-amino acids could not be found. The racemization of Asp residues in proteins does not occur uniformly but does so at specific Asp residues on the basis of the sequence context or structural considerations that make the specific residues more susceptible to the reaction than others. It is therefore necessary to determine the nature of the Asp residues at specific sites within particular proteins.
Conventional enantioseparation of free amino acids by gas chromatography (GC) or reversed-phase high-performance liquid chromatography (RP-HPLC) is easier than looking for d-amino acids in the context of an intact protein. Identification of a very small quantity of d-amino acids at specific sites in proteins comprised almost entirely of l-amino acids is similar to looking for a needle in a haystack. In order to analyze the specific sites of protein-bound d-amino acids, several complex steps such as (1) purification of the protein, (2) enzymatic digestion of the protein, (3) separation and identification of the enzyme-digested peptides, (4) hydrolysis of the enzyme-digested peptides, (4) derivatization of the amino acids to diastereoisomers, (5) application of the diastereoisomers to reversed-phase high-performance liquid chromatography (RP-HPLC), and determination of the D/L ratio of amino acids by analysis of the respective peak areas. Recently, we proposed a new method of analysis for determining the Asp isomers at individual sites in a protein with decreased complexity using LC-MS systems.
This review deals with (1) the presence of d-amino acid residues in proteins, (2) the mechanism of how d-aspartate residues spontaneously occur in proteins under physiological conditions, (3) the influence of d-Asp on protein structure and function, and (4) the recent advances in d-amino acid analysis in proteins.
1.1 The Presence of d-Amino Acid Residues in Proteins
Although proteins consist exclusively of l-amino acids, d-amino acids have been detected in various tissues as described in the Introduction. Table 15.1 shows that d-amino acids have been found in many proteins from various tissues. It is therefore no longer uncommon to find d-amino acids in living organisms. Almost all d-amino acids found are d-Asp since Asp is the most easily racemizable amino acid. Earlier studies only showed that d-Asp accumulated in proteins of tissues with age. Because d-Asp was detected in homogenates of tissues, it could not be determined whether all of the aspartic acid in the protein was racemized uniformly or whether particular aspartic acid residues had a greater tendency to racemize in specific proteins.
Recent studies clearly indicate that Asp residues of proteins are not racemized uniformly but that d-Asp residues may be present at some specific sites in some particular proteins such as eye lens crystallins (Fujii et al. 1994a, b; Fujii et al. 2011, 2012), β-amyloid protein (Roher et al. 1993), histone H2B (Young et al. 2005), type I collagen (Cloos and Fledelius 2000), etc. In addition to these proteins, recent studies reported that Asn-127 in mouse lysozyme quickly racemizes after incubation (pH 7 and 37 °C) for 8 weeks, Cys220 in the hinge sequence of immunoglobulin gamma 1 (IgG1) quickly racemizes after storage for 6 months at 40 °C (Amano et al. 2011), and Asp-24 in the heavy chain peptide H5 is highly racemized (Zhang et al. 2011) (Table 15.1). These studies clearly indicate that racemization to d-amino acids occurs more easily than may have been expected.
1.2 How Do d-Aspartate Residues Spontaneously Occur at Specific Sites in Proteins Under Physiological Conditions?
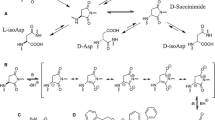
We found several specific d-Asp sites in aged human lens proteins and have studied the mechanism of the spontaneous isomerization of proteins under physiological conditions. Human lens proteins are composed of three major structural proteins, namely, α-, β-, and γ-crystallins. α-Crystallin functions like a chaperone, binding to nonnative or unfolded proteins and protecting them against aggregation induced by heat, reduction, and chemical modification (Horwitz 1992). The chaperone-like activity of α-crystallin might play an important role in preventing the aggregation and insolubilization of other lenticular proteins, thereby maintaining the transparency of the eye lens. Alpha-crystallin is a polymer consisting of two subunits, αA and αB. We previously reported the presence of d-isomers at Asp-58, Asp-76, Asp-84, and Asp-151 in αA-crystallin (Fujii et al. 1994b, 2012); at Asp-36, Asp-62, and Asp-96 in αB-crystallin (Fujii et al. 1994a, 2012); and at Asp-4 in βB2-crystallin from aged human lenses (Fujii et al. 2011). d-Asp formation was accompanied by isomerization from the natural α-Asp to the abnormal β-Asp (Fujii et al. 1999). Racemization begins when the hydrogen atom attached to the α-carbon atom is released. Usually, this reaction proceeds with difficulty in mild conditions, such as those found in the body. However, Asp residues in proteins are susceptible to racemization because Asp has a carboxyl group in its side chain. Inversion and isomerization of Asp residues in proteins are considered to proceed via a succinimide intermediate. As shown in Fig. 15.1, the simultaneous formation of β- and d-Asp residues in the protein can be explained as follows: (1) When the lone-pair electron of the nitrogen atom of the amino acid residue following the Asp residue attacks the carboxyl group of the side chain of the Lα-Asp residue, l-succinimide is generated by intramolecular cyclization. (2) l-Succinimide can be converted to d-succinimide via an intermediate through keto–enol tautomerism. (3) d-Succinimide is then hydrolyzed at either side of its two carbonyl groups to form Dα- and Dβ-Asp; similarly, l-succinimide is hydrolyzed to form Lα- and Lβ-Asp. Thus, four isomers, Lα-Asp, Lβ-Asp, Dα-Asp, and Dβ-Asp, are simultaneously formed in the protein. The difference in abundance of the Asp isomers in the protein may be due to the rate constants for the formation of the isomers. The rate constant for hydrolysis from succinimidyl peptide to β-Asp peptide is about 5 times higher than the rate constant for hydrolysis from l-succinimidyl peptide to l-α-Asp peptide. Thus, of these Asp isomers, large amounts of d-β- and l-β-isomers are present, but the amount of d-α-isomer is not significant (Aki and Fujii 2013).
The rate of succinimide formation is considered to depend on the residue neighboring the Asp residue. When the neighboring amino acid has a small side chain, as found in glycine (Gly), alanine (Ala), or serine (Ser), succinimide forms easily because there is no steric hindrance (Geiger and Clarke 1987; Fujii et al. 1999). In fact, as shown in Table 15.2, many Gly, Ser, and Ala residues were found to be the residue adjacent to d-Asp sites. In addition to the effects of the adjacent residues, Asp may also be susceptible to racemization when the residues are located in flexible regions as suggested in Table 15.2. These observations indicate that formation of succinimide in proteins depends both on the amino acids neighboring the Asp residues and on the higher-order structure of the protein.
1.3 The Influence of d-Asp on Protein/Peptide Structure and Function
1.3.1 d-β-Asp in Protein Promotes Massive and Heterogeneous Aggregation
The appearance of d-Asp isomers in a protein can cause major changes in the 3-D structure because the different side-chain orientation may induce an abnormal peptide backbone. In addition to d-Asp formation, the β-linkage of Asp may affect the quaternary structure because the main chain of the protein is elongated. Therefore, the presence of these isomers may be one of the triggers for abnormal aggregation. Moreover, these processes can induce partial unfolding of the corresponding proteins, leading to a disease state. In fact, samples of αA-crystallin containing large amounts of d-β-Asp obtained from donors of ~80 years of age have been shown to undergo abnormal aggregation to form massive and heterogeneous aggregates (Fujii et al. 2007). Specifically, αA-crystallin from normal young individuals (1-year-old, non-racemized samples) had an average sedimentation coefficient of 17 S at 37 °C, whereas the same protein from elderly individuals had an average sedimentation coefficient of 30 S (range, 20–60 S). Changes in the self-association of α-crystallin aggregates have also been correlated to changes in chaperone activity. α-Crystallin from young donors displays chaperone activity, but this activity is reduced by 60 % in aged α-crystallin aggregates (Fujii et al. 2007) (Table 15.3a).
This chaperone activity plays an important role in preventing the aggregation and insolubilization of other lenticular proteins. Hence, the loss of this activity adversely affects maintenance of the transparency of the eye lens.
1.3.2 A Single Substitution of an Asp Isomer in a Peptide Induces a Large Change in the Properties of the Peptide
As described in Sect. 3.1, the appearance of d- and β-Asp in a protein potentially induces large changes to its higher-order structure as well as to its function. However, it remains unclear whether the formation of the Asp isomer is the direct trigger for such a change. In order to clarify the effect of the inversion to d-isomers in a protein, we synthesized peptides corresponding to the 70–88 (KFVIFLDVKHFSPEDLTVK) fragment of human αA-crystallin, which is known to have chaperone function (Tanaka et al. 2008). The Lα-Asp corresponding to position 76 was replaced by diastereoisomers Lβ-Asp, Dα-Asp, and Dβ-Asp, and the biochemical properties of the four different peptides were then compared. The peptides containing abnormal isomers (Lβ-Asp, Dα-Asp, and Dβ-Asp) were more hydrophilic than the normal peptide (containing Lα-Asp) and adopted a random coil structure, rather than the normal β-sheet motif. The normal peptide promoted the aggregation of insulin, while the other three isomers suppressed its aggregation (Fujii et al. 2010).
This result clearly indicates that a single substitution of an Asp isomer in a peptide induces a large change in the properties of the peptide (Table 15.3b).
1.4 Recent Advances in d-Amino Acid Analysis in Proteins
The racemization of Asp residues in proteins does not occur uniformly but does so at specific Asp residues on the basis of the sequence context or structural considerations that make the specific residues more susceptible to reaction than others. Therefore, it is necessary to determine the nature of the Asp residues at specific sites within particular proteins.
The separation of the optical isomers of amino acids has previously been considered to be difficult because the physical and chemical properties of the optical isomers are the same. In addition to this, enantioseparation of the bound form of amino acids requires the hydrolysis of the protein/peptide before the analysis of the enantiomers. Conventional enantioseparation of amino acids has been performed using gas chromatography (GC) or reversed-phase high-performance liquid chromatography (RP-HPLC). GC analysis requires nanomole levels of sample, while picomole levels are required for reversed-phase high-performance liquid chromatography (RP-HPLC) analysis. GC analysis is direct enantioseparation through the use of a chiral capillary column, while reversed-phase high-performance liquid chromatography (RP-HPLC) analysis is indirect enantioseparation based on the analysis of the diastereoisomeric derivatives of the amino acid samples produced by chiral derivatizing reagents. Both methods require the appropriate amino acid derivatization or preparation in advance of the analysis, the former requiring changing the samples into the gaseous state before injection onto the GC and the latter requiring production of diastereoisomeric derivatives in the case of the non-chiral column. The process is very complex for free d-amino acid analysis. In addition, in order to analyze the specific sites of d-amino acids in protein, more complicated steps are required other than free d-amino acid analysis: (1) the protein is digested with an appropriate enzyme. (2) The resulting peptides are separated by reversed-phase high-performance liquid chromatography (RP-HPLC). (3) The peptides are identified by mass analysis and/or protein sequencing. (4) The α- or β-isomer of the identified peptides is determined by Edman degradation reaction. (5) The D/L ratio of the identified peptides is determined after hydrolysis with 6 N HCl and derivatization. (6) The diastereoisomers are analyzed by reversed-phase high-performance liquid chromatography (RP-HPLC) and the D/L ratio of amino acids determined by analysis of the respective peak areas. The resulting analysis of the isomerization of Asp residues in a protein can be accurate but it is a technically demanding process. Consequently, there has been little study of the presence and function of d-amino acids in living organisms.
In the review we propose a new accurate and quick LC-MS-based analysis for determining the specific sites having Asp isomers and quantifying the amounts of Asp isomers at the individual sites of all lens crystallins in the water-insoluble (WI) and the water-soluble (WS) fractions without the need for complicated purification from the lens tissues. Figure 15.2a, b shows a typical full LC-MS chromatogram of the tryptic peptides from the WI and WS lens proteins. Generally, each peptide would be expected to elute as one peak with one mass number; however, peptides which contain Asp isomers were separated into multiple peaks, and they eluted at different retention times during the LC-MS run even though they had entirely the same sequences. Using this property, we are able to identify peptides which have isomeric Asp residues. For example, the peptide predicted to correspond to positions 55–65 of αA-crystallin (αA 55–65; TVLDSGISEVR: [M+2H]2+=588.3) as identified by the database was mainly separated into four different peaks which eluted at different times as shown in Fig. 15.3a, b. Figure 15.3c, d shows the LC-MS chromatogram of αB 57–69 (APSWFDTGLSEMR: [M+2H]2+=748.8) from the WI and WS fractions, respectively. This peptide was also separated into several peaks.
LC-MS chromatogram of the tryptic peptides of water-insoluble (WI) and water-soluble (WS) fractions of lens proteins from elderly donor. (a) and (b): MS range 588–589.5 m/z (αA-crystallin 55–65) of WI and WS fractions, respectively. (c) and (d): mass range 748.5–750 m/z (αB-crystallin 57–69) of WI and WS fractions, respectively
The number of peptides from αA 55–65 and αB 57–69 was greater from WI protein (Fig. 15.3a, c) than from WS protein (Fig. 15.3b, c). A similar multiple separation of the various peptides containing Asp residues was obtained from all crystallins, that is, αA-, αB-, βA3-, βA4-, βB1-, βB2-, and γS-crystallin in both WI and WS proteins with the amounts of isomeric peptides in the WI fractions being greater than in the WS fractions. The results are summarized in Fig. 15.4. Figure 15.4 shows the amounts of the four Asp isomers of αA- and αB-crystallins from the WI and WS fractions. The amount of normal l-α-Asp is dramatically decreased, while the other isomeric ratios increased at all Asp sites in the WI fraction compared to the WS fraction.
2 Prospects
Racemization and isomerization of amino acids in proteins can cause major changes in structure, since different side-chain orientations can induce an abnormal peptide backbone. Therefore, these posttranslational modifications can induce the partial unfolding of protein leading to a disease state. Thus, it is necessary to determine the levels of isomeric Asp residues at specific sites in any protein. Here, we describe a convenient and robust biochemical method for identifying the isomeric Asp sites in proteins using LC-MS systems (Fig. 15.5). There are many advantages to this new method: (1) No requirement for large amounts of sample proteins. (2) No requirement for the purification of lens proteins from the WI and WS fractions. (3) No requirement for complicated analytical steps which usually include the hydrolysis of the peptides followed by derivatization to the diastereoisomers of amino acids. This new method is able to search comprehensively for the Asp isomers in damaged or aged proteins from all living tissues and cells. Furthermore, the isomeric Asp sites can be determined, and the amounts of the Asp isomers can be quantified quickly and accurately at the femtomole level. This new method therefore improves the study of the isomerization of any amino acid which occurs spontaneously in living tissues or cells.
References
Aki K, Fujii N (2013) Kinetics of isomerization and inversion of aspartate 58 of alphaA-crystallin peptide mimics under physiological conditions. PLoS One 8(3), e58515. doi:10.1371/journal.pone.0058515
Amano M, Hasegawa J, Kobayashi N, Kishi N, Nakazawa T, Uchiyama S, Fukui K (2011) Specific racemization of heavy-chain cysteine-220 in the hinge region of immunoglobulin gamma 1 as a possible cause of degradation during storage. Anal Chem 83(10):3857–3864
Broccardo M, Erspamer V, Falconieri Erspamer G, Improta G, Linari G, Melchiorri P, Montecucchi PC (1981) Pharmacological data on dermorphins, a new class of potent opioid peptides from amphibian skin. Br J Pharmacol 73(3):625–631
Cloos PA, Fledelius C (2000) Collagen fragments in urine derived from bone resorption are highly racemized and isomerized: a biological clock of protein aging with clinical potential. Biochem J 345(Pt 3):473–480
Fujii N, Ishibashi Y, Satoh K, Fujino M, Harada K (1994a) Simultaneous racemization and isomerization at specific aspartic acid residues in alpha B-crystallin from the aged human lens. Biochim Biophys Acta 1204(2):157–163
Fujii N, Satoh K, Harada K, Ishibashi Y (1994b) Simultaneous stereoinversion and isomerization at specific aspartic acid residues in alpha A-crystallin from aged human lens. J Biochem 116:663–669
Fujii N, Harada K, Momose Y, Ishii N, Akaboshi M (1999) d-amino acid formation induced by a chiral field within a human lens protein during aging. Biochem Biophys Res Commun 263(2):322–326
Fujii N, Tajima S, Tanaka N, Fujimoto N, Takata T, Shimo-Oka T (2002) The presence of D-beta-aspartic acid-containing peptides in elastic fibers of sun-damaged skin: a potent marker for ultraviolet-induced skin aging. Biochem Biophys Res Commun 294(5):1047–1051
Fujii N, Shimmyo Y, Sakai M, Sadakane Y, Nakamura T, Morimoto Y, Kinouchi T, Goto Y, Lampi K (2007) Age-related changes of alpha-crystallin aggregate in human lens. Amino Acids 32(1):87–94
Fujii N, Fujii N, Kida M, Kinouchi T (2010) Influence of Lbeta-, Dalpha- and Dbeta-Asp isomers of the Asp-76 residue on the properties of alphaA-crystallin 70–88 peptide. Amino Acids 39(5):1393–1399
Fujii N, Kawaguchi T, Sasaki H, Fujii N (2011) Simultaneous stereoinversion and isomerization at the Asp-4 residue in betaB2-crystallin from the aged human eye lenses. Biochemistry 50(40):8628–8635
Fujii N, Sakaue H, Sasaki H (2012) A rapid, comprehensive liquid chromatography-mass spectrometry (LC-MS)-based survey of the Asp isomers in crystallins from human cataract lenses. J Biol Chem 287(47):39992–40002. doi:10.1074/jbc.M112.399972
Geiger T, Clarke S (1987) Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem 262(2):785–794
Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K (1992) The presence of free d-serine in rat brain. FEBS Lett 296(1):33–36
Hashimoto A, Nishikawa T, Oka T, Takahashi K (1993) Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J Neurochem 60(2):783–786
Helfman PM, Bada JL (1976) Aspartic acid racemisation in dentine as a measure of ageing. Nature 262(5566):279–281
Horwitz J (1992) Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A 89(21):10449–10453
Jolles P (1998) d-amino acids in sequences of secreted peptides of multicellular organisms. Birkhauser Verlag, Basel, Switzerland, Basel, Boston, Berlin
Katane M, Homma* H (2011) d-Aspartate oxidase: the sole catabolic enzyme acting on free d-Aspartate in mammals. In: d-Amino acids in chemistry, life sciences, and biotechnology. Willey-VHC, Zürich
Masters PM, Bada JL, Zigler JS Jr (1977) Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature 268(5615):71–73
Masuda W, Nouso C, Kitamura C, Terashita M, Noguchi T (2002) d-Aspartic acid in bovine dentine non-collagenous phosphoprotein. Arch Oral Biol 47(11):757–762
Montecucchi PC, de Castiglione R, Piani S, Gozzini L, Erspamer V (1981) Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa sauvagei. Int J Pept Protein Res 17(3):275–283
Powell JT, Vine N, Crossman M (1992) On the accumulation of d-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis 97(2–3):201–208
Ritz S, Turzynski A, Schutz HW, Hollmann A, Rochholz G (1996) Identification of osteocalcin as a permanent aging constituent of the bone matrix: basis for an accurate age at death determination. Forensic Sci Int 77(1–2):13–26
Ritz-Timme S, Laumeier I, Collins MJ (2003) Aspartic acid racemization: evidence for marked longevity of elastin in human skin. Br J Dermatol 149(5):951–959
Roher AE, Lowenson JD, Clarke S, Wolkow C, Wang R, Cotter RJ, Reardon IM, Zurcher-Neely HA, Heinrikson RL, Ball MJ, Greenberg BD (1993) Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J Biol Chem 268(5):3072–3083
Tanaka N, Tanaka R, Tokuhara M, Kunugi S, Lee YF, Hamada D (2008) Amyloid fibril formation and chaperone-like activity of peptides from alphaA-crystallin. Biochemistry 47(9):2961–2967
Young GW, Hoofring SA, Mamula MJ, Doyle HA, Bunick GJ, Hu Y, Aswad DW (2005) Protein L-isoaspartyl methyltransferase catalyzes in vivo racemization of Aspartate-25 in mammalian histone H2B. J Biol Chem 280(28):26094–26098
Zhang J, Yip H, Katta V (2011) Identification of isomerization and racemization of aspartate in the Asp-Asp motifs of a therapeutic protein. Anal Biochem 410(2):234–243
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Fujii, N., Takata, T., Fujii, N., Aki, K., Sakaue, H. (2016). d-Amino Acid Residues in Proteins Related to Aging and Age-Related Diseases and a New Analysis of the Isomers in Proteins. In: Yoshimura, T., Nishikawa, T., Homma, H. (eds) D-Amino Acids. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56077-7_15
Download citation
DOI: https://doi.org/10.1007/978-4-431-56077-7_15
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56075-3
Online ISBN: 978-4-431-56077-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)